Evaluating the readiness for ultra-hypofractionated prostate and breast radiotherapy in sub-Saharan Africa: a strategic needs-assessment of six leading African institutions
Joseph Weygand1, Yao Hao2, Munir Awol3, Adedayo O Joseph4,5, Solomon Kibudde6, Abba Malloum7,8, Twalib A Ngoma9,10, Samuel O Adeneye4,5, Kavuma Awusi6, Jumaa D Kisukari10, Thokozani Mkhize11,12, Maureen Bilinga Tendwa13, Victoria Ainsworth14, Azeezat Ajose5, William Swanson15, Stephen Avery16, Rohini Bhatia15, Frank Chinegwundoh17,18, Curtiland Deville19, Mohammed Saiful Huq20, Heng Li19, Joerg Lehmann21,22,23, Christopher F Njeh24, Krishni Wijesooriya25, Wilfred Ngwa19, Katy Graef26, Janine Simons27, Onyinye Balogun28 and Luca Incrocci27
1Department of Radiation Oncology and Applied Sciences, Dartmouth College, Lebanon, NH 03766, USA
2Department of Radiation Oncology, Washington University School of Medicine, St. Louis, MO 63130, USA
3Department of Oncology, School of Medicine, Addis Ababa University, Addis Ababa 1165, Ethiopia
4Department of Radiation Biology, Radiotherapy and Radiodiagnosis, College of Medicine, University of Lagos, Lagos 102215, Nigeria
5NSIA-LUTH Cancer Centre, Lagos University Teaching Hospital, Lagos 102215, Nigeria
6Uganda Cancer Institute, Kampala 759125, Uganda
7Department of Radiotherapy and Oncology, University of KwaZulu-Natal, Durban 4041, South Africa
8Department of Oncology, Inkosi Albert Luthuli Central Hospital, Durban 4138, South Africa
9Department Clinical Oncology, Muhimbili University of Health and Allied Sciences, Dar es Salaam 11000, Tanzania
10Ocean Road Cancer Institute, Dar es Salaam 11000, Tanzania
11Department of Nuclear Medicine, University of KwaZulu-Natal, Durban 4041, South Africa
12Department of Nuclear Medicine, Inkosi Albert Luthuli Central Hospital, Durban 4138, South Africa
13South Africa Health Product Regulatory Authority, Pretoria 0007, South Africa
14Department of Physics and Applied Physics, University of Massachusetts Lowell, Lowell, MA 01854, USA
15Department of Radiation Oncology, Emory University, Atlanta, GA 30322, USA
16Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA 19104, USA
17Department of Urology, Barts Health NHS Trust, London E1 2ES, UK
18City St. George’s, University of London, London SW17 0RE, UK
19Department of Radiation Oncology and Molecular Radiation Sciences, Johns Hopkins University, Baltimore, MD 21218, USA
20Department of Radiation Oncology, University of Pittsburgh School of Medicine and UPMC Hillman Cancer Center, Pittsburgh, PA 15260, USA
21Department of Radiation Oncology, Calvary Mater Newcastle, Newcastle 2298, Australia
22School of Information and Physical Sciences, University of Newcastle, Newcastle 2308, Australia
23Institute of Medical Physics, University of Sydney, Sydney 2006, Australia
24Department of Radiation Oncology, Indiana University School of Medicine, Indianapolis, IN 47405, USA
25Department of Radiation Oncology, University of Virginia School of Medicine, Charlottesville, VA 22903, USA
26BIO Ventures for Global Health, Seattle, WA 98121, USA
27Department of Radiotherapy, Erasmus Medical Center, Rotterdam 3015, The Netherlands
28Department of Radiation Oncology, Weill Cornell Medicine, New York, NY 10021, USA
Abstract
Sub-Saharan Africa (sSA) continues to face a critical shortage in radiotherapy resources, exacerbating the region’s growing cancer burden. One potential strategy that can partially offset this problem is the increased adoption and broader implementation of ultra-hypofractionated radiotherapy (UHFRT), whereby a smaller number of treatment sessions are required since each session administers higher doses of radiation (to an equivalent biological dose) compared to conventional fractionation. UHFRT techniques have been widely adopted in Europe and North America, particularly for prostate and breast treatments, but differences in the available technology and demographics and biology in sSA necessitate rigorous evaluation of the existing infrastructure and clinical workflows before its widespread implementation in these settings. This study makes a first attempt to interrogate the readiness of six leading sSA institutions for the transition toward UHFRT treatment regimens. The survey was structured into five sections which assessed (1) general clinical capacity and infrastructure, (2) the clinical breast cancer treatment program, (3) the clinical prostate cancer treatment program, (4) medical physics support and quality management and (5) research capacity. The survey responses revealed a strong willingness among African clinicians to adopt UHFRT treatment regimens and generally sufficient supporting infrastructure (i.e., equipment, staffing, quality assurance programs and research support) already in place. However, some technical gaps were identified such as the lack of employment of breath-hold techniques in treating breast cancer and nonutilisation of fiducial markers and perirectal spacers in treating prostate cancer. All six responding institutions expressed enthusiasm to participate in a training course aimed at addressing these technical gaps. These findings underscore the potential for the successful implementation of breast and prostate cancer UHFRT in sSA, provided that targeted training and technical support are delivered. Addressing the identified gaps will be critical in ensuring the safe and effective adoption of this advanced treatment technique across the region.
Keywords: ultra-hypofractionated radiotherapy, radiotherapy capacity building, sub-Saharan Africa, prostate cancer, breast cancer, needs assessment
Correspondence to: Joseph Weygand
Email: joseph.weygand@hitchcock.org
Published: 20/02/2025
Received: 10/10/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Cancer has emerged as a significant and escalating health crisis in sub-Saharan Africa (sSA). Between 1990 and 2017, sSA experienced an almost two-fold increase in cancer incidence [1], with projections indicating that both incidence and cancer-related mortality in sSA could double once more between 2020 and 2040 [2]. Radiation therapy, indicated in over half of all cancer patients [3], is a crucial treatment modality that can partially alleviate this crisis [4]. Despite this, countries in sSA have historically lacked sufficient capacity to meet local radiotherapy demands [5, 6]. According to a 2015 Lancet Oncology Commission report [7], over 25 countries in sSA lacked access to radiation therapy altogether and many of the countries in sSA that do provide radiotherapy services only have the capacity to treat a small fraction of the country’s cancer patients. More recently, a 2022 Lancet Oncology Commission report [8] demonstrated only marginal improvements in radiotherapy access across the region. The report estimated that over 5,000 treatment units would need to be installed in sSA over the next 15 years to achieve equitable cancer care, a target that is unfortunately improbable, given current resource constraints in the region.
In addition to equipment shortages, sSA faces a critical deficit in the radiotherapy workforce [9, 10]. Offering radiotherapy services requires radiation therapists, radiation or clinical oncologists and medical physicists [11], the latter two roles requiring several years of specialised education and training. As an example, the International Atomic Energy Agency (IAEA) recommends that there be at least one radiation oncologist per 250–300 patients annually [12]. This, however, is an unattainable benchmark in most countries in sSA where there are often only one or two oncologists available to serve disproportionately large patient volumes. This imbalance can lead to substandard care and, as a result, poor clinical outcomes [13].
Until the dearth of radiotherapy equipment and the radiation oncology workforce in sSA are adequately addressed, there remains an urgent need for strategies that can increase patient throughput, partially alleviating this crisis. A frequently proposed solution is the increased utilisation of hypofractionated treatment regimens [14–18] whereby a reduced number of treatment sessions deliver larger doses of radiation (to an equivalent biological dose) relative to conventional fractionation [19]. The lower α/β ratio in certain disease sites, such as prostate [20] and breast [21], renders the radiobiological rationale of hyperfractionation irrelevant, allowing for the possibility to safely hypofractionate [22]. Large multicenter randomised clinical trials performed in both North America and Europe have demonstrated the noninferiority of moderate- and ultra-hypofractionated radiotherapy (UHFRT) in both prostate [23–26] and breast cancer [27–30], precipitating the adoption of hypofractionated radiotherapy regimens as the standard of care for localised disease in high-income countries [31, 32].
Moderate hypofractionation, however, can only marginally increase access to radiotherapy in sSA. By contrast, UHFRT, defined herein as dose-fractionation regimens with fractional doses exceeding 5 Gy [33], on the other hand, has the potential to markedly increase access to radiotherapy in sSA. As an example, a UHFRT regimen of 42.7 Gy in seven fractions could theoretically treat more than five prostate cancer patients for every one prostate patient treated with a conventionally fractionated regimen of 78 Gy in 39 fractions since the number of fractions is reduced by a factor of greater than 5.5 when transitioning from conventional fractionation to UHFRT techniques [34]. Similar gains could be realised by utilising a UHFRT regimen of 26 Gy in five fractions rather than more conventional regimens for breast patients [35] such as of 50 Gy in 25 fractions. Randomised clinical trials ran in Sweden [36] and the UK [37] for prostate and breast, respectively, have established the noninferiority of these UHFRT techniques.
However, the establishment of noninferiority in a European and North American setting does not necessarily confirm noninferiority in an African setting where the patient populations and, in some instances, the radiation treatment technology are different. This necessitates the local exploration of the feasibility, safety and impact on outcomes of implementing ultra-hypofractionation (UHF) radiotherapy in the sSA context. The multi-center HypoAfrica study [38], modelled on the CHHiP trial [23], is underway to assess the feasibility of moderate hypofractionation in prostate cancer in multiple centers throughout sSA. Additional clinical trials to similarly assess hypofractionation in breast and cervical cancer by the same sites are in development [39]. As moderate hypofractionated techniques gain traction in sSA the logical next step is to begin to explore the feasibility of UHFRT techniques in an African setting. This study makes the first attempt to assess the readiness of UHFRT in an African setting by reporting the results of a comprehensive needs-assessment survey on the implementation of UHFRT carried out at six leading cancer treatment institutions in sSA.
Methods
A needs-assessment survey was developed by a panel of radiotherapy experts, namely physicists and oncologists, from sites in Africa, the United States, Europe and Australia. The survey was created as a Microsoft Word document and distributed to the various sites via email. The target population included clinical sites in Africa that participated in the first HypoAfrica study and those who are starting to engage in the HypoAfrica network. The survey was administered to six sites in six different African countries: (1) the Nigeria Sovereign Investment Authority and Lagos University Teaching Hospital Cancer Center in Lagos, Nigeria, (2) Ocean Road Cancer Institute in Dar es Salaam, Tanzania, (3) Inkosi Albert Luthuli Central Hospital in Durban, South Africa, (4) Uganda Cancer Institute in Kampala, Uganda, (5) Black Lion Hospital in Addis Ababa, Ethiopia and (6) Parirenyatwa Hospital Radiotherapy Centre in Harare, Zimbabwe. The geographical distribution of the surveyed sites throughout Africa is illustrated in Figure 1. As can be seen, one of the sites is located in West Africa, two are in Southern Africa and three are in East Africa (one of which is in the Horn of Africa). All centers with the exception of Black Lion Hospital in Ethiopia are in Anglophone Africa.
