Challenges of osteosarcoma care in Africa: a scoping review of the burden, management and outcome
Oluwaseun J Olajugba, Emmanuel O Oladeji, Damilola Adesola, Ridwanullah O Abdullateef, Godwin Rockson, Abdul K Bah and Oluwatobi O Olayode
Trauma and orthopaedics, Surgery Interest Group of Africa, Lagos 105101, Nigeria
Abstract
Osteosarcoma has the highest incidence among individuals of African descent, with growing evidence suggesting ethnic and racial genetic underpinning. Hence, it presents a grave public health challenge in Africa given the widening inequities in access to cancer care. This scoping review addresses the critical gap in the availability of locally relevant data on the magnitude of the burden and challenges relating to the management and outcome of African centres. This study included 1,374 patients from eighteen studies. 81% presented with locally advanced or metastatic disease. While surgical treatment for osteosarcoma is shifting toward limb salvage on a global scale, amputation remains preponderant in Africa as only 53% underwent limb salvage operations. The pooled 5-year overall survival was 49.1%. Late presentation, workforce and infrastructural shortage, cultural beliefs, patronage of unorthodox medicine practitioners and high healthcare costs were the barriers driving poor outcomes in African centres. Strategies to improve outcomes should focus on addressing these barriers.
Keywords: osteosarcoma, oncology care inequity, Africa, SDG, public health
Correspondence to: Emmanuel O Oladeji
Email: olusolaoladejiemma@gmail.com
Published: 23/01/2025
Received: 28/05/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Globally, osteosarcoma is the most prevalent form of primary bone malignancy in children and adolescents, with an annual incidence of 5.6 cases per million children and a peak incidence in the second decade of life [1, 2]. It predominantly affects the extremities, threatening life and limb [2].
Although rare on a global scale, the incidence is highest among individuals of African descent, with growing evidence suggesting ethnic and racial genetic underpinning [3, 4]. Hence, it poses a grave public health challenge in Africa as the inequities in access to cancer care continue to widen [1, 2, 5, 6].
The clinical presentations of osteosarcoma are heterogeneous, and so are the imaging, pathological and biological behaviours [2]. Advances in cancer chemotherapy and surgical oncological techniques over the past decades have transformed the care delivered to osteosarcoma patients with a global trend toward limb salvage surgeries and overall improved survival outcomes and quality of life for patients [2]. The experience is however different in Africa where presentation with advanced disease remains rife, mortality is high and the limb is often beyond salvage [7].
Despite the increasing health burden, osteosarcoma, such as other cancers, receives distressingly little attention from continental and global policymakers and easily gets overshadowed by the health burden of infectious diseases [1, 8]. This is partly reinforced by the limited availability of locally relevant data on the magnitude of the problem and its impact on victims and their families. This lack of information tends to obscure the severity of the problem, underscoring the limited attention paid to addressing this burdensome socio-economic and public health problem, and the inherent tendency to undermine Goal 3 of the Sustainable Development Goals (SDG) [9].
To address this critical gap and identify the unique challenges that hinder the delivery of quality care to patients with osteosarcoma in African centres, we conducted a scoping review evaluating the burden of osteosarcoma in Africa, the management practices and the management outcomes. In addition to closing identified gaps in the literature, this review hopes to impact local policy and practice in African centres by providing further insights to guide healthcare resource allocation, evidence-based decision-making and policy impact assessment. We present this article following the preferred reporting systems for systematic review and meta-analysis extension for scoping reviews (PRISMA) guidelines.
Methods
An electronic database search was performed on PubMed, African Journal Online, Web of Science, Google Scholar, Cochrane Library and Scopus using a combination of entry terms, text words and MeSH terms about the burden, management and outcome of osteosarcoma in African centres, from database’s inception to 30 June 2023. A manual search of the reference lists of identified articles was conducted to obtain relevant additional literature.
Published articles retrievable in English on the burden, management and outcome of osteosarcoma of the appendicular skeleton were included in the review. Articles not available in full text, comments, letters to the editor, opinion pieces, case reports and reviews were excluded. Titles and/or abstracts of all relevant studies returned from the different databases were deduplicated and screened independently by two reviewers, followed by full article screening. A third reviewer resolved any disagreements between the first two reviewers.
Relevant data were extracted in two stages – a pilot stage and the main stage, using a data extraction proforma developed on Microsoft Excel. Pooled statistics related to different characteristics were estimated. The review adopted the methodological guidance proposed by Peters et al [10] in the scoping review process. Neither ethical approval nor informed consent was required for this study.
Results
Eighteen papers were eligible for inclusion in this review. A summary of the sequential screening process for article selection is presented in Figure 1.
The majority of the papers were from Egypt (n = 9) and South Africa (n = 4), while Tunisia (n = 3), Kenya (n = 1) and Nigeria (n = 1) contributed the remaining papers. (Figure 2) The articles were published over 35 years, from 1985 to 2020, with most of them (n = 15) being published after 2010 (Table 1). All the articles were observational studies and the majority (n = 15) were conducted retrospectively (Table 1).
