The impact of the Oncotype DX Recurrence Score on treatment decisions and clinical outcomes in patients with early breast cancer: the Maccabi Healthcare Services experience with a unified testing policy
Nava Siegelmann-Danieli1, Barbara Silverman1, Aviad Zick2, Anat Beit-Or1, Itzhak Katzir1 and Avi Porath1
1 Maccabi Healthcare Services, 27 Hamered Street, Tel Aviv 68125, Israel
2 Hadassah Medical Center, Ein Kerem, Jerusalem 91120, Israel
Correspondence to: Nava Siegelmann-Danieli. Email: danieli_na@mac.org.il
Abstract
The Oncotype DX Recurrence Score is a validated prognosticator in oestrogen receptor positive (ER ) breast cancer. Our retrospective analysis of a prospectively defined cohort summarises the clinical implications associated with Oncotype DX testing according to the Maccabi Healthcare Services (MHS) policy. The MHS eligibility criteria for testing included ER N0/pN1mic invasive tumours, discussion of test implications with an oncologist, ductal carcinoma 0.6–1 cm Grade 2–3, HER2 negative ductal carcinomas with 1.1–4.0 cm Grade 1–2, or lobular carcinoma. Large (> 1 cm) Grade 3 tumours could have grade reassessed. We linked Recurrence Score results with patients’ information and used chi-squared tests to assess the associations thereof. Between January 2008 and December 2011, tests were performed on 751 patients (MHS-eligible, 713); 54%, 38%, and 8% of patients had low, intermediate, and high Recurrence Score results, respectively. Recurrence Score distribution varied significantly with age (P = 0.002), with increasing Recurrence Score values with decreasing age. The proportion of patients with high Recurrence Score results varied by grade/size combination and histology, occurring in 32% of small (≤ 1 cm) Grade 3 and 3% of larger (1.1–4 cm) Grade 1 ductal tumours and only in 2% of lobular carcinomas. Chemotherapy was administered to 1%, 13%, and 61% of patients with low, intermediate, and high Recurrence Score results, respectively (P < 0.0001), but only to 2% of intermediate score patients ≥ 65 years. Luteinising-hormone-releasing hormone agonists with tamoxifen were used in 27% of low Recurrence Score patients ≤ 50 years. With a median follow-up of 26 months, no systemic recurrences were documented, whereas four patients exhibited locoregional recurrences. In summary, in this low-to-moderate risk patient population, testing identified 46% of patients as intermediate/high risk. Treatment decisions were influenced by Recurrence Score results and patients’ age. The current MHS policy seems to achieve the goal of promoting chemotherapy use according to the test results in a prespecified patient population.
Keywords: biomarker, breast cancer, chemotherapy, health policy, personalised medicine.
Background
Molecular testing in oncology is a highly evolving field with a potential impact on early detection, diagnosis, and risk assessment, as well as on establishing prognosis and predicting both responses to therapy and drug toxicity. Tests that are necessary for treatment decisions, such as HER2 levels in breast cancer (for HER2-directed therapies) and anaplastic lymphoma kinase rearrangement in lung adenocarcinoma (for crizotinib therapy), are scored high for clinical utilities and are covered by most modern insurance plans. However, other molecular tests that are intended to assist in the prognostic and/or predictive evaluation of early breast tumours, such as the Oncotype DX breast cancer assay (Genomic Health Inc., Redwood City, California, United States) and MammaPrint 70 gene assay (Agendia, Amsterdam, The Netherlands), may be considered to have lower scores for clinical utility and, therefore, are not uniformly covered by providers.