Figure 1. Geographic distribution of the six African institutions participating in the needs assessment survey.
The survey was constructed to interrogate all relevant aspects of the respondents’ UHFRT programs. It consisted of an introductory section followed by five substantive sections. The introductory section collected demographic information including respondent’s name and contact information, the name and location of the institution and whether the institution is academic, government-owned or private practice. Section I evaluated the general clinical capacity of each institution and was to be completed by clinical directors. This section assessed the clinical infrastructure, radiotherapy equipment and techniques, staffing, training capacity and referral patterns. To provide context for UHFRT readiness, Section I also established a benchmark of each institution's general clinical capacity and infrastructure. These data offered insights into foundational resources, such as imaging capabilities and staffing, that are essential for the safe implementation of UHFRT.
Section II investigated the clinical breast cancer treatment program and was to be filled out by a clinical or radiation oncologist responsible for treating breast cancer. This section assessed the institution’s current breast cancer treatment infrastructure and capacity, current dose-fractionation patterns in use, utilisation of breath-hold techniques and willingness of clinicians to consider UHFRT regimens for clinical presentations requiring whole breast radiation, regional nodal irradiation or chest wall radiation. Section III investigated the institution’s clinical prostate cancer treatment program and was to be filled out by a clinical or radiation oncologist responsible for treating prostate cancer. This section assessed the current prostate cancer treatment infrastructure of the respondent’s institution, dose-fractionation patterns currently used to treat prostate cancer, the utilisation of implanted fiducial markers [40] and perirectal spacers [41] and clinical considerations of using UHFRT techniques in prostate cancer. Section IV investigated the medical physics aspects of UHFRT and was to be filled out by the chief medical physicist. This section assessed the quality management program [42–44], the image-guided techniques [45–47] employed and the utilisation of artificial intelligence (AI) [48] of the respondent’s institution. The final section, Section V, investigated the institution’s research capacity and was to be filled out by clinical directors. This section assessed the previous research and clinical trial experience, management of the research program and institutional review board (IRB) considerations. When quantitative results were available, the mean and standard deviation were calculated. This study was reviewed by the IRB at the University of Massachusetts Lowell and was determined to be exempt from further review due to its minimal risk to participants.
Results
Responses were received from each of the six institutions to whom the survey was distributed. 83.3% of respondents (5/6) identified their institution as academic, while 50.0% of respondents (3/6) stated that their institution was government-owned. All facilities surveyed utilise an electronic medical record system. Half of the facilities exclusively use electronic records, while the other half use a combination of electronic and paper records.
Section I: General clinical capacity and infrastructure
There was a wide range in the size of the radiotherapy departments of the respondents and the equipment that they had at their disposal. Figure 2 illustrates the quantities of the equipment offered by each institution. As can be seen, five of the six (83.3%) institutions possess computed tomography (CT) simulators, while half of the institutions (50.0%) utilise two-dimensional simulators, one site (16.7%) exclusively and two (33.3%) in addition to their CT simulators. All institutions (100.0%) offer treatment with linear accelerators, although there is variation in the number possessed: one institution (16.7%) has one, two institutions (33.3%) have two, two institutions (33.3%) have three and one institution (16.7%) has more than five. Additionally, half of the institutions (50.0%) also utilise cobalt-based teletherapy. Each center (100.0%) employs high-dose rate (HDR) brachytherapy in addition to external beam services. CT is the most prevalent advanced imaging technique [49] used among the surveyed institutions, as all centers (100.0%) possess at least one CT scanner. Magnetic resonance imaging (MRI) scanners [50, 51], positron emission tomography (PET) scanners and gamma cameras used for nuclear medicine are rarer in sSA, as evidenced by the fact that only two (33.3%), three (50.0%) and two (33.3%) of the surveyed institutions offered these imaging services, respectively. All centers (100.0%) offer mammography services, and four of the centers (66.7%) have on-board kV portal imaging in conjunction with their treatment units.
Each institution (100.0%) surveyed has training programs for both radiation oncologists and radiation therapists. Half of the institutions (50.0%) surveyed had training programs for medical physicists and nurses. Only one institution (16.7%) had a training program for medical dosimetrists. Each center (100.0%) employed either four or more than five radiation oncologists, four or more than five physicists, more than five radiation therapists and more than five nurses. Dosimetrists (1.5 ± 2.0), statisticians (1.8 ± 1.7), data assistants (2.0 ± 1.9) and research coordinators (2.2 ± 1.7) were relatively less common.
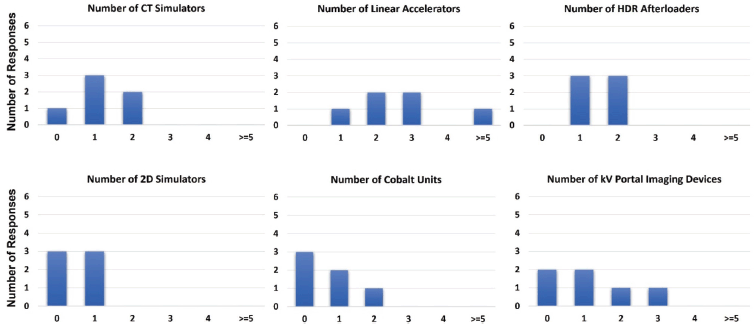
Figure 2. Radiotherapy equipment capacity among surveyed institutions.
All centers (100.0%) stated that they employ three-dimensional conformal radiotherapy and electron-based radiotherapy. 66.7% of the surveyed centers (4/6) employ intensity-modulated radiotherapy (IMRT)-based treatments, while 16.7% (1/6) of the surveyed centers employed stereotactic techniques. All centers use Eclipse by Varian Medical Systems (Palo Alto CA, USA) as their primary external-beam treatment planning system, while one site additionally uses Monaco by Elekta (Stockholm, Sweden). SagiPlan by BEBIG Medical GmbH (Berlin, Germany) and BrachyVision by Varian were used by four (66.7%) and two centers (33.3%), respectively, for brachytherapy planning. In addition to radiotherapy, all surveyed institutions offer laboratory testing, biopsy, histology [52–55] and chemotherapy services. 83.3% (5/6) of surveyed institutions offered immunohistochemistry and 66.7% (4/6) offered genetic testing. All surveyed institutions additionally employ medical oncologists, breast surgeons and urologists.
Section II: Clinical breast cancer treatment program
Enthusiasm to utilise UHFRT for breast cancer was found. Figure 3 illustrates the oncologists’ willingness to utilise UHFRT in a variety of clinical situations. As can be seen, 66.7% of the respondents (4/6) were willing to use UHFRT techniques for whole-breast radiotherapy following breast conservation therapy, whole-breast radiotherapy with an indication for a boost to the tumour bed post-lumpectomy and post-mastectomy chest wall radiotherapy. 50.0% of the respondents (3/6) indicated a willingness to use UHFRT techniques for patients with an indication of regional radiation. Moreover, it was demonstrated that three of the centers (50.0%) are already employing fractional doses greater than or equal to 5 Gy for some patients with doses of either 26 Gy in five fractions or 25 Gy in five fractions. Other dose-fractionation schemes commonly employed were 50 Gy in twenty-five fractions, 40.05 Gy in fifteen fractions or 42.67 Gy in sixteen fractions.
Reasons cited for hesitancy toward UHFRT for breast cancer were concerns over motion management (66.7%, 4/6), inadequate technology (16.7%, 1/6), personal comfort (66.7%, 4/6), acute normal tissue toxicities (16.7%, 1/6), late normal tissue toxicities (50.0%, 3/6), a lack of evidence (33.3%, 2/6) and cost and reimbursement (16.7%, 1/6). The term ‘personal comfort’ reflects clinicians' familiarity and confidence in using UHFRT regimens for breast cancer, as these techniques represent a significant shift from conventional practices. Only one (16.7%) out of the six respondents indicated that their clinic employs breath-hold techniques. 66.7% (4/6) of respondents stated that their contours (both targets and organs-at-risk) undergo a peer review, while 83.3% (5/6) of respondents stated that their radiation treatment plans undergo a peer review.
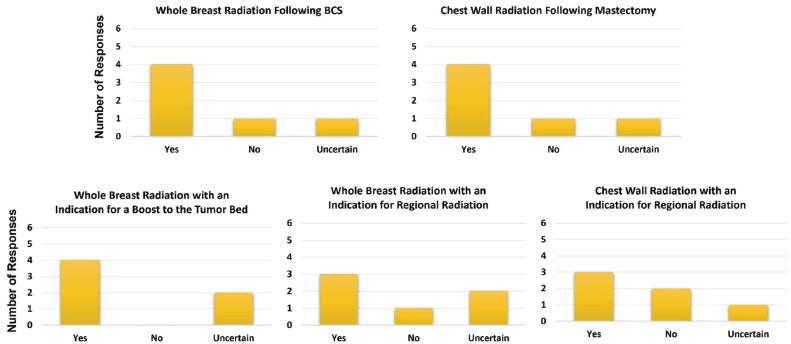
Figure 3. Willingness to utilise ultra-hypofractionated treatment techniques in a variety of clinical scenarios in breast cancer.
Section III: Clinical prostate cancer treatment program
For prostate cancer, none of the respondents reported employing UHFRT dose-fractionation regimens, although half of the respondents (50.0%) reported employing the more moderate hypofractionation regimen of 60 Gy in 20 fractions. This is not surprising since none of the respondents reported using either implanted fiducial markers or perirectal spacers to aid in the localisation and reduction of normal tissue toxicities, respectively, of their prostate cancer patients. Other fractionation schemes that were reportedly employed were 78 Gy in 39 fractions (33.3%, 2/6), 70 Gy in 28 fractions (66.7%, 4/6), 74 Gy in 34 fractions (16.7%, 1/6), 60 Gy in 20 fractions (50.0%, 3/6) and 70 Gy in 35 fractions (16.7%, 1/6).
Section IV: Radiotherapy quality assurance (QA) programs
Figure 4 illustrates the perceived benefits of adopting UHFRT techniques among the surveyed clinicians. All respondents (100.0%) indicated an inclination towards UHFRT techniques citing enhanced treatment capacity, as well as reduced treatment duration for patients, and lower associated costs. Furthermore, five of the six respondents (83.3%) indicated that UHFRT techniques would reduce the burden on the treatment machines and/or institutions, with half (50%) indicating their belief that UHFRT techniques would also lower costs to providers and/or institutions.
All respondents (100.0%) reported the existence of a quality management program at their respective institutions. 83.3% of respondents (5/6) reported that their radiotherapy programs engage in external dosimetry auditing, through the IAEA [56] in Vienna, the Imaging and Radiation Oncology Core [57] in Houston or other national regulatory bodies. All respondents (100.0%) confirmed that patient-specific QA [58–60] is performed for each patient receiving IMRT, five of which (83.3%) use portal dosimetry [61], while one (16.7%) uses log file-based [62] analysis methods. 83.3% of respondents (5/6) indicated that medical physicists perform initial plan checks before treatment. Additionally, 83.3% of respondents (5/6) performed peer review on their contour volumes (both targets and organs-at-risk) and on their treatment plans. However, only 33.3% of respondents (2/6) indicated that medical physicists perform weekly and end-of-treatment chart reviews. Similarly, only 33.3% of respondents (2/6) claimed that radiation oncologists perform daily image reviews for their image-guided treatments. 66.7% of the respondents (4/6) indicated that their institution utilised AI-based auto-contouring [63] software, although there is stark heterogeneity as to which software option is being used amongst the surveyed institutions.
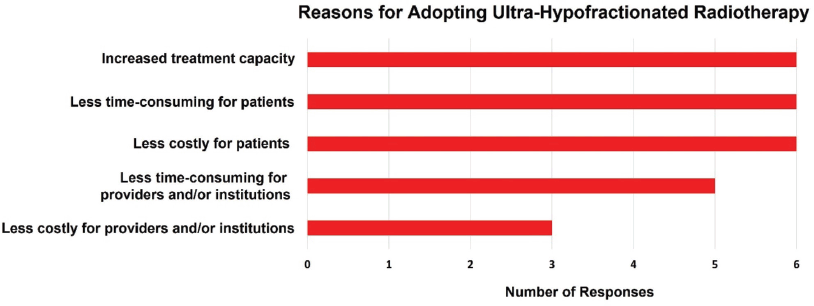
Figure 4. Reasons for adopting UHFRT.
