Gastric cancer in Central America: a scoping review
Jade Tso1, Mustafa Al-Qaraghli2, Susana Galeas3, Mustafa Faleh Abidalhassan4 and Cameron E Gaskill2
1Davis School of Medicine, University of California, Sacramento, CA 95817, USA
2Davis Department of Surgery, Division of Surgical Oncology, University of California, Sacramento, CA 95817, USA
3Clínica Esperanza, Roatán 34101, Honduras
4Northwell Health Department of Surgery, Lake Success, New York, NY 11042, USA
Abstract
Stomach cancer is the second most common cause of cancer-related death in Central Latin America and the fifth most common cancer by incident in the region. Understanding the epidemiology of stomach cancer is crucial to the appropriate planning, implementation and evaluation of comprehensive cancer control programs. The objective of this scoping review was to quantify the population-based incidence of stomach cancer in Central America from available data, identify reported risk factors, presentation and oncologic stage and explore the frequency of treatment used and survival outcomes for stomach cancer in Central America. Primary reports, cancer registries, hospital registries, endoscopy registries, case studies and case series focusing on the epidemiology of gastric cancer in Belize, Costa Rica, El Salvador, Guatemala, Honduras, Nicaragua and Panama, along with its treatment modalities and mortality rates were included. After identifying 616 citations, 20 studies met the inclusion criteria and were selected for data extraction. 12 were from Costa Rica and 5 from Honduras, with few studies from other countries such as Belize, El Salvador and Panama. Crude rates of gastric adenocarcinoma varied widely across different studies, with rates ranging from 0.09/100,000 to 32.04/100,000 in Costa Rica between 1996 and 2015. Overall, there was a general decrease in crude rates over recent study periods. Studies in El Salvador and Panama reported lower crude rates compared to Costa Rica. Non-cardia cancers were more common than cardia cancers. Surgery was the main treatment discussed in the reviewed papers. Mortality data were limited. Our review highlights the need for reliable cancer registries in this region. Often, cancer registries provide the only opportunity for properly assessing the extent and nature of cancer burdens in developing countries. This information is crucial in creating priorities for cancer control public health programs.
Keywords: gastric cancer, Central America, review
Correspondence to: Jade Tso
Email: jtso@ucdavi
Published: 23/01/2025
Received: 04/10/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Gastric adenocarcinoma (GC) is the third most common cause of cancer-related mortality worldwide and the leading infection-associated cancer [1, 2]. In 2019, there were an estimated 957,000 deaths and 1.27 million incident cases of stomach cancer worldwide. The burden of disease shows wide geographical variation, with more than 70% of total cases occurring in low and middle-income countries (LMICs) [3]. As a result of the various interacting risk factors for GC, the burden of disease is clustered into epidemiological pockets throughout the world, with emerging evidence that the largest pocket in the Western hemisphere is located in Central America. It is estimated that stomach cancer is the second most common cause of cancer-related death in Central Latin America and the cancer with the fifth most absolute number of incident cases in the region [1]. While there have been steady declines in GC worldwide in the past few decades [4] stomach cancer is evident to be a growing concern in Central America. The Central America Four (CA-4) region, including El Salvador, Guatemala, Honduras and Nicaragua is the largest low- and middle-income country region in the Western Hemisphere with a growing cancer burden that is expected to increase by 73% by 2030 [5]. Previous studies have observed that two-thirds of GC incident cases in the region are male and half of the patients come from rural areas [6]. The population of GC patients in Central America tends to be younger and the non-cardia and intestinal subtypes tend to be more common, which is similar to other low and middle-income regions [6]. The high prevalence of intestinal subtypes can be explained by earlier lifelong H. pylori infection as well as other host and environmental factors [6].
Understanding the epidemiology of stomach cancer is a crucial element of appropriate planning, implementation and evaluation of comprehensive cancer control programs. However, there are large disparities in the availability and quality of data on cancer burden globally. There is a paucity of literature regarding gastric cancer epidemiology in Central America with the exception of Costa Rican and Panamanian national cancer registries. Population-based cancer registries (PBCRs) and hospital-based cancer registries are especially limited in the CA-4.
The scoping review had multiple objectives. The first was to quantify the population-based incidence of stomach cancer in Central America from all available data. Second was to identify reported risk factors, presentation and oncologic stage for stomach cancer in Central America. Third was to explore the frequency of treatment used and survival outcomes for stomach cancer in Central America. This paper aimed to synthesise current literature describing the epidemiology of gastric cancer in Central America. We present this article in accordance with the preferred reported items for systematic reviews and meta-analyses (PRISMA)-ScR reporting checklist.
Methods
We conducted a scoping review following Arksey and O’Malley’s [7] expanded framework to systematically search and explore research on gastric cancer incidence in Central America as well as treatment and outcomes. Research ethics approval was not required. This scoping review is reported according to the PRISMA and an extension of reporting scoping reviews, PRISMA-Scr [8]. This study was originally designed as a systematic review and registered through PROSPERO (PROSPERO 2022 CRD42022342989) [9].
Research questions
- What is the population-based incidence and age-standardised rate of stomach cancer in Central America?
- What are the reported risk factors, associated symptoms and stage of presentation for stomach cancer in Central America?
- What is the frequency of treatment used for stomach cancer in Central America?
- What are the mortality rates for stomach cancer in Central America?
Search strategy
We searched for relevant documents in PubMed, Embase, Web of Science, Global Health (CAB Direct) and the Latin American and Caribbean Health Sciences Literature databases. In consultation with a health sciences librarian, we developed a search strategy using MeSH headings for gastric cancer and Central America. The final search strategy for all databases can be found in Appendix 1.
