Postoperative simple biochemical markers for prediction of bone metastases in Egyptian breast cancer patients
Nadia YS Morcos 1, Nadia I Zakhary 2, Mahmoud M Said 1 and May MM Tadros 1
1 Biochemistry Department, Faculty of Science, Ain Shams University, Egypt
2 Cancer Biology Department, National Cancer Institute, Cairo University, Egypt
Correspondence to: Nadia YS Morcos. Email: nadiamrcs@googlemail.com
Abstract
Objective: The present study was undertaken to identify patient populations at high risk for bone metastases (BM) at any time after diagnosis of operable breast cancer.
Subjects and methods: A total number of 59 cases with breast cancer after mastectomy was subdivided into two main groups that included 30 patients with radiologically confirmed BM and 29 patients with no bone metastasis (NBM). Patients with NBM were formerly observed for a one-year follow-up interval to monitor the development of bone metastasis (new BM). Parameters included a full blood picture, tumour markers (carcinoembryonic antigen and CA 15.3) and some biochemical markers (vascular endothelial growth factor and zinc levels, as well as tartrate-resistant acid phosphatase and alkaline phosphatase activities).
Results: A significant elevation was recorded in carcinoembryonic antigen level and alkaline phosphatase activity, as well as inflammation and vascularisation markers at the time of primary diagnosis in patients with BM, compared with those without BM. CA 15.3 was significantly higher in the new BM group as compared with the other two groups (patients free of bone metastasis [free BM] and BM). According to the likelihood ratio, a panel of single, calculated as well as combined markers was proposed to predict BM within one year in breast cancer patients.
Conclusion: Vascularisation and inflammation markers, as well as CA 15.3 are predictive of bone recurrence within one year in breast carcinoma patients. We suggest that in cancer validation studies it is imperative to search for markers that link to the premetastatic process and to determine what type of mechanism is active in each stage.
Keywords: Breast cancer, bone metastasis, inflammation markers, vascularisation markers
Introduction
Breast cancer in women is the most common type of cancer-related mortality among Egyptian women worldwide [1]. According to GLOBOCAN 2008, breast cancer accounts for 38% of all new cancer cases in Egypt [2]. The median age at diagnosis is one decade younger than in Europe and North America and a good number of patients are premenopausal [3]. Sadly, the lack of public awareness and the financial shortages that impede screening and diagnostic services make the chances of survival lower and mortality rates correspondingly high [4].
The process of breast cancer metastasis, including tumour cell seeding, tumour dormancy, and metastatic growth, is only partly understood [5]. Bone is the first site of distant disease in 25–40% of patients with metastatic breast cancer, and up to 60–80% of patients with recurrent breast cancer eventually show evidence of skeletal involvement [6].
Complex biological pathways, including inflammation [7, 8], angiogenesis [9], invasion [10], osteoclastic activation, and bone matrix degradation, are involved in the formation of bone metastases [5]. Although the current diagnosis of bone metastases relies on bone imaging techniques, they are not sensitive enough for early detection and are invasive and expensive to use [11].
A flurry of surrogate biomarkers to detect micrometastasis has been developed in the last decade. These biomarkers open avenues for understanding cancer dormancy and metastasis, and may help predict outcome and therapeutic decisions at diagnosis and during follow-up of cancer patients [12].
The aim of the present study is to investigate prospectively whether simple markers and their combination could be used as a tool to detect and predict bone metastasis in breast carcinoma patients.
Patients and methods
Patients
Egyptian women aged 30–69 years were recruited for this study (2007–2011) and selected from the outpatient clinic at the Clinical Pathology Department of the National Cancer Institute (NCI), Cairo University, Egypt. Physical examinations were processed by physicians in the Medical Oncology Unit, and routine clinical and pathological examinations were done. Once they were fully diagnosed and clinical staging of the disease was done according to the TNM classification of the American Joint Committee on Cancer (AJCC) TNM system, 59 patients were mastectomised and then scheduled for the appropriate treatment protocol of chemotherapy and radiotherapy. Of these patients, 29 had NBM and 30 had radiologically confirmed BM. Chemotherapy regimens included either FAC (5-fluorouracil adriamycin cyclophosphamide) or FEC (5-fluorouracil epirubicin cyclophosphamide) intravenously every 3 weeks for six cycles, and calcium supplements. Patients with BM received bisphosphonates, vitamin D, and calcium. Other therapeutic modalities included tamoxifen for hormone responsive patients, and Herceptin for human epidermal growth factor receptor-2 (HER2)-positive patients. Patients with NBM were observed for a one-year follow-up interval by bone scan to monitor the development of BM. All subjects gave written informed consent to participate in the study, which was carried out in accordance with the Helsinki Declaration.