Maccabi Healthcare Services (MHS) is the second largest health maintenance organisation (HMO) in Israel, insuring close to two million members and caring for over 8,700 new cases of adults with nonhaematology invasive tumours annually. Among these cases, approximately 1,000 are breast cancer patients, the majority of which (97%) are diagnosed with nonmetastatic disease (MHS members participate in the National Breast Cancer Early Detection Programme in Israel). HMO resources in Israel have been increasingly strained since 1995, with changes in state reimbursement following the introduction of the Israeli National Health Insurance Law. While modern oncology drugs are often covered under the law, certain molecular technologies, including breast cancer profiling, are not reimbursed by the state. In January 2008, MHS elected to approve and completely cover molecular profiling for newly diagnosed breast cancer patients according to a prespecified policy. This policy aimed to identify patients whose likelihood (prior to molecular profiling) of being offered chemotherapy in addition to hormonal therapy is low-to-moderate and whose lives may be saved by early chemotherapy use. Oncotype DX, a 21-gene real-time reverse transcription polymerase chain reaction assay, that provides numerical results in the form of a Recurrence Score [1], was elected as the molecular test of choice, as it can be performed on fixed paraffin-embedded tissues, is considered highly accurate with repetitive tests [2, 3], is supported by an extensive database [4], and its prognostic and predictive utility in oestrogen receptor positive (ER ) node negative patients has been demonstrated in several large cohorts using prospectively designed, retrospective analyses of archival samples, with prespecified statistical analysis plans (NSABP B-14 and NSABP B-20 [1, 5]; there are now more validation studies with consistent results) [6–10].
This article summarises the experience of MHS with Oncotype DX testing from 2008 to 2011, and addresses the association of the Recurrence Score results with clinical and pathological features, treatment approaches, and clinical outcomes.
Methods
Patients
This retrospective analysis of a prospectively defined cohort evaluated all MHS patients who underwent Oncotype DX testing from 1 January 2008 to 31 December 2011. All tests were approved by MHS headquarters. The approval policy required patients to meet the following criteria: (a) diagnosis of ER primary breast cancer and node negative or microscopic nodal involvement only; (b) having discussed the implications of the test with an oncologist and being prepared to undergo chemotherapy should it be recommended; and (c) having ductal carcinoma 0.6–1.0 cm with Grade 2–3 histology (if HER2 positive, not being considered for immediate trastuzumabcontaining therapy), OR HER2 negative 1.1–4.0 cm with Grade 1–2 ductal histology, OR infiltrating lobular tumours. Large (> 1.0 cm) ductal tumours of Grade 3 histology were not approved; however, the grade could be reassessed and the test was approved if histology grade was then determined as 1–2. Genetic predisposition to breast cancer was not taken into consideration for testing approval (MHS headquarters personnel had no information on patients’ BRCA 1/2 mutation status at the time of testing approval). In rare cases, testing was also approved for patients not fulfilling the MHS criteria, mostly due to unique medical conditions. The study was approved by the Ethics Committee of Assuta Hospital. All patients signed informed consent forms prior to Oncotype DX testing.
Data collection
Individual information for all breast cancer patients who underwent Oncotype DX testing was extracted from the MHS database, including pathology information, which was reviewed by an oncology physician, and demographic, vital status, pharmacy purchasing, and hospital claims data, which were all extracted electronically for the period from the date of testing through 30 June 2012. Ki-67 data were available for patients who had a core biopsy or breast surgery at MHS-affiliated facilities (i.e., when tumour samples were sent to the MHS central pathology lab for analysis). There, Ki-67 levels were measured using an anti-Ki-67 antibody (SP6, Thermo Scientific, Rockford, Illinois, United States) and the BenchMark XT staining platform (Ventana, Tucson, Arizona, United States). Teva Pharmaceutical Industries Ltd, the distributer of Oncotype DX testing in Israel, provided the Recurrence Score results, which were categorised as follows: low (< 18); intermediate (18–30); and high (≥ 31). The patients may have been treated by any oncologist in Israel (either affiliated with MHS or not) according to their personal preferences. The treatments received (chemotherapy, endocrine therapy, or both) were ascertained by combining data from pharmacy purchases and hospital claims. The current analysis defined patients as having received systemic chemotherapy if chemotherapy-specific hospital bills were submitted for services provided within 12 months of testing. Pharmacy purchasing records provided information on hormonal therapy with tamoxifen; aromatase inhibitors (AIs), including anastrozole, letrozole, and exemestane; and luteinising-hormonereleasing hormone (LHRH) agonists. The clinical documents for all patients who died or had subsequent (≥ 12 months) chemotherapy or late mastectomy (over six months from the date of testing) were reviewed to assess systemic and locoregional recurrences.