Study selection process
The inclusion and exclusion criteria for studies followed the population, concept and context categories for scoping reviews. Literature was included if they were primary reports, cancer registries, hospital registries, endoscopy registries, case studies and case series that described the epidemiology of gastric cancer in Central America, its treatment and outcomes (including survival and post-operative complication rates). Research and reports set in Belize, Costa Rica, El Salvador, Guatemala, Honduras, Nicaragua and Panama only were included. Nonprimary reports, consolidated data, systematic reviews and conference abstracts were excluded. Reports published over 25 years ago (before 1997) were also excluded. Reports available in English or Spanish were included.
An initial screen of titles and abstracts was conducted to determine eligibility. Duplicates were removed. All results were tracked using a systematic review platform, Covidence (Melbourne Australia). A reason for excluding each article after full-text review was recorded based on the eligibility criteria.
Charting the data
After initial title and abstract screening, two authors (JT & MA) reviewed the full-text articles to determine inclusion/exclusion. Differences were resolved by discussion. The full texts of included studies were reviewed and data were extracted from eligible studies using a prestructured data collection form, with one team member extracting data and another team member checking the extracted data for reliability and accuracy. The following data were extracted: bibliographic information, country of study, location of study, study type, type of registry, total patients, number of gastric cancer cases, age-standardised ratio or crude rate (if given), listed risk factors, location of cancer, treatment provided, listed barriers to care and outcomes of treatment (survival rate and postoperative complication rate). To calculate crude rates for studies that only gave a number of cases of gastric cancer during a specified time period in a specified location, population data (including percentage of population by sex by year and total population by year) was pulled from publicly available databases or local censuses [10–12]
Collating, summarising and reporting findings
Study characteristics were summarised using frequencies and percentages. Study findings were synthesised narratively. Qualitative and quantitative study findings were reported separately.
Critical appraisal
Critical appraisals of each of the included papers were done according to criteria from the Joanna Briggs Institute [13].
Results
The literature search yielded a total of 616 citations (Figure 1). After deduplication, 385 articles were included for screening. Following a review of titles and abstracts, a total of 83 articles that met initial inclusion criteria were included for a full-text review. Reference lists from these articles were examined for potential inclusion of additional citations. After a full-text review, 20 studies were included for data extraction.
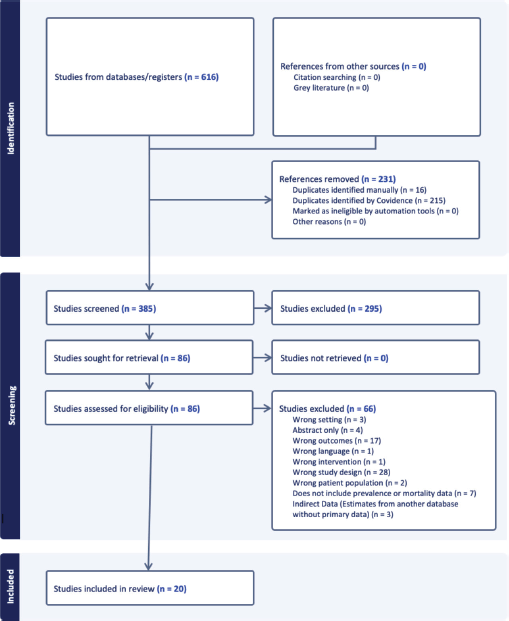
Figure 1. Study selection process.
Study characteristics
Study characteristics are summarised in Table 1. Details on individual studies are provided in Table 2. The majority of studies were published between 2015 and 2020 (53%), n = 19. Six studies were cross sectional, four cohorts, three case control, one prevalence study, one cancer registry and three other study types. Other study types included a summary analysis of cancer registries, hospital registries, prospective population-based studies that used endoscopy and pathology registries, a non-randomised community control trial and a description of a screening program. 58% of the studies were focused in Costa Rica and 26% in Honduras. There was one paper that included data from Costa Rica, El Salvador, Honduras and Panama. Notably, none of the included studies described the epidemiology of gastric cancer in Belize. All included studies were assessed using the Joanna Briggs critical appraisal checklists and were deemed appropriate to include in this review.
Table 1. Characteristics of included studies.
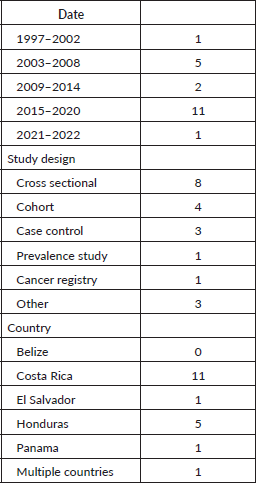
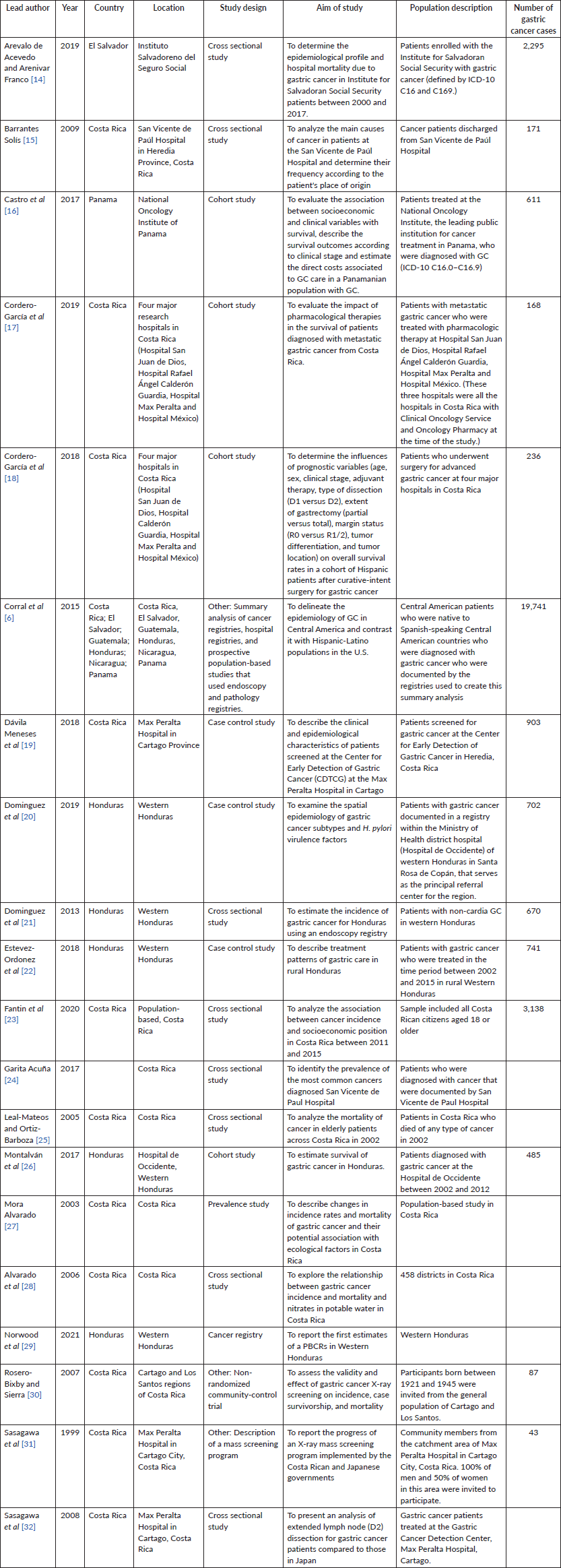
Table 2. Individual study information for included articles.