Laboratory evaluations
Serum alkaline phosphatase (ALP) was determined by a kinetic method using an assay kit provided by Globe Diagnostic (Italy), plasma tartrate-resistant acid phosphatase (TRAP5b) activity by an immunoassay method using an ELISA kit (Immunodiagnostic Systems, UK) and plasma carcinoembryonic antigen (CEA) level by an electrochemiluminescence immunoassay ‘ECLIA’ method using an assay kit provided from Roche Diagnostics, USA. Serum vascular endothelial growth factor (VEGF) and CA 15.3 levels were determined by the double-antibody enzyme immunoassay methods using ELISA kits (Signosis, USA). Serum zinc concentration was evaluated by atomic absorption spectrophotometry using a 3100 Perkin Elmer Atomic Absorption Spectrometer (USA) in the Central Laboratory, Faculty of Science, Ain Shams University.
A combination of previously reported markers was used in the present study that included monocytes% (monocyte count [107/L]/WBC count [109/L]) × 100 [13] and P2ms [(platelet count [109/L])2/(monocyte fraction [%] × segmented neutrophil fraction [%])] [14]. Similarly, two new markers were proposed that include ALP/mono% (alkaline phosphatase/monocytes%) and VEGF/mono% (vascular endothelial growth factor/monocytes%).
Statistical methods
The results were computed and statistics analysed using SPSS statistical software version 17.0 (SPSS Inc., Chicago, IL, USA). The quantitative results were expressed as median with minimum–maximum values. The non-parametric
Results
Characteristics of patients are depicted in Table 1. There were no significant correlations between serum biomarkers and patient characteristics. Two breast cancer patients out of 30 from the BM group had visceral metastasis, which did not affect the overall result of the study. Markers chosen for the present study were selected to cover cancer dormancy and dissemination (CA 15.3 and CEA), angiogenesis and inflammation (VEGF, platelets, monocytes%, neutrophils%, zinc and ALP) and bone markers (TRAP5b and ALP). The results revealed that the four patients who developed bone metastasis within one year (new BM), although initially categorised with the NBM group, had a biochemical profile closer to those with radiologically confirmed bone metastasis (BM). Consequently, the results were compared once between the initial two groups (NBM versus BM), then recalculated by excluding the data of the four new BM patients from the NBM group. As a result, an assortment of data was attained, justifying the importance of choosing the appropriate conditions and samples for accurate validation.
Table 1: Baseline characteristics of breast cancer patients included in the study.
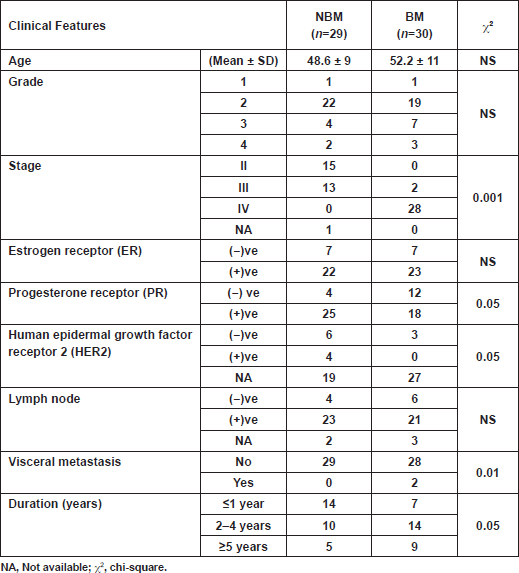
Analyses showed significantly elevated CEA level and ALP activity, as well as inflammation (ALP/mono% and P2ms), and vascularisation (VEGF/mono%) markers, and in contrast a significant decrease in monocytes% at the time of primary diagnosis in patients with BM, compared with those with NBM (Table 2).The CA 15.3 was significantly higher in the new BM group as compared with the other two groups (free BM and BM; p < 0.01) (Table 3). A cutoff value of 106 U/ml for CA 15.3 significantly differentiated between patients that newly developed BM within a one-year follow-up interval (100% of new BM patients had CA 15.3 values ≥ 106 U/ml) and those without bone involvement or initially diagnosed with BM (100% and 90% of free BM and BM patients had CA 15.3 < 106 U/ml, respectively) (χ2<0.001) (Table 4). No significant differences in TRAP5b, VEGF and zinc levels were recorded in all comparisons.