The range of linear accelerator QA tests is illustrated in Figure 5. Here, the specific tests inquired about are shown as different rows, and the frequency at which the test is performed is color coded (blue – performed daily; orange – performed monthly; green – performed annually). As can be seen, general radiation safety tests are performed daily at each institution. Radiation output is measured daily, monthly and annual at each institution. Flatness and symmetry are measured both monthly and annually at each institution but are only measured daily at 66.7% of the institutions (4/6). The lasers, the position of the radiation isocenter and the motion of the treatment couch are being monitored monthly and annually at each institution (100.0%) but only daily at some institutions. Notably, positioning/repositioning tests are only being performed daily at two of the six institutions (33.3%) despite recommendations [64] that state that they should be performed on a daily basis. Monitor unit linearity, the coincidence of the radiation and mechanical isocenter and end-to-end tests verifying localisation and dosimetric delivery are performed annually by each institution. However, small field dosimetry measurements [65, 66] are only performed annually by 33.3% of the institutions (2/6).
Section V: Clinical research environment
83.3% of the respondents (5/6) reported institutional experience in running clinical trials [67] for either breast or prostate cancer. All of the respondents (100.0%) reported that their institution has previous clinical research experience, although the number of previous studies varied widely: from 1 previous study reported by 1 institution (16.7%) to over 50 reported by 2 institutions (33.3%). Among the responding institutions, the management of the research budget is handled by either the principal investigator, the university or some combination of the two. All institutions (100.0%) have an IRB, although the cost of an IRB application varies widely among the responding institutions: from 100 to 700 USD. All institutions provide some level of financial reimbursement for travel to patients on the protocol.
Four of the six respondents (66.7%) stated that additional equipment was needed for the execution of UHFRT clinical trials, and one respondent (16.7%) stated that they were uncertain. Anticipated challenges in the execution of UHFRT clinical trials in an African setting were numerous and included treatment costs, patient hesitancy, downtime of the linear accelerators, patient follow-up due to economic reasons and data handling. Moreover, all respondents expressed interest in a course covering the clinical and technical aspects pertinent to UHFRT. Suggested topics included clinical decision-making for breast, gynecological and genitourinary cancers [68], radiation biology [69], contouring, treatment planning, treatment delivery and verification and QA.
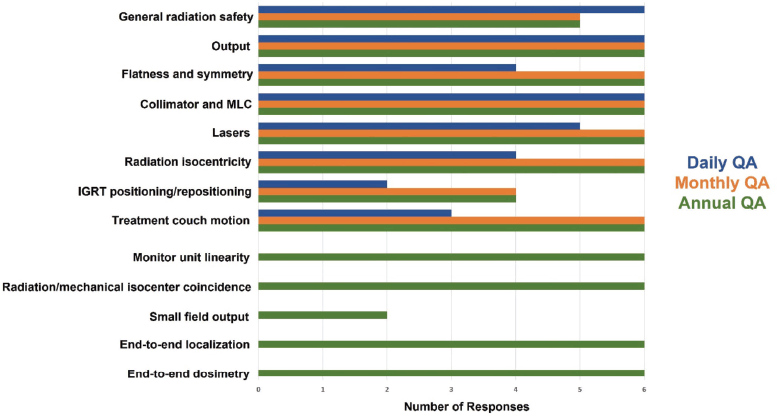
Figure 5. Linear accelerator QA tests and associated frequencies.
Discussion
In this survey, the feasibility of the application of UHFRT was assessed. Results indicated that there is strong interest in UHFRT in sSA due to its potential to increase treatment capacity and reduce costs. However, certain challenges remain to be addressed, such as limited access to advanced technology, concerns over motion management, potential toxicities, insufficient training and the need for additional equipment/processes to safely implement UHFRT in sSA. All respondents indicated a willingness to participate in dedicated practical training courses on the implementation of UHFRT to treat breast and prostate cancer.
This study highlights UHFRT as a strategy to improve access to radiotherapy in SSA, but its implementation must be contextualised. While UHFRT reduces the number of treatment sessions, other resource considerations – such as preparation time for fiducial marker placement and perirectal spacer application – can offset these gains. To address this, the broader adoption of moderate hypofractionation and single-fraction palliative radiotherapy should also be emphasised as complementary approaches. Additionally, infrastructure enhancements, such as streamlined workflows and better utilisation of available equipment, will be critical in ensuring that UHFRT can be both feasible and impactful.
Historically, radiation therapy has been delivered over a large number of low-dose (~ 2 Gy) treatment fractions [70] as a means to maximise the therapeutic ratio, which increases as the number of fractions increase. This was first experimentally observed nearly a century ago by Coutard [71] and given theoretical justification decades later with the development of the linear-quadratic model [72, 73]. This advantage is based on the larger α/β ratio of tumours relative to normal tissues. Some tumour types, however, such as breast and prostate tumours, have a lower α/β ratio than other tumours. For these malignancies, the α/β ratio is on the order of the associated late normal tissue toxicities, so that the advantage of hyperfractionation is negated [74]. This understanding has driven the shift towards shorter treatment courses for these disease sites in high-income countries over the past decade and a half and is the motivation for testing its utility in sSA where the conventional (traditionally longer) treatment courses limit access to radiotherapy treatment.
Given the substantial proportion of cancer patients in sSA presenting with advanced-stage disease, the demand for effective palliative radiotherapy remains pressing. Hypofractionated regimens, particularly single-fraction treatments for conditions such as painful bone metastases, spinal cord compression or other oncologic emergencies, must remain a cornerstone of care delivery. These approaches not only address critical symptom management but also offer a resource-efficient means of alleviating patient suffering within the context of severely constrained radiotherapy capacities. Ensuring that the implementation of UHFRT does not divert resources or attention away from these essential palliative services is imperative. Instead, a synergistic model that integrates hypofractionated palliative treatments with the broader adoption of UHFRT can help establish a more holistic and equitable radiotherapy framework. Such a balanced approach would optimise the use of existing resources while addressing both the curative and palliative needs of cancer patients in the region.
A key requirement for the safe implementation of UHFRT techniques is a high degree of spatial precision [75]. Delivering larger fractional doses can pose risks to patients without the utmost confidence that the delivered radiation field conforms to the tumour. It is no surprise that the UHFRT courses in high-income countries became common at roughly the same time that the availability of image-guided techniques [76–79] became widespread. If UHFRT techniques are going to be applied in an African setting, it is important that similarly stringent requirements for spatial localisation are maintained. Two survey respondents indicated that their treatment units lacked on-board kV imaging, precluding the standard UHFRT setup strategy for prostate cancer commonly used in high-income countries, where alignment is typically ensured via cone-beam CT (CBCT) image matching with the aid of fiducials implanted in the prostate. Three-dimensional alignment is still possible using orthogonal views on the megavoltage (MV) portal imaging device but is more challenging due to the reduced contrast at MV energies. In lower resource settings, novel solutions based on the available technology can often provide viable ways forward. Despite this, transitioning to higher risk treatment techniques like UHF necessitates rigorous evaluation of any proposed solutions to ensure patient safety.
Another identified deficiency was the underutilisation of implanted fiducial markers and perirectal spacer gel for prostate radiotherapy. The prostate exhibits significant interfraction motion [80–82], typically on the order of 2–4 mm in the anterior-posterior and superior-inferior axes and 1–2 mm laterally [83], although significantly larger motion has been observed [84]. First introduced in Canada nearly 30 years ago [85], fiducial markers have gained widespread application, as they aid in the three-dimensional localisation of the prostate. Perirectal spacer gel can also be inserted between the prostate and rectum to mitigate rectal toxicity by increasing the separation between the planning target volume and the anterior rectal wall [86]. First introduced in Europe in 2007 [87] this technique has become commonplace, particularly in prostate cancer patients with minimal anatomic separation between the prostate and rectum. Studies show that the use of perirectal spacer gels increases the separation between the prostate and rectum, reduces the mean rectal dose and minimises acute rectal toxicity [88]. None of the African institutions surveyed in this study used implanted fiducials or perirectal spacers. Thus, this presents a prime opportunity to train African clinical/radiation oncologists and/or urologists to use these tools but also provides an opportunity to intentionally implement strategies for safer delivery of prostate radiotherapy, navigating barriers in procurement and sustainability of radiotherapy accessories. The successful implementation of this technique will also necessitate the involvement of urologists, as their expertise is critical for the accurate placement of fiducials and perirectal spacers. Collaboration between radiation oncologists and urologists will be essential to ensure the safe and effective integration of these tools into prostate cancer treatment protocols in the region.
With this survey, the lack of utilisation of breath-hold techniques for breast cancer radiotherapy was identified. Breath-hold techniques, particularly deep inspiration breath-hold (DIBH), reduce radiation exposure to the heart and lungs during breast radiotherapy. During deep inspiration, the lungs expand, and the diaphragm moves downward. This expansion physically moves the heart away from the chest wall and out of the radiation field [89]. Also, since the lungs expand, the volume of the lungs increases, which reduces lung toxicity since the lungs are parallel organs [90] and are subject to volumetric constraints. Although it is not uncommon to treat patients in free breathing mode [91], it has been shown that the use of DIBH reduces the V30 of the lung and all heart dosimetric parameters when treated with left-sided breast patients with UHFRT regimens [92]. Only one of the six surveyed African centers utilised breath-hold techniques. Thus, this represents another training opportunity that can lead to the application of a technique in African centers that improves dosimetry in UHFRT (and also in moderate and conventional fractionated) breast treatments. Unlike fiducial markers and perirectal spacers, breath-hold techniques do not require the use of consumable supplies and, hence, will not add cost to the treatment process once the staff is properly trained on the use of breath-hold techniques and the clinical procedure is in place.
The survey revealed notable variability in the availability of technologies relevant to UHFRT, such as breath-hold capabilities and trans-rectal ultrasound. These tools, critical for advanced treatment techniques like UHFRT, were available at only one or two centers, highlighting significant disparities in infrastructure across the surveyed institutions. The limited availability of these technologies underscores the need for targeted investments and capacity-building initiatives to standardise access to advanced radiotherapy tools. This variability also reflects the broader challenges faced by institutions in sSA, where resource constraints often limit the adoption of cutting-edge practices despite the enthusiasm and willingness of clinicians to implement them.