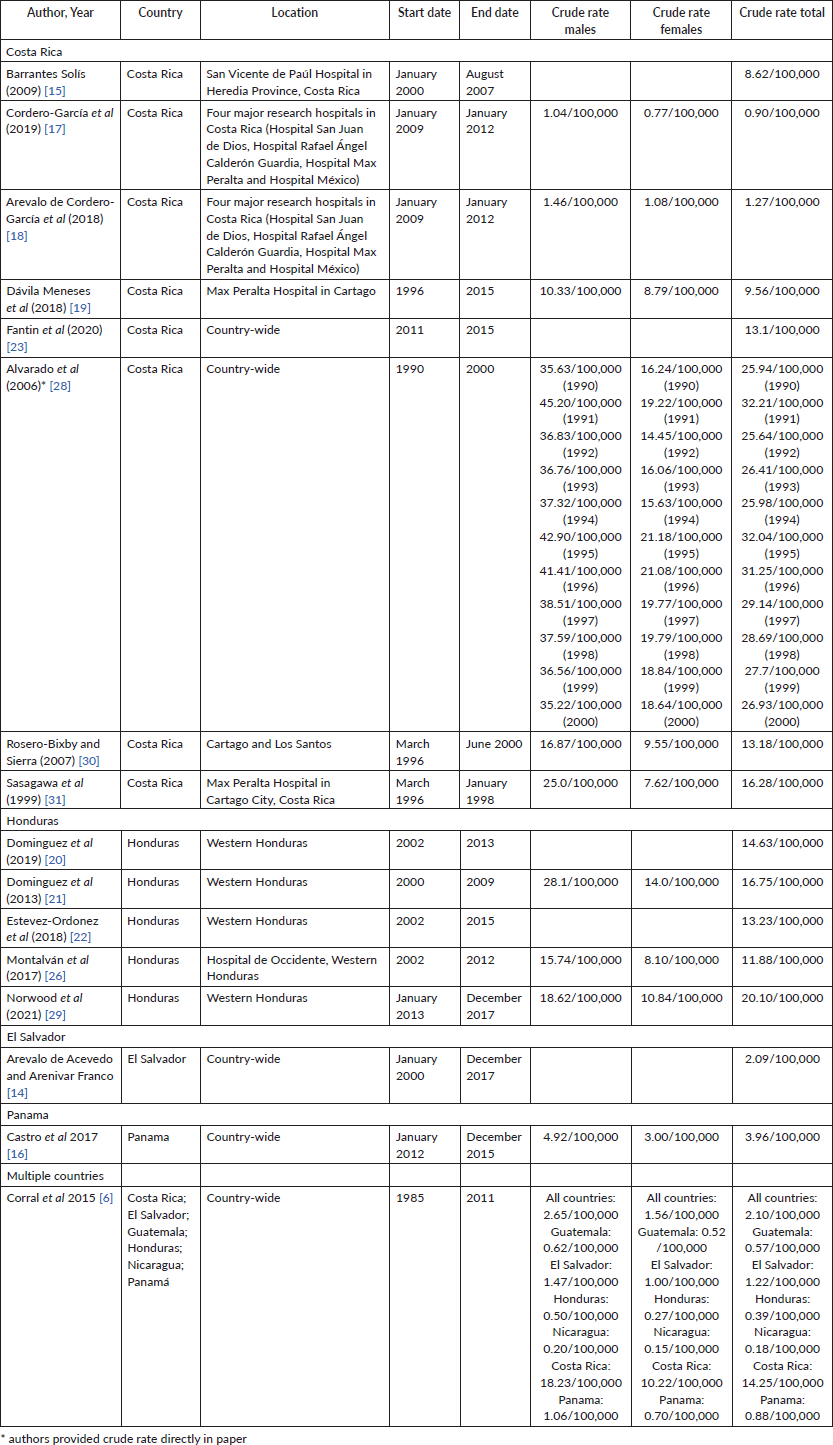
Crude rates
Crude rates were directly provided by authors in 1 paper [28]. The remaining papers included in Table 3 provided total GC cases for a certain time period. Total GC cases were divided by the sum of the population in the study’s catchment area over the time period reported. Rates were reported by 100,000 people and delineated by sex if allowed by data given in the original study.
