Treatment of early-stage HER2+ breast cancer—an evolving field
Arlindo R Ferreira1, 2, Kamal S Saini3 and Otto Metzger-Filho1
1Dana-Farber Cancer Institute, 450 Brookline Ave, Boston MA 02215, USA
2Hospital de Santa Maria and Instituto de Medicina Molecular, Universidade de Lisboa, Av. Prof. Egas Moniz, 1649-035—Lisbon, Portugal
3Institut Jules Bordet, Université Libre de Bruxelles, Boulevard de Waterloo 121, 1000, Brussels, Belgium
Correspondence to: Otto Metzger-Filho. Email: otto_metzger@dfci.harvard.edu
Abstract
The evolving field of HER2-targeted therapy has significantly improved the outcome of women diagnosed with HER2-positive invasive breast cancer. In this review, we sought to summarise the efficacy of trastuzumab-based regimens in the adjuvant and neoadjuvant setting with a special emphasis on relevant clinical questions: treatment duration, sequence of trastuzumab administration, toxicity, the role of anthracycline-based regimens, and optimal management of small HER2+ tumours. Controversial topics are discussed taking into consideration the development of modern anti-HER2 agents.
Keywords: breast neoplasm; trastuzumab; neoadjuvant therapy; adjuvant therapy; antibodies, monoclonal, humanised
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Published: 16/04/2015; Received: 16/01/2015
Background
Breast cancer (BC) is a heterogeneous disease with several biological subtypes. Human epidermal growth factor receptor 2 (HER2) is a strong phenotype determinant present in approximately 20% [1] of all BCs. Directed therapy towards this receptor with trastuzumab, a humanised monoclonal antibody, decisively contributed to a prognostic improvement. This was first demonstrated in the metastatic setting and subsequently in early-stage BC. In this article, the authors review the role of trastuzumab in early-stage BC.
Trastuzumab: clinical efficacy in the adjuvant setting
In the adjuvant setting, anti-HER2-targeted therapy with trastuzumab greatly contributed to improvement of clinical outcomes, as measured by disease-free survival (DFS) and overall survival (OS). Several multicentre international trials were designed to assess the role of trastuzumab treatment in high-risk early-stage BC patients, defined as node positive or node negative with tumours larger than 1 cm [2–5] or 2 cm [6, 7] (largely patients with stage II or stage III BC). The key features of these trials are summarised in Table 1.
Table 1. Summary of results from major trials addressing trastuzumab benefit in the adjuvant treatment of BC.
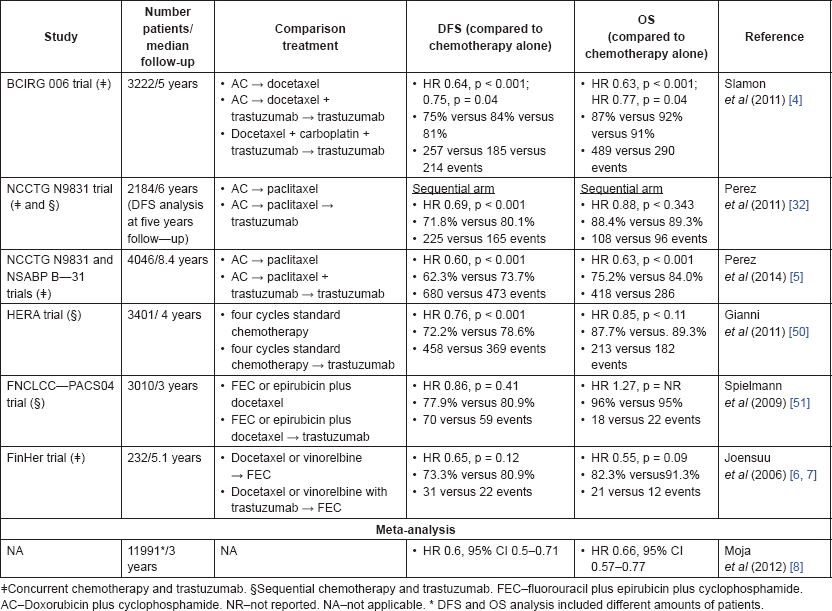
These findings were further scrutinised in a meta-analysis that gathered the results of eight trials (total 11991 patients) testing the benefit of trastuzumab when added to adjuvant chemotherapy versus chemotherapy alone [8]. With a median follow-up of three years, trastuzumabcontaining treatments significantly improved OS (hazard ratio [HR] 0.66, 95% confidence interval (CI) 0.57–0.77, and disease-free survival (DFS) (HR 0.60, 95% CI 0.5–0.71). Despite efficacy improvement, cardiac toxicity was documented in a minority of cases, with trastuzumabtreated patients being more likely to suffer from congestive heart failure (CHF; relative risk [RR] 5.11, 90% CI 3–8.72) and left ventricular ejection fraction (LVEF) decline (RR 1.83, 90% CI 1.36–2.47). Currently, trastuzumab is the only anti-HER2 drug that has demonstrated a survival benefit in the adjuvant setting.
Recently, trastuzumab was also prospectively tested in a cohort of patients mainly with stage I HER2-positive BC [9]. The anticipated small absolute benefit and the expected adverse events led to the exclusion of most of these patients in the pivotal studies of trastuzumab. In this phase II, uncontrolled, single-arm and multicentre study, 406 patients with tumours with less than 3 cm and mostly node negative disease (1.5% patients had N1mic) were treated with adjuvant paclitaxel (80 mg weekly) plus trastuzumab (4 mg/kg loading dose followed for 2 mg/kg weekly) for 12 weeks, followed by trastuzumab (either 2mg/kg weekly or 6 mg/kg every three weeks) for a total of 40 weeks, thus omitting any anthracycline or platinum agent. With a median follow-up of four years, the investigators reported an invasive disease-free survival at three years of 98.7% (95% CI 97.6–99.8). Among the patients with disease recurrence, two (0.4%) had distant events. A 6% withdrawal rate because of adverse events was also reported. The most relevant adverse events included grade 3 neuropathy (3.2%; 95% CI 1.7–5.4), symptomatic but reversible congestive heart failure (0.5%; 95% CI 0.1–1.8) and asymptomatic declines in ejection fraction (3.2%; 95% CI 1.7–5.4). Based on the comparison to historical data, the authors argue that the risk of cancer recurrence and serious toxic events were low.
Finally, population-based studies reported reassuring patient safety outcomes and treatment compliance [10–13].