The absence of advanced technologies such as fiducial markers and breath-hold techniques highlights the critical need for carefully designed, targeted training programs. These programs must transcend technical instruction to encompass broader considerations of cost-effectiveness, feasibility and patient safety within resource-constrained settings. For example, the adoption of breath-hold techniques in breast cancer radiotherapy represents a particularly promising intervention. This method not only improves dosimetric outcomes by reducing radiation exposure to critical organs such as the heart and lungs but also requires no consumable materials, making it a cost-neutral enhancement to clinical practice. Such strategies are uniquely suited to the sSA context, where economic and infrastructural constraints demand innovative solutions that maximise clinical impact without imposing significant financial burdens. Tailored training initiatives that address these multifaceted needs can facilitate the safe and effective integration of these techniques, ultimately improving patient outcomes while respecting local resource limitations.
Because of the more stringent spatial precision requirements of UHFRT, the importance of machine QA becomes magnified. The survey responses generally indicated a strong commitment to treatment unit QA across the six institutions. A notable area requiring improvement is the execution of image-guided positioning/repositioning tests. These tests should be performed daily [93] for each imaging system (MV planar, kV planar, kV CBCT) that is used in the setup of patients. However, it was shown that only one-third of the surveyed centers are actually performing these tests. This can be addressed through more rigorous training on how to perform these tests, interpret image data and appropriate actions when addressing setup variation, and didactic discussions on the importance of these tests, highlighting the clinical implications if the repositioning functionality malfunctions and a patient being treated with UHF is erroneously treated with a significant systematic shift in their position. Additionally, it was highlighted that small field dosimetry measurements are not being verified on an annual basis at most of the surveyed centers. This does not have severe implications in the context here since prostate and breast fields are not typically in the small field regime. However, it can be argued that IMRT treatments consist of many small fields. Also, if the centers are currently employing or plan to start employing stereotactic treatments, recent guidelines by the IAEA [65] and the American Association of Physicists in Medicine [66] serve as an excellent resource.
A distinction between mandatory QA elements and nonessential enhancements is crucial for understanding the feasibility of implementing UHFRT in resource-limited settings. Mandatory QA components for UHFRT include rigorous treatment planning, machine-specific QA and daily image guidance to ensure accurate patient setup and dose delivery. For example, daily imaging is critical for verifying target positioning and compensating for anatomical changes between fractions. These elements form the foundation of safe and effective UHFRT delivery and must be prioritised in any implementation strategy. In contrast, optional QA enhancements, such as AI-driven auto-contouring, represent technological advancements that, while beneficial, are not prerequisites for UHFRT. These tools can enhance efficiency and capacity by streamlining workflows and reducing planning times but are less critical in settings where foundational infrastructure remains a primary limitation. Among the surveyed centers, variability in QA infrastructure was evident, with some institutions lacking daily image guidance capabilities, which may limit their readiness for UHFRT adoption. By focusing on mandatory requirements while gradually incorporating optional enhancements, centers can develop a phased approach to implementing UHFRT, optimising the use of existing resources while building capacity for future advancements.
The limited adoption of UHFRT for prostate cancer in the surveyed institutions appears to stem from infrastructure gaps and misconceptions about its requirements. While tools such as fiducial markers and rectal spacers are often recommended to enhance precision and reduce rectal toxicity, they are not mandatory for the safe and effective delivery of UHFRT. Data from the PACE-B trial [94], a large, multicenter, randomised study, demonstrate that UHFRT can achieve high efficacy and safety standards without the universal use of these tools. In the trial, fiducial markers were recommended but not required and rectal spacers were not utilised. Instead, the critical component for delivering UHFRT was robust daily image guidance, such as CBCT, combined with adherence to stringent planning and QA protocols. Among the surveyed centers, the inconsistent availability of daily imaging and limited familiarity with UHFRT techniques likely pose more significant barriers to adoption than the absence of fiducial markers or rectal spacers. Additionally, the survey responses did not explicitly assess enthusiasm for prostate UHFRT or explore clinicians’ perceptions of its feasibility, highlighting an area for future investigation. Addressing these barriers through targeted training, investment in image-guidance technologies and education about evidence-based UHFRT practices could facilitate broader implementation in resource-limited settings.
This survey identified AI integration in four of the six African clinics, specifically in auto-segmentation applications. The delineation of normal structures can be a time-intensive, repetitive task that limits patient throughput by taking away valuable time from either the clinician or the planner [95]. Normal structures, while exhibiting some variation from patient to patient, all possess fairly similar geometries. Thus, auto-segmentation software presents as an attractive option to increase efficiency in high-income countries and throughput in more resource-constrained settings. In recent years, multiple companies have developed auto-segmentation technologies, leading to significant heterogeneity in software solutions across different clinics. This was evident amongst the surveyed clinics, where the four institutions utilising auto-segmentation tools reported five different software solutions in use. As with any novel technology introduced into clinical practice, rigorous evaluation of these tools before introduction into clinical practice is necessary. The responsibility for commissioning such tools, ensuring their seamless integration and maintaining an ongoing QA program lies with the medical physicist. Accuracy of delineation, however, is the responsibility of the clinical or radiation oncologist. The incorporation of peer-review assessments through QA contouring chart rounds has been well documented [96] and implementing similar practices to evaluate auto-segmentation results could ensure the overall safety of radiotherapy treatments in which these tools are used. AI utilisation is set to increase in the coming years, leading to the further automation of the treatment planning process. One such application, the radiation planning assistant (RPA), first uses AI to contour the targets and normal structures and then to define the orientation, shape and intensity of the radiation beams to create an acceptable treatment plan [97]. As AI solutions such as the RPA are more fully incorporated into clinical practice, the treatment planning workflow of clinics in sSA is likely to change considerably.
All respondents expressed keen interest in participating in a practical course on hypofractionation. A wide variety of topics were suggested including the radiobiology of hypofractionation, patient eligibility criteria and selection for hypofractionated treatment regimens, clinical evidence supporting hypofractionation in breast, prostate and gynecological cancers, AI applications of auto-segmentation and auto-planning software, as well as immobilisation, breath-hold techniques and QA. This kind of course could address some of the identified knowledge gaps discussed above, enhance the proficiency in applying these techniques and ensure safety and efficacy of application within the African context. To address this, a practical course on hypofractionation is being developed covering the topics listed above along with the utilisation of implanted fiducials and perirectal spacers for prostate cancer.
Economic evaluations must extend beyond the straightforward comparison of treatment sessions to encompass the full spectrum of costs associated with implementing UHFRT. This includes expenses related to pre-treatment preparations, such as the placement of fiducial markers or perirectal spacers, as well as the logistical and financial burdens of patient accommodations, particularly for those traveling long distances to access care. Furthermore, a comprehensive cost-benefit analysis must also account for the infrastructure enhancements and QA protocols essential for the safe and effective delivery of UHFRT. By presenting a more nuanced and multifaceted understanding of the financial implications, the study acknowledges the intricate challenges inherent in deploying advanced radiotherapy techniques within resource-limited settings. This approach ensures that economic considerations align with practical realities, fostering strategies that balance cost-efficiency with clinical efficacy and equity.
A number of limitations should be acknowledged in this study which may impact the generalisability and comprehensiveness of the findings. First, the survey was conducted at only six institutions across sSA, potentially skewing results towards better-resourced centers and limiting insights from smaller or less-equipped facilities. Additionally, the data were self-reported by the participating institutions, introducing the potential for reporting bias and variability in the accuracy of the responses. Next, due to its prospective nature, the study did not assess clinical outcomes that are critical towards confirming the safety and efficacy of UHFRT in African populations, as existing data from high-income settings may not directly apply to this context. Finally, the fact that the survey was to be filled out by various members of the radiation oncology teams (clinical directors, oncologists and physicists) rather than a single person at each site may have affected the inherent consistency of the data obtained from a given institution. For example, only four of the responding clinical directors indicated that their center employs IMRT, while all six responding physicists referenced their centers’ IMRT QA capabilities. Similar inconsistencies exist with respect to the application of 3D conformal radiotherapy. Despite these limitations, the findings of this study remain valuable as they provide a first comprehensive assessment of the readiness of key radiotherapy institutions in sSA for the implementation of UHFRT. The identification of both strengths and specific gaps, such as the need for technical upgrades and targeted training, offers actionable insights that can guide future efforts to enhance radiotherapy capacity in the region. Moreover, the strong willingness of institutions to adopt UHFRT highlights the potential for positive impact, especially with the provision of appropriate support and resources.
Conclusion
This comprehensive needs assessment has highlighted the potential for the adoption of UHFRT in sSA. While infrastructure and clinical workflows at leading institutions appear generally sufficient, targeted training and technical upgrades are necessary to bridge identified gaps, such as breath-hold techniques in breast cancer treatments and the lack of fiducial and perirectal spacer utilisation in prostate cancer treatments. The willingness of all institutions to participate in educational programs reflects the region’s enthusiasm to embrace these advanced treatment techniques. With appropriate support and training, UHF radiotherapy could significantly enhance radiotherapy access in the region, ultimately leading to increased radiotherapy treatment capacity and more effectively addressing the region’s growing cancer burden.