A total of 1,374 patients from the eighteen eligible studies were included in this review (Table 1). The incidence of osteosarcoma was reported in only three studies and ranged from 1.2 to 3.2 cases per million population [11–13] Twelve studies reported the mean age of the study participants ranging from 13 to 42 years [11, 13–23] and the pooled mean was 17.6 years. Fifteen studies reported the sex distribution of the study participants, showing a pooled male (n = 508) to female (n = 452) ratio of 1.1 (Table 1).
Thirteen papers reported the anatomical location of osteosarcoma among the study participants (n = 572). The femur was the most affected in 289 cases, the tibia came a distant second (n = 166), while the axial skeleton was involved in 41 (7.2%) persons (Table 2). Pain, swelling, constitutional symptoms, ulcers and pathological fractures were the reported clinical features at presentation [14, 16, 22, 23]. The mean time from onset of symptoms to presentation was reported in five studies and ranged from 3.4 to 14.1 months with a pooled mean of 4.5 months [14–16, 22–24].
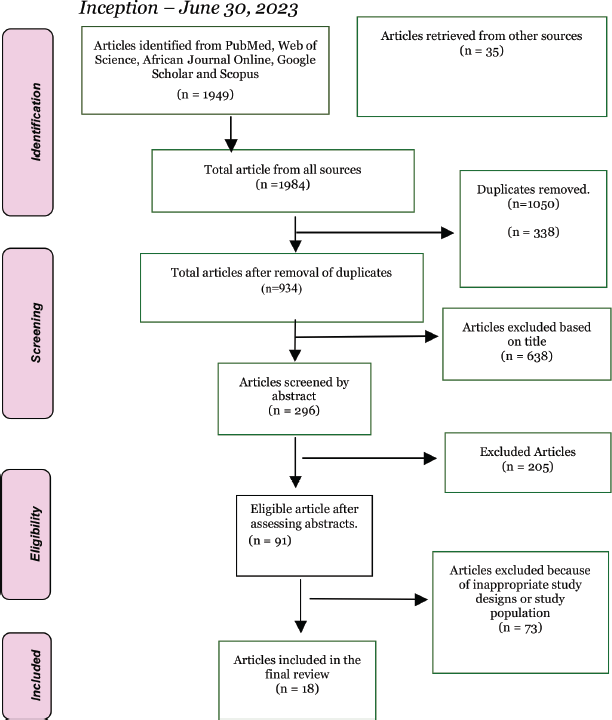
Figure 1. PRISMA flowchart of article identification and selection process.
Data on the Enneking Staging System was available from seven studies. This review grouped the reported stages into localised (I and II) and metastatic (III) disease. 44.7% of the cases (n = 317) had the metastatic disease while the rest had localised disease – with the tumour locally advanced in about one-third (36.4%). The most common site of metastasis was the lungs. Using the adapted WHO classification of malignant primary bone tumours, [25] thirteen studies reported high-grade osteosarcoma (n = 482, 70.6%) as the most common pathological type among the study participants.
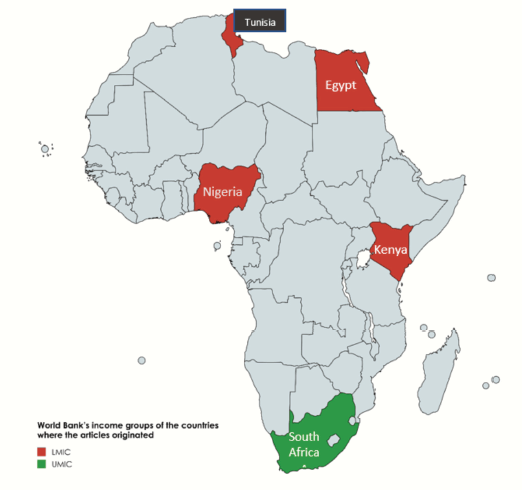
Figure 2. Map showing countries where the articles in this review originated and their income classification. LMIC: Lower middle-income country, UMIC: Upper middle-income country.
Radiological assessment with plain radiographs was undertaken in all patients, however computerised tomography scan or magnetic resonance imaging (MRI) was conducted in only two-thirds of the participants. Additionally, 16 persons required further assessment with a bone scan and angiography [26, 27].
Therapeutic modalities were chemotherapy, surgery, radiotherapy, cryotherapy and palliative care. Surgical procedures reported from 9 studies include limb salvage operations in 53% and amputation in the rest (Table 3) [14, 16, 17, 23, 24, 27–29]. The details of limb salvage surgery undertaken were only available in three papers. This included wide excision with tumour prosthesis (with the use of megaprosthesis total knee replacement described in one), and tumour resection followed by reimplantation of frozen autograft [15, 23, 27]. Three patients with lung metastasis underwent pulmonary metastatectomy [16].