Analyses
The entire cohort was used to evaluate the relationship between Recurrence Score categories and treatments received. The relationship between score categories and clinicopathological characteristics was limited to the 95% of patients who met the MHS eligibility criteria for testing. The Mantel–Haenszel chi-squared test was used to test for the association between score and age categories and the Cochran– Mantel–Haenszel row mean score chi-squared test was used to test for the association between score category and nominal clinicopathological characteristics and treatments received. Where appropriate, post hoc pairwise comparisons were corrected for multiple testing by the Bonferroni method. A multivariable logistic regression model was used to assess the probability of receiving chemotherapy as a function of score category, age, and the interactions thereof. All analyses were conducted using SAS statistical software version 9. 2 (SAS Institute Inc., Cary, North Carolina, United States); P < 0.05 was considered significant.
Results
Patient population
A total of 751 MHS patients who underwent Oncotype DX testing between 1 January 2008 and 31 December 2011 were included, 713 of whom (95%) met the MHS criteria for testing. The majority of our cohort (485 patients, 64.6%) was 45–64 years of age, 138 patients (18.4%) were 18–44 years of age, and 128 patients (17.0%) were ≥ 65 years. Recurrence Score distribution showed that 407 patients (54.2%) had a low Recurrence Score result, 285 patients (37.9%) had an intermediate Recurrence Score result, and 59 patients (7.9%) had a high Recurrence Score result.
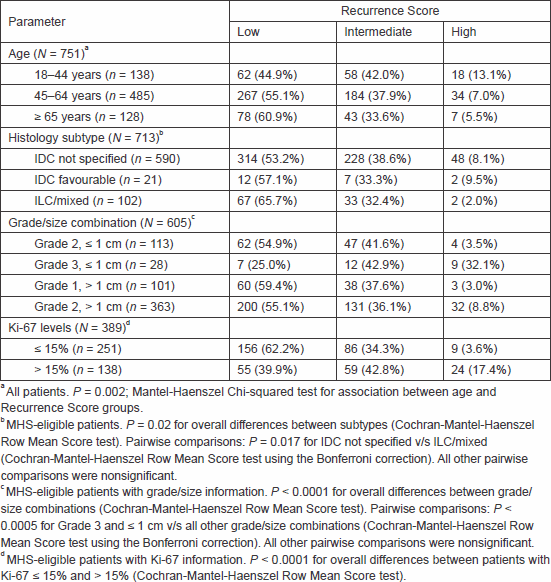
Recurrence Score distribution remained stable over the years (P = 0.27; chi-squared test, data not shown). It varied significantly, however, with age (P = 0.002; Mantel–Haenszel chi-squared test), with increasing Recurrence Score values with decreasing age (Table 1).
Recurrence score distribution and pathological characteristics
Analysis was conducted for the MHS-eligible patients. The majority (590 patients, 82.7%) had invasive ductal carcinoma (IDC) not specified, 21 patients (3.0%) had favourable IDC histological subtypes (mucinous, tubular, and medullary [11]), and 102 patients (14.3%) had invasive lobular carcinoma (ILC) alone or mixed with IDC (87 patients with ILC alone, 15 with mixed; all considered ILC for the current analysis). The Recurrence Score distribution differed significantly between the histological subtypes (P = 0.02; Cochran–Mantel–Haenszel row mean score test; Table 1). In pairwise comparisons, the only significant difference was between the IDC not specified and the ILC groups (P = 0.017; Cochran–Mantel–Haenszel row mean score test using the Bonferroni correction). Intermediate and high Recurrence Score rates in the IDC not specified group were 38.6% and 8.1%, respectively, and in the ILC group, these rates were 32.4% and 2.0%. The high Recurrence Score category in the ILC group referred to only two patients, both of whom had microscopic nodal involvement and primary tumour size ≥ 2 cm. Of note, Recurrence Score distribution in the IDC favourable group was not significantly different from that in the IDC not specified group (P = 0.86).