Trastuzumab: clinical efficacy in the neoadjuvant setting
Neoadjuvant therapy is the standard approach for treating locally advanced and inflammatory BCs; however, it can be also considered an equally valid strategy for the treatment of early-stage BCs [14]. In HER2-positive BCs, a pathologic complete response (pCR) rate of around 31–50% (for hormone receptor-positive and negative respectively) is expected [15]. In these cases, pCR is a prognostic marker of favourable long-term outcomes, as measured by event free survival (EFS; HR 0.39; 95% CI 0.31–0.50) and OS (HR 0.34; 95% CI 0.24–0.47) [15].
In the neoadjuvant setting, trastuzumab has been tested in combination with chemotherapy and other anti-HER2 agents (Table 2). The ‘first generation’ trials addressed the efficacy of trastuzumab plus chemotherapy when compared to chemotherapy alone. The MD Anderson study [16] was a single centre, phase III trial that randomly assigned 42 of 164 planned patients with stage II–IIIA invasive but non-inflammatory BC to either paclitaxel followed by fluorouracil, epirubicin, and cyclophosphamide (FEC) with or without trastuzumab. The primary endpoint was pCR rate. The data and safety monitoring board stopped this study early given the significant differences in clinical response between arms favouring the use of trastuzumab: pCR rate of 65.2% (95% CI, 43–84%) versus 26.3% (95% CI, 9–51%), p = 0.016. At a median follow-up of three years, no DFS events were recorded in the trastuzumab arm, while in the arm without trastuzumab 94.7% (95% CI, 85.2–100%) were DFS-free at one year and 85.3% (95% CI, 67.6–100%) were DFS-free at three years (p = 0.041) (17). No patients receiving chemotherapy plus trastuzumab developed clinical cardiac dysfunction or cardiac related deaths. The NOAH study [18] provided further insight into the efficacy of trastuzumab. This multicentre, open-label, phase III trial randomly assigned 235 patients with locally advanced or inflammatory BC to either neoadjuvant trastuzumab plus a chemotherapy regimen based on doxorubicin, paclitaxel, cyclophosphamide, methotrexate, and fluorouracil followed by adjuvant trastuzumab, or neoadjuvant chemotherapy only. It is noteworthy that patients allocated to chemotherapy alone later received one year of adjuvant trastuzumab after the final positive results of the trastuzumab arm. With a median follow-up of three years, the neoadjuvant trastuzumab arm demonstrated a 41% event-free survival (EFS) improvement (HR 0.59, 95% CI 0.38–0.90; p = 0.013) for primary endpoint. The pCR rate was also higher in the trastuzumab arm (38% versus 19%, p = 0.001). Long term results with a median follow-up of 5.4 years confirmed the previous EFS results (46% improvement; 95% CI 7–56%) and reported an 41% improvement in five-years BC specific survival (BCSS; HR 0.59, p = 0.023) [19]. Finally, the GeparQuattro [20] was a prospective study that enrolled 1509 patients with locally advanced BC, from which 451 HER2-positive and 1058 HER2-negative, to receive epirubicin and cyclophosphamide (EC) and then be randomly assigned to either docetaxel, docetaxel plus capecitabine, or docetaxel followed by capecitabine. Those patients with HER2-positive BC further received concomitant trastuzumab for a total period of one year. A pCR (primary endpoint) rate of 31.7% was observed in those patients receiving trastuzumab (with HER2-positive disease). In comparison, only 15.7% of the patients achieved a pCR in the reference group of HER2-negative patients. These trials supported the current use of trastuzumab in association with chemotherapy in the neoadjuvant setting.
Table 2. Summary of results from major trials addressing trastuzumab benefit in the neoadjuvant treatment of BC.
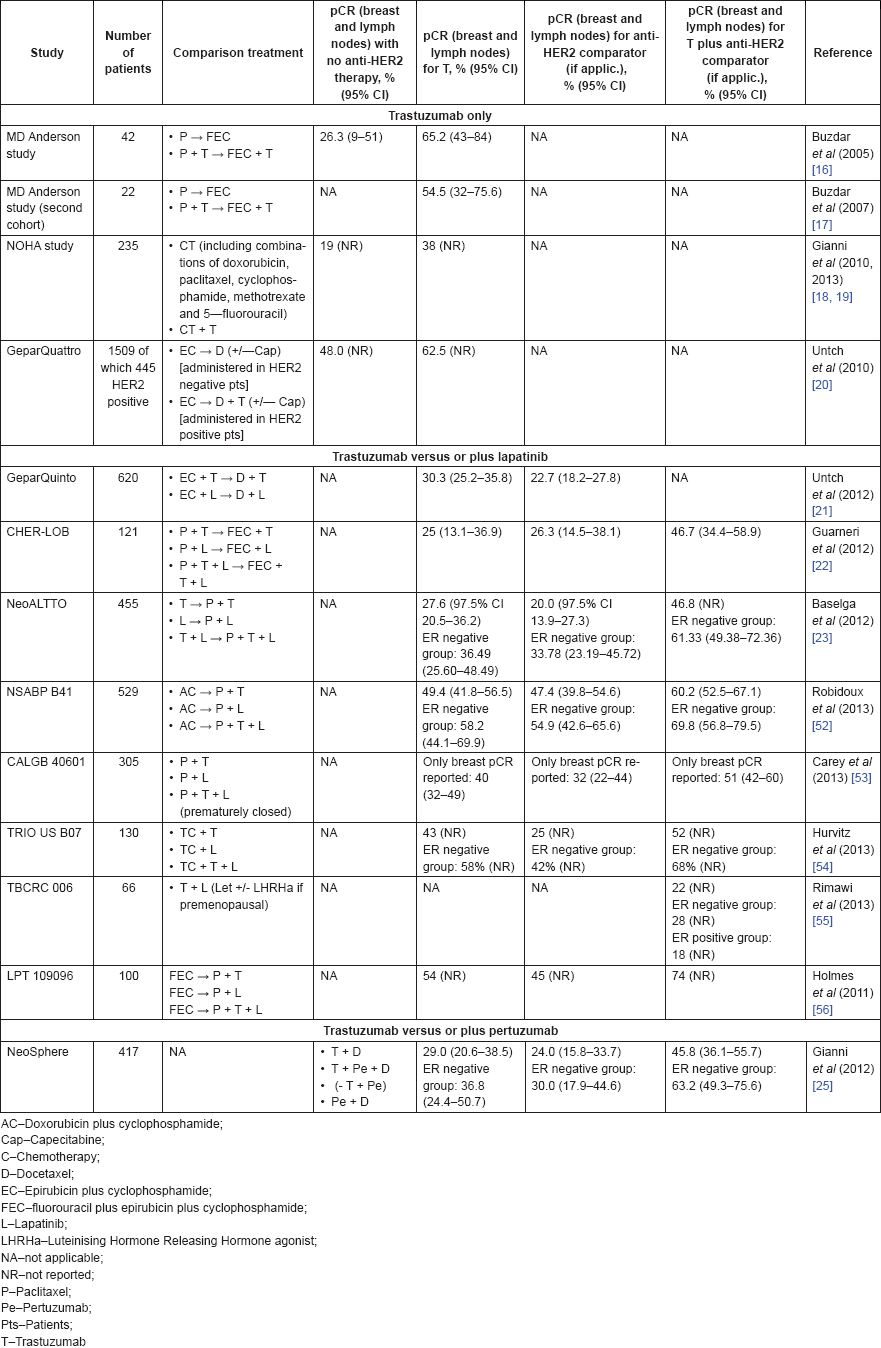