Acknowledgments
None.
Conflicts of interest
There are no conflicts of interest to declare.
Funding
Research reported in this publication was partially supported by BIO Ventures for Global Health’s AC3T Study Pool and the National Institutes of Health under Award Number R01CA239042 and supplement for implementation research and R13 grant supporting the Global Health Catalyst summits R13CA257481. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health.
Author contributions
MA, AOJ, SK, AM, TAN, MBT, AA, RB, CD, KW, JS, OB and LI contributed to the clinical aspects of the survey design. JW, YH, SOA, KA, JDK, TM, VA, WS, SA, MSH, HL, JL, CFN and WN contributed to the technical (medical physics) aspects of the survey design. VA, WS and KG led the IRB application process. MA, AOJ, SK, AM and TAN facilitated the collection of the clinical aspects of the data. SOA, KA, JDK and TM facilitated the collection of the technical aspects of the data. FC provided urology expertise. JW and YH analysed the data, and all authors participated in the interpretation of the results. WN, KG, JS, OB and LI provided the vision for the project. JW executed the project. All authors contributed to writing the manuscript.
References
1. Gouda HN, Charlson F, and Sorsdahl K, et al (2019) Burden of non-communicable diseases in sub-Saharan Africa, 1990–2017: results from the Global Burden of Disease Study 2017 Lancet Glob Health 7(10) e1375–e1387 https://doi.org/10.1016/S2214-109X(19)30374-2 PMID: 31537368
2. Sharma R, Fronterre C, and Ssentongo AE, et al (2022) Mapping cancer in Africa: a comprehensive and comparable characterization of 34 cancer types using estimates from GLOBOCAN 2020 Front Public Health 10 839835 https://doi.org/10.3389/fpubh.2022.839835
3. Barton MB, Jacob S, and Shafiq J, et al (2014) Estimating the demand for radiotherapy from the evidence: a review of changes from 2003 to 2012 Radiother Oncol 112(1) 140–144 https://doi.org/10.1016/j.radonc.2014.03.024 PMID: 24833561
4. Irabor OC, Swanson W, and Shaukat F, et al (2020) Can the adoption of hypofractionation guidelines expand global radiotherapy access? An analysis for breast and prostate radiotherapy JCO Glob Oncol 6 667–678 https://doi.org/10.1200/JGO.19.00261 PMID: 32343628 PMCID: 7193821
5. Grover S, Xu MJ, and Yeager A, et al (2015) A systematic review of radiotherapy capacity in low- and middle-income countries Front Oncol 4 380 https://doi.org/10.3389/fonc.2014.00380 PMID: 25657930 PMCID: 4302829
6. Abdel-Wahab M, Bourque JM, and Pynda Y, et al (2013) Status of radiotherapy resources in Africa: an International Atomic Energy Agency analysis Lancet Oncol 14(4) e168–e175 https://doi.org/10.1016/S1470-2045(12)70532-6 PMID: 23561748
7. Atun R, Jaffray DA, and Barton MB, et al (2015) Expanding global access to radiotherapy Lancet Oncol 16(1) 1153–1186 https://doi.org/10.1016/S1470-2045(15)00222-3 PMID: 26419354
8. Ngwa W, Addai B, and Adewole I, et al (2022) Cancer in sub-Saharan Africa: a Lancet Oncology Commission Lancet Oncol 23(6) e251–e312 https://doi.org/10.1016/S1470-2045(21)00720-8 PMID: 35550267 PMCID: 9393090
9. Tsapaki V, Tabakov S, and Rehani MM (2018) Medical physics workforce: a global perspective Phys Med 55 33–39 https://doi.org/10.1016/j.ejmp.2018.10.012 PMID: 30471817
10. Elmore SNC, Prajogi GB, and Rubio JAP, et al (2019) The global radiation oncology workforce in 2030: estimating physician training needs and proposing solutions to scale up capacity in low-and middle-income countries Appl Rad Oncol 8(2) 10–16 https://doi.org/10.37549/ARO1193
11. Lief E, Weygand J, and Parker SA, et al (2023) Global representatives’ initiative of the American Association of Physicists in Medicine Med Phys Int 11(1) 16–21
12. Ameri A, Barzegartahamtan M, and Ghavamnasiri M, et al (2018) Current and future challenges of radiation oncology in Iran: a report from the Iranian Society of Clinical Oncology Clin Oncol 30(4) 262–268 https://doi.org/10.1016/j.clon.2017.12.021
13. Vanderpuye V, Hammad N, and Martei Y, et al (2019) Cancer care workforce in Africa: perspectives from a global survey Infect Agent Cancer 14 1–8 https://doi.org/10.1186/s13027-019-0227-8
14. Swanson W, Samba RN, and Lavelle M (2021) Practical guidelines on implementing hypofractionated radiotherapy for prostate cancer in Africa Front Oncol 11 725103 https://doi.org/10.3389/fonc.2021.725103 PMID: 34926247 PMCID: 8673781
15. Swanson W, Kamwa F, and Samba R, et al (2021) Hypofractionated radiotherapy in African cancer centers Front Oncol 10 618641 https://doi.org/10.3389/fonc.2020.618641 PMID: 33680940 PMCID: 7933544
16. Kraus RD, Weil CR, and Abdel-Wahab M (2022) Benefits of adopting hypofractionated radiotherapy as a standard of care in low-and middle-income countries JCO Glob Oncol 8 e2200215 https://doi.org/10.1200/GO.22.00215 PMID: 36525619 PMCID: 10166538
17. Mushonga M, Weiss J, and Liu ZA, et al (2023) Hypofractionation in breast cancer radiotherapy across World Bank income groups: results of an international survey JCO Glob Oncol 9 e2200127 https://doi.org/10.1200/GO.22.00127 PMID: 36706350 PMCID: 10166450
18. Parker SA, Weygand J, and Bernat BG, et al (2024) Assessing radiology and radiotherapy needs for cancer care in low-and-middle-income countries: insights from a global survey of departmental and institutional leaders Adv Rad Oncol 9(11) 101615 https://doi.org/10.1016/j.adro.2024.101615
19. Höcht S, Aebersold DM, and Albrecht C, et al (2017) Hypofractionated radiotherapy for localized prostate cancer Strahlenther Onkol 193(1) 1–12 https://doi.org/10.1007/s00066-016-1041-5
20. Miralbell R, Roberts SA, and Zubizarreta E, et al (2012) Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets Int J Radiat Oncol Biol Phys 82(1) e17–e24 https://doi.org/10.1016/j.ijrobp.2010.10.075
21. Yarnold J, Bentzen SM, and Coles C, et al (2011) Hypofractionated whole-breast radiotherapy for women with early breast cancer: myths and realities Int J Radiat Oncol Biol Phys 79(1) 1–9 https://doi.org/10.1016/j.ijrobp.2010.08.035
22. Shaitelman SF, Lei X, and Thompson A, et al (2018) Three-year outcomes with hypofractionated versus conventionally fractionated whole-breast irradiation: Results of a randomized, noninferiority clinical trial J Clin Oncol 36(35) 3495–3503 https://doi.org/10.1200/JCO.18.00317 PMCID: 6286164
23. Dearnaley D, Syndikus I, and H Mossop, et al (2016) Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, noninferiority, phase 3 CHHiP trial Lancet Oncol 17(8) 1047–1060 https://doi.org/10.1016/S1470-2045(16)30102-4 PMID: 27339115 PMCID: 4961874
24. Incrocci L, Wortel RC, and Alemayehu WG, et al (2016) Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial Lancet Oncol 17(8) 1061–1069 https://doi.org/10.1016/S1470-2045(16)30070-5 PMID: 27339116
25. Lee WR, Dignam JJ, and Amin MB, et al (2016) Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer J Clin Oncol 34(20) 2325–2332 https://doi.org/10.1200/JCO.2016.67.0448 PMID: 27044935 PMCID: 4981980
26. Catton CN, Lukka H, and Gu CS, et al (2017) Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer J Clin Oncol 35(17) 1884–1890 https://doi.org/10.1200/JCO.2016.71.7397 PMID: 28296582
27. Whelan T, MacKenzie R, and Julian J, et al (2002) Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer J Natl Cancer Inst 94(15) 1143–1150 https://doi.org/10.1093/jnci/94.15.1143 PMID: 12165639
28. Bentzen SM, Agrawal RK, and Aird EG, et al (2008) The UK standardisation of breast radiotherapy (START) trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial Lancet Oncol 9(4) 331–341 https://doi.org/10.1016/S1470-2045(08)70077-9 PMID: 18356109 PMCID: 2323709
29. Bentzen SM, Agrawal RK, and Aird EG, et al (2008) The UK standardisation of breast radiotherapy (START) trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial Lancet 371(9618) 1098–1107 https://doi.org/10.1016/S0140-6736(08)60348-7 PMID: 18355913 PMCID: 2277488
30. Offersen BV, Alsner J, and Nielsen HM, et al (2020) Hypofractionated versus standard fractionated radiotherapy in patients with early breast cancer or ductal carcinoma in situ in a randomized phase III trial: the DBCG HYPO trial J Clin Oncol 38(31) 3615–3625 https://doi.org/10.1200/JCO.20.01363 PMID: 32910709
31. Morgan SC, Hoffman K, and Loblaw A, et al (2018) Hypofractionated radiation therapy for localized prostate cancer: an ASTRO, ASCO, and AUA evidence-based guideline J Clin Oncol 36(34) 3411–3430 https://doi.org/10.1200/JCO.18.01097 PMCID: 6269129
32. Smith BD, Bellon JR, and Blitzblau R, et al (2018) Radiation therapy for the whole breast: executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline Pract Radiat Oncol 8(3) 145–152 https://doi.org/10.1016/j.prro.2018.01.012 PMID: 29545124
33. Lehrer EJ, Kishan AU, and Yu JB, et al (2020) Ultrahypofractionated versus hypofractionated and conventionally fractionated radiation therapy for localized prostate cancer: a systematic review and meta-analysis of phase III randomized trials Radiother Oncol 148 235–242 https://doi.org/10.1016/j.radonc.2020.04.037 PMID: 32505965
34. Sabbagh A, Weiss J, and Tawk B, et al (2023) Hypofractionation adoption in prostate cancer radiotherapy: results of an international survey JCO Glob Oncol 9 e2300046 https://doi.org/10.1200/GO.23.00046 PMID: 37319396 PMCID: 10497301
35. Busschaert SL, Kimpe E, and Barbé K, et al (2024) Introduction of ultra-hypofractionation in breast cancer: implications for costs and resource use Radiother Oncol 190 110010 https://doi.org/10.1016/j.radonc.2023.110010
36. Widmark A, Gunnlaugsson A, and Beckman L, et al (2019) Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial Lancet 394(10196) 385–395 https://doi.org/10.1016/S0140-6736(19)31131-6 PMID: 31227373
37. Brunt AM, Haviland JS, and Wheatley DA, et al (2020) Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial Lancet 395(10237), P1613–P1626
38. Olatunji E, Swanson W, and Patel S, et al (2023) Challenges and opportunities for implementing hypofractionated radiotherapy in Africa: lessons from the HypoAfrica clinical trial Ecancermedicalscience 17 1508 https://doi.org/10.3332/ecancer.2023.1508 PMID: 37113724 PMCID: 10129374
39. Amjad R, Moldovan N, and Raziee H, et al (2024) Hypofractionated radiotherapy in gynecologic malignancies – a peek into the upcoming evidence Cancers 16(2) 362 https://doi.org/10.3390/cancers16020362
40. Shinohara K and Roach III M (2008) Technique for implantation of fiducial markers in the prostate Urology 71(2) 196–200 https://doi.org/10.1016/j.urology.2007.10.011 PMID: 18308082
41. Mariados N, Sylvester J, and Shah D, et al (2015) Hydrogel spacer prospective multicenter randomized controlled pivotal trial: dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy Int J Radiat Oncol Biol Phys 92(5) 971–977 https://doi.org/10.1016/j.ijrobp.2015.04.030 PMID: 26054865
42. Kutcher GJ, Coia L, and Gillin M, et al (1994) Comprehensive QA for radiation oncology: report of AAPM radiation therapy committee task group 40 Med Phys 21(4) 581–618 https://doi.org/10.1118/1.597316 PMID: 8058027
43. Huq MS, Fraass BA, and Dunscombe PB, et al (2016) The report of task group 100 of the AAPM: application of risk analysis methods to radiation therapy quality management Med Phys 43(7) 4209–4262 https://doi.org/10.1118/1.4947547 PMID: 27370140 PMCID: 4985013
44. Krauss RF, Balik S, and Cirino ET, et al (2023) AAPM medical physics practice guideline 8. b: linear accelerator performance tests J Appl Clin Med Phys 24(11) e14160 https://doi.org/10.1002/acm2.14160 PMID: 37793084 PMCID: 10647991
45. Weygand J, Fuller CD, and Ibbott GS, et al (2016) Spatial precision in magnetic resonance imaging–guided radiation therapy: the role of geometric distortion Int J Radiat Oncol Biol Phys 95(4) 1304–1316 https://doi.org/10.1016/j.ijrobp.2016.02.059 PMID: 27354136
46. Bryant JM, Weygand J, and Keit E, et al (2023) Stereotactic magnetic resonance-guided adaptive and non-adaptive radiotherapy on combination MR-linear accelerators: current practice and future directions Cancers 15(7) 2081 https://doi.org/10.3390/cancers15072081 PMID: 37046741 PMCID: 10093051