When only IDC patients were assessed, grade/size combination was significantly associated with Recurrence Score results: despite performing the test only for small (≤ 1 cm) Grade 3 tumours, high Recurrence Score results occurred in 32.1% of those cases, as opposed to 3.0–8.8% of Grade 1–2 tumours sized 0.6–4.0 cm (P < 0.0005 for Grade 3 and ≤ 1 cm versus all other grade/size combinations; Cochran–Mantel–Haenszel row mean score test using the Bonferroni correction; Table 1).
Next, we examined the association between the Recurrence Score results and the level of the proliferation biomarker Ki-67, which was available for 389 patients (55.0%). There was a weak but statistically significant positive correlation between the two parameters (r = 0.32, 95% confidence interval, 0.23–0.41; Pearson correlation). The Recurrence Score distribution differed significantly between patients with Ki-67 ≤ 15% and > 15% (P < 0.0001; Cochran–Mantel–Haenszel row mean score test; Table 1), but there was a wide distribution of Recurrence Score results in both groups.
Table 1. Distribution of Recurrence Score categories by patient/tumour characteristics.
Recurrence score results and treatments received
Of the 751 patients in our cohort, 18 patients (2%) received no chemotherapy and did not purchase any hormonal agents. Altogether, 77 patients (10%) received systemic chemotherapy. High Recurrence Score patients received chemotherapy at a greater rate than intermediate and low Recurrence Score patients (61%, 13%, and 1%, respectively; P < 0.0001; chi-squared test) overall and even in the older age group (≥ 65 years), in which 43% of high Recurrence Score patients received chemotherapy. For intermediate Recurrence Score patients, older age was significantly associated with a decreased use of chemotherapy (29%, 10%, and 2% of patients 18–44, 45–64, and ≥ 65 years received chemotherapy, respectively; P < 0.0001 for comparing chemotherapy use across age groups; chi-squared test; Figure 1).
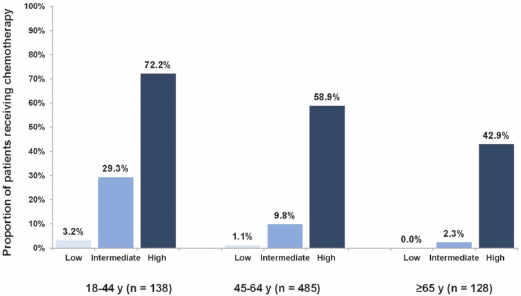
Figure 1. Proportions of patients receiving chemotherapy by age group and Recurrence Score category. Increase from each Recurrence Score level to the next (P < 0.0001); decrease from 18–44 years to 45–64 years (P = 0.0043), from 45–64 years to ≥ 65 years (P = 0.039); logistic regression.
In a multivariable logistic regression analysis in which the probability of receiving chemotherapy was modelled as a function of Recurrence Score category, age, and the interaction thereof, the interactions were found to be nonsignificant. Recurrence Score category and age group were found to be independent predictors of chemotherapy use (Table 2).
Of the 751 patients in our cohort, 730 patients (97%) purchased endocrine therapy agents (tamoxifen, AIs, LHRH agonists, or their combinations) from MHS-owned/affiliated pharmacies. Overall, tamoxifen was purchased for 659 patients (88%), AIs for 238 patients (32%), and LHRH agonists for 90 patients (12%). For the most part, the purchase/use of endocrine therapy was similar across the Recurrence Score categories; the range of endocrine therapy use across the Recurrence Score categories was 78–90% for tamoxifen, 31–34% for AIs, and 9–13% for LHRH agonists.