Subsequently, the ‘second generation’ trials compared trastuzumab to other anti-HER2 agents or the added benefit of other anti-HER2 agents to trastuzumab plus chemotherapy. The GeparQuinto trial [21] performed a head-to-head comparison between trastuzumab and lapatinib. This was a multicentre, open-label, phase III trial that randomly assigned 620 patients with locally advanced BC to epirubicin plus cyclophosphamide and docetaxel with either trastuzumab or lapatinib. The primary endpoint was the pCR rate, which was of 30.3% in the trastuzumab arm, and of 22.7% in the lapatinib arm (OR 0.68, 95% CI 0.47–0.97; p = 0.04) revealing a higher activity of trastuzumab. Lapatinib was also tested in association with trastuzumab. The CHER-LOB study [22] was a phase II trial that randomised 121 stage II to IIIA operable BC patients to trastuzumab, lapatinib, or both. The primary endpoint was the rate of pCR, which was superior in the combination arm (46.7%, 90% CI 34.4–58.9%). The trastuzumab only arm had a pCR of 25% (90% CI 13.1–36.9%) and the lapatinib only arm 23.6% (90% 14.5–38.1%). The association lapatinib-trastuzumab was further tested in the NeoALTTO study [23], a multicentre, open-label, phase III trial that randomly assigned 455 patients with tumours greater than 2 cm to either lapatinib, trastuzumab, or both. The primary endpoint was pCR rate, which was higher in the group receiving combination therapy (51.3%, 95% CI 43.1–59.5; versus 29·5%, 95% CI 22.4–37.5 in the trastuzumab arm; p = 0·0001 for the difference). No significant difference was documented between the trastuzumab and lapatinib arms (lapatinib pCR of 24.7%, 95% CI 18.1–32.3; p = 0.34 for the difference). The authors concluded that the dual inhibition with trastuzumab and lapatinib might be a valid option in the neoadjuvant setting. Of note, less impressive findings were subsequently documented in the ALTTO study that tested the adjuvant use of the same combination of trastuzumab plus lapatinib in the adjuvant setting (discussed ahead) [24]. Other studies testing this combination are summarised in Table 2.
Besides lapatinib, trastuzumab was also tested in combination with pertuzumab. The NeoSphere trial [25] was a multicentre, open-label, phase II trial that randomly assigned 417 patients with early, locally advanced, or inflammatory BC to four therapeutic groups: trastuzumab plus docetaxel (group A), pertuzumab and trastuzumab plus docetaxel (group B), pertuzumab and trastuzumab (group C), and pertuzumab plus docetaxel (group D). After surgery, patients who received docetaxel, further received FEC, and then completed trastuzumab, while the group C received docetaxel, FEC, and afterwards completed one year of trastuzumab. The primary endpoint was pCR (in the breast). The trial revealed that patients treated with docetaxel, trastuzumab, and pertuzumab (group B) had a more favourable pCR rate (45.8%, 95% CI 36.1–55.7) when compared with those treated with docetaxel plus trastuzumab (group A, 29.0%, 95% CI 20.6–38.5; p = 0·014 for the difference). Groups C and D had a pCR of 16.8% (95% CI 10.3–25.3) and 24.0% (15.8–33.7), respectively. In this study, the combination of trastuzumab to pertuzumab did not increase cardiac toxicity. Besides showing that the combination of two anti-HER2 agents (trastuzumab and pertuzumab) improves the rates of pCR, it was also noteworthy that an arm with dual HER2 blockage (trastuzumab + pertuzumab; group C) is per se effective in achieving pCR in a subset of patients without any additional chemotherapy.
Optimal duration of therapy
Only recently was the treatment duration of trastuzumab more clearly established. The pivotal trials studying the role of trastuzumab in early BC used an empirical reference therapy duration of one year [2–4]. However, the potential for improved efficacy versus the need for reduced toxicity led to an exploration of different treatment durations.
The FinHER trial [6] tested a shorter duration of adjuvant trastuzumab in a subset of early BC patients with HER2-positive high-risk cancers (axillary-node-positive or node-negative with breast tumour mass ≥2 cm). In this multicentre, open-label, phase III trial, 1010 patients were randomly assigned to adjuvant docetaxel or vinorelbine followed by FEC with or without nine cycles of weekly trastuzumab in the subset of HER2-positive breast cancer (232 patients, 22.97%). The primary endpoint was recurrence-free survival (RFS). At a median follow-up of 37 months, trastuzumab was an effective therapy (RFS HR 0.41, 95% CI 0.21–0.83, p = 0.01; OS HR 0.41, 95% CI 0.16–1.08, p = 0.07). None of the women exposed to trastuzumab had heart failure. The documented clinical improvement with the addition of trastuzumab was similar to those trials with standard one-year therapy [2, 3], and with a potential improvement in the toxicity profile.