Table 2: Level of biochemical markers at the time of diagnosis in breast cancer patients.
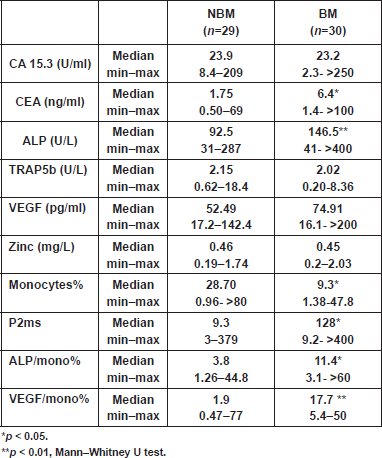
Table 3: Level of biochemical markers at the time of diagnosis of breast cancer patients who remained free, developed or had bone metastases within one year.
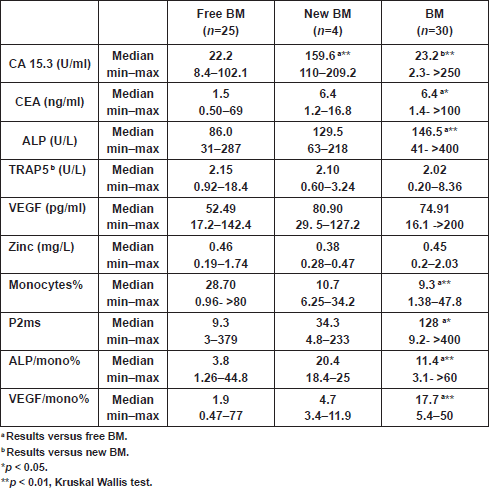
By using the cutoff value of 100 pg/ml for VEGF, it was possible to sort out 88.5% of free BM patients (<100 pg/ml), from 50% of patients who newly developed BM (≥100 pg/ml) (χ2<0.05) (Table 4). Consequently, we combined the VEGF and monocytes% as representative biomarkers for tumour angiogenesis. From ROC analysis, a ratio between VEGF and monocytes% (VEGF/mono%) was considered as an angiogenic marker with a cutoff value of 3.26. Cross-tabulation revealed that all patients who newly developed BM and those with initial BM had VEGF/mono% values ≥3.26, compared with only 26.7% of patients who remained free of BM (χ2<0.002) (Table 4).
Table 4: Cross-tabulation showing the reliability of significant markers (single, calculated and combined) in predicting bone metastasis within one year among all groups.
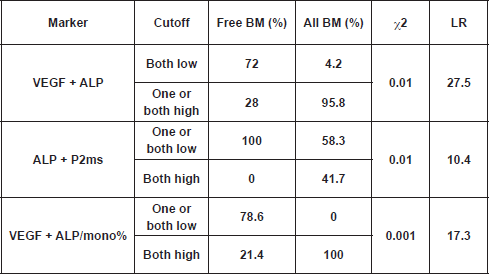
From the present study, we found that total ALP was elevated in all patients with bone metastasis; however, a significant increase was recorded in the BM group only, as compared with the free BM group (Table 3). A cutoff value of 119.2 U/L for serum ALP significantly differentiated between patients with BM and those without bone involvement (NBM; χ2 <0.001) at the initial diagnosis. Accordingly, by calculating the ratio between ALP and monocytes%, and using a cutoff value of 4.9 for such ratio, we were able to differentiate between 72.7% of patients who were initially diagnosed with NBM (<4.9) and 90% of patients who had BM (≥4.9) (χ2<0.001). However, such differences did not reach significance when we compared free BM with new BM groups, probably due to the small number of patients in the latter group. Interestingly, by using the cutoff values of ALP P2ms, we found that all patients without bone metastasis (free BM) had one or both markers below cutoff levels, whereas 66.7% of patients with new BM or 41.7% of all patients with bone metastasis (all BM) had both markers above the specified cutoff values. On the other hand, all patients with BM had both VEGF ALP/mono% above cutoff levels, while 78.6% of free BM patients had one or both of the aforementioned parameters below the specified cutoff levels (χ2 <0.001). In addition, cross-tabulation revealed that 95.8% of all BM patients had one or both of VEGF ALP above cutoff levels, and in contrast 72% of free BM patients had both indices below cutoff levels (χ2 < 0.01) (
The result of the current study revealed that the strength of markers (according to the likelihood ratio) in predicting bone metastasis within one year is in the following descending order: VEGF ALP; VEGF ALP/mono%; VEGF/mono%; monocytes%; CA 15.3; ALP P2ms; P2ms and VEGF (Table 4).