The chemotherapeutic regimens varied across studies with high-dose methotrexate (HDMTX) adopted in only one study (Table 3). None of the articles reported the use of a second-line chemotherapy regimen and details of radiotherapy treatment were lacking from papers that reported using this treatment modality.
None of the papers suggested the involvement of multidisciplinary team discussion before the commencement of care. Eight studies reported refusal of treatment by the patient, carer or family representative in 14.6% of participants. The reasons for refusal of treatment included cultural beliefs, objecting to amputation, perceived futility of treatment and inability to afford the cost of care.
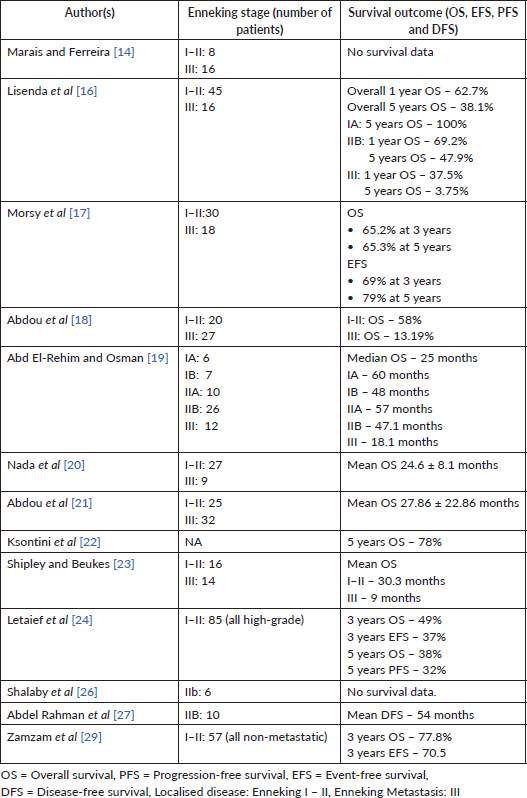
Eleven of the eighteen studies reported survival outcomes. Outcome endpoints varied across studies and included overall survival (OS), event-free survival (EFS), disease-free survival (DFS) and progression-free survival (PFS) (Table 2). 3- and 5-year OS ranged from 40.7% to 79% and 38% to 78%, respectively, with a pooled OS of 49.1%, while the corresponding 3-year and 5-year EFS ranged from 43.5% to 70.5% and 40.7% to 65.2% [17, 28, 29]. Postoperative functional scores of 70%–82.4% were reported from two studies using a modified system of the Musculoskeletal System Society [26, 27].
Table 1. Study characteristics.
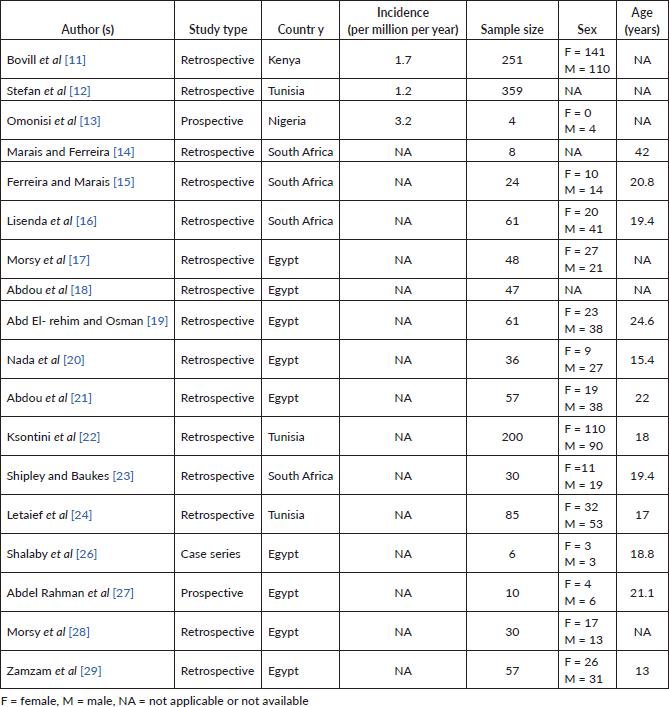
Table 2. Chemotherapy regimens and surgical procedures.
Table 3. Outcomes of care.
Evidence of metastasis was the most common poor clinical prognostic factor, while high serum alkaline phosphatase and lactate dehydrogenase levels were identified as poor biochemical prognostic factors [14, 24, 29]. Additionally, a cytoplasmic pattern of Ephrin A4, high ADAM8 expression, down-regulated HLA Class I antigen expression and overexpression or co-expression of Ezrin and HER2 were the poor immunohistochemical prognostic factors [18–20, 26].