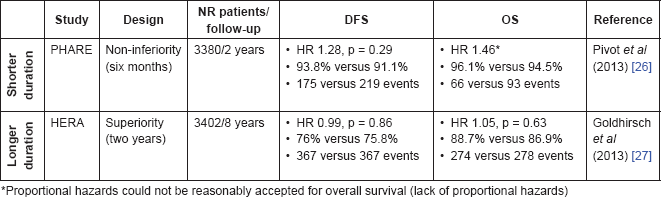
Moreover, the phase III study PHARE [26] randomly assigned 1693 HER2-positive high-risk patients (axillary-disease positive) that had already received at least four cycles of chemotherapy and six months of trastuzumab to continue trastuzumab for an additional period of six months or to discontinue trastuzumab. This was a non-inferiority trial with DFS as primary outcome. At a median follow-up of three-and-onehalf years, six months of trastuzumab could not demonstrate non-inferiority (DFS HR 1.28, 95% CI 1.05–1.56, p for non-inferiority = 0.29) (Table 3). Furthermore, fewer patients in the 12-month arm had distant recurrences as first DFS event (108 versus 141 events; HR 1.33, 95% CI 1.04–1.71). These unfavourable results were also observed for OS (66 versus 93 events; HR 1.46, 95% CI 1.06–2.01), despite the need for additional accumulation of events. The trial had preplanned sub-group analyses by hormone-receptor status and timing of administration of chemotherapy and trastuzumab (sequential or concomitant). The ER-negative cancers treated with sequential chemotherapy and trastuzumab had the worst two year DFS (89.8%, 95% CI 85.8–92.7 versus 84.5%, 95% CI 80.0–88.1), which the authors argue contributed more decisively to the unfavourable results of six months of trastuzumab. Concerning safety, similar rates of serious adverse events were documented; however early stopping of trastuzumab because of cardiac toxicity was more common in the 12-month arm (103 events or 6.1% versus 32 events or 1.9%). Finally, other studies under development are further testing shorter treatment regimens of adjuvant trastuzumab. Comparing 6 to 12 months: the Hellenic group trial (NCT00615602; enrolled 489 patients) and the Persephone study (NCT00712140; plans to enrol 4000 patients); while comparing 3 to 12 months: the SOLD study (NCT00593697; enrolled 2168 patients) and Short-Her study (NCT00629278; plans to enrol 2500 patients).
In search of improved efficacy, the HERA trial, an international, multicentre, open-label phase III trial, besides comparing one year of adjuvant trastuzumab with observation after standard adjuvant chemotherapy, also enrolled patients to a third arm treated with two years of trastuzumab [27]. This trial was designed to detect DFS superiority of the two-year treatment arm and included patients without evidence of disease at 12 months after randomisation (landmark analysis). At a median follow-up of eight years, the results showed no significant difference between groups in terms of DFS (HR 0.99, 95% CI 0.85–1.14, p = 0.86) or OS (HR 1.05, 95% CI 0.86–1.28, p = 0.63) (Table 3). The results were further explored in preplanned secondary analysis by hormone receptor status, without any difference between subgroups. Interestingly, the hormone-receptor-negative subgroup receiving two years of trastuzumab had a transient but not statistically significant improvement in DFS. The authors propose that this may represent a short-term augmented risk of relapse during a period when no adjuvant therapy is given to these patients (no hormone, nor trastuzumab therapy). The authors argue that this finding is specially supported by the fact that the cohort of hormone-receptor-negative patients receiving a second year of trastuzumab closely resembles that of the hormone-receptor-positive groups. Regarding safety, severe symptomatic cardiac endpoints (CHF NYHA III-IV with a LVEF decline ≥10% from baseline and to an absolute LVEF <50%, or cardiac death) were similar between both arms (14 events or 0.8% versus 16 events or 1%). On the other hand, asymptomatic or mildly symptomatic cardiac endpoints (CHF NYHA I-II with a LVEF decline ≥10% from baseline and to an absolute LVEF <50%) were more common in the two-year arm (69 events or 4.1% versus 120 events or 7.2%). Based on these results the authors concluded that two years of adjuvant trastuzumab has an unfavourable risk-benefit ratio.
Grounded in the current evidence, one year of adjuvant trastuzumab remains the standard treatment duration.
Table 3. Optimal treatment duration, results compared to one year standard therapy.
Anthracycline use and treatment sequence
Anthracycline-based chemotherapy regimens were used in the majority of the trials evaluating trastuzumab in the adjuvant setting (Table 1). As previously discussed, cardiotoxicity, manifested as CHF or asymptomatic LVEF decline, was identified as a source of concern with adjuvant trastuzumab therapy, which is aggravated with the use of anthracycline-based chemotherapy regimens [28–30]. With the rationale of enhancing cardiac safety of HER2-positive BC adjuvant therapy, the BCIRG-006 study [4] tested the efficacy of an anthracycline-free chemotherapy regimen. This international, multicentre, open-label, phase III trial randomised 3222 patients with HER2-positive high-risk T1–T3 breast cancer to receive a standard adjuvant anthracycline-taxane containing chemotherapy regimen (AC-T), the same chemotherapy regimen plus trastuzumab (AC-TH), or an anthracycline-free chemotherapy regimen containing docetaxel, carboplatin, and trastuzumab (TCH). In this trial, high risk was defined as node positive or node negative with high-risk features (hormone receptor negative, histologic grade 2–3, tumour >2 cm, and age at diagnosis <35 years). The primary endpoint was DFS. With a median follow-up of approximately five years, AC-T, AC-TH, and TCH arms had an estimated DFS of 75%, 84%, and 81%, respectively; the HR for DFS comparing AC-T to AC-TH and AC-T to TCH were 0.64 and 0.63, respectively (both p < 0.001). The AC-T, AC-TH, and TCH arms had an OS of 87%, 92%, and 91%, respectively; the HR for OS comparing AC-T to AC-TH and AC-T to TCH were 0.63 (p < 0.001) and 0.77 (p = 0.04), respectively. The numerical differences in OS and DFS between AC-TH and TCH were statistically non-significant. Concerning safety, the occurrence of CHF was more common in the group exposed to AC-TH (2.0%) than in AC-T (0.7%) or TCH (0.4%). This difference was also evident for asymptomatic decline in LVEF (>10% drop). The group receiving AC-TH had the highest incidence of LVEF decline (18.6%) when compared to AC-T (11.2%) and TCH (9.4%). Differences in CHF and LVEF decline between in AC-TH and TCH groups were statistically different (p < 0.001). Neutropaenia and leukopaenia were also more frequent in the AC-TH arm (without an impact on febrile neutropaenia), while thrombocytopaenia and anaemia were more frequent in the TCH arm. Sensory and motor neuropathy was more frequent in the AC-TH arm. Grounded on these results, the authors highlighted potential advantages of the TCH approach over AC-TH: similar efficacy between the two regimens (while recognising that the study was not powered for this comparison), reduced CHF and haematologic toxicity (myelodysplasia and acute leukaemia), and shorter adjuvant treatment duration (12 versus 16 weeks). This evidence was sufficient for the authors to conclude ‘the risk-benefit ratio favoured the non-anthracycline TCH regimen over AC-T plus trastuzumab’. Others [31] disagree with this conclusion, based mainly on lack of power of BCIRG-006 trial to demonstrate equivalence or non-inferiority between the two chemotherapy regimens; the absence of other prospective studies demonstrating the same conclusion; and the uncertain benefit from carboplatin when compared to anthracyclinetaxane based chemotherapy in HER2-positive BC.