Table 5: Cross-tabulation showing the reliability of combined markers in predicting bone metastasis within one year (free BM versus all BM [BM new BM]).
Discussion
Metastasis is the leading cause of cancer death, and the metastatic cascade is a complex, yet inefficient process that we have only begun to understand recently [16]. Recent studies delineate a key role for the tumour microenvironment [17, 18] and cancer dormancy in cancer progression, and in consequence how to diagnose and treat the disease [19].
Many prognostic biomarkers discovered to date are usually evaluated as single markers, which lack sufficient specificity. We concur with Chechlinska’s argument that most cancer prognostic biomarker studies using modern technologies are methodologically flawed as they compare samples from cancer patients with those of healthy, inflammation-free people [20] or at best with those of patients with already metastasised tumours. In validation studies, it is important to determine whether the patient has dormant disease, and what type of mechanism is active, together with his or her systemic inflammatory response [21].
Comparing CA 15.3 and CEA tumour markers in NBM versus BM patients revealed that only CEA showed a significant (
Our results concur with several studies, signifying that CA 15.3, which detects soluble forms of MUC1 oncoprotein, is valuable for monitoring therapy in patients with metastatic disease [24–27]. We also found that the rise in CA 15.3 was independent of tumour size, axillary nodal status and other sites of metastasis, which is in line with previous results [22, 28]. Contrary to Duffy
Inflammation and angiogenesis are hallmarks of cancer, and together they play important roles at all stages of cancer progression, where it is difficult to separate them from one another. Inflammation incites and promotes carcinogenesis by creating a tissue microenvironment that can enhance invasion, migration and angiogenesis [29]. The change in tumour phenotype from angiostatic to angiogenic is known as the angiogenic switch, which helps tumour to escape from dormancy. Under this concept, the balance of proangiogenic and antiangiogenic factors would ultimately determine the activation status of the switch [30]. Most solid tumours are infiltrated by different cells such as tumour-associated macrophages (TAMs), monocytes, neutrophils and platelets. It is speculated that the recruited monocytes differentiate into macrophages, which release numerous factors including VEGF that facilitates invasion [31, 32]. Similarly, neutrophils are recruited by tumour cells, where they secrete powerful proangiogenic factors including VEGF [33].
Different studies evaluated VEGF and monocytes percentage separately as vascularisation markers [20, 30, 34–36]. To the best of our knowledge, we proposed herein a ratio between VEGF and monocytes percentage as a new vascularisation marker for breast cancer. However, this ratio warrants further validation studies.
Zinc (Zn) is an antioxidant, or free radical scavenger. Changes in zinc level in the blood of cancerous patients may be the cause of malignant tumour occurrence [37]. Another important role of zinc is in the process of angiogenesis, where it activates the matrix metalloproteinases (MMPs), especially under hypoxic conditions [38]. In the present study, although the blood zinc level was lower in patients with new BM, the differences among the studied groups did not reach significance.
The contribution of platelets to the progression of cancer is an emerging area of research interest. Complex interactions between tumour cells and circulating platelets play an important role in cancer growth and dissemination, and a growing body of evidence supports a role for physiologic platelet receptors and platelet agonists in cancer metastases and angiogenesis [19, 39–41]. Boucharaba
The inverted correlation between platelets and monocytes% and segmented neutrophils% (known as P2ms) was recently used as a non-invasive test for hepatic fibrosis [14, 43, 44]. Correspondingly, we considered the possibility of using P2ms as a predictive marker of inflammation in breast cancer patients. To our knowledge, this is the first study validating the diagnostic accuracy of P2ms for detecting inflammation in breast cancer with BM. Cross-tabulation revealed that a cutoff value of 105.76 for P2ms significantly differentiated between patients who remained free of BM (88.9% of free BM patients had values <105.76) and those that newly developed or initially diagnosed with BM (66.7% of both patient groups had values ≥105.76).
