Intramedullary spinal cord metastases from breast cancer: detection with 18F-FDG PET/CT
Laura Gilardi, Stefano Vassallo, Marzia Colandrea, Laura Lavinia Travaini and Giovanni Paganelli
Division of Nuclear Medicine, European Institute of Oncology, Milan, Italy
Correspondence to: Giovanni Paganelli. Email: divisione.medicinanucleare@ieo.it
Abstract
A 35-year-old woman, already treated with surgery, chemotherapy, and radiotherapy for a ductal carcinoma of the left breast, underwent an 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) scan for an increase of the serum markers carcinoembryonic antigen (CEA) and cancer antigen 15.3 (CA15.3). The scan showed multiple FDG-avid lesions in the liver and bone. The images also detected two areas of uptake in the dorsal and lumbar spinal cord, which were suspicious for metastases; magnetic resonance imaging (MRI) confirmed these lesions.
Keywords: spinal cord metastases, breast cancer, PET/CT, 18F-FDG
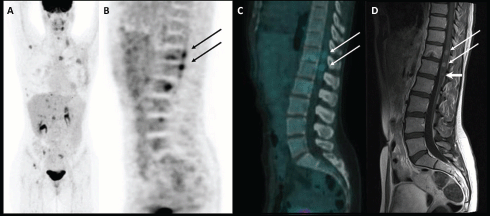
Figure 1: Maximum intensity projection image of 18F-FDG PET/CT scan (A); PET, CT, and MRI sagittal images of spinal cord metastases (B, C, and D respectively).
A 35-year-old woman underwent a left mastectomy in November 2011 and subsequent chemotherapy and radiotherapy for a ductal breast carcinoma. An adjuvant hormonal treatment with tamoxifen and Luteinizing Hormone-Releasing Hormone analogues was then started. In September 2012, a follow-up blood test revealed an increase of the serum markers CEA and CA15.3, so she underwent an 18F-FDG PET/CT scan (injected dose: 171 MBq; serum glucose level: 70 mg/dl; time between injection and acquisition: 73 min) that showed multiple FDG-avid lesions in the liver and bone (maximum intensity projection image in Figure 1A). A sagittal PET and fused PET/CT images also detected two areas of focal uptake in the dorsal and lumbar spinal cord (SUVbw max 4.1 and 4.7, respectively; see Figure 1B and C—arrows), which were suspicious for metastases. An MRI confirmed these lesions, which had maximal diameters of 8 and 9 mm (Figure 1D—long arrows), and also detected a 4-mm metastasis (Figure 1D—short arrow), which did not show significant radiotracer uptake due to its limited dimension. In this case, the sensitivity was higher for the MRI than for the PET/CT, but the latter is usually performed from the skull base to the mid-thigh, allowing the detection of distant metastases anywhere in the body, even in unusual sites [1]. Indeed, intramedullary spinal metastatic lesions are exceedingly rare: they account for 3.4–5% of myelopathies in cancer patients and comprise 1–3% of all intramedullary spinal cord tumours. Lung cancer, especially small-cell carcinoma, accounts for the majority of cases (54%). Other common culprits are breast carcinoma (13%), melanoma (9%), lymphoma (5%), and renal cell carcinoma (4%) [2, 3]. The metastatic lesions are usually solitary but may be multifocal in 15% of cases [4]. In three-fourths of reported patients, the time from the onset of neurological symptoms to the development of the full neurological deficit is less than one month [5, 6]. The identification of intramedullary spinal cord metastases in an asymptomatic patient, as occurred in our case, allows treatments to be performed early in the course of the disease, improving the patient’s prognosis and quality of life. Detection of these rare metastases through 18F-FDG PET/CT has already been reported in the literature for lung cancer and renal cell carcinoma [7–10]. To the best of our knowledge, this is the first report on spinal cord metastases from breast cancer detected by PET/CT.
Conflicts of interests
The authors declare that they have no conflicts of interest.
References
1. Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, Coleman RE, Wahl R, Paschold JC, Avril N, Einhorn LH, Suh WW, Samson D, Delbeke D, Gorman M and Shields AF (2008) Recommendations on the use of 18F-FDG PET in oncology J Nucl Med 49 480–508 DOI: 10.2967/jnumed.107.047787 PMID: 8287273
2. Schiff D and O’Neill BP (1996) Intramedullary spinal cord metastases: clinical features and treatment outcome Neurology 47 906–12 DOI: 10.1212/WNL.47.4.906 PMID: 8857717
3. Mut M, Schiff D and Shaffrey ME (2005) Metastasis to nervous system: spinal epidural and intramedullary metastases J Neurooncol 75 43–56 DOI: 10.1007/s11060-004-8097-2 PMID: 16215815
4. Chi JH and Parsa AT (2006) Intramedullary spinal cord metastasis: clinical management and surgical considerations Neurosurg Clin N Am 17 45–50 DOI: 10.1016/j.nec.2005.10.003 PMID: 16448907
5. Grem JL, Burgess J and Trump DL (1985) Clinical features and natural history of intramedullary spinal cord metastasis Cancer 5 2305–14 DOI: 10.1002/1097-0142(19851101)56:9<2305::AID-CNCR2820560928>3.0.CO;2-X
6. Jellinger K, Kothbauer P, Sunder-Plassmann E and Weiss R (1979) Intramedullary spinal cord metastases J Neurol 220 31–41 DOI: 10.1007/BF00313146 PMID: 84065
7. Jayasundera MV, Thompson JF and Fulham MJ (1997) Intramedullary spinal cord metastasis from carcinoma of the lung: detection by positron emission tomography Eur J Cancer 33 508–9 DOI: 10.1016/S0959-8049(97)89034-X PMID: 9155543
8. Komori T and Delbeke D (2001) Leptomeningeal carcinomatosis and intramedullary spinal cord metastases from lung cancer. Detection with FDG Positron Emission Tomography Clin Nucl Med 26 905–7 DOI: 10.1097/00003072-200111000-00001 PMID: 11595839
9. Nguyen NC, Sayed MM, Taalab K, et al (2008) Spinal cord metastases from lung cancer. Detection with F-18 FDG PET/CT Clin Nucl Med 33 356–8 DOI: 10.1097/RLU.0b013e31816a784c PMID: 18431157
10. Poggi MM, Patronas N, Buttman JA, Hewitt SM and Fuller B (2001) Intramedullary spinal cord metastasis from renal cell carcinoma. Detection by positron emission tomography Clin Nucl Med 26 837–9 DOI: 10.1097/00003072-200110000-00006 PMID: 11564920