Whereas some of the pivotal trials opted to give trastuzumab after chemotherapy (sequential approach), others preferred to administer trastuzumab concurrent with paclitaxel (Table 1). The NCCTG N9831 study design evaluated the sequential versus concurrent approach [32]. In this multicentre, open-label, phase III study, 2448 patients received adjuvant doxorubicin and cyclophosphamide every three weeks for four cycles, and they were then randomly assigned to receive one of three options: weekly paclitaxel for 12 weeks (arm A), weekly paclitaxel plus sequential weekly trastuzumab for 52 weeks (arm B), or weekly paclitaxel plus concurrent trastuzumab for 12 weeks followed by weekly trastuzumab for 40 weeks (arm C). The primary endpoint was DFS. After a median follow-up of six years and when comparing arm B (n = 954) to C (n = 949), those receiving concurrent trastuzumab (arm C) presented a strong trend towards a reduction in the risk of DFS (HR 0.77, 99.9% CI 0.53–1.11; p-value 0.0216; the p-value superior to the prespecified boundary of 0.00116 for interim analysis).
Finally, some studies also assessed the sequence of chemotherapies (i.e. anthracycline and taxane) given in combination with trastuzumab. A randomised phase III neoadjuvant clinical trial [33] tested anthracycline followed by taxane or the reverse order, concomitantly with trastuzumab. A total of 282 patients were randomly assigned (1:1 ratio) to four cycles of FEC followed by weekly paclitaxel plus trastuzumab or the reverse sequence, with continued trastuzumab during FEC. No significant differences in pCR in the breast (primary outcome) were noted (56.5% versus 54.2% in the reverse sequence/concurrent trastuzumab arm). Grade 3–4 neutropaenia, LVEF decline, fatigue, and neurosensory disability were more frequent in reverse sequence arm.
Formulation differences
The studies leading to the approval of trastuzumab used an intravenous (IV) formulation of the drug. While IV trastuzumab has already been shown to be a cost-effective therapy [34], its administration still requires a considerable amount of time, as the establishment of an IV line has its implications on hospitals’ logistics, finances, and patients’ quality of life. These obstacles opened the opportunity for newer formulations. In the Hannah study [35] a newer subcutaneous (SC) formulation was explored. In this phase III non-inferiority trial that included 596 BC patients eligible for neoadjuvant therapy, an IV formulation (loading dose of 8 mg/kg over 90 minutes followed by 6 mg/kg over 30–90 minutes) was compared to a SC formulation (600 mg fixed dose administered in the thigh for over about five minutes), both every three weeks and in combination with docetaxel times four followed by FEC times four (1:1 ratio between arms). Following surgery both anti-HER2 agents were continued till the completion of one year. The primary outcome was pCR and serum trough concentration at a pre-dose cycle eight before surgery. A pCR rate of 40.7% versus 45.4% was obtained in the IV versus SC formulations (95% CI for the difference -4.0 to 13.4). Moreover, a non-inferior serum trough concentration at pre-dose cycle eight before surgery was also obtained (69.0 μg/mL versus 51.8 μg/mL in the IV group; geometric mean ratio of C (trough) 1.33, 90% CI 1.24–1.44). Hence, SC trastuzumab was considered non-inferior to the IV formulation after meeting both co-primary endpoints. However, a higher rate of patients in the SC arm had serious adverse events (SAE; 21% versus 12% in the IV arm), including febrile neutropaenia (5.7% versus 3.4% in the IV arm) and infections (8.1% versus 4.4% in the IV arm). Four of these SAEs led to death, three of which in the SC arm and of which two considered being treatment related. Results from this trial led to regulatory approval of the SC formulation by the European Medicines Agency. More recently, the PrefHer study revealed that 91% of the patients’ preferred the SC formulation [36].
Early predictors of response to trastuzumab or other anti-HER2 therapies
HER2 overexpression remains the only validated predictive marker of response to anti-HER2 therapy, even though the response to anti-HER2 directed therapies based on this marker is not homogeneous. The development of markers that would allow selecting patients with the highest likelihood to respond would be of much benefit to maximise benefit and decrease harm.
In the neoadjuvant setting, 18F-FDG positron emission tomography (PET)/computed tomography (CT) imaging is being tested as a predictor of the likelihood of obtaining a pCR. The NeoALTTO trial [23] tested the predictive value of 18F-FDG PET/CT to identify those patients with an increased likelihood of obtaining a pCR [37]. This trial had a key design feature consisting of a six week ‘biologic window period’, during which only anti-HER2 drugs were provided. Only after this period could a chemotherapy begin (paclitaxel). During this six-week interval three 18F-FDG PET/CT were performed: at baseline, two and six weeks. From a sample of 86 patients, a total of 62 completed the three evaluations. After two weeks of anti-HER2 therapy metabolic response was evident in the tumour and the degree of response at two weeks correlated strongly with the metabolic response at six weeks (R2 0.81). PET/CT responders had a two-fold likelihood of achieving a pCR (week two: 42% versus 21%, p = 0.12; week six: 44% versus 19%, p = 0.05). PET/CT may therefore be a useful non-invasive marker of anti-HER2 response. These results are being further explored in a subset of patients in the ongoing NeoPHOEBE study (NCT01816594), a phase II study evaluating the role of a new oral PI3K inhibitor in the neoadjuvant treatment of BC.
In the metastatic setting, HER2 molecular imaging using 89Zirconium-labeled trastuzumab is being tested as a noninvasive whole-body imaging technique to determine tumour HER2 expression status and the localisation of tumour lesions HER2-positive, especially those inaccessible to biopsy [38], The IJBMNZrT003 trial (NCT01420146) is a phase I trial testing this diagnostic potential of HER2-positive tumours using 89Zirconium-labeled trastuzumab.
Other anti-HER2 therapies
Despite trastuzumab efficacy in the treatment of HER2-positive BC, tumour relapse and resistance to therapy is common [39]; hence, other agents targeting HER2 receptor were developed.