According to the data emerging from several studies, markers of bone metabolism may be an additional, useful, noninvasive diagnostic and prognostic tool to improve cancer disease management [45, 46]. Two different types of bone metabolism markers can be analysed: markers of bone resorption (such as tartrate-resistant acid phosphatase) and markers of bone formation (such as alkaline phosphatase).
Tartrate-resistant acid phosphatase isoform 5b (TRAP5b) is a biochemical marker of osteoclast number and activity. Mounting evidence has demonstrated serum TRAP5b as a useful marker of bone resorption and is among one of the many markers that have been studied to be a surrogate marker of BM in cancer patients [45, 47, 48].
It was not possible to demonstrate a statistically significant difference in TRAP5b activity among all groups in the present study. Hung and Oremek [48] reported a similar conclusion. Other authors examined healthy women and breast cancer patients at primary diagnosis without signs of osseous metastases and did not find any difference in TRAP5b activity, whereas a significant difference was observed in patients in whom bone metastases were newly diagnosed [47, 49, 50]. These differences were explicated by Wu
Total alkaline phosphatase (ALP) is a bone formation marker, used as a routine marker for skeletal involvement; however, different publications have pointed to the fact that bone-specific ALP is a better choice as an index of bone formation due to its higher specificity for bone [52]. Total ALP has been the most used marker for detecting increased bone formation in metastatic prostate and breast cancer [45, 46, 53]. ALP is also considered an inflammatory marker in cancer patients, and its elevation correlates with other inflammatory markers such as high C-reactive protein, low blood zinc levels, and reduction in overall survival of cancer patients [54–56]. In accordance with previous studies [54–56], our results show that inflammatory markers could be used for the prediction of bone recurrence in breast cancer patients.
Conclusion
Our relatively small study population limits the predictive power of the panels presented here, but the benefits of a serum biomarker and multimarker approach are clearly illustrated, and further studies utilising larger clinical cohorts are well warranted. This study demonstrates that a simple blood sample harbours information of tumour relapse in breast cancer patients and brings benefit to existing clinical predictors. We prove the importance of determining whether the patient has dormant disease, together with her systemic inflammatory response in order to improve the predictive marker validation.
Acknowledgments
The authors would like to thank Dr. Ola Mohamed Reda Khorshid, Assistant Professor of Medical Oncology, National Cancer Institute, Cairo University, for her cooperation and kind help in samples’ collection.
References
1. Salem AAS, Salem MAE and Abbas H (2010) Breast cancer: surgery at the South Egypt Cancer Institute Cancers 2 1771–8 DOI: 10.3390/cancers2041771
2. Ferlay J, Shin HR, Bray F, Forman D, Mathers C and Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008 Int J Cancer 12 72893–917 DOI: 10.1002/ijc.25516
3. Dey S, Soliman AS, Hablas A, Seifeldein IA, Ismail K, Ramadan M, El-Hamzawy H, Wilson ML, Banerjee M and Boffetta P (2010). Urban-rural differences in breast cancer incidence in Egypt (1999–2006) Breast 19 417–23 DOI: 10.1016/j.breast.2010.04.005 PMID: 20452771
4. Zawilla N (2011) Breast cancer in Egypt: a fact sheet The Health 2 8–10
5. Feller L, Kramer B and Lemmer J (2011) A short account of metastatic bone disease Cancer Cell Int 11 24–9 DOI: 10.1186/14752867-11-24 PMID: 21794164 PMCID: 3160351