Next, we evaluated the treatment approach in 293 patients aged ≤ 50 years, and assessed physicians’ choice of chemotherapy and/or LHRH agonists in addition to hormonal therapy with tamoxifen (LHRH agonists are considered as a potential substitute for chemotherapy in ER premenopausal women [12–17]) versus none. In this age group, overall, a more aggressive treatment approach was noted, and even in the low Recurrence Score group, close to one-third of patients received chemotherapy and/or LHRH agonists. Still, the proportion of patients receiving LHRH agonists and/or chemotherapy varied significantly across Recurrence Score groups (P < 0.0001 for comparison across the three groups; chi-squared test; Figure 2).
Table 2. Odds ratios for receiving chemotherapy (logistic regression analysis) (N = 751).
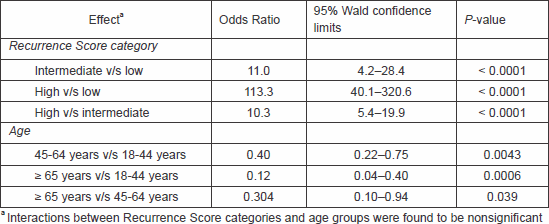
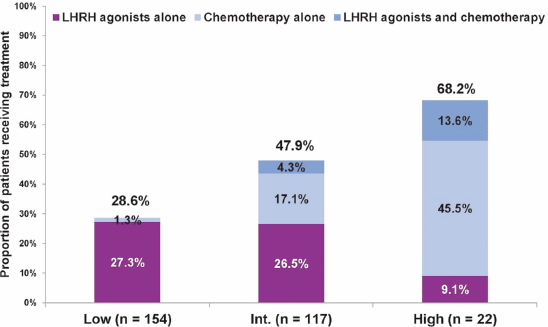
Figure 2. Treatment with LHRH agonists, chemotherapy, or both, in addition to tamoxifen by Recurrence Score category in patients ≤ 50 years. For treatment with LHRH agonists and/or chemotherapy: P < 0.0001 for comparing all three groups (chi-squared test).
Clinical outcomes and subsequent events
Median follow-up for the entire cohort was 26 months. We explored the rate of systemic recurrence by examining the MHS database to identify patients whose chemotherapy hospital services were provided more than one year from the date of Oncotype DX testing. Six such patients (0.8%) were identified. These patients included one with ovarian cancer, one with colorectal cancer, one with acute myeloid leukaemia, and one with clear cell carcinoma of the uterus. In addition, two patients received late chemotherapy for locoregional recurrences in ipsilateral axillary lymph nodes. Notably, these two patients did not receive chemotherapy; one did not meet the MHS criteria due to involved axillary nodes and had a low Recurrence Score result, and the other was 43 years old with borderline eligibility (Grade 3, 1 cm) and an intermediate Recurrence Score result.
We also examined the MHS database to identify patients that had a mastectomy after more than six months from the date of Oncotype DX testing. Five such patients (0.7%) were identified, of whom only only patient with high Recurrence Score result, who was treated with lumpectomy, radiation therapy, and chemotherapy, followed by tamoxifen, developed local breast recurrence 27 months after her primary surgery. Another patient, with intermediate Recurrence Score result who had lumpectomy followed by radiation, underwent contralateral mastectomy for newly diagnosed contralateral breast cancer 17 months from her initial surgery. The remaining three identified patients had mastectomies as a prophylactic measure or to facilitate obtaining symmetry in breast reconstruction.
Four patients died during follow-up: one due to advanced biliary tract malignancy, one from intracerebral haemorrhage, and one at the age of 86 with severe aortic stenosis; all were breast cancer free. The fourth patient who had an intermediate Recurrence Score result expired due to recurrent metastatic cervical cancer with extensive abdominal and pelvic involvement proved histologically at the time of small bowel resection surgery; several weeks prior to her death, the patient also had a biopsy-proven axillary nodal recurrence of ILC.
