Outcomes following CyberKnife robotic radiosurgery for pituitary adenomas—a large single-centre study
Kamran Saeed1, Kaynat Siddiqui2, Hafiza Fatima Aziz2, Fatima Shaukat1, Shazia Kadri1, Aneeta Ghulam Muhammad1, Aneela Darbar3 and Tariq Mahmood1
1Cyberknife and Tomotherapy Center, Jinnah Postgraduate Medical Center (JPMC), Karachi 75510, Pakistan
2Dr. Ziauddin Hospital, Karachi 74700, Pakistan
3The Aga Khan University Hospital, Karachi 74800, Pakistan
Abstract
Introduction: The role of stereotactic radiosurgery (SRS) in pituitary adenomas (PAs) is evolving especially considering its safety. Existing literature is hampered by limited sample sizes and short-term follow-ups, impeding its preeminence in the clinical and radiological outcomes. We propose a comprehensive, single-centred study to evaluate the outcomes following CyberKnife stereotactic radiosurgery (CK SRS) for PAs in a larger patient population, incorporating meticulous clinical and radiological follow-up.
Methods: This is a retrospective cohort study of 278 cases of PAs that underwent CK SRS from 2013 to 2021. Based on their endocrinology profile, they were classified as functional adenomas (FA) and non-functional adenomas (NFA). We assessed pre and post-CK SRS clinical, visual, hormonal and radiological parameters and the associated toxicity. Where applicable, data were compared using the Independent t-test, chi-square test, Fisher Exact and Mann-Whitney U test. A p-value <0.05 was considered significant.
Results: The median age of the patients was 40.13 ± 12.61 years (111 female and 167 male patients). The median prescribed radiosurgery dose was 25.0 ± 5.0 Gy into 3 or 5 fractions. The median follow-up time was 12 months (IQR 20). Data were grouped into NFA (169, 60.8%) and FA (109, 39.2%). After adjusting for patients lost to follow-up, post-CK SRS visual perimetry improved in 80.4% of patients and tumour size reduced in 78.6% of the study population. Seventeen patients with NFA and nine with FA manifested new-onset hormonal deficiencies. No statistically significant differences were seen in post-CK SRS visual outcomes and hormone deficiency groups.
Conclusion: CK SRS is effective and safe for managing PAs, achieving tumour control and preserving visual function with minimal toxicity. Extended follow-up is needed to evaluate post-SRS toxicity and hypopituitarism.
Keywords: CyberKnife, radiosurgery, pituitary adenomas, acromegaly, Cushing’s disease, nonfunctional adenomas
Correspondence to: Kaynat Siddiqui
Email: Kaynat.smc@gmail.com
Published: 28/11/2024
Received: 12/07/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Pituitary adenomas (PAs) constitute a heterogeneous group of intracranial tumours, representing approximately 14%–22% of primary benign brain tumours [1]. Their classification as functional or non-functional is contingent upon their hormonal secretions. Incidental discovery or clinical attention is prompted by symptoms arising from mass effects, such as headaches, visual field defects, apoplexy or endocrinopathies [2].
Surgery is the gold standard modality for non-functional and functional adenomas (FA) except in cases of prolactinomas that require mainly medical management and often yield optimal curative outcomes. The selection of an optimal treatment modality is contingent upon factors such as the tumour’s anatomical location, its proximity to optic pathways and the patient’s clinical status. However, stereotactic radiosurgery (SRS) has demonstrated promise in managing residual and recurrent adenomas, particularly in cases where surgery is declined or tumours are deemed inoperable [3, 4]. Among SRS modalities, CyberKnife stereotactic radiosurgery (CK SRS) stands out as an advanced technology that uses robotics to deliver radio surgical doses in a frameless manner, eliminating the need for skull fixation and minimal patient discomfort, distinguishing it from gamma knife procedures [5].
While prior studies have indicated favourable outcomes for CK SRS in both FA and non-functional adenomas (NFA) on tumour reduction or stability, vision preservation and neuroendocrine control [6–8] – existing literature is hampered by limited sample sizes and short-term follow-ups, impeding the definitive establishment of CK SRS preeminence in the clinical and radiological outcomes of PAs. Consequently, we propose a comprehensive, single-centred study to evaluate the outcomes following CK SRS for PAs in a larger patient population, incorporating meticulous clinical and radiological follow-up.
Methods
Patient information
The data were retrospectively gathered from the clinical records of 278 patients from 2013 to 2021 at the CyberKnife Robotic Radiosurgery Centre in Karachi, Pakistan. All data were collected following Institutional Review Board guidelines. All patients received CK SRS as an adjunct for residual or recurrent tumours or upfront in cases where surgery was not considered as a first-line treatment modality due to co-morbidities, critical location or clinical status of the patient.