The timely management of patients who presented with osteosarcoma was hindered by several factors, including late presentation, poor access to oncology care, workforce and infrastructural shortage, cultural perceptions about limb loss leading to refusal of amputation, high patronage of unorthodox medical practitioners and spiritual healing centres, inability to afford the cost of care due to high out-of-pocket expenditure on healthcare and loss to follow up [14, 16, 17, 23, 24]. The mean interval from onset of symptoms to presentation was 4.5 months and limited access to MRI caused a delay in diagnosis and commencement of treatment [16]. Late presentation with advanced disease and pathological fracture was high and precluded limb salvage surgeries [14, 15]. Additionally, some patients had defaulted from treatment at a different hospital while others declined treatment for localised disease, only to present later with metastatic disease [14, 16].
Discussion
This scoping review presents a summary of the current state of epidemiology, management practices and outcomes of osteosarcoma in Africa while highlighting the challenges that hinder the delivery of quality care. While previous reviews have addressed osteosarcoma burden and care from a global perspective, [2, 30] this is the first review to provide a summary of the literature from the African viewpoint. Despite the relatively higher disease burden of osteosarcoma in people of African descent [3], the vast majority of the studies identified during our literature search were conducted in high-income countries which reflects the generally low research output from Africa, especially in the sub-Saharan region [31]. This paucity of publications underscores the need to generate locally applicable and globally relevant data that can drive improvement in management practices and lead to better outcome profiles of patients managed for osteosarcoma in African centres.
Africa is a diverse continent with countries at various stages of cancer transition. Although sub-Saharan Africa bears a higher health burden of osteosarcoma, [8] most of the studies included in this review were conducted in Northern Africa, which may reflect the better allocation of resources for healthcare and cancer research in this African sub-region [5]. The incidence of 1.2–3.2 cases per million population found in this review is far lower than the 6.8 cases per million persons reported among African Americans by the US Cancer Statistics Working Group and the estimated 4.2 cases per million per year in sub-Saharan Africa reported by Rojas et al [30, 32]. This wide disparity mirrors the lack of effective population-based and hospital-based cancer registries in Africa in addition to the generally lower incidence of cancers in Northern Africa [5, 8, 33 34]. Although there were more males than females affected in our review, a male-to-female ratio of 1.1 suggests almost equal sex distribution, contrary to previous findings of male preponderance [2, 30, 35]. Deliberate investment in cancer registry and clinical services is key to obtaining accurate data on the magnitude of the disease and improving access to care. HDMTX chemotherapy regimen is an effective approach for treating osteosarcoma and is well-established in developed countries [36] Nevertheless, HDMTX therapy has a narrow therapeutic index and thus can cause significant toxicity leading to morbidities and occasionally mortality if MTX levels are not monitored rigorously [36]. This potentially deters using HDMTX in low-resource areas such as sub-Saharan African (SSA) and India, [37] as seen in this review where only two studies reported the use of MTX [24, 29], and just one of them adopted HDMTX (Table 3) [29]. Abdel Rahman et al [27] and Letaief et al [24] from studies conducted in Egypt and Tunisia, acknowledged the lack of resources for monitoring MTX levels thereby precluding HDMTX as a chemotherapy option in managing patients with osteosarcoma.
Access to oncological services is limited in Africa, which results in delayed cancer diagnosis and treatment [38–40]. This is due to an inadequate specialised workforce and deficient pathological, radiological and nuclear imaging infrastructures required to arrive at a diagnosis and properly stage cancers [16, 41, 42]. This was corroborated by Lisenda et al [16] who reported delays in diagnosis and treatment due to limited access to MRI. There is an acute shortage of MRI and computed tomography (CT) scanners in low- and middle-income countries (LMICs) with less than one CT scanner per 1 million population and an even wider gap for MRI machines [43]. This lack of workforce and infrastructure makes it challenging to complete the diagnosis timely [41, 44]. Building capacity through regional, continental and international collaborations is a proven strategy for tackling this problem.
Late presentation of cancer patients is an important problem in Africa, with the interval between the onset of symptoms and first presentation as high as 400 days in this review [24]. This may explain the high proportion of patients who presented with metastatic disease and the high rate of amputation performed instead of limb salvage operation which is now the global standard [2, 35].
The high cost of cancer care constitutes a major barrier to timely diagnosis, particularly in many SSA countries where patients incur out-of-pocket expenses for both direct and indirect costs of treatment [43, 45]. With little or no financial protection mechanisms, this situation is dire for many who live below the poverty line and families are hesitant to spend scarce resources on a disease that is perceived to be incurable in the first instance [43, 45]. This is comparable to the challenges faced in India in managing osteosarcoma as reported by Mittal et al [37] SSA and other LMIC countries such as India have poor surgical infrastructures, a shortage of skilled surgeons and require out-of-pocket healthcare financing, which can be a determining factor in accessing care by indigent patients [37].