Discussion
The current retrospective analysis of a prospectively defined cohort evaluated the association of Recurrence Score results, clinicopathological features, treatments received, and clinical outcomes in a large cohort of N0/Nmic ER primary breast cancer patients who underwent Oncotype DX testing. The cohort included patients with low and intermediate risk pathological categories reflecting the MHS policy. As may be expected, the rate of high Recurrence Score results in our cohort (8%) was substantially lower than that observed in cohorts of ER node negative and node positive patients with unspecified histology grade (25–33%) [1, 5, 9]; still, high and intermediate Recurrence Score categories occurred in almost half of our population (46%).
We observed the associations between Recurrence Score results and age, tumour grade/size, and histology subtype. Specifically, high Recurrence Score was twice as common in younger compared with older age groups, consistent with the findings in [5]. Up to one-third of patients with Grade 3 ≤ 1 cm tumours had high Recurrence Score and a weak but statistically significant association between Recurrence Score and Ki-67 was observed, consistent with prior reports [18, 19]. Looking into the associations between histological subtypes and Recurrence Score yielded two observations: Recurrence Score distribution in patients with IDC with favourable features (mucinous, tubular, and medullary [11]) and IDC not specified was not statistically different, although the number of patients in the IDC favourable group was limited; and high Recurrence Score was extremely rare (2%) in patients with ILC histology, as compared with a rate of 8% in the IDC not specified group. The latter findings are supported by two other reports, the first by Asad et al [20] who observed a significant difference in Recurrence Score distribution between only eight patients with ILC and 77 patients with IDC with a higher proportion of low Recurrence Score patients in the ILC group; and the other by Baehner et al [21] who evaluated Recurrence Score results from more than 25,000 Genomic Health-tested commercial samples (and potentially associated with selection bias) and found a significantly lower median Recurrence Score result in ILC than IDC tumours (17.5 versus 18.2; P < 0.05).
This analysis is retrospective and conclusions regarding clinical outcomes and subsequent events are currently limited by a relatively short follow-up duration (median of 26 months). Nonetheless, the analysis has several strengths: data on treatments received and outcome were complete using MHS resources and all pathological reports were reviewed by an oncology physician. Furthermore, treatment decisions reflect national treatment trends as the majority of MHS patients are still treated in government hospitals due to a shortage of MHS oncologists (as no national treatment recommendations exist, all oncologists, regardless of HMO affiliation, are expected to use international treatment guidelines in their clinical practice). The logistic regression analysis demonstrated that both Recurrence Score category and age were independent factors impacting treatment decisions, with higher Recurrence Score and younger age increasing the likelihood of treatment with chemotherapy. Interestingly, our data suggest that in younger patients (≤ 50 years) with low Recurrence Score results, physicians may be inclined to administer more than ‘only tamoxifen’ and use an LHRH agonist despite the lack of evidence for its benefit [22]. In the high Recurrence Score group, approximately two-thirds of patients received chemotherapy, which is considerably less than optimal given the MHS eligibility criteria with request to discuss chemotherapy use in cases found to be at high recurrence risk. This apparent gap may suggest that, in some cases, the oncologist and/or patient may have changed their mind about chemotherapy treatment after the Recurrence Score results became available.
The rationale for the MHS policy is to identify patients whose lives may be saved by early chemotherapy use and who may not otherwise be considered for chemotherapy by the treating physicians. Indeed, follow-up data on this cohort, despite being limited (median of 26 months), with the various treatment approaches as detailed, showed only four locoregional recurrences, one in a patient borderline for eligibility and another in a noneligible patient. The MHS policy excludes patients with large Grade 3 tumours based on findings from the original Paik et al [1] report that assessed 668 tamoxifen-treated patients in the NSABP B-14 study and showed that high grade still had significant prognostic value in multivariable analysis, including the Recurrence Score. While in that report, concordance for histology grade was only 59–65% between two pathologists, it was highest for poorly differentiated tumours (kappa, 0.61) [1]. It should be noted, however, that low Recurrence Score may occur in about 20% of patients with Grade 3 tumours [1, 5], and that a Recurrence Score–pathology-clinical (RSPC) assessment was found to be more prognostic for distant recurrence than Recurrence Score alone [23]; nonetheless, the NSABP B20 data analysis suggests that Recurrence Score is still the best predictor of chemotherapy benefit (RSPC was not predictive of chemotherapy benefit [23]). We expect the data from a prospective trial that incorporates both Recurrence Score results and grade (the ongoing TAILORx trial), to best define their relative significance. Another molecular classification method using genomic grading index also supports the use of molecular classification mostly in Grade 2 tumours [24, 25]. To overcome potential inaccuracies in Grade 3 tumour readings, the MHS policy allows pathological reviews of all large Grade 3 tumours and approves Oncotype DX testing if one of the reviewers considers the tumour to be of lower grade.