Lapatinib is a small molecule that operates as a dual tyrosine kinase inhibitor blocking HER2 and epidermal growth factor receptor (EGFR). Lapatinib is currently not indicated in the treatment of HER2-positive BC in the adjuvant setting either as a monotherapy or in combination with trastuzumab. The TEACH investigators addressed the role of adjuvant lapatinib as a monotherapy in a randomised, placebo-controlled, phase III, and multinational trial that assigned 3161 women to lapatinib or placebo (1:1 ratio). With a median follow-up of almost four years, only a non-statistically significant trend towards lapatinib was found in terms of DFS (HR 0.83, 95% CI 0.70–1.00, p = 0.053; primary endpoint). However, no difference in terms of OS was observed (HR 0.99, 95% CI 0.74–1.31, p = 0.96). Lapatinib was also tested in association with trastuzumab in non-metastatic BC in the ALTTO study, a randomised open-label phase III trial [24]. As defined by the study group [40], the study has three study designs: ‘In Design 1, all (neo)adjuvant chemotherapy is completed prior to administration of the study treatments. In Design 2, all anthracycline-based (neo)adjuvant chemotherapy is completed prior to administration of the study treatments, while taxane is given concurrently with the study treatments. In Design 2B, a non-anthracycline regimen containing docetaxel and carboplatin is given concurrently with study treatments’. Within each of the design options patients were randomised to receive one of the following treatments: trastuzumab alone; lapatinib alone (until August 2011); trastuzumab followed by lapatinib; or lapatinib in combination with trastuzumab. The primary endpoint was DFS. An interim analysis after a median follow-up of four-and-one-half years and 555 DFS events revealed a non-significant trend towards improved DFS in the combination arm (HR 0.84, 97.5% CI 0.70–1.02; P = 0.048; four years DFS 88% versus 86%). This result contrasted with the significant improvement in terms of pCR of the combination therapy in the NeoALTTO study [23]. In light of the ALTTO study, there is no current support for the use of lapatinib in the adjuvant setting.
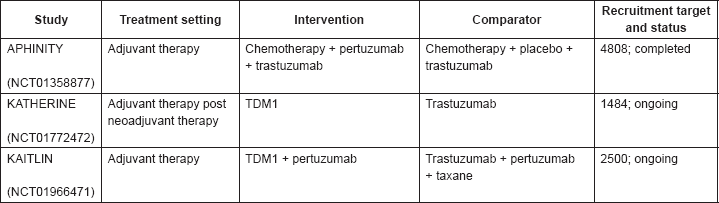
Pertuzumab is a recombinant humanised monoclonal antibody that targets the extracellular subdomain II of HER2 blocking receptor dimerisation (Table 4) [41]. Its mechanism of action is considered complementary to that of trastuzumab [42]. The CLEOPATRA trial [43] compared the progression-free-survival (PFS) between the combination of pertuzumab plus trastuzumab plus docetaxel and the combination of placebo plus trastuzumab plus docetaxel in 808 patients with HER2-positive metastatic BC. In the updated results from ESMO 2014 at a median follow- up of 50 months, this randomised, phase III trial demonstrated a benefit from pertuzumab when compared to the placebo (OS HR 0.68, 95% CI 0.56–0.84, p < 0.001; 56.6 months versus 40.8 months respectively) without increased cardiac toxicity. The PERUSE (NCT01572038) and VELVET (NCT01565083) studies are currently testing pertuzumab in metastatic BC in association with other chemotherapy regimens. In the neoadjuvant setting two phase II trials, the NeoSphere [25] (previously discussed) and TRYPHAENA trials [44], showed promising results in the pertuzumab plus trastuzumab plus docetaxel association when compared to the same schema without pertuzumab. Given the positive results of pertuzumab in association with trastuzumab in the metastatic and neoadjuvant setting, this combination is being further explored in the adjuvant setting. The APHINITY trial (NCT01358877) is a phase III, multinational, multicentre, double-blind, placebo-controlled trial comparing the efficacy and safety of chemotherapy plus trastuzumab and pertuzumab with that of chemotherapy plus trastuzumab and placebo as adjuvant therapy in patients with primary HER2-positive BC. The trial has completed recruitment (n = 4805) in August 2013. The primary endpoint is invasive DFS (IDFS).
Trastuzumab emtansine (T-DM1) is a conjugate between trastuzumab antibody and emtansine (DM1), a cytotoxic drug (Table 4) [45]. In the metastatic setting, the EMILIA trial compared the combination of lapatinib plus capecitabine versus T-DM1 in 991 patients with HER2-positive BC previously treated with trastuzumab and a taxane in terms of PFS. This randomised, phase III trial demonstrated an improvement from the T-DM1 arm when compared to lapatinib plus capecitabine, not only in terms of PFS (9.6 months versus 6.4 months, respectively; HR 0.65, 95% CI 0.55–0.77, p < 0.001) but also OS (30.9 months versus 25.1 months, respectively; HR 0.68, 95% CI 0.5–0.85, p < 0.001) in patients previously treated with trastuzumab. In the adjuvant setting, the KATHERINE trial (NCT01772472, started recruiting in January 2013) compares the DFS (primary endpoint) between T-DM1 versus trastuzumab as adjuvant therapy in patients with HER2-positive BC who have residual disease in the breast or axillary lymph nodes after preoperative therapy. The estimated recruitment target is 1484 patients (1:1 ratio). Also in the adjuvant setting, the KAITLIN study (NCT01966471, started recruiting in January 2014) is comparing T-DM1 plus pertuzumab to trastuzumab plus pertuzumab plus a taxane, both followed by an anthracycline. The estimated recruitment target is 2500 patients (1:1 ratio). Of note, the comparator arm (trastuzumab plus pertuzumab plus a taxane) is not a standard of care and is being currently tested in the APHINITY trial.
Table 4. Summary of pertuzumab and trastuzumab emtansine (T-DM1) (neo)adjuvant phase III studies.
Trastuzumab plus other anti-HER2 therapies (without chemotherapy)
As previously discussed, trastuzumab significantly enhanced the clinical outcomes in patients with ‘high risk’ HER2-positive BCs, i.e. tumours larger than 1 cm [2–4] or 2 cm [6]. Historically, tumours of less than 1 cm size (T1a-b) were considered to be ‘low risk’ tumours and often considered not to benefit from anti-HER2 directed therapy. However, some retrospective studies [46–48] and a prospective phase II study questioned this ‘low risk’ assumption [9]. Yet, standard trastuzumab plus chemotherapy regimens have well-documented toxicities, thus making the prospect of regimens containing anti-HER2 therapies with less intense chemotherapy regimens, even if applicable only in a minority of the BC patient population [49].