As shown in Table 3, crude rates varied considerably by sex, country and time period studied. Overall, crude rates were higher in males than in females for all the studies in which rates could be separated by sex. Total crude rates in Costa Rica ranged from 0.09/100,000 to 32.04/100,000. Overall, crude rates appeared to decrease over more recent study periods. Total crude rates in Honduras ranged from 11.88/100,000 to 20.10/100,000, with the latter being from a more recent paper with a study period of 2013 to 2017. While this trend could be indicative of a potential true rise in rates in Western Honduras, it could also be indicative of registries becoming more robust and increased access to hospital services and the ability to be diagnosed. The single study conducted in El Salvador and a single study conducted in Panama yielded total crude rates of 2.09/100,000 and 3.96/100,000, respectively. Corral et al [6] provided case numbers for Guatemala, El Salvador, Honduras, Nicaragua, Costa Rica and Panama for the time period 1985 through 2011, with a calculated crude rate for the region of 2.65/100,000 for males and 1.56/100,000 for females.
Table 3. Calculated crude rates.
Characteristics of gastric cancer and listed risk factors
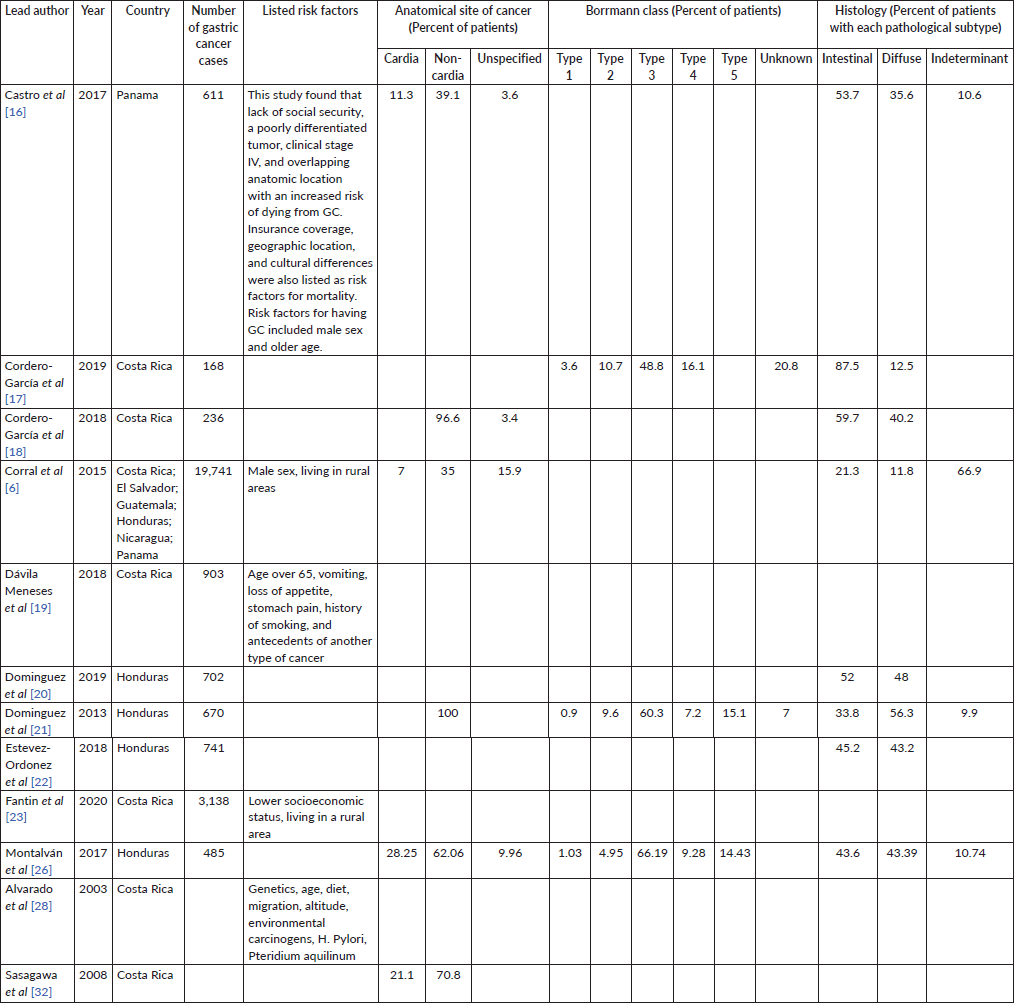
Twelve papers described the characteristics of gastric cancer in their study population. Six studies reported on anatomical sites–each study listed non-cardia as being the most common site with rates ranging between 35% and 100% of cases. Three studies reported Borrmann class, with type 3 being the most commonly reported with rates ranging from 48.88% to 66.19%. Histology varied among studies. Five studies reported intestinal pathological subtype to be more common and one reported diffuse to be more common, while two reported similar percentages of intestinal and diffuse pathologies. Five studies reported on risk factors and symptoms. Factors including male sex, older age, lower socioeconomic status and living in a rural area were reported as increasing risk for having GC. Antecedent symptoms such as vomiting, loss of appetite and stomach pain were also listed as risk factors. Lack of social security, a poorly differentiated tumour, clinical stage IV and overlapping anatomic location with an increased risk of dying from GC. Insurance coverage, geographic location and cultural differences were also listed as risk factors for mortality (Table 4).
Table 4. Stated risk factors and characteristics of gastric cancer.
Treatments and listed barriers to care
Eight papers included information on GC treatment, with all including surgery as a provided treatment amongst their study population. Chemotherapy, radiation and palliative support were also described in five studies. Reported barriers to care included lack of social security, living in a rural or remote area, political turmoil and poverty (Table 5).