47. Bryant JM, Doniparthi A, and Weygand J, et al (2023) Treatment of central nervous system tumors on combination MR-linear accelerators: review of current practice and future directions Cancers 15(21) 5200 https://doi.org/10.3390/cancers15215200 PMID: 37958374 PMCID: 10649155
48. Thompson RF, Valdes G, and Fuller CD, et al (2018) Artificial intelligence in radiation oncology: a specialty-wide disruptive transformation? Radiother Oncol 129(3) 421–426 https://doi.org/10.1016/j.radonc.2018.05.030 PMID: 29907338 PMCID: 9620952
49. Kozee M, Weygand J, and Andreozzi JM, et al (2023) Methodology for computed tomography characterization of commercially available 3D printing materials for use in radiology/radiation oncology J Appl Clin Med Phys 24(6) e13999 https://doi.org/10.1002/acm2.13999 PMID: 37096305 PMCID: 10243336
50. Jalloul M, Miranda‐Schaeubinger M, and Noor AM, et al (2023) MRI scarcity in low‐and middle‐income countries NMR Biomed 36(12) e5022 https://doi.org/10.1002/nbm.5022 PMID: 37574441
51. Wang J, Weygand J, and Hwang KP, et al (2016) Magnetic resonance imaging of glucose uptake and metabolism in patients with head and neck cancer Sci Rep 6(1) 30619
52. Salzillo TC, Hu J, and Nguyen L, et al (2016) Interrogating metabolism in brain cancer Magn Reson Imaging Clin N Am 24(4) 687–703 https://doi.org/10.1016/j.mric.2016.07.003 PMID: 27742110 PMCID: 5091807
53. Weygand J, Carter SE, and Salzillo TC, et al (2017) Can an organoid recapitulate the metabolome of its parent tissue? A pilot NMR spectroscopy study J Cancer Prev Curr Res 8(7) 00307
54. Dutta P, Perez MR, and Lee J, et al (2019) Combining hyperpolarized real-time metabolic imaging and NMR spectroscopy to identify metabolic biomarkers in pancreatic cancer J Proteome Res 18(7) 2826–2834 https://doi.org/10.1021/acs.jproteome.9b00132 PMID: 31120258
55. Salzillo TC, Mawoneke V, and Weygand J (2021) Measuring the metabolic evolution of glioblastoma throughout tumor development, regression, and recurrence with hyperpolarized magnetic resonance Cells 10(10) 2621 https://doi.org/10.3390/cells10102621 PMID: 34685601 PMCID: 8534002
56. Izewska J, Lechner W, and Wesolowska P (2018) Global availability of dosimetry audits in radiotherapy: the IAEA dosimetry audit networks database Phys Imaging Radiat Oncol 5 1–4 https://doi.org/10.1016/j.phro.2017.12.002 PMID: 33458360 PMCID: 7807735
57. Alvarez P, Kry SF, and Stingo F, et al (2017) TLD and OSLD dosimetry systems for remote audits of radiotherapy external beam calibration Radiat Meas 106 412–415 https://doi.org/10.1016/j.radmeas.2017.01.005 PMID: 29230093 PMCID: 5722458
58. Low DA, Harms WB, and Mutic S, et al (1998) A technique for the quantitative evaluation of dose distributions Med Phys 25(5) 656–661 https://doi.org/10.1118/1.598248 PMID: 9608475
59. Low DA, Moran JM, and Dempsey JF, et al (2011) Dosimetry tools and techniques for IMRT Med Phys 38(3) 1313–1338 https://doi.org/10.1118/1.3514120 PMID: 21520843
60. Miften M, Olch A, and Mihailidis D, et al (2018) Tolerance limits and methodologies for IMRT measurement-based verification QA: recommendations of AAPM Task Group No. 218 Med Phys 45(4) e53–e83 https://doi.org/10.1002/mp.12810 PMID: 29443390
61. Dogan N, Mijnheer BJ, and Padgett K, et al (2023) AAPM task group report 307: use of EPIDs for patient-specific IMRT and VMAT QA Med Phys 50(8) e865–e903 https://doi.org/10.1002/mp.16536 PMID: 37384416 PMCID: 11230298
62. Sun B, Rangaraj D, and Boddu S, et al (2012) Evaluation of the efficiency and effectiveness of independent dose calculation followed by machine log file analysis against conventional measurement based IMRT QA J Appl Clin Med Phys 13(5) 140–154 https://doi.org/10.1120/jacmp.v13i5.3837
63. Cardenas CE, Yang J, and Anderson BM, et al (2019) Advances in auto-segmentation Semin Radiat Oncol 29(3) 185–197 https://doi.org/10.1016/j.semradonc.2019.02.001 PMID: 31027636
64. Klein EE, Hanley J, and Bayouth J, et al (2009) Task group 142 report: quality assurance of medical accelerators Med Phys 36(9) 4197–4212 https://doi.org/10.1118/1.3190392 PMID: 19810494
65. Palmans H, Andreo P, and Huq MS, et al (2018) Dosimetry of small static fields used in external photon beam radiotherapy: Summary of TRS-483, the IAEA–AAPM International Code of Practice for reference and relative dose determination Med Phys 45(11) e1123–e1145 https://doi.org/10.1002/mp.13208 PMID: 30247757
66. Das IJ, Francescon P, and Moran JM, et al (2021) Report of AAPM task group 155: megavoltage photon beam dosimetry in small fields and non-equilibrium conditions Med Phys 48(10) e886–e921 https://doi.org/10.1002/mp.15030 PMID: 34101836
67. Naghavi AO, Bryant JM, and Kim Y, et al (2024) Habitat escalated adaptive therapy (HEAT): a phase 2 trial utilizing radiomic habitat-directed and genomic-adjusted radiation dose (GARD) optimization for high-grade soft tissue sarcoma BMC Cancer 24(1) 1–16 https://doi.org/10.1186/s12885-024-12151-7
68. Liveringhouse C, Netzley A, and Bryant JM, et al (2023) Trimodal therapy using an MR–guided radiation therapy partial bladder tumor boost in muscle invasive bladder cancer Adv Rad Oncol 8(6) 101268 https://doi.org/10.1016/j.adro.2023.101268
69. Brand DH, Kirby AM, and Yarnold, et al (2022) How low can you go? The radiobiology of hypofractionation Clin Oncol 34(5) 280–287 https://doi.org/10.1016/j.clon.2022.02.009
70. Moonen L and Bartelink H (1994) Fractionation in radiotherapy Cancer Treat Rev 20(4) 365–378 https://doi.org/10.1016/0305-7372(94)90018-3 PMID: 7954492
71. Coutard H (1934) Principles of x ray therapy of malignant diseases Lancet 224(5784) 1–8 https://doi.org/10.1016/S0140-6736(00)90085-0
72. Elkind MM and Sutton H (1960) Radiation response of mammalian cells grown in culture. 1. Repair of X-ray damage in surviving Chinese hamster cells Radiat Res 13(4) 556–593 https://doi.org/10.2307/3570945 PMID: 13726391
73. Fowler JF (1989) The linear-quadratic formula and progress in fractionated radiotherapy Br J Radiol 62(740) 679–694 https://doi.org/10.1259/0007-1285-62-740-679 PMID: 2670032
74. Nahum AE (2015) The radiobiology of hypofractionation Clin Oncol 27(5) 260–269 https://doi.org/10.1016/j.clon.2015.02.001
75. Joint Head and Neck Radiotherapy-MRI Development Cooperative (2017) Prospective analysis of in vivo landmark point-based MRI geometric distortion in head and neck cancer patients scanned in immobilized radiation treatment position: results of a prospective quality assurance protocol Clin Transl Radiat Oncol 7 13–9
76. Jaffray DA, Siewerdsen JH, and Wong JW, et al (2002) Flat-panel cone-beam computed tomography for image-guided radiation therapy Int J Radiat Oncol Biol Phys 53(5) 1337–1349 https://doi.org/10.1016/S0360-3016(02)02884-5 PMID: 12128137
77. Jaffray DA (2012) Image-guided radiotherapy: from current concept to future perspectives Nat Rev Clin Oncol 9(12) 688–699 https://doi.org/10.1038/nrclinonc.2012.194 PMID: 23165124
78. Weygand J, Armstrong T, and Bryant JM, et al (2023) Accurate, repeatable, and geometrically precise diffusion-weighted imaging on a 0.35 T magnetic resonance imaging-guided linear accelerator Phys Imaging Radiat Oncol 28 100505 https://doi.org/10.1016/j.phro.2023.100505
79. Keit E, Liveringhouse C, and Figura N, et al (2023) Feasibility and toxicity of full-body volumetric modulated arc therapy technique for high-dose total body irradiation Technol Cancer Res Treat 22 1–9 https://doi.org/10.1177/15330338231180779
80. Huang E, Dong L, and Chandra A, et al (2002) Intrafraction prostate motion during IMRT for prostate cancer Int J Radiat Oncol Biol Phys 53(2) 261–268 https://doi.org/10.1016/S0360-3016(02)02738-4 PMID: 12023128
81. Beltran C, Herman MG, and Davis BJ (2008) Planning target margin calculations for prostate radiotherapy based on intrafraction and interfraction motion using four localization methods Int J Radiat Oncol Biol Phys 70(1) 289–295 https://doi.org/10.1016/j.ijrobp.2007.08.040
82. Ng M, Brown E, and Williams A, et al (2014) Fiducial markers and spacers in prostate radiotherapy: current applications BJU Int 113 13–20 https://doi.org/10.1111/bju.12624 PMID: 24894851
83. Schiffner DC, Gottschalk AR, and Lometti M, et al (2007) Daily electronic portal imaging of implanted gold seed fiducials in patients undergoing radiotherapy after radical prostatectomy Int J Radiat Oncol Biol Phys 67(2) 610–619 https://doi.org/10.1016/j.ijrobp.2006.09.042 PMID: 17236978
84. Wu J, Haycocks T, and Alasti H, et al (2001) Positioning errors and prostate motion during conformal prostate radiotherapy using on-line isocentre set-up verification and implanted prostate markers Radiother Oncol 61(2) 127–133 https://doi.org/10.1016/S0167-8140(01)00452-2 PMID: 11690677
85. Crook JM, Raymond Y, and Salhani D, et al (1995) Prostate motion during standard radiotherapy as assessed by fiducial markers Radiother Oncol 37(1) 35–42 https://doi.org/10.1016/0167-8140(95)01613-L PMID: 8539455
86. Susil RC, McNutt TR, and DeWeese TL, et al (2010) Effects of prostate-rectum separation on rectal dose from external beam radiotherapy Int J Radiat Oncol Biol Phys 76(4) 1251–1258 https://doi.org/10.1016/j.ijrobp.2009.07.1679
87. Prada PJ, Fernández J, and Martinez AA, et al (2007) Transperineal injection of hyaluronic acid in anterior perirectal fat to decrease rectal toxicity from radiation delivered with intensity modulated brachytherapy or EBRT for prostate cancer patients Int J Radiat Oncol Biol Phys 69(1) 95–102 https://doi.org/10.1016/j.ijrobp.2007.02.034 PMID: 17707267
88. Wilder RB, Barme GA, and Gilbert RF, et al (2010) Cross-linked hyaluronan gel reduces the acute rectal toxicity of radiotherapy for prostate cancer Int J Radiat Oncol Biol Phys 77(3) 824–830 https://doi.org/10.1016/j.ijrobp.2009.05.069 PMID: 20510195
89. Boda-Heggemann J, Knopf AC, and Simeonova-Chergou A, et al (2016) Deep inspiration breath hold-based radiation therapy: a clinical review Int J Radiat Oncol Biol Phys 94(3) 478–492 https://doi.org/10.1016/j.ijrobp.2015.11.049 PMID: 26867877
90. Hopewell JW and Trott KR (2000) Volume effects in radiobiology as applied to radiotherapy Radiother Oncol 56(3) 283–288 https://doi.org/10.1016/S0167-8140(00)00236-X PMID: 10974376
91. Tokuda PJK, Mitsuyoshi T, and Ono Y, et al (2024) Acute adverse events of ultra-hypofractionated whole-breast irradiation after breast-conserving surgery for early breast cancer in Japan: an interim analysis of the multi-institutional phase II UPBEAT study Breast Cancer 31 643–648 https://doi.org/10.1007/s12282-024-01577-3 PMID: 38607499 PMCID: 11194189
92. Hafez A, Abdelaziz DM, and Khalil MM, et al (2020) The necessity of using deep inspiration breath-hold in the radiotherapy of left breast cancer patients who undergo the UK FAST trial Biomed Phys Eng Express 7(1) 015004 https://doi.org/10.1088/2057-1976/abc9f7
93. Hanley J, Dresser S, and Simon W, et al (2021) AAPM task group 198 report: an implementation guide for TG 142 quality assurance of medical accelerators Med Phys 48(10) e830–e885 https://doi.org/10.1002/mp.14992 PMID: 34036590
94. van As N, Griffin C, and Tree A, et al (2024) Phase 3 trial of stereotactic body radiotherapy in localized prostate cancer N Engl J Med 391(15) 1413–1425 https://doi.org/10.1056/NEJMoa2403365 PMID: 39413377 PMCID: 7616714
95. Chen Z, King W, and Pearcey R, et al (2008) The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature Radiother Oncol 87(1) 3–16 https://doi.org/10.1016/j.radonc.2007.11.016
96. Cox BW, Kapur A, and Sharma A, et al (2015) Prospective contouring rounds: a novel, high-impact tool for optimizing quality assurance Pract Radiat Oncol 5(5) e431–e436 https://doi.org/10.1016/j.prro.2015.05.005 PMID: 26215585
97. Court L, Aggarwal A, and Burger H, et al (2023) Addressing the global expertise gap in radiation oncology: the radiation planning assistant JCO Glob Oncol 9 e2200431 https://doi.org/10.1200/GO.22.00431 PMID: 37471671 PMCID: 10581646
Appendix: Survey
General information
This data will be anonymised.
1. Institution representative name (First and Last)