6. Korde LA and Gralow JR (2011) Can we predict who’s at risk for developing bone metastases in breast cancer? J Clin Oncol 29 3600–4 DOI: 10.1200/JCO.2011.35.7038 PMID: 21859994
7. Mantovani A, Allavena P, Sica A and Balkwill F (2008) Cancer-related inflammation Nature 454 436–44 DOI: 10.1038/nature07205 PMID: 18650914
8. Zhao Y, Kong X, Li X, Yan S, Yuan C, Hu W and Yang Q (2011) Metadherin mediates lipopolysaccharide-induced migration and invasion of breast cancer cells PLoS One 6 e29363 DOI: 10.1371/journal.pone.0029363 PMID: 22195048 PMCID: 3241708
9. Hillen F and Griffioen AW (2007) Tumor vascularization: sprouting angiogenesis and beyond Cancer Metastasis Rev 26 489–502 DOI: 10.1007/s10555-007-9094-7 PMID: 17717633
10. Kumar S and Weaver VM (2009). Mechanics, malignancy, and metastasis: the force journey of a tumor cell Cancer Metastasis Rev 28113-27 DOI: 10.1007/s10555-008-9173-4 PMID: 19153673 PMCID: 2658728
11. Huang Q and Ouyang X (2012) Biochemical markers for the diagnosis of bone metastasis: a clinical review Cancer Epidemiol 36 94–8 DOI: 10.1016/j.canep.2011.02.001
12. Gerges N and Jabado N (2010) Biomarkers in cancer micrometastasis: where are we at? Bioanalysis 2 881–99 DOI: 10.4155/ bio.10.49 PMID: 21083219
13. Elias EG, Leuchten JM, Buda BS and Brown SD (1986) Prognostic value of initial mononucleated cell percentages in patients with epidermoid carcinoma of the head and neck Am J Surg 152 487–90 PMID: 3777326
14. Kwak MS, Lee JH, Chung GE, Yu SJ, Jung EU, Kim BH, Choi DH, Kim HY, Kim SH, Lee JM, Lee JY, Kim YJ, Yoon JH and Lee HS (2011) Low P2/MS value is an independent risk factor for early recurrence of hepatocellular carcinoma after radiofrequency ablation therapy Hepatogastroenterology 58 147–52 PMID: 21510303
15. Pinsky PF and Zhu CS (2011) Building multi-marker algorithms for disease prediction—the role of correlations among markers Biomark Insights 6 83–93 DOI: 10.4137/BMI.S7513 PMID: 21918599 PMCID: 3169344
16. Mina LA and Sledge GW Jr (2011) Rethinking the metastatic cascade as a therapeutic target Nat Rev Clin Oncol 83 25–32
17. Ungefroren H, Sebens S, Seidl D, Lehnert H and Hass R (2011) Interaction of tumor cells with the microenvironment Cell Commun Signal 9 18 DOI: 10.1186/1478-811X-9-18 PMID: 21914164 PMCID: 3180438
18. Fan F, Schimming A, Jaeger D and Podar K (2012) Targeting the tumor microenvironment: focus on angiogenesis J Oncol 2012 281261 DOI: 10.1155/2012/281261
19. Calorini L and Bianchini F (2010) Environmental control of invasiveness and metastatic dissemination of tumor cells: the role of tumor cell-host cell interactions Cell Commun Signal 8 24 PMID: 20822533 PMCID: 2945354
20. Chechlinska M, Kowalewska M and Nowak R (2010) Systemic inflammation as a confounding factor in cancer biomarker discovery and validation Nat Rev Cancer 10 2–3 PMID: 20050335
21. Carlsson A, Wingren C, Kristensson M, Rose C, Fernö M, Olsson H, Jernström H, Ek S, Gustavsson E, Ingvar C, Ohlsson M, Peterson C and Borrebaeck CA (2011) Molecular serum portraits in patients with primary breast cancer predict the development of distant metastases Proc Natl Acad Sci USA 108 14252–7 DOI: 10.1073/pnas.1103125108 PMID: 21844363 PMCID: 3161545
22. Duffy MJ (2006) Serum tumor markers in breast cancer: are they of clinical value? Clin Chem 52 345–51 DOI: 10.1373/ clinchem.2005.059832 PMID: 16410341
23. Evangelista L, Baretta Z, Vinante L, Cervino AR, Gregianin M, Ghiotto C, Bozza F and Saladini G (2011) Could the serial determination of CA15.3 serum improve the diagnostic accuracy of PET/CT?: results from small population with previous breast cancer Ann Nucl Med 25 469–77 DOI: 10.1007/s12149-011-0488-9 PMID: 21476056