The NeoSphere trial [25] (previously discussed) randomly assigned patients with early, locally advanced, and inflammatory BC to trastuzumab plus docetaxel or pertuzumab and trastuzumab plus docetaxel or pertuzumab and trastuzumab or pertuzumab plus docetaxel. The pCR rates between arms were 29%, 45.8%, 16.8%, and 24%, respectively. It was promising to note the low but significant proportion of patients receiving only trastuzumab plus pertuzumab without chemotherapy that achieved a pCR (16.8%), which raised the possibility that a small minority of patients don’t benefit from chemotherapy when receiving trastuzumab plus pertuzumab. This observation was especially valid for ER-negative patients, who achieved a pCR in 29% of the cases. Other predictive biomarkers of response could help selecting those patients that do not benefit from chemotherapy.
Conclusion and future perspectives
HER2 blockade has brought significant clinical benefit with acceptable toxicity, leading to a paradigm shift in the management of this population with hitherto poor prognosis. The preliminary data from combining new anti-HER2 therapies with trastuzumab are exciting, and several large studies are underway to validate these in the neoadjuvant and adjuvant settings.
Conflict of interest
The authors declare no conflicts of interest.
References
1. Owens MA, Horten BC and Da Silva MM (2004) HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues Clin Breast Cancer 5(1) 63–9 DOI: 10.3816/CBC.2004.n.011 PMID: 15140287
2. Romond EH et al (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer N Engl J Med 353(16) 1673–84 DOI: 10.1056/NEJMoa052122 PMID: 16236738
3. Piccart-Gebhart MJ et al (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer N Engl J Med 353(16) 1659–72 DOI: 10.1056/NEJMoa052306 PMID: 16236737
4. Slamon D et al (2011) Adjuvant trastuzumab in HER2-positive breast cancer N Engl J Med 365(14) 1273–83 DOI: 10.1056/NEJMoa0910383 PMID: 21991949 PMCID: 3268553
5. Perez EA et al (2014) Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2–positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831 J Clin Oncol 32(33) 3744–52 DOI: 10.1200/JCO.2014.55.5730 PMID: 25332249 PMCID: 4226805
6. Joensuu H et al (2006) Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer N Engl J Med 354(8) 809–20 DOI: 10.1056/NEJMoa053028 PMID: 16495393
7. Joensuu H et al (2009) Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer trial J Clin Oncol 27(34) 5685–92 DOI: 10.1200/JCO.2008.21.4577 PMID: 19884557
8. Moja L et al (2012) Trastuzumab containing regimens for early breast cancer Cochrane Database Syst Rev 4 Cd006243 DOI: 10.1002/14651858.CD006243.pub2 PMID: 22513938
9. Tolaney SM et al (2015) Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer N Engl J Med 372(2) 134–41 DOI: 10.1056/NEJMoa1406281 PMID: 25564897 PMCID: 4313867
10. Campiglio M et al (2013) Effect of adjuvant trastuzumab treatment in conventional clinical setting: an observational retrospective multicenter Italian study Breast Cancer Res Treat 141(1) 101–10 DOI: 10.1007/s10549-013-2658-z PMID: 23942848 PMCID: 3758836
11. Tripathy D et al (2013) First-line treatment patterns and clinical outcomes in patients with HER2-positive and hormone receptor-positive metastatic breast cancer from registHER Oncologist 18(5) 501–10 DOI: 10.1634/theoncologist.2012-0414 PMID: 23652380 PMCID: 3662840
12. Vaz-Luis I et al (2014) Duration and toxicity of adjuvant trastuzumab in older patients with early-stage breast cancer: a population-based study J Clin Oncol 32(9) 927–34 DOI: 10.1200/JCO.2013.51.1261 PMID: 24516021 PMCID: 3948095
13. Adamo V et al (2014) The risk of toxicities from trastuzumab, alone or in combination, in an elderly breast cancer population Oncology 86(1) 16–21 DOI: 10.1159/000353450
14. Mauri D, Pavlidis N and Ioannidis JPA (2005) Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis J Natl Cancer Inst 97(3) 188–94 DOI: 10.1093/jnci/dji021 PMID: 15687361
15. Cortazar P et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis Lancet 384(9938) 164–72 DOI: 10.1016/S0140-6736(13)62422-8 PMID: 24529560
16. Buzdar AU et al (2005) Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2–positive operable breast cancer J Clin Oncol 23(16) 3676–85 DOI: 10.1200/JCO.2005.07.032 PMID: 15738535
17. Buzdar AU et al (2007) Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2–positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen Clin Cancer Res 13(1) 228–33 DOI: 10.1158/1078-0432.CCR-06-1345 PMID: 17200359
18. Gianni L et al (2010) Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort Lancet 375(9712) 377–84 DOI: 10.1016/S0140-6736(09)61964-4 PMID: 20113825
19. Gianni L et al (2014) Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort Lancet Oncol 15(6) 640–7 DOI: 10.1016/S1470-2045(14)70080-4 PMID: 24657003
20. Untch M et al (2010) Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study J Clin Oncol 28(12) 2024–31 DOI: 10.1200/JCO.2009.23.8451 PMID: 20308670
21. Untch M et al (2012) Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial Lancet Oncol 13(2) 135–44 DOI: 10.1016/S1470-2045(11)70397-7 PMID: 22257523
22. Guarneri V et al (2012) Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2–positive operable breast cancer: Results of the randomized phase II CHER-LOB Study J Clinical Oncol 30(16) 1989–95 DOI: 10.1200/JCO.2011.39.0823
23. Baselga J et al (2012) Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial Lancet 379(9816) 633–40 DOI: 10.1016/S0140-6736(11)61847-3 PMID: 22257673
24. Piccart-Gebhart MJ et al (2014) First results from the phase III ALTTO trial (BIG 2-06; NCCTG [Alliance] N063D) comparing one year of anti-HER2 therapy with lapatinib alone (L), trastuzumab alone (T), their sequence (T→L), or their combination (T+L) in the adjuvant treatment of HER2-positive early breast cancer (EBC) ASCO Annual Meeting Proceedings
25. Gianni L et al (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial Lancet Oncol 13(1) 25–32 DOI: 10.1016/S1470-2045(11)70336-9