Radiosurgery technique and parameters
Patients underwent treatment on the CyberKnife G4 machine model equipped with independent rapid integrated shaping. It utilises a 6- mega-voltage lightweight linear accelerator mounted on a fully articulated robotic arm. Treatment planning involved using Multiplan software (Accuray precision 3.3) for inverse planning. Patients were positioned supine and immobilised using a thermoplastic face mask. Treatment plans were generated at a workstation using image data from contrast-enhanced computed tomography (CT) scans with a slice thickness of 1 mm, supplemented with gadolinium-enhanced magnetic resonance imaging (MRI). The gross tumour volume (GTV) and critical structures were delineated in each consecutive CT slice. No additional margin was applied to the GTV to derive the clinical target volume and planning target volume.
Clinical assessment
The initial assessment incorporated formal visual field testing (perimetry), conducted by an ophthalmologist, gadolinium-enhanced MRI scans and hormonal assessment by an endocrinologist. In our clinic, all patients had follow-ups, which included MRI with contrast and perimetry. For non-functional pituitary adenomas, follow-ups were conducted at 3 or 6-month intervals during the first 2 years, followed by annual evaluations thereafter. In contrast, patients with functional pituitary adenomas (FPA) were monitored every 8 months until hormonal remission was achieved, after which they transitioned to annual follow-ups. We evaluated all post-CK SRS variables at the latest follow-up. The departmental radiologist evaluated the MRI scans. Any reduction in tumour size on MRI was considered tumour reduction, while the absence of any visible contrast-enhancing lesion on MRI was taken as complete tumour resolution. Tumour stabilisation was defined as no change in tumour size compared to pre-CK SRS scans. Similarly, vision changes detected through perimetry were classified as improved, stable or deteriorated accordingly.
Hormonal assessment
The post-treatment endocrine follow-up was determined by the patient’s endocrinologist, considering the pre-treatment endocrine status and the adenoma’s functional status. We evaluated the latest hormonal profile. In our study, we defined biochemical remission in FPA as the normalisation of hormone levels. Partial reduction was defined as a decrease in specific hormone levels in FPA, although not within their normal ranges. Additionally, an increase in hormone levels was defined as levels exceeding their pre-CK SRS values. All patients underwent an evaluation for post-treatment hypopituitarism.
Statistical analysis
Data were analysed by using SPSS version 26. For quantitative variables mean ± SD or median (IQR) were reported based on normality. However, qualitative variables were reported as frequency and percentages. Statistical comparisons were done using parametric or non-parametric tests, as appropriate. Continuous variables were analysed using the independent T-test or Mann–Whitney U test depending on the normality of the distribution. Normality was examined by the use of the Shapiro–Wilk test. Categorical variables were analysed by Pearson’s chi-squared test or Fisher’s exact test, as appropriate. For the comparison of post-CK perimetry and post-CK radiology with continuous variables, ANOVA or Kruskal–Wallis tests were applied, as appropriate.
Results
At the time of treatment, the median age of the patients was 40.13 ± 12.61 years, ranging from 8 to 79 years. The cohort comprised 111 female and 167 male patients. Eighty-four percent (n = 234) of the study population had previous surgery. The median follow-up time was 12 (IQR 20) months. Detailed patient demographic data is presented in Table 1.
The study included 169 (60.8%) NFA and 109 (39.2%) FA (Figure 1). The most common FA was growth hormone (GH)-secreting (n = 89, 81.7%) followed by Adrenocorticotrophic hormone (ACTH)-secreting adenomas (n = 17, 15.6%) and medically refractory prolactinomas (n = 3, 2.8%) (Figure 2). The median prescribed dose was 25.0 ± 5.0 Gy into 3 or 5 fractions. The median GTV was 7,143.5 (IQR 5937.6) mm.
Visual outcomes
Among a cohort of 278 patients, the pre-CK SRS perimetry was available for 265 patients. Out of these, partial visual impairment was present in 239 (86%) cases. However, only 189 (67.9%) patients provided post-CK SRS perimetry for comparison. Among these, the visual field improved in 152 (80.4%), while remained stable in 35 (18.5%) patients. Two (1.1%) instances of deteriorated vision were recorded post-intervention, one of which was attributed to pituitary apoplexy. Although post-CK SRS perimetry improved in patients with larger GTV (p- value = 0.18), we found no statistically significant correlation between post-CK SRS perimetry and tumour type, history of previous surgery, gender, age and radiosurgery parameters (Table 2).
Table 1. Baseline and treatment characteristics of patients undergoing fractionated CyberKnife radiosurgery for PAs.

Figure 1. Types of PAs.