Other reasons for delayed diagnosis include poor coordination between healthcare sectors and continued patronage of traditional healers and complementary medicine practitioners [43]. Addressing these barriers requires multidisciplinary policy formulation and commitment to implementation on a systemic level to achieve the SDG goal of reducing mortality from non-communicable diseases by one-third [9].
Of the 52 patients who refused treatment, 24 were offered amputation, either as a form of curative treatment or as part of a palliative care plan to treat pain and improve quality of life. Refusal to accept treatment suggests that even when confronted with a life-threatening condition, amputation as a treatment option is not culturally acceptable in some parts of Africa [46, 47]. Poor limb prosthetic services and the lack of well-coordinated pre- and post-amputation psychosocial support may additionally be the underpinning reasons why patients decline amputation [48]. Early presentation of patients and availability of specialised workforce and infrastructure for appropriate oncological surgery would significantly change the narrative toward performing more limb salvage operations. Culturally sensitive discussions with patients about treatment options by healthcare professionals can improve the treatment uptake.
In our review, the 5-year survival rate for osteosarcoma ranged between 38% and 78% with a pooled OS of 49.1%. This survival outcome is lower than survival rates of 60.17%, >65% and 68% reported from global studies [2, 30, 42]. It is important to note that most studies that reported outcome survival were from Northern Africa, where the provision of cancer care and availability of research resources appear better than most SSA. The implication is that the actual survival rate in Africa may be more disconcerting [8]. The prognostic factors identified in this review are consistent with findings from global studies [30, 42].
There were some limitations to this study. Most of the studies identified were hospital-based studies, predominantly from Northern and Southern Africa, which may have underestimated the burden of the disease. Also, only studies published in English were included in this review, potentially leaving out relevant information published in other languages. Additionally, case reports, conference proceedings and reviews were excluded, implying that relevant data may have been missed. One major challenge with making outcome comparisons between studies was the varying endpoints adopted by different authors, which were either not defined or only done vaguely, highlighting the need for transparent reporting of the component events and endpoints.
Conclusion
This review highlighted the challenges compromising the management and outcome of osteosarcoma in Africa. While surgical treatment for osteosarcoma is shifting toward limb salvage on a global scale, amputation remains common in Africa and is an important reason why patients decline treatment. Late presentation, poor access to oncology care, workforce and infrastructural shortage, refusal of amputation, high patronage of unorthodox medical practitioners and spiritual healing centres and inability to afford the cost of care were the barriers limiting the delivery of appropriate care and driving the significant poorer outcomes. Overcoming these barriers will require a multifaceted approach matching policy formulation with sustainable implementation; human capital development with infrastructural upscaling; and international collaborations with optimisation of local resources.
Recommendations
Addressing the barriers to osteosarcoma care in Africa requires a comprehensive and multifaceted approach incorporating targeted interventions at institutional, national and continental levels. A critical step in this direction is an accurate estimation of the disease burden to provide contextually appropriate data that would inform research priorities, allocation of resources and planning for future needs. Establishing and reinforcing cancer registries across Africa and fostering a culture of collaboration and information-sharing among researchers would ensure valuable data on incidence, outcomes and treatment efficacy are generated.
Improved capabilities to expand access to appropriate diagnostic infrastructure is vital to ensuring timely diagnosis while expanding regional specialised oncology facilities and addressing the shortage of specialised healthcare workers. Creating partnerships with international organisations and non-governmental organisations can help equipment procurement and support training programs.
Chemotherapy is a cornerstone of osteosarcoma treatment. Hence, there is an imperative to standardise chemotherapy protocols to minimise variability in treatment strategies and ensure consistent, high-quality care across the continent. The limitation posed by the lack of capacity to monitor MTX levels in many African centres can be circumvented by adopting non-HDMTX regimens such as OGS-12 which has demonstrated comparable outcomes with acceptable toxicity [49]. Future research collaborations should explore the adaptation and effectiveness of these protocols in the African context.
Additionally, there is a need for enhanced capacity building through training programs for orthopaedic oncologists and other allied specialised healthcare workers, with a particular focus on limb salvage interventions that are feasible in low-resource settings, in addition to improving access to and the quality of prosthetic and psychological support services pre and post amputation. This can be achieved through partnerships between specialised centres across Africa and international collaboration initiatives.
Without mitigating the high cost of cancer care which typifies the lack of healthcare financial safety mechanisms in SSA countries, delayed diagnoses will continue to impact treatment outcomes adversely. Strategies to alleviate the high out-of-pocket expenses faced by patients and their families include increasing access to health insurance at national levels especially outside the formal sectors, adopting a plurality of health insurance schemes with each targeting different groups and innovative health financing strategies for cancer care through a partnership with international cancer care vanguards, industry and non-governmental organisations [50, 51].