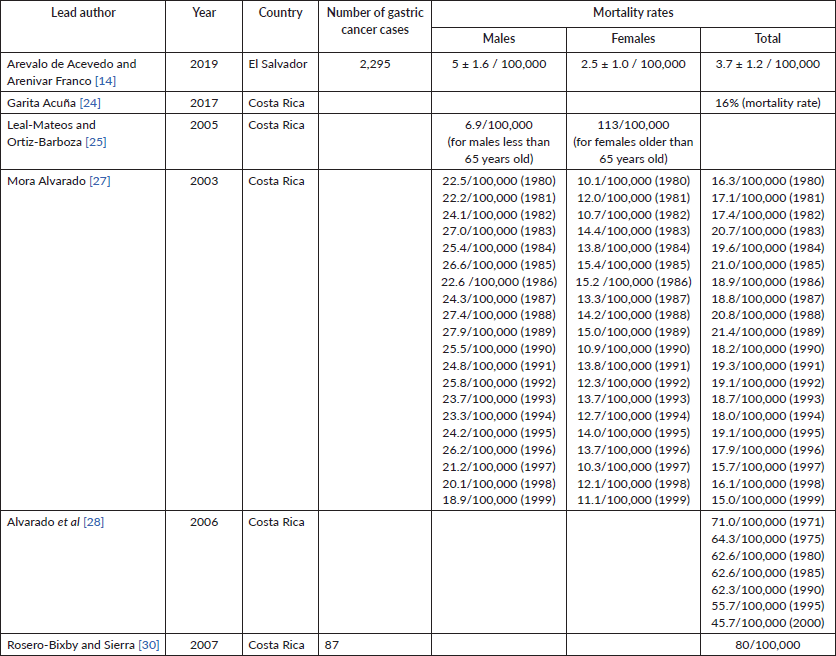
Mortality rates
Six papers reported mortality rates, with five being from Costa Rica. Arevalo de Acevedo and Arenivar Franco [14] in El Salvador reported a mortality rate of 5/100,000 for males, 2.5/100,000 for females and 3.7/100,000 overall. The remaining four papers were based in Costa Rica. According to Mora Alvarado 2003 and 2006, mortality rates have overall decreased in the final two decades of the twentieth century (Table 6).
Table 5. Treatments and stated barriers to care.
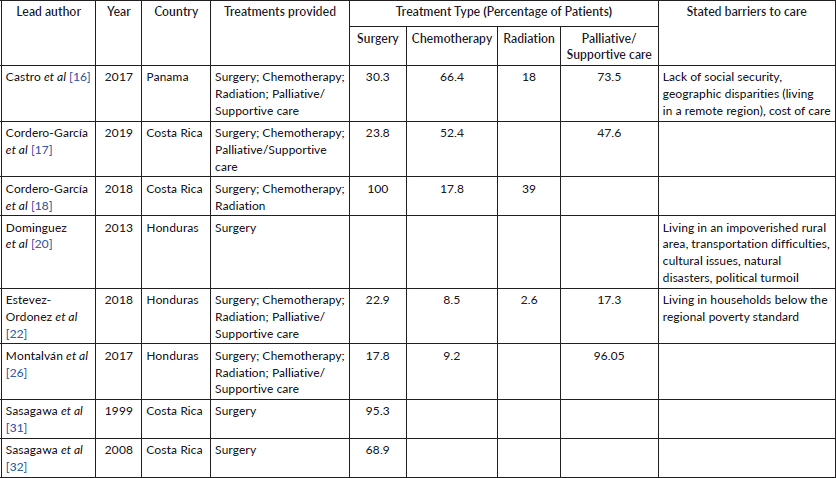
Table 6. Mortality rates.
Discussion
Our review identified a total of 20 publications discussing the epidemiology, treatment and outcomes of gastric cancer in Central America during the time period between 1997 and 2022. This points to the paucity of literature on this topic, particularly in Central American countries outside of Costa Rica. 12 of the 20 publications were from Costa Rica and 5 were from Honduras (with the majority coming from Western Honduras). Notably, there were no papers focusing on the epidemiology of GC in Belize alone and only one in El Salvador and one in Panama. Estimated crude rates were far ranging, particularly in Costa Rica for which there were the most studies available. The discrepancies in these rates may be attributable to limited data availability as well as studies representing a large geographical area – with some being nationwide versus others focusing on specific provinces. Total crude rates ranged from 0.09/100,000 to 32.04/100,000 in the time periods covered by the studies (1996–2015). Overall, crude rates appeared to decrease over more recent study periods. This is consistent with global trends of GC declining over the past three decades [33].
Overwhelmingly, non-cardia cancers were found to be more common than cardia cancers in the region. This is expected given the strong positive association between H. pylori and non-cardiac gastric cancer. A previous study found the odds ratio for the association between H. pylori and non-cardiac gastric cancer to be 4.79, consistent across sociodemographic, clinical and lifestyle factors [34] A recent study done in Western Honduras and Central Guatemala found H. pylori seropositivity to be 87% with 83% of the nearly 1,150 subjects to have active H. pylori infection [35]. This finding also may explain the observed decreased incidence of GC in the region, as improved access to clean water and sanitation decreases H. pylori infections.
The incidence and mortality rates of this study were similar to those found in previous studies. Sierra et al [36] compiled crude rates and mortality rates in Central and South America using population-based registries and nation-wide cancer deaths from the World Health Organisation’s mortality database up to 2007. This study found that crude rates in Costa Rica were 22.0/100,000 and 14.4/100,000 for males and females, respectively, during the 2003–2007 period, consistent with our review [36]. El Salvador had crude rates of 2.8/100,000 for males and 1.8/100,000 for females in the 1999–2003 period, close to our total crude rate of 2.09/100,000 total crude rate [36]. Mortality rates in El Salvador differed: our review found 3.7/100,000 for males and 2.5/100,000 for females, while Sierra et al [36] reported 8.8/100,000 for males and 7.6/100,000 for females. In Costa Rica, Sierra et al [36] reported crude death rates at 17.1/100,000 for males and 9.4 for females from 2003 through 2007, while studies in our review found rates that varied from 8/100,000 to 62.6/100,000, likely due to the broader time period studied. Similar to Sierra et al [36], our review also found non-cardia cancers to be more common than cardia cancers.