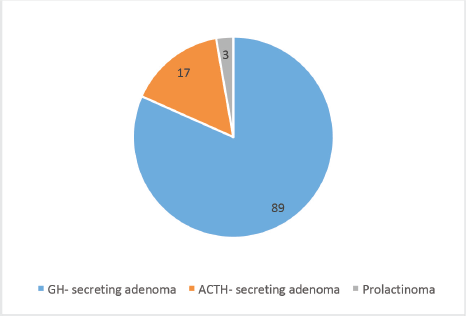
Figure 2. Sub-groups of FA.
Table 2. Correlation of post radiosurgery visual outcomes with other variables.
Radiological outcomes
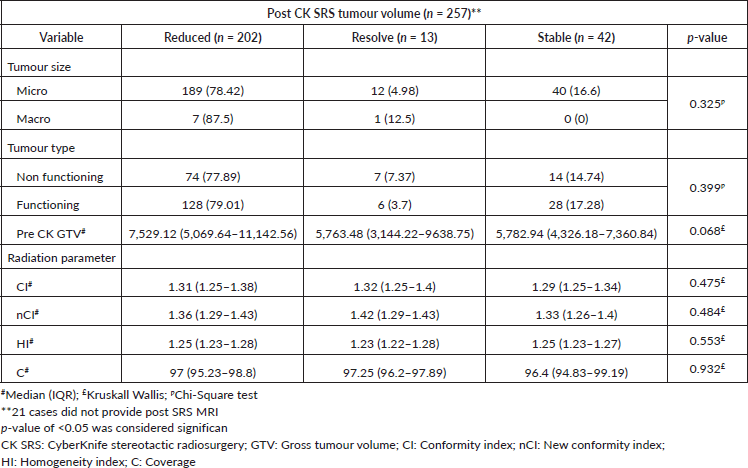
During the follow-up period, only 257 participants provided MRI scans, representing 92.4% of the cohort. Among these individuals, 202 (78.6%) had a reduction in tumour volume, 13 (5.1%) had complete resolution of enhancing lesion and 42 (16.3%) had no interval change in tumour size throughout the follow-up period (Figure 3a and b). We observed that post-CK SRS tumour reduction was more in the larger GTV group (p-value = 0.068). Furthermore, there was no statistically significant correlation between post-CK SRS radiological outcomes and tumour type or radiosurgery parameters (Table 3).
Hormonal outcomes
Among the cohort of 278 patients, 87 individuals (32%) exhibited pre-CK SRS hormonal deficiencies, primarily resulting from the tumour or post-surgical resection. Notably, following the treatment, 224 (80.2%) patients followed with the endocrinologist and, therefore, were assessed for any new onset hypopituitarism. We found that 17 (10.1%) cases of NFA and 9 (8.3%) cases of FA manifested new-onset hormonal deficiencies and 199 (71.58%) patients had normal endocrine profiles in the post-CK SRS follow-up. Moreover, there is no statistically significant correlation with age or previous surgery (Table 4).
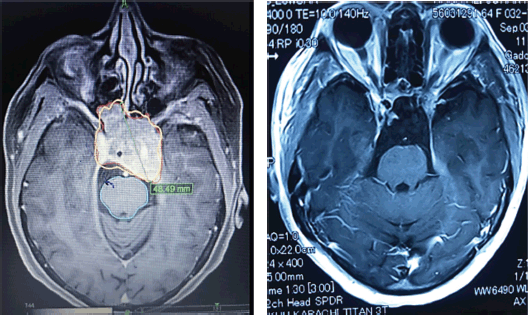
Figure 3. (a): A case of large pituitary macroadenoma before receiving CyberKnife radiosurgery. (b): Near complete resolution of pituitary macroadenoma of the same patient after receiving CyberKnife radiosurgery in the follow-up period.
Table 3. Factors associated with change in tumour volume (post CK SRS radiology).
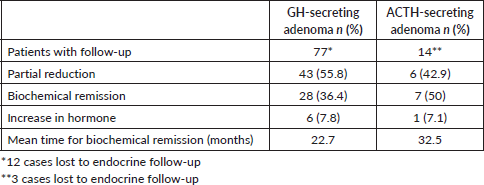
The endocrinological monitoring of acromegaly patients involved the assessment of insulin like growth factor -1 and GH levels. Among the 77 cases included in the follow-up analysis, biochemical remission was observed in 28 cases (36.4%), while 43 cases (55.8%) showed a partial reduction in hormone levels. Conversely, hormone level elevation was documented in 6 patients (7.8%). Similarly, 14 individuals with Cushing’s disease underwent follow-up evaluations based on serum cortisol levels. Among these cases, seven (50%) achieved biochemical remission, while six (42.9%) exhibited a partial reduction in hormone levels. However, in one instance (7.1%), there was an observed increase in hormone levels during the follow-up period. Furthermore, two (66.7%) cases of medically refractory prolactinoma demonstrated a partial reduction in hormone levels followed by a singular case of biochemical remission following CK SRS (Table 5).