Finally, improved awareness through educational campaigns and culturally sensitive dialogues highlighting the dangers of delayed or improper treatment targeting patients, communities in rural and underserved areas and primary healthcare providers are crucial to earlier detection and changing cultural perceptions and stigma surrounding the uptake of treatment modalities like amputation.
By implementing these recommendations, African countries can build a sustainable framework to improve osteosarcoma care and outcomes, advancing both immediate patient support and a broader healthcare system.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This research did not receive any specific grant funding agencies in the public, commercial or not-for-profit sectors.
Author contributions
Oluwaseun Joshua Olajugba and Emmanuel Olusola Oladeji contributed equally to this work.
Oluwaseun Joshua Olajugba: Conceptualised the work, carried out literature search and data analysis, drafted the manuscript, critically revised it and approved it for final submission.
Emmanuel Olusola Oladeji: Conceptualised and supervised the work, carried out literature search and data analysis, drafted the manuscript, critically revised it and approved it for final submission.
Damilola Adesola: Data collection and analysis, critically revised the manuscript and approved it for final submission.
Ridwanullah Olamide Abdullateef: Data collection and analysis, critically revised the manuscript and approved it for final submission.
Godwin Rockson: Data collection, analysis, critically revised the manuscript and approved it for final submission.
Abdul Karim Bah: Data collection, analysis, critically revised the manuscript and approved it for final submission.
Oluwatobi Olayode: Data analysis, critically revised the manuscript and approved it for final submission.
References
1. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
2. Misaghi A, Goldin A, and Awad M, et al (2018) Osteosarcoma: a comprehensive review SICOT J 4 12 https://doi.org/10.1051/sicotj/2017028 PMID: 29629690 PMCID: 5890448
3. Cole S, Gianferante DM, and Zhu B, et al (2022) Osteosarcoma: a surveillance, epidemiology, and end results program-based analysis from 1975 to 2017 Cancer 128(11) 2107–2118 https://doi.org/10.1002/cncr.34163 PMID: 35226758
4. Sadykova LR, Ntekim AI, and Muyangwa-Semenova M, et al (2020) Epidemiology and risk factors of osteosarcoma Cancer Invest 38(5) 259–269 https://doi.org/10.1080/07357907.2020.1768401 PMID: 32400205
5. Hamdi Y, Abdeljaoued-Tej I, and Zatchi AA, et al (2021) Cancer in Africa: the untold story Front Oncol 11 650117 https://doi.org/10.3389/fonc.2021.650117 PMID: 33937056 PMCID: 8082106
6. ScienceDirect Topics (2016) Disease burden –an overview https://www.sciencedirect.com/topics/medicine-and-dentistry/disease-burden
7. Edmund M, Buunaaim ADB, and Mikdad R, et al (2019) The limb beyond salvage: a case report on two cases of fibroblastic variants of osteosarcoma J Orthop Case Rep 9(4) 96–100 PMID: 32405499 PMCID: 7210912
8. Bray F, Parkin DM, and African Cancer Registry Network (2022) Cancer in sub-Saharan Africa in 2020: a review of current estimates of the national burden, data gaps, and future needs Lancet Oncol 23(6) 719–728 https://doi.org/10.1016/S1470-2045(22)00270-4 PMID: 35550275
9. Department of Economic and Social Affairs Transforming Our World: The 2030 Agenda for Sustainable Development (New York: Department of Economic and Social Affairs) https://sdgs.un.org/2030agenda
10. Peters MDJ, Marnie C, and Tricco AC, et al (2020) Updated methodological guidance for the conduct of scoping reviews JBI Evid Synth 18(10) 2119–2126 https://doi.org/10.11124/JBIES-20-00167 PMID: 33038124
11. Bovill EG Jr, Kung'u A, and Bencivenga A, et al (1985) An epidemiological study of osteogenic sarcoma in Kenya: the variations in incidence between ethnic groups and geographic regions, 1968–1978 Int Orthop 9(1) 59–63 https://doi.org/10.1007/BF00267039 PMID: 3860481
12. Stefan DC, Stones DK, and Wainwright RD, et al (2015) Childhood cancer incidence in South Africa, 1987–2007 S Afr Med J 105(11) 939–947 https://doi.org/10.7196/SAMJ.2015.v105i11.9780 PMID: 26632323