Our findings were similar to previous studies that pulled from World Health Organisation cancer registries. Variations amongst crude rates and crude death rates between studies can likely be attributed to the incompleteness of the data available. The overall geographic and temporal variation of stomach cancer rates observed in this review may be explained by variations in the prevalence of H. pylori, improvements in sanitation, preservation and storage of foods and smoking patterns [33] Additionally, the included studies may have underestimated incidence of gastric cancer–in absence of a systematic screening program for GC, only symptomatic cases are detected and asymptomatic cases may go unreported. For patients with limited access to healthcare, particularly those living in poverty or those living in rural areas, symptomatic patients may never make it to a health facility and their cases may go unreported [20].
The crude rates and mortality rates found in the current study point to the emerging epidemiological hotspot for GC in Central America. Comparatively, East Asia has the highest incidence rates of GC for both males and females at 32.5 and 13.2 per 100,000, respectively. While the rates seen in Central America are not as high as East Asia, they remain significantly higher than in North America (5.4 per 100,000 for males and 3.1 per 100,000 for females) [37]. Mortality rates for GC are also estimated to be higher in Central America than North America [37].
Our review highlights the need for reliable cancer registries in the Central American region. Often, cancer registries provide the only opportunity for properly assessing the extent and nature of cancer burdens in developing countries. Population-based GC registries are valuable for identifying demographic and geographic differences in incidences and mortality rates [38]. Hospital-, clinic- or laboratory-level data give more details for public health officials to understand local epidemiology and risk factors [38]. However, there are a number of hurdles that exist to advancing the collection of this data in LMICs including traditional Ministry of Health priorities, budget constraints and pathology service limitations [6]. In the Central Latin American context, cancer registry success may be possible by focusing attention on local-level data collection, with the Western Honduras Copán registry serving as a good example. These local registries can be monitored by national data collection guidelines [38]. Investing in GC surveillance programs and training health workers to collect accurate data can prove to be a valuable investment for regional and national health systems. This information is crucial in creating priorities for cancer control public health programs [39].
Eight of the 20 included studies discussed treatment, and surgery was provided to patients. Studies have described substantial barriers to care for GC in Central America, particularly in rural areas of the CA-4. Estevez-Ordonez et al [22] found that only 1 in 5 patients received curative or palliative surgical treatment, and 1 in 12 patients received chemotherapy in rural western Honduras, with living in households below the regional poverty standard and distance from a facility offering chemotherapy listed as major barriers [22]. Mortality data were scarce, only included in 5 of the 20 studies, with crude death rates being far-ranging. These factors point to the need for increased capacity in the region, both in terms of being able to provide far-reaching surgical treatment as well as chemotherapy, particularly to rural patients.
This paper is the first to compile published data regarding the epidemiology and treatment of GC in Central America using primary reports. While one of the strengths of this paper is that it highlights the need for additional research in regard to this topic, the small number of publications (20) limits the ability to do subsequent meta-analysis of GC incidence, treatment or outcomes in the region. Additionally, as the majority of the papers did not give a calculated crude rate, the authors of the current manuscript used the number of GC cases stated in the papers to calculate crude rates from publicly available population data. This additional step of estimating crude rates introduces a margin of error that accompanies using population data estimates.
Conclusion
Stomach cancer is one of the most frequently diagnosed cancers and the leading cause of cancer death in Central America, with current literature pointing to the region becoming an emerging hotspot. The current literature review is the first of its kind to consolidate information on epidemiology, treatment and mortality rates of GC in Central America from existing literature over the past 25 years. The crude rates we calculated and mortality rates found from our review were comparable to the rates calculated in other studies using WHO registries. The authors of the studies we pulled from also discussed similar known risk factors including age, sex, low socioeconomic factors and living in a rural area. This review highlights the need for increased data collection on gastric cancer prevalence, treatment and outcomes in Central America.
Acknowledgments
The authors would like to thank Amy Studer for her assistance in crafting the search strategy for this scoping review.
Conflicts of interest
The authors declare no conflicts of interest.
Funding
The authors declare no funding sources for this study.
References