24. Bernier AJ, Zhang J, Lillehoj E, Shaw AR, Gunasekara N and Hugh JC (2011) Non-cysteine linked MUC1 cytoplasmic dimers are required for Src recruitment and ICAM-1 binding induced cell invasion Mol Cancer 10 93 DOI: 10.1186/1476-4598-10-93 PMID: 21798038 PMCID: 3161956
25. Duffy MJ, Evoy D and McDermott EW (2010) CA 15.3: uses and limitation as a biomarker for breast cancer Clin Chim Acta 411 1869–74 DOI: 10.1016/j.cca.2010.08.039 PMID: 20816948
26. Kufe DW (2009) Mucins in cancer: function, prognosis and therapy Nat Rev Cancer 9 874–85 DOI: 10.1038/nrc2761 PMID: 19935676
27. Zhao X, Xu X, Zhang Q, Jia Z, Sun S, Zhang J, Wang B, Wang Z and Hu X (2011) Prognostic and predictive value of clinical and biochemical factors in breast cancer patients with bone metastases receiving “metronomic” zoledronic acid BMC Cancer 11 403 DOI: 10.1186/1471-2407-11-403 PMID: 21936956 PMCID: 3196722
28. Yerushalmi R, Tyldesley S, Kennecke H, Speers C, Woods R, Knight B and Gelmon KA (2012) Tumor markers in metastatic breast cancer subtypes: frequency of elevation and correlation with outcome Ann Oncol 23 338–45 DOI: 10.1093/annonc/mdr154
29. Kelly-Spratt KS, Pitteri SJ, Gurley KE, Liggitt D, Chin A, Kennedy J, Wong CH, Zhang Q, Buson TB, Wang H, Hanash SM and Kemp CJ (2011) Plasma proteome profiles associated with inflammation, angiogenesis, and cancer PLoS One 6 e19721 DOI: 10.1371/ journal.pone.0019721 PMID: 21589862 PMCID: 3093388
30. Ch’ng ES, Jaafar H and Tuan Sharif SE (2011) Breast tumor angiogenesis and tumor-associated macrophages: histopathologist’s perspective Patholog Res Int 2011 572706
31. Murdoch C, Giannoudis A and Lewis CE (2004) Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues Blood 104 2224–34 DOI: 10.1182/blood-2004-03-1109 PMID: 15231578
32. Rogers TL and Holen I (2011) Tumor macrophages as potential targets of bisphosphonates J Transl Med 9 177 DOI: 10.1186/1479 5876-9-177 PMID: 22005011 PMCID: 3215187
33. Tazzyman S, Lewis CE, Murdoch C (2009) Neutrophils: key mediators of tumor angiogenesis Int J Exp Pathol 90 222–31 DOI: 10.1111/j.1365-2613.2009.00641.x PMID: 19563607
34. Rüegg C (2006) Leukocytes, inflammation, and angiogenesis in cancer: fatal attractions J Leukoc Biol 80 682–4 DOI: 10.1189/ jlb.0606394 PMID: 16849612
35. Angelillo-Scherrer A (2012) Leukocyte-derived microparticles in vascular homeostasis Circ Res 110 356–69 DOI: 10.1161/ CIRCRESAHA.110.233403 PMID: 22267840
36. Porrata LF, Ristow K, Colgan JP, Habermann TM, Witzig TE, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Nowakowski GS, Thompson C and Markovic SN (2012) Peripheral blood lymphocyte/monocyte ratio at diagnosis and survival in classical Hodgkin’s lymphoma Haematologica 97 262–9 DOI: 10.3324/haematol.2011.050138 PMCID: 3269488
37. Milde D, Altmannova K, Vyslouzil K and Stuzka V (2005) Trace element levels in blood serum and colon tissue in colorectal cancer Chemical Papers-Slovak Academy of Sciences 59 157–60
38. Sheffer M, Simon AJ, Jacob-Hirsch J, Rechavi G, Domany E, Givol D and D’Orazi G (2011) Genome-wide analysis discloses reversal of the hypoxia-induced changes of gene expression in colon cancer cells by zinc supplementation Oncotarget 2 1191–202 PMID: 22202117
39. Belting M, Ahamed J and Ruf W (2005) Signaling of the tissue factor coagulation pathway in angiogenesis and cancer Arterioscler Thromb Vasc Biol 25 1545–50 DOI: 10.1161/01.ATV.0000171155.05809.bf PMID: 15905465
40. Peddareddigari VG, Wang D and Dubois RN (2010) The tumor microenvironment in colorectal carcinogenesis Cancer Microenviron 3 149–66 DOI: 10.1007/s12307-010-0038-3
41. Bambace NM and Holmes CE (2011) The platelet contribution to cancer progression J Thromb Haemost 9 237–49 DOI: 10.1111/ j.1538-7836.2010.04131.x