2. Institution representative email address

3. Name of institution

4. Name of Potential HypoAfrica PI (s) in your institution.
If more than one, please designate one as a contact PI

5. Contact PI phone number

6. Institution address

7. Where is your radiation facility located (country)?

8. Where is your radiation facility located (city)?

9. Which of the following best describes your radiation facility? (Select all that apply).

Section I: General clinical capacity and infrastructure
1. Does your institution use paper or electronic records?

2. For the following radiotherapy equipment, how many units of each does your facility have access to?
3. If you answered ‘other’ in the above question, please specify the other types of radiotherapy equipment available at your facility.

4. What types of radiotherapy techniques does your facility practice? (Select all that apply).
5. What facilities does your center have? (Select all that apply.)
6. For each imaging equipment, how many units does your facility have access to?
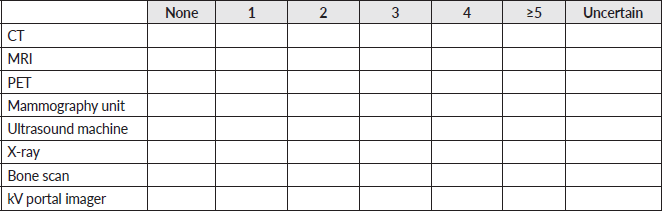
7. What external beam treatment planning system does your facility use? (Select all that apply).

8. Please specify the dose calculation algorithm and whether dose to water or dose to medium is calculated (if applicable).

9. What brachytherapy planning system does your facility use? (Select all that apply).

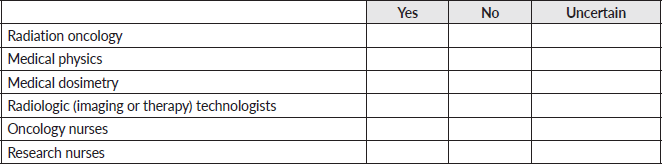
10. Does your facility have a training program for the following specialties?
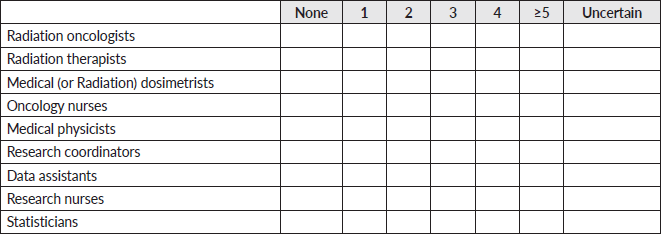
11. For each clinical personnel, how many individuals does your facility have access to?
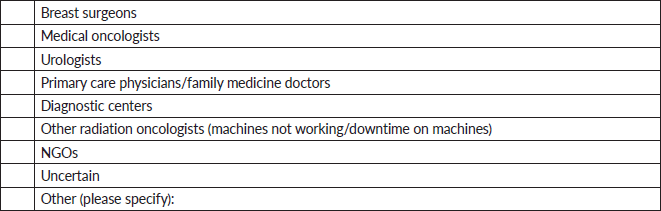
12. How are patients referred to your facility for radiation therapy? (Select all that apply).
13. Would your clinic be interested in participating in a course on hypofractionated radiotherapy?

14. If you answered ‘yes’ in the above question, please specify any topics of interest that you would like the course to cover.

15. Please provide any additional information regarding this section that you wish to share.

Section II: Clinical breast cancer treatment program
1. How many breast surgeons do you have at your institution? Please state ‘Uncertain’ if not known.

2. Approximately how many breast surgeons refer patients to you? Please state ‘Uncertain’ if not known.

3. Who else do you receive referrals from for breast radiation? Please state ‘Uncertain’ if not known.

4. What fractionation regimens are you currently utilising for whole-breast radiation therapy? (Select all that apply).
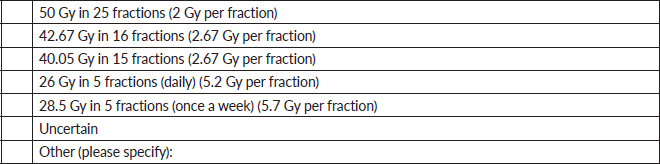
5. For a T1-3N0-1 breast cancer, would you utilise ultra-hypofractionation (>5 Gy per fraction) in the below scenarios?
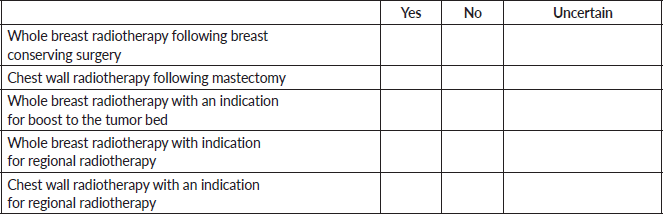
6. If you do not provide ultra-hypofractionation, can you briefly indicate the reason(s) why (e.g., most of my patients are not eligible for ultra- hypofractionation or concerns regarding safety)? (Select all that apply).
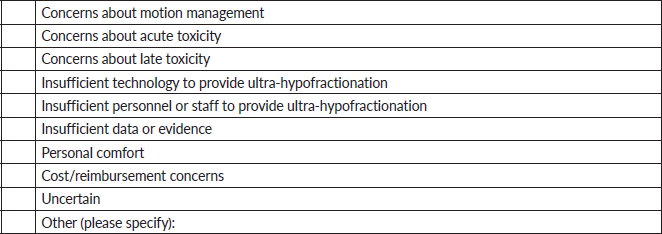
7. If you do not provide ultra-hypofractionation now, would you consider treating T1-3N0-1 breast cancer patients with ultra-hypofractionation in the future?

8. If you answered ‘no’ in the previous question, please explain why.

9. Do you use breath hold techniques for breast treatment? (Select all that apply).
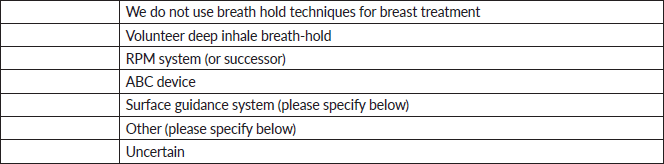
10. If you answered ‘surface guidance system’ or ‘other’ in the previous question, please specify.

11. What systems do you have at the CT simulator to monitor patient’s breath hold amplitude? (Select all that apply).

12. If you answered ‘surface guidance system’ or ‘other’ in the previous question, please specify.

13. What imaging technique do you use for breath hold verification during treatment? (Select all that apply).
14. If you answered ‘surface imaging’ or ‘other’ in the previous question, please specify.

15. How do you verify the correct CT data set was used during planning (DIBH versus free breathing), and treatment? Please state ‘Uncertain’ if not known.

16. Approximately how many patients are treated for breast cancer at your clinic on a monthly basis? Please state ‘Uncertain’ if not known.

17. Approximately what percentage of the patients that you see have had a sentinel lymph node biopsy only (without axillary lymph node dissection)? Please state ‘Uncertain’ if not known.