1. Ferlay J, Ervik M, and Lam F, et al (2024) Global Cancer Observatory: Cancer Today (Lyon: International Agency for Research on Cancer) [https://gco.iarc.who.int/today] Date accessed: 07/11/24
2. de Martel C, Georges D, and Bray F, et al (2020) Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis Lancet Glob Health 8(2) e180–e190 https://doi.org/10.1016/S2214-109X(19)30488-7
3. Ferlay J, Soerjomataram I, and Dikshit R, et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012 Int J Cancer 136(5) E359–E386 https://doi.org/10.1002/ijc.29210
4. Sitarz R, Skierucha M, and Mielko J, et al (2018) Gastric cancer: epidemiology, prevention, classification, and treatment Cancer Manag Res 10 239–248 https://doi.org/10.2147/CMAR.S149619 PMID: 29445300 PMCID: 5808709
5. Piñeros M, Frech S, and Frazier L, et al (2018) Advancing reliable data for cancer control in the Central America four region J Glob Oncol 4 1–11
6. Corral JE, Delgado Hurtado JJ, and Domínguez RL, et al (2015) The descriptive epidemiology of gastric cancer in Central America and comparison with United States Hispanic populations J Gastrointest Cancer 46(1) 21–28 https://doi.org/10.1007/s12029-014-9672-1 PMCID: 4503236
7. Arksey H and O’Malley L (2005) Scoping studies: towards a methodological framework Int J Soc Res Methodol 8(1) 19–32 https://doi.org/10.1080/1364557032000119616
8. Tricco AC, Lillie E, and Zarin W, et al (2018) PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation Ann Intern Med 169(7) 467–473 https://doi.org/10.7326/M18-0850 PMID: 30178033
9. Schiavo JH (2019) PROSPERO: an international register of systematic review protocols Med Ref Serv Q 38(2) 171–180 https://doi.org/10.1080/02763869.2019.1588072 PMID: 31173570
10. Data Commons [https://datacommons.org]
11. World Bank [https://data.worldbank.org]
12. Instituto Nacional De Estadística (2013) Censo de Poblacion XVII y Vivienda VI [https://scholar.google.com/scholar_lookup?title=Censo+de+Pobacion+XVII+y+Vivienda+V I&publication_year=2013]
13. Joanna Briggs Institute (2020) Critical Appraisal Tools (Adelaide: Joanna Briggs Institute) [https://jbi.global/critical-appraisal-tools]
14. Arevalo de Acevedo CE and Arenivar Franco ME (2019) Mortalidad del Cáncer Gástrico en Instituto Salvadoreño del Seguro Social 2000-2017: Perfil Epidemiológico (San Salvador: Universidad de El Salvador) [https://repositorio.ues.edu.sv/items/19f83eb6-3a55-4138-a271-239ef1af633a]
15. Barrantes Solís T (2009) Principales causas de cáncer atendidas en un centro hospitalario: Costa Rica 2003–2007 Rev Costarric Salud Pública 18(1) 37–42 [http://www.scielo.sa.cr/scielo.php?script=sci_arttext&pid=S1409-14292009000100007&lng=en]
16. Castro F, Shahal D, and Tarajia M, et al (2017) Baseline characteristics, survival and direct costs associated to treatment of gastric cancer patients at the National Oncology Institute of Panama from 2012 to 2015: a hospital-based observational study BMJ Open 7(9) e017266 https://doi.org/10.1136/bmjopen-2017-017266 PMID: 28947456 PMCID: 5623512
17. Cordero-García E, Ramos-Esquivel A, and Alpízar-Alpízar W (2019) Evaluation of pharmacological therapies used in Costa Rica in patients with metastatic gastric cancer: a retrospective study J Gastrointest Oncol 10(3) 523–528 https://doi.org/10.21037/jgo.2019.01.18 PMID: 31183203 PMCID: 6534705
18. Cordero-García E, Ramos-Esquivel A, and Alpízar-Alpízar W (2018) Predictors of overall survival after surgery in gastric cancer patients from a Latin-American country J Gastrointest Oncol 9(1) 64–72 https://doi.org/10.21037/jgo.2017.10.07 PMID: 29564172 PMCID: 5848039
19. Dávila Meneses A, Quintanilla Retana F, and Castillo Araya K (2018) Caracterización clínica y epidemiológica de la población tamizada en el centro de detección temprana de cáncer gástrico, Costa Rica: período 1996–2015 Rev Costarric Salud Pública 27(2) 68–81 [http://www.scielo.sa.cr/scielo.php?script=sci_arttext&pid=S1409-14292018000200068&lng=en]
20. Dominguez RL, Cherry CB, and Estevez-Ordonez D (2019) Geospatial analyses identify regional hot spots of diffuse gastric cancer in rural Central America BMC Cancer 19(1) 545 https://doi.org/10.1186/s12885-019-5726-x PMID: 31174492 PMCID: 6554991
21. Dominguez RL, Crockett SD, and Lund JL, et al (2013) Gastric cancer incidence estimation in a resource-limited nation: use of endoscopy registry methodology Cancer Causes Control 24(2) 233–239 https://doi.org/10.1007/s10552-012-0109-5
22. Estevez-Ordonez D, Montalvan-Sanchez EE, and Wong RE, et al (2018) Health barriers and patterns of gastric cancer care in Rural Central American Resource-limited settings JAMA Oncol 4(8) 1131–1133 https://doi.org/10.1001/jamaoncol.2018.2570 PMID: 29978182 PMCID: 6396808
23. Fantin R, Ulloa CS, and Solís CB (2020) Social gradient in cancer incidence in Costa Rica: findings from a national population-based cancer registry Cancer Epidemiol 68 101789 https://doi.org/10.1016/j.canep.2020.101789 PMID: 32795947
24. Garita Acuña M (2017) Prevalencia del cáncer en la provincia de Heredia, Costa Rica Rev Costarric Salud Pública 26(2) 141–147 [http://www.scielo.sa.cr/scielo.php?script=sci_arttext&pid=S1409-14292017000200141&lng=en]
25. Leal-Mateos M and Ortiz-Barboza A (2005) Mortalidad por cáncer en la persona adulta mayor de Costa Rica Acta Méd Costarric 47(1) 43–46 [http://www.scielo.sa.cr/scielo.php?script=sci_arttext&pid=S0001-60022005000100007&lng=en]
26. Montalván EE, Montalván DM, and Urrutia SA, et al (2017) Survival of gastric cancer in Western Honduras pilot study: 2002–2012 Rev Med Hondur 85(1) 6–10