MHS, as a large HMO with limited resources, examines outcomes repeatedly, and reevaluates its policies accordingly. Currently, MHS is considering revising its Oncotype DX eligibility criteria to exclude patients for whom testing is less likely to modify treatment decisions (i.e. patients with Grade 1 tumours and no micrometastases, were 0% are found to be high risk), in order to free resources that could be used to expand the eligibility criteria to potential other patient groups.
Conclusions
Our results demonstrate that in a cohort of N0/Nmic ER primary breast cancer patients with low or intermediate risk by traditional parameters, 46% of patients had intermediate or high Recurrence Score results (38% and 8%, respectively). Recurrence Score findings along with patients’ age impacted treatment decisions, an approach that was potentially associated with favourable outcomes. Thus, using Oncotype DX testing in this patient population may help to identify a clinically relevant proportion of patients that are likely to benefit from chemotherapy treatment.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Acknowledgments
The authors thank Lior Soussan-Gutman, Shira Yaari, Gabriel Hodik, and Tamar Peretz Yablonski for their contributions to data collection and analysis. Avital Bareket-Samish provided medical writing assistance, which was funded by Genomic Health Inc.
References
1. Paik S et al (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer N Engl J Med 351 2817–26 DOI: 10.1056/NEJMoa041588 PMID: 15591335
2. Cronin M et al (2007) Analytical validation of the Oncotype DX genomic diagnostic test for recurrence prognosis and therapeutic response prediction in node-negative, estrogen receptor-positive breast cancer Clin Chem 53 1084–91 DOI: 10.1373/clinchem.2006.076497 PMID: 17463177
3. Baehner FL et al (2011) Consistency and control in clinical assay technology over time: the Oncotype DX Recurrence Score and assessment of single gene expression levels [abstract] Cancer Res 71 (25 Suppl) nr P1-07-11 DOI: 10.1158/0008-5472.SABCS11-P1-07-11
4. Habel LA et al (2006) A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients Breast Cancer Res 8 R25 DOI: 10.1186/bcr1412 PMID: 16737553 PMCID: 1557737
5. Paik S et al (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer J Clin Oncol 24 3726–34 DOI: 10.1200/JCO.2005.04.7985 PMID: 16720680
6. Goldstein LJ et al (2008) Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features J Clin Oncol 26 4063–71 DOI: 10.1200/JCO.2007.14.4501 PMID: 18678838 PMCID: 2654377
7. Albain KS et al (2010) Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial Lancet Oncol 11 55–65 DOI: 10.1016/S1470-2045(09)70314-6
8. Dowsett M et al (2010) Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study J Clin Oncol 28 1829–34 DOI: 10.1200/JCO.2009.24.4798 PMID: 20212256
9. Toi M et al (2010) Clinical significance of the 21-gene signature (Oncotype DX) in hormone receptor-positive early stage primary breast cancer in the Japanese population Cancer 116 3112–18 DOI: 10.1002/cncr.25206 PMID: 20564629
10. Mamounas EP et al (2012) Association between the 21-Gene Recurrence Score (RS) and benefit from adjuvant paclitaxel (Pac) in node-positive (N ), ER-positive breast cancer patients (pts): results from NSABP B-28 SABCS: San Antonio Breast Cancer Symp. (San Antonio, TX, 4–8 Dec.)