42. Boucharaba A, Serre CM, Grès S, Saulnier-Blache JS, Bordet JC, Guglielmi J, Clézardin P and Peyruchaud O (2004) Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer J Clin Invest 114 1714–25 PMID: 15599396 PMCID: 535068
43. Yu SJ, Lee JH, Chung GE, Lee CH, Cho EJ, Jang ES, Kwak MS, Kim YJ, Yoon JH,Jang JJ andLee HS (2010) Validation of P2/ MS for reflecting hepatic fibrosis in patients with hepatocellular carcinoma Korean J Hepatol 16 389–96 DOI: 10.3350/ kjhep.2010.16.4.389
44. Yu SJ, Kim DH, Lee JH, Chung GE, Yim JY, Park MJ, Kim YJ, Yoon JH, Jang JJ and Lee HS (2011) Validation of P2/MS and other noninvasive fibrosis scoring systems in the Korean population with nonalcoholic fatty liver disease Korean J Gastroenterol 57 19–27 DOI: 10.4166/kjg.2011.57.1.19 PMID: 21258197
45. Voorzanger-Rousselot N, Juillet F, Mareau E, Zimmermann J, Kalebic T and Garnero P (2006) Association of 12 serum biochemical markers of angiogenesis, tumor invasion and bone turnover with bone metastases from breast cancer: a crosssectional and longitudinal evaluation Br J Cancer 95 506–14 DOI: 10.1038/sj.bjc.6603285 PMID: 16880790
46. Uccello M, Malaguarnera G, Vacante M and Motta M (2011) Serum bone sialoprotein levels and bone metastases J Cancer Res Ther 7115–9 DOI: 10.4103/0973-1482.82912 PMID: 21768695
47. Chao TY, Wu YY and Janckila AJ (2010) Tartrate-resistant acid phosphatase isoform 5b (TRACP5b) as a serum maker for cancer with bone metastasis Clin Chim Acta 411 1553–64 DOI: 10.1016/j.cca.2010.06.027 PMID: 20599857
48. Hung MJ and Oremek GM (2011) Value of TRACP5b as a diagnostic marker for detection of bone metastases in patients with breast cancer Eur J Gynaecol Oncol 32 615–8
49. Capeller B, Caffier H, Sütterlin MW and Dietl J (2003) Evaluation of tartrate-resistant acid phosphatase (TRAP) 5b as serum marker of bone metastases in human breast cancer Anticancer Res 231011–16
50. Chao TY, Ho CL, Lee SH, Chen MMJ, Janckila AJ and Yam LT (2004) Tartrate-resistant acid phosphatase 5b as a serum marker of bone metastasis in breast cancer patients J Biomed Sci 11511–16
51. Wu YY, Janckila AJ, Ku CH, Yu CP, Yu JC, Lee SH, Liu HY, Yam LT and Chao TY (2010) Serum tartrate-resistant acid phosphatase 5b activity as a prognostic marker of survival in breast cancer with bone metastasis BMC Cancer 10 158 DOI: 10.1186/14712407-10-158 PMID: 20416078 PMCID: 2873389
52. Leeming DJ, Hegele A, Byrjalsen I, Hofmann R, Qvist P, Karsdal MA, Schrader AJ, Wagner R and Olbert P (2008) Biochemical markers for monitoring response to therapy: evidence for higher bone specificity by a novel marker compared with routine markers Cancer Epidemiol Biomarkers Prev 17 1269–76 DOI: 10.1158/1055-9965.EPI-07-2697 PMID: 18483350
53. Fohr B, Dunstan CR and Seibel MJ (2003) Clinical review 165: markers of bone remodeling in metastatic bone disease J Clin Endocrinol Metab 88 5059–75 DOI: 10.1210/jc.2003-030910 PMID: 14602728
54. McMillan DC, Sattar N, Talwar D, O’Reilly DS and McArdle CS (2000) Changes in micronutrient concentrations following antiinflammatory treatment in patients with gastrointestinal cancer Nutrition 16 425–8 DOI: 10.1016/S0899-9007(00)00270-7 PMID: 10869897
55. Brown DJ, Milroy R, Preston T and McMillan DC (2007) The relationship between an inflammation-based prognostic score (Glasgow Prognostic Score) and changes in serum biochemical variables in patients with advanced lung and gastrointestinal cancer J Clin Pathol 60 705–8 DOI: 10.1136/jcp.2005.033217
56. Proctor MJ, Morrison DS, Talwar D, Balmer SM, O’Reilly DS, Foulis AK, Horgan PG and McMillan DC (2011) An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumor site: a Glasgow Inflammation Outcome Study Br J Cancer 104 726–34 DOI: 10.1038/sj.bjc.6606087 PMID: 21266974 PMCID: 3049591