27. Mora Alvarado D (2003) Evolución de algunos aspectos epidemiológicos y ecológicos del cáncer gástrico en Costa Rica Rev Costarric Salud Pública 12(21) 7–17 [http://www.scielo.sa.cr/scielo.php?script=sci_arttext&pid=S1409-14292003000100004&lng=en]
28. Alvarado D, Garcia H, and Solano A (2006) Estudio exploratorio sobre la incidencia de cáncer gástrico y los contenidos de nitratos en el agua potable en Costa Rica Rev Costarric Salud Publica 15 17–28
29. Norwood DA, Montalvan-Sanchez EE, and Corral JE, et al (2021) Western Honduras Copán population-based cancer registry: initial estimates and a model for rural Central America JCO Glob Oncol 7 1694–1702 https://doi.org/10.1200/GO.21.00273 PMID: 34914550 PMCID: 8691495
30. Rosero-Bixby L and Sierra R (2007) X-ray screening seems to reduce gastric cancer mortality by half in a community-controlled trial in Costa Rica Br J Cancer 97(7) 837–843 https://doi.org/10.1038/sj.bjc.6603729 PMID: 17912238 PMCID: 2360405
31. Sasagawa T, Solano H, and Mena F (1999) Gastric cancer in Costa Rica Gastrointest Endosc 50(4) 594–595 https://doi.org/10.1016/S0016-5107(99)70095-7 PMID: 10502192
32. Sasagawa T, Solano H, and Vega W, et al (2008) The effectiveness of extended lymph node dissection for gastric cancer performed in Costa Rica under the supervision of a Japanese surgeon: a comparison with surgical results in Japan Am J Surg 195(1) 53–60 https://doi.org/10.1016/j.amjsurg.2007.01.028
33. Ferro A, Peleteiro B, and Malvezzi M, et al (2014) Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype Eur J Cancer 50(7) 1330–1344 https://doi.org/10.1016/j.ejca.2014.01.029 PMID: 24650579
34. Morais S, Costa A, and Albuquerque G, et al (2022) “True” Helicobacter pylori infection and non-cardia gastric cancer: a pooled analysis within the stomach cancer pooling (StoP) project Helicobacter 27(3) e12883 https://doi.org/10.1111/hel.12883 PMID: 35235224
35. Kim DH, Montalvan-Sanchez EE, and Alvarez CS, et al (2022) High seroprevalence of Helicobacter pylori and CagA/VacA virulence factors in northern Central America Am J Gastroenterol 117(10S) e1153–e1154 https://doi.org/10.14309/01.ajg.0000863088.15690.58
36. Sierra MS, Cueva P, and Bravo LE, et al (2016) Stomach cancer burden in Central and South America Cancer Epidemiol 44(Suppl 1) S62–S73 https://doi.org/10.1016/j.canep.2016.03.008 PMID: 27678324
37. Morgan E, Arnold M, and Camargo MC, et al (2022) The current and future incidence and mortality of gastric cancer in 185 countries, 2020–2040: a population-based modelling study EClinicalMedicine 47 101404 https://doi.org/10.1016/j.eclinm.2022.101404
38. Ramadhar A, Miller PN, and Muchengeti M, et al (2024) Gastric cancer in Sub-Saharan Africa –a systematic review of primary data Ecancermedicalscience 18 1680 https://doi.org/10.3332/ecancer.2024.1680
39. dos Santos Silva I (1999) The role of cancer registries Cancer Epidemiology: Principles and Methods ed dos Santos Silva I (Lyon: International Agency for Research on Cancer) pp 385–403
Appendix 1. Final search strategies.
PubMed
((‘Central America’[MeSH Terms]) OR (‘central america*’[All Fields] OR ‘belize*’[All Fields] OR ‘costa rica*’[All Fields] OR ‘el salvador*’[All Fields] OR ‘salvadoran*’[All Fields] OR ‘salvadorian*’[All Fields] OR ‘salvadorean*’[All Fields] OR ‘guatemala*’[All Fields] OR ‘hondura*’[All Fields] OR ‘nicaragua*’[All Fields] OR ‘panama*’[All Fields])) AND (((stomach[All Fields] OR gastric[All Fields]) AND (neoplasms[All Fields] OR
cancer*[All Fields] OR carcinoma*[All Fields] OR neoplas*[All Fields] OR adenocarcinoma*[All Fields] OR tumour*[All Fields] OR tumour*[All Fields])) OR (‘stomach neoplasms’[MeSH])).
Embase
(‘stomach tumour’/exp OR ((stomach:au,ti,kw OR gastric:au,ti,kw) AND (cancer*:au,ti,kw OR carcinoma*:au,ti,kw OR neoplas*:au,ti,kw OR adenocarcinoma*:au,ti,kw))) AND (‘central america’/de OR ‘belize’/exp OR ‘costa rica’/exp OR ‘el salvador’/exp OR ‘guatemala’/exp OR ‘honduras’/exp OR ‘nicaragua’/exp OR ‘panama’/exp OR ‘central american’/de OR ‘belizean’/exp OR ‘costa rican’/exp OR ‘guatemalan’/exp OR ‘honduran’/exp OR ‘nicaraguan’/exp OR ‘panamanian’/exp OR ‘salvadoran’/exp OR ‘central america*’:ti,ab,kw OR belize*:ti,ab,kw OR ‘costa rica*’:ti,ab,kw OR ‘el salvador*’:ti,ab,kw OR salvadoran*:ti,ab,kw OR salvadorian*:ti,ab,kw OR salvadorean*:ti,ab,kw OR guatemala*:ti,ab,kw OR hondura*:ti,ab,kw OR nicaragua*:ti,ab,kw OR panama*:ti,ab,kw) NOT (‘conference abstract’/it OR ‘conference paper’/it OR ‘conference review’/it).
Web of Science
TS=((stomach OR gastric) AND (neoplas* OR cancer* OR carcinoma* OR adenocarcinoma*)) AND TS=(‘central america*’ OR belize* OR ‘costa rica*’ OR ‘el salvador*’ OR salvadoran* OR salvadorian* OR salvadorean* OR guatemala* OR hondura* OR nicaragua* OR panama*).
Global Health (CAB Direct)
(de:(‘Central America’ OR ‘Belize’ OR ‘Costa Rica’ OR ‘El Salvador’ OR ‘Guatemala’ OR ‘Honduras’ OR ‘Nicaragua’ OR ‘Panama’) OR (‘central america*’ OR belize* OR ‘costa rica*’ OR ‘el salvador*’ OR salvadoran* OR salvadorian* OR salvadorean* OR guatemala* OR hondura* OR nicaragua* OR panama*)) AND (de:(‘stomach cancer’) OR ((stomach OR gastric) AND (neoplas* OR cancer* OR carcinoma* OR adenocarcinoma*))).
Latin America and Caribbean Health Sciences Literature
(((stomach OR gastric) AND (neoplas$ OR cancer$ OR carcinoma$ OR adenocarcinoma$))) AND (((central america$) OR (belize$) OR (costa rica$) OR (el salvador$)) OR (salvadoran$ OR salvadorian$ OR salvadorean$ OR guatemala$ OR hondura$ OR nicaragua$ OR panama$)).