18. Approximately what percentage of the patients that you see get an axillary lymph node dissection prior to their consult with you? Please state ‘Uncertain’ if not known.

19. Approximately what percentage of the patients that you see receive neoadjuvant chemotherapy? Please state ‘Uncertain’ if not known.

20. Do target and OAR contours get peer-reviewed by another physician before treatment planning?

21. Do plans undergo a peer-review assessment before beginning of treatment?

22. Would you be interested in participating in a course on hypofractionated radiotherapy?

23. If you answered ‘yes’ in the above question, please specify any topics of interest that you would like the course to cover.

24. Please provide any additional information regarding this section that you wish to share.

Section III: Clinical prostate cancer treatment program
1. How many urologists do you have at your institution? Please state ’Uncertain’ if not known.

2. Approximately how many urologists refer patients to you? Lease state ‘Uncertain’ if not known.

3. What fractionation regimens are you currently utilising for localised prostate cancer external beam radiation therapy? (Select all that apply).
4. Which patient and tumour characteristics do you consider in decision-making regarding ultra-hypofractionation? (Select all that apply).
5. Which reasons do you consider relevant to opt for ultra-hypofractionation? (Select all that apply).
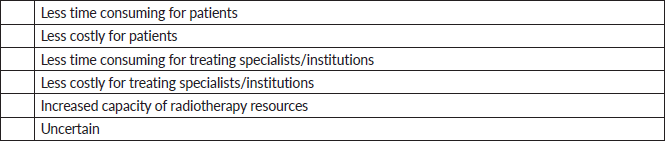
6. Do you have access to trans-rectal ultrasound in your clinic?

7. Do you use implanted fiducials for motion management in prostate treatment?

8. If you answered ‘yes’ to the previous question, what is the size of the fiducial that you use? Please state ‘Uncertain’ if not known.

9. If your center uses fiducials, what type of material (e.g., gold) are the fiducials made of? Please state ‘Uncertain’ if not known know. Please state ‘Not applicable’ if fiducials are not used.

10. If your center uses fiducials, please state the name of the manufacturer of the fiducials. Please state ‘Uncertain’ if not known. Please state ‘Not applicable’ if fiducials are not used.

11. If your center uses fiducials, please note the average cost of the fiducials (please note currency – e.g., USD, NGN). Please State ‘Uncertain’ if not known. Please state ‘Not applicable’ if fiducials are not used.

12. What imaging verification technique do you use for fiducials? (Select all that apply).

13. What is the tolerance for fiducial matching during prostate treatment at your facility? (Select all that apply.)

14. Do you use hydrogel or other rectal spacers (i.e., balloons) to create separation between the prostate and rectum for prostate treatment?

15. If you answered ‘yes’ to the previous question, please specify what type of rectal spacer (e.g., hydrogel, balloon and so on) your center uses.

16. Who performs hydrogel and or fiducial placement procedures at your center? (Select all that apply).

17. Does your center perform peer review for every patient’s treatment charts?

18. Do target and OAR contours get peer-reviewed by another physician before treatment planning?

19. Do plans undergo a peer-review assessment before beginning of treatment?

20. Would you be interested in participating in a course on hypofractionated radiotherapy?

21. If you answered ‘yes’ in the above question, please specify any topics of interest that you would like the course to cover.

22. Please provide any additional information regarding this section that you wish to share.

Section IV: Radiotherapy QA programs
1. Is there a quality management program in place for your radiation treatment systems?

2. Has an external body audited or accredited your radiation treatment program?

3. If you answered ‘yes’ to the previous question, please specify the name of the auditing/accrediting organisation and the most recent audit/accreditation date.

4. How often does a qualified medical physicist perform dosimetry checks on your external beam treatment machines (linear accelerator or Co-60)? (Select all that apply).

5. How often does personnel other than a qualified medical physicist (e.g., a radiation therapist or a technician) perform dosimetry checks on your external beam treatment machines (linear accelerator / Co-60)? (Select all that apply).

6. Does your center perform pre-treatment patient specific QA (PSQA) for every IMRT and/or VMAT patient?

7. If you answered ‘No’ to the previous question, please specify for what percentage of patients/treatment plans that PSQA is performed.

8. What type of pre-treatment patient specific QA (PSQA) does your center perform for IMRT and/or VMAT patients? (Select all that apply).

9. If you answered ‘Measurement based PSQA’ or ‘Other’ in the previous question, please specify.

10. Do medical physicists perform pre-treatment, weekly and end of treatment chart reviews at your center?

11. Do radiation oncologists perform pre-treatment, weekly and end of treatment chart reviews at your center?

12. Do radiation oncologists perform daily image reviews at your center?

13. If you answered ‘No’ to the above question, how often is imaging review performed at your center? Please state ‘Uncertain’ if not known.

14. For ultra-hypofractionation, are radiation oncologists and medical physicists available for image reviews at the treatment console during each fraction at your center?

15. Will you or your staff be able to transfer the digital treatment records such as DICOM treatment plan and dose files to another location? (Instructions would be provided.)

16. What types of IGRT are used at your center? (Select all that apply).
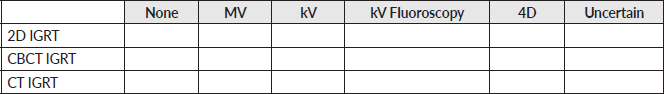
17. Please list the model and manufacturer of each IGRT system used at your center. Please state ‘Uncertain’ if not known.

18. What registration methods are used at your center? (Select all that apply).

19. What type of alignment does your site perform for each site? (Select all that apply).
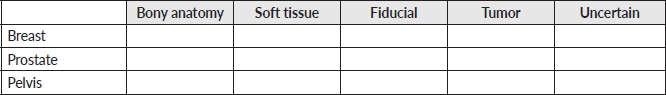
20. Does your center perform the following imaging QA processes? (Select all that apply).
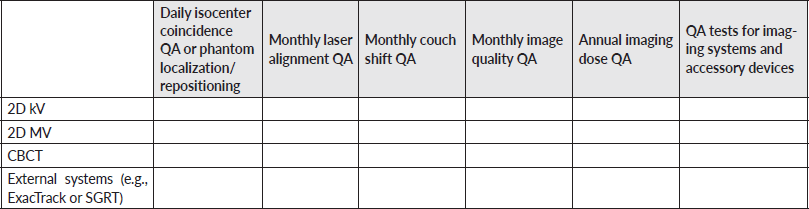
21. What is your center's typical IGRT frequency and imaging method? (Select all that apply).
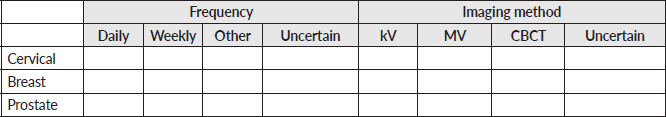
22. What is your center's tolerance level (in mm) for patient repositioning? Please specify for each imaging method, as applicable.
23. Does your center reimage after shifting the patient? If yes, please describe the circumstances when your center reimages. Please state ‘Uncertain’ if not known.

24. In what situations does your center reimage the patients during the treatment? Please state ‘Uncertain’ if not known.

25. Is the treatment couch at your center able to rotate?

26. If you answered ‘Yes’ in the previous question, what is your center's rotational tolerance? Please state ‘Uncertain’ if not known.

27. What do the daily QA tests of your treatment machine include? (Select all that apply).

28. What do the monthly QA tests of your treatment machine include? (Select all that apply).

29. What do the annual QA tests of your treatment machine include? (Select all that apply).
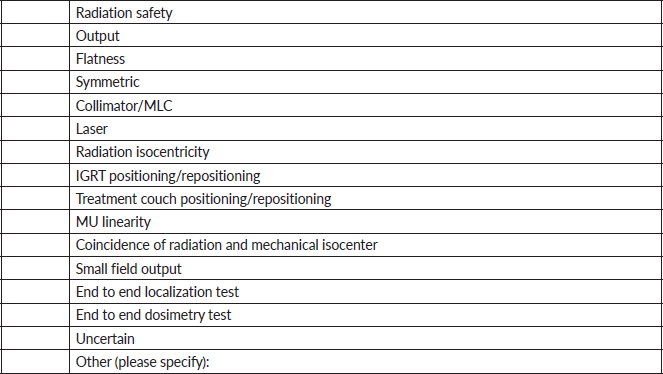
30. Does your center use auto-contouring software?

31. If you answered ‘yes’ to the previous question, what auto-contouring software does your center use? Please state ‘Uncertain’ if not known.

32. Do target and OAR contours get peer-reviewed by another physician before treatment planning?

33. Do plans undergo a peer-review assessment before beginning of treatment?

34. Would you be interested in participating in a course on hypofractionated radiotherapy?

35. If you answered ‘yes’ in the above question, please specify any topics of interest that you would like the course to cover.

36. Please provide any additional information regarding this section that you wish to share.

Section V: Clinical research environment
1. Are there any ongoing/completed trials at your center?

2. If you answered ‘Yes’ to the previous question, please state the name and objectives of the trial(s). Please state ‘Uncertain’ if not known.

3. Are there any ongoing/completed clinical trials for breast or prostate cancer at your center?

4. If you answered ‘yes’ to the previous question, please state the name and objectives of the trial(s). Please state ‘Uncertain’ if not known.

5. Have you previously conducted clinical research studies?

6. If you answered ‘yes’ to the previous question, please state the total number of studies. Please state ‘Uncertain’ if not known.

7. For recruiting to research trials, what would be the source of your participant population? Please indicate the estimated percentage for each source type.
8. Who handles budget and contract negotiations? Please state ‘Uncertain’ if not known.

9. What is your institution’s grant overhead rate? Please state ‘Uncertain’ if not known.

10. What fees would you require in a trial contract (outside per-patient fees). Please state all fees. Please state ‘Uncertain’ if not known.

11. Do you provide reimbursement for travel expenses? Please state ‘Uncertain’ if not known.

12. Would you need to buy any equipment/supplies to conduct this study (medical devices, BP machines, laptops, radiotherapy machines)? If yes, please provide details. Please state ‘Uncertain’ if not known.

13. What challenges do you anticipate with patient enrollment (such as conflicts of interest, site issues or other enrolling studies, country-specific requirements or potential difficulties)? Please state ‘Uncertain’ if not known.

14. Do you have access to IRB or Ethics Committee at the site? If no, how would you get ethical approval for the study? Please state ‘Uncertain’ if you do not know.

15. How frequently does your local IRB meet? Please state ‘Uncertain’ if not known.

16. What are the submission requirements for your local IRB? Please state ‘Uncertain’ if not known.

17. What are the costs required for IRB application? Please state ‘Uncertain’ if not known.

18. How long does it take from initial IRB submission to approval? Please state ‘Uncertain’ if not known.

19. Do you need the informed consent form, handouts or advertisements translated to any other language? If yes, please state which language and how the document will be translated. Please state ‘Uncertain’ if not known.

20. Would you be interested in participating in a course on clinical trials?

21. If you answered ‘Yes’ in the above question, please specify any topics of interest that you would like the course to cover.

22. Please provide any additional information regarding this section that you wish to share.