26. Pivot X et al (2013) 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial Lancet Oncol 14(8) 741–8 DOI: 10.1016/S1470-2045(13)70225-0 PMID: 23764181
27. Goldhirsch A et al (2013) 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial Lancet 382(9897) 1021–8 DOI: 10.1016/S0140-6736(13)61094-6 PMID: 23871490
28. Perez EA et al (2008) Cardiac safety snalysis of doxorubicin and cyclophosphamide Followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 Adjuvant Breast Cancer Trial J Clin Oncol 26(8) 1231–8 DOI: 10.1200/JCO.2007.13.5467 PMID: 18250349 PMCID: 4048960
29. Tan-Chiu E et al (2005) Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2–overexpressing breast cancer: NSABP B-31 J Clin Oncol 23(31) 7811–9 DOI: 10.1200/JCO.2005.02.4091 PMID: 16258083
30. Procter M et al (2010) Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial J Clin Oncol 28(21) 3422–8 DOI: 10.1200/JCO.2009.26.0463 PMID: 20530280
31. Burstein HJ et al (2012) Choosing the best trastuzumab-based adjuvant chemotherapy regimen: should we abandon anthracyclines? J Clin Oncol 30(18) 2179–82 DOI: 10.1200/JCO.2012.42.0695 PMID: 22614986
32. Perez EA et al (2011) Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer J Clin Oncol 29(34) 4491–7 DOI: 10.1200/JCO.2011.36.7045 PMID: 22042958 PMCID: 3236650
33. Buzdar AU et al (2013) Fluorouracil, epirubicin, and cyclophosphamide (FEC-75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by FEC-75 plus trastuzumab as neoadjuvant treatment for patients with HER2-positive breast cancer (Z1041): a randomised, controlled, phase 3 trial Lancet Oncol 14(13) 1317–25 DOI: 10.1016/S1470-2045(13)70502-3 PMID: 24239210 PMCID: 4176878
34. Liberato NL, Marchetti M and Barosi G (2007) Cost effectiveness of adjuvant trastuzumab in human epidermal growth factor receptor 2–positive breast cancer J Clin Oncol 25(6) 625–33 DOI: 10.1200/JCO.2006.06.4220 PMID: 17308267
35. Ismael G et al (2012) Subcutaneous versus intravenous administration of (neo) adjuvant trastuzumab in patients with HER2-positive, clinical stage I–III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial Lancet Oncol 13(9) 869–78 DOI: 10.1016/S1470-2045(12)70329-7 PMID: 22884505
36. Pivot X et al (2013) Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study Lancet Oncol 14(10) 962–70 DOI: 10.1016/S1470-2045(13)70383-8 PMID: 23965225
37. Gebhart G et al (2013) 18F-FDG PET/CT for early prediction of response to neoadjuvant lapatinib, trastuzumab, and their combination in HER2-positive breast cancer: results from Neo-ALTTO J Nucl Med 54(11) 1862–8 DOI: 10.2967/jnumed.112.119271 PMID: 24092940
38. Dijkers EC et al (2010) Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer Clin Pharmcol Ther 87(5) 586–92 DOI: 10.1038/clpt.2010.12
39. Saini KS et al (2011) Beyond trastuzumab: new treatment options for HER2-positive breast cancer Breast 20 Supplement 3(0) S20–7 DOI: 10.1016/S0960-9776(11)70289-2 PMID: 22015288
40. GlaxoSmithKline ALTTO Trial–Trial Overview 2012 [October 19 2013] available from: http://alttotrials.com/hcp.php
41. Franklin MC et al (2004) Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex Cancer Cell 5(4) 317–28 DOI: 10.1016/S1535-6108(04)00083-2 PMID: 15093539
42. Scheuer W et al (2009) Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models Cancer Res 69(24) 9330–6 DOI: 10.1158/0008-5472.CAN-08-4597 PMID: 19934333
43. Baselga J et al (2012) Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer New Engl J Med 366(2) 109–19 DOI: 10.1056/NEJMoa1113216
44. Schneeweiss A et al (2013) Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol 24(9) 2278–84 DOI: 10.1093/annonc/mdt182 PMID: 23704196
45. Lewis Phillips GD et al (2008) Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate Cancer Res 68(22) 9280–90 DOI: 10.1158/0008-5472.CAN-08-1776 PMID: 19010901
46. Vaz-Luis I et al (2014) Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: a multi-institutional study J Clinical Oncol 32(20) 2142–50 DOI: 10.1200/JCO.2013.53.1608
47. Gonzalez-Angulo AM et al (2009) High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller J Clin Oncol 27(34) 5700–6 DOI: 10.1200/JCO.2009.23.2025 PMID: 19884543 PMCID: 2792998
48. Chia S et al (2008) Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers J Clin Oncol 26(35) 5697–704 DOI: 10.1200/JCO.2007.15.8659 PMID: 19001334
49. Constantinidou A and Smith I (2011) Is there a case for anti-HER2 therapy without chemotherapy in early breast cancer? Breast 20 Supplement 3(0) S158–61 DOI: 10.1016/S0960-9776(11)70316-2 PMID: 22015286
50. Gianni L et al (2011) Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial Lancet Oncol 12(3) 236–44 DOI: 10.1016/S1470-2045(11)70033-X PMID: 21354370
51. Spielmann M et al (2009) Trastuzumab for patients with axillary node–positive breast cancer: results of the FNCLCC-PACS 04 Trial J Clin Oncol 27(36) 6129–34 DOI: 10.1200/JCO.2009.23.0946 PMID: 19917839
52. Robidoux A et al (2013) Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial Lancet Oncology 14(12) 1183–92 DOI: 10.1016/S1470-2045(13)70411-X
53. Carey L, Berry D and Ollila D (2013) Clinical and translational results of CALGB 40601: A neoadjuvant phase III trial of weekly paclitaxel and trastuzumab with or without lapatinib for HER2-positive breast cancer J Clin Oncol 31(15 Suppl) 500
54. Hurvitz S et al (2013) Abstract S1-02: Final analysis of a phase II 3-arm randomized trial of neoadjuvant trastuzumab or lapatinib or the combination of trastuzumab and lapatinib, followed by six cycles of docetaxel and carboplatin with trastuzumab and/or lapatinib in patients with HER2+ breast cancer (TRIO-US B07) Cancer Res 73(24 Supplement) S1–2 DOI: 10.1158/0008-5472.SABCS13-S1-02
55. Rimawi MF et al (2013) Multicenter phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2–overexpressing breast cancer: TBCRC 006 J Clin Oncol 31(14) 1726–31 DOI: 10.1200/JCO.2012.44.8027 PMID: 23569315 PMCID: 3641695
56. Holmes FA et al (2011) Correlation of molecular effects and pathologic complete response to preoperative lapatinib and trastuzumab, separately and combined prior to neoadjuvant breast cancer chemotherapy J Clin Oncol 29 (suppl abstr 506)