13. Omonisi AE, Komolafe AO, and Adefehinti O, et al (2020) Cancer in ife-ijesha, Southwestern Nigeria: an account of a 20-year cancer registration from ife-ijesha cancer registry Int J Intern Med 9 11–19
14. Marais LC and Ferreira N (2013) Osteosarcoma in adult patients living with HIV/AIDS ISRN Oncol 2013 219369 PMID: 23762607 PMCID: 3612482
15. Ferreira N and Marais LC (2012) Osteosarcoma presentation stages at a tumour unit in South Africa S Afr Med J 102(8) 673–676 https://doi.org/10.7196/SAMJ.5835 PMID: 22831944
16. Lisenda L, Linda ZA, and Snyman FPJ, et al (2017) Osteosarcoma patient outcomes at a South African tertiary hospital S Afr Med J 107(9) 754–757 https://doi.org/10.7196/SAMJ.2017.v107i9.11424 PMID: 28875882
17. Morsy AM, Abdelgawad MI, and Ahmed BM, et al (2019) Pediatric osteosarcoma of extremities: a 15-year experience from a tertiary care cancer center in upper Egypt J Pediatr Hematol Oncol 41(6) e371–e383 https://doi.org/10.1097/MPH.0000000000001407 PMID: 30629005
18. Abdou AG, Abd el-Wahed MM, and Asaad NY, et al (2010) Ephrin A4 expression in osteosarcoma, impact on prognosis, and patient outcome Indian J Cancer 47(1) 46–52 https://doi.org/10.4103/0019-509X.58859 PMID: 20071790
19. Abd El-Rehim DM and Osman NA (2015) Expression of a disintegrin and metalloprotease 8 and endostatin in human osteosarcoma: implication in tumor progression and prognosis J Egypt Natl Canc Inst 27(1) 1–9 https://doi.org/10.1016/j.jnci.2014.11.001
20. Nada OH, Ahmed NS, and Abou Gabal HH (2014) Prognostic significance of HLA EMR8-5 immunohistochemically analyzed expression in osteosarcoma Diagn Pathol 9 72 https://doi.org/10.1186/1746-1596-9-72
21. Abdou AG, Kandil M, and Asaad NY, et al (2016) The prognostic role of Ezrin and HER2/neu expression in osteosarcoma Appl Immunohistochem Mol Morphol 24(5) 355–363 https://doi.org/10.1097/PAI.0000000000000197
22. Ksontini FL, Guermazi F, and Khadija M, et al (2018) Descriptive epidemiology of malignant primary osteosarcoma in Tunisia 1980–2016 Asian Pac J Cancer Care 3(4) 81 https://doi.org/10.31557/apjcc.2018.3.4.81
23. Shipley J and Beukes C (2012) Outcomes of osteosarcoma in a tertiary hospital SA Orthopaed J 11 18–22
24. Letaief F, Khrouf S, and Yahiaoui Y, et al (2020) Prognostic factors in high-grade localized osteosarcoma of the extremities: the Tunisian experience J Orthop Surg 28(3) 2309499020974501 https://doi.org/10.1177/2309499020974501
25. Gerrand C, Athanasou N, and Brennan B, et al (2016) UK guidelines for the management of bone sarcomas Clin Sarcoma Res 6 7 https://doi.org/10.1186/s13569-016-0047-1 PMID: 27148438 PMCID: 4855334
26. Shalaby S, Shalaby H, and Bassiony A (2006) Limb salvage for osteosarcoma of the distal tibia with resection arthrodesis, autogenous fibular graft and Ilizarov external fixator J Bone Joint Surg Br 88(12) 1642–1646 https://doi.org/10.1302/0301-620X.88B12.17879 PMID: 17159179
27. Abdel Rahman M, Bassiony A, and Shalaby H (2009) Reimplantation of the resected tumour-bearing segment after recycling using liquid nitrogen for osteosarcoma Int Orthop 33(5) 1365–1370 https://doi.org/10.1007/s00264-009-0773-6 PMID: 19370347 PMCID: 2899142
28. Morsy AM, Ahmed BM, and Rezk KM, et al (2020) Age and tumor location predict survival in nonmetastatic osteosarcoma in upper Egypt J Pediatr Hematol Oncol 42(2) e66–e78 https://doi.org/10.1097/MPH.0000000000001506
29. Zamzam MA, Moussa EAH, and Ghoneimy AM, et al (2018) Outcomes and Prognostic Factors for Non-metastatic Osteosarcoma of the Extremity
30. Ottaviani G and Jaffe N (2009) The epidemiology of osteosarcoma Cancer Treat Res 152 3–13 https://doi.org/10.1007/978-1-4419-0284-9_1
31. Marincola E and Kariuki T (2020) Quality research in Africa and why it is important ACS Omega 5(38) 24155–24157 https://doi.org/10.1021/acsomega.0c04327 PMID: 33015430 PMCID: 7528179
32. Rojas GA, Hubbard AK, and Diessner BJ, et al (2021) International trends in incidence of osteosarcoma (1988–2012) Int J Cancer 149(5) 1044–1053 https://doi.org/10.1002/ijc.33673 PMID: 33963769 PMCID: 9137041
33. Curado MP (2019) Importance of hospital cancer registries in Africa Ecancermedicalscience 13 948 https://doi.org/10.3332/ecancer.2019.948 PMID: 31552121 PMCID: 6722112
34. The Cancer Atlas Northern Africa, West and Central Asia http://canceratlas.cancer.org/dKL