11. Li CI (2010) Risk of mortality by histologic type of breast cancer in the United States Horm Cancer 1 156–65 DOI: 10.1007/s12672-010-0016-8
12. Schmid P et al (2007) Leuprorelin acetate every-3-months depot versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant treatment in premenopausal patients with node-positive breast cancer: the TABLE study J Clin Oncol 25 2509–15 DOI: 10.1200/JCO.2006.08.8534 PMID: 17577027
13. LHRH-agonists in Early Breast Cancer Overview group (2007) Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials Lancet 369 1711–23 PMID: 17512856
14. Roche H et al (2006) Complete hormonal blockade versus epirubicin-based chemotherapy in premenopausal, one to three node-positive, and hormone-receptor positive, early breast cancer patients: 7-year follow-up results of French Adjuvant Study Group 06 randomised trial Ann Oncol 17 1221–7 DOI: 10.1093/annonc/mdl107 PMID: 16731539
15. International Breast Cancer Study Group (2003) Adjuvant chemotherapy followed by goserelin versus either modality alone for premenopausal lymph node-negative breast cancer: a randomized trial J Natl Cancer Inst 95 1833–46 PMID: 14679153
16. Kaufmann M et al (2003) Survival analyses from the ZEBRA study. goserelin (Zoladex) versus CMF in premenopausal women with node-positive breast cancer Eur J Cancer 39 1711–17 DOI: 10.1016/S0959-8049(03)00392-7 PMID: 12888366
17. Jonat W et al (2002) Goserelin versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy in premenopausal patients with node-positive breast cancer: the Zoladex Early Breast Cancer Research Association Study J Clin Oncol 20 4628–35 DOI: 10.1200/JCO.2002.05.042 PMID: 12488406
18. Williams DJ et al (2011) Proliferation (Ki-67 and phosphohistone H3) and oncotype DX recurrence score in estrogen receptor-positive breast cancer Appl Immunohistochem Mol Morphol 19 431–6 DOI: 10.1097/PAI.0b013e318206d23d PMID: 21297447
19. Gluz O et al (2012) Prospective comparison of Recurrence Score and different definitions of luminal subtypes by central pathology assessment of single markers in early breast cancer: results from the phase III WSG-planB trial SABCS: San Antonio Breast Cancer Symp. (San Antonio, TX, 4–8 Dec.)
20. Asad J et al (2008) Does oncotype DX recurrence score affect the management of patients with early-stage breast cancer? Am J Surg 196 527–9 DOI: 10.1016/j.amjsurg.2008.06.021 PMID: 18809056
21. Baehner FL et al (2007) Gene expression by standardized quantitative RT-PCR in the special histologic subtypes of estrogen receptor positive invasive breast cancer SABCS: San Antonio Breast Cancer Symp. (San Antonio, TX, 13–16 Dec.)
22. Johnston SR (2012) Controversies in adjuvant endocrine therapy for breast cancer ASCO educational article http://www.asco.org/ASCOv2/Education & Training/Educational Book?&vmview=edbk_detail_view&confID=114&abstractID=87. Accessed January 3 2013
23. Tang G et al (2011) Risk of recurrence and chemotherapy benefit for patients with node-negative, estrogen receptor-positive breast cancer: recurrence score alone and integrated with pathologic and clinical factors J Clin Oncol 29 4365–72 DOI: 10.1200/JCO.2011.35.3714 PMID: 22010013 PMCID: 3221521
24. Loi S et al (2007) Definition of clinically distinct molecular subtypes in estrogen receptor-positive breast carcinomas through genomic grade J Clin Oncol 25 1239–46 DOI: 10.1200/JCO.2006.07.1522 PMID: 17401012
25. Sotiriou C et al (2006) Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis J Natl Cancer Inst 98 262–72 DOI: 10.1093/jnci/djj052 PMID: 16478745