35. Prater S and McKeon B (2023) Osteosarcoma. BTI – StatPearls (St. Petersburg: Statpearls Publishing)
36. Dupuis C, Mercier C, and Yang C, et al (2008) High-dose methotrexate in adults with osteosarcoma: a population pharmacokinetics study and validation of a new limited sampling strategy Anticancer Drugs 19(3) 267–273 https://doi.org/10.1097/CAD.0b013e3282f21376 PMID: 18510172
37. Mittal A, Pushpam D, and Ganguly S, et al (2022) Controversies and challenges in the management of osteosarcoma-an Indian perspective Indian J Surg Oncol 13(4) 939–955 https://doi.org/10.1007/s13193-021-01486-3
38. Omotoso O, Teibo JO, and Atiba FA, et al (2023) Addressing cancer care inequities in sub-Saharan Africa: current challenges and proposed solutions Int J Equity Health 22(1) 189 https://doi.org/10.1186/s12939-023-01962-y PMID: 37697315 PMCID: 10496173
39. Azevedo MJ (2017) The state of health system(s) in Africa: challenges and opportunities Historical Perspectives on the State of Health and Health Systems in Africa, Volume II. African Histories and Modernities (Cham: Palgrave Macmillan)
40. Benson E (2022) These 20 African Countries are on the World Bank's Low Income List, Due to Low GNI Per Capita https://africa.businessinsider.com/local/markets/these-20-african-countries-are-on-the-world-banks-low-income-list-due-to-low-gni-per/n03hbdc Date accessed: 10/12/2023
41. Vanderpuye V, Hammad N, and Martei Y, et al (2019) Cancer care workforce in Africa: perspectives from a global survey Infect Agent Cancer 14 11 https://doi.org/10.1186/s13027-019-0227-8 PMID: 31139248 PMCID: 6528232
42. Nguyen JC, Baghdadi S, and Pogoriler J, et al (2022) Pediatric osteosarcoma: correlation of imaging findings with histopathologic features, treatment, and outcome Radiographics 42(4) 1196–1213 https://doi.org/10.1148/rg.210171 PMID: 35594197
43. Hricak H, Abdel-Wahab M, and Atun R, et al (2021) Medical imaging and nuclear medicine: a lancet oncology commission Lancet Oncol 22(4) e136–e172 https://doi.org/10.1016/S1470-2045(20)30751-8 PMID: 33676609 PMCID: 8444235
44. Manirakiza AVC, Rubagumya F, and Mushonga M, et al (2023) The current status of National Cancer Control Plans in Africa: data from 32 countries J Cancer Policy 37 100430 https://doi.org/10.1016/j.jcpo.2023.100430 PMID: 37392842
45. McCormack V and Newton R (2019) Research priorities for social inequalities in cancer in sub-Saharan Africa Reducing Social Inequalities in Cancer: Evidence and Priorities for Research eds Vaccarella S, Lortet-Tieulent J, and Saracci R, et al (Lyon: International Agency for Research on Cancer)
46. Morgado Ramirez DZ, Nakandi B, and Ssekitoleko R, et al (2022) The lived experience of people with upper limb absence living in Uganda: a qualitative study Afr J Disabil 11 890 https://doi.org/10.4102/ajod.v11i0.890 PMID: 35747758 PMCID: 9210140
47. Owolabi EO and Chu KM (2022) Knowledge, attitude and perception towards lower limb amputation amongst persons living with diabetes in rural South Africa: a qualitative study Afr J Prim Health Care Fam Med 14(1) e1–e10 https://doi.org/10.4102/phcfm.v14i1.3398 PMID: 36226936 PMCID: 9623825
48. Yinusa W and Ugbeye ME (2003) Problems of amputation surgery in a developing country Int Orthop 27(2) 121–124 https://doi.org/10.1007/s00264-002-0421-x PMID: 12700939 PMCID: 3460656
49. Bajpai J, Chandrasekharan A, and Talreja V, et al (2017) Outcomes in non-metastatic treatment naive extremity osteosarcoma patients treated with a novel non-high dosemethotrexate-based, dose-dense combination chemotherapy regimen 'OGS-12' Eur J Cancer 85 49–58 [doi: 10.1016/j.ejca.2017.08.013] https://doi.org/10.1016/j.ejca.2017.08.013 PMID: 28888849
50. Ifeagwu SC, Yang JC, and Parkes-Ratanshi R, et al (2021) Health financing for universal health coverage in Sub-Saharan Africa: a systematic review Glob Health Res Policy 6(1) 8 https://doi.org/10.1186/s41256-021-00190-7 PMCID: 7916997
51. Fenny AP, Yates R, and Thompson R (2021) Strategies for financing social health insurance schemes for providing universal health care: a comparative analysis of five countries Global Health Action 14(1) 1868054 https://doi.org/10.1080/16549716.2020.1868054 PMID: 33472557 PMCID: 7833020