Table 4. Factors associated with new onset hormone deficiency in the follow-up period.
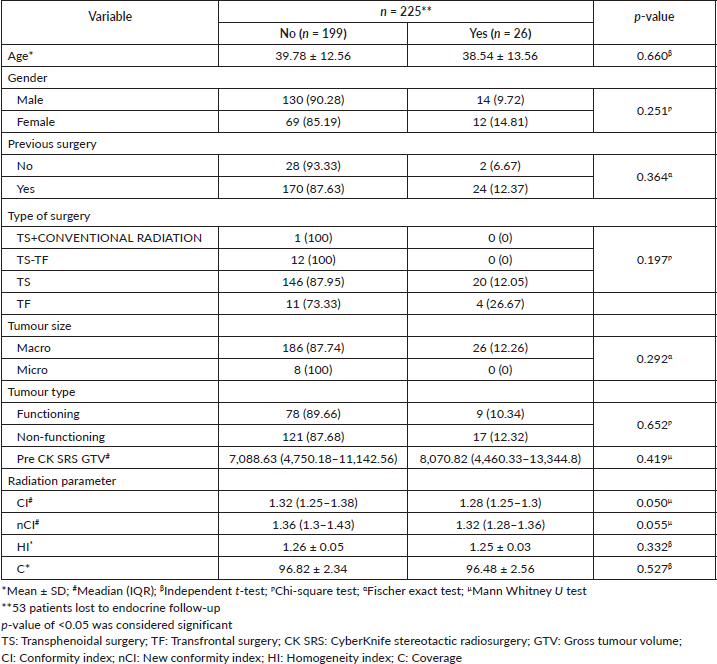
Table 5. Hormonal outcomes of FPA.
Post SRS toxicity
Among the 278 cases, post-SRS complications were encountered in 6 patients (2.6%). These complications encompassed mild sensory disturbances in the facial region or occurrences of sharp intermittent pain at the lateral facial region that was managed conservatively. Importantly, no instances of extraocular motor deficits were observed in any patient during the follow-up period. Five patients experienced mortality during the follow-up period. One patient succumbed to end-stage renal disease, while another developed uncontrolled diabetes leading to fatal complications. Both of these patients had co-morbid conditions prior to CK SRS. Additionally, a patient with Cushing’s disease died due to uncontrolled ACTH levels as he did not seek further treatment. Two patients experienced cerebrovascular accidents, leading to subsequent mortality.
Discussion
The management of FA requires a multidisciplinary approach. In the case of prolactinomas, medical interventions serve as the primary treatment modality, with further intervention considered only when the condition becomes medically refractory. Conversely, for acromegaly and Cushing’s disease, surgery is considered the gold standard of treatment. After initial therapeutic measures, residual or recurrent disease is effectively managed through SRS. In instances where surgical intervention is contraindicated due to coexisting medical conditions, patient refusal or heightened surgical risks attributed to critical tumour locations, radio-surgical intervention is employed as a primary treatment modality for these tumours [4].
We observed that a significant number of cases in our study had improved perimetry in the follow-up period which may be due to the hypofractionated schedule of the CK- SRS as highlighted by Meral et al [9]. In their study of 31 patients with acromegaly treated with hypofractionated CK SRS, they observed 100% tumour control along with 86.7% hormonal remission. Furthermore, three patients had improved optic neuropathy. However, 32.3% of patients developed hypopituitarism in the follow-up period [9]. In another study by Abdali et al [10] 41 post-surgical residual ACTH-secreting adenomas, were treated with single fraction SRS. They reported that at a median follow-up of 48.8 months, none of their patients had any visual deterioration and 60.97% of cases achieved biochemical remission at 14 months [10]. Similarly, a cohort of 53 patients with perioptic PAs within 3 mm of optic apparatus, were treated with fractionated CK SRS where none experienced visual decline [4]. The authors also reported a case of pituitary apoplexy, which was observed in our study as well. Sala et al [7] shared their experience with CK SRS for invasive GH-secreting macroadenomas, where they observed no visual deterioration during the follow-up period of 43.2 months. Furthermore, Kajiwara et al [11] studied 21 patients with PAs who underwent CK SRS with a mean dose of 14.3 ± 4.5 Gy at a mean follow–up of 35.3 ± 10.7 months. They reported worsened visual acuity in one patient 2 years post-CK SRS due to cystic expansion of the tumour [11]. However, a study of 20 consecutive patients, treated with hypofractionated CK SRS, was followed for 26.6 ±10.5 months and did not report any visual deterioration post SRS [12]. In a retrospective study of ACTH-secreting adenomas, 7 cases were observed for different outcome measures where none reported worsening of vision in the follow-up period [13]. In a large study of perioptic tumours treated with hypofractionated CK SRS, it was observed that among 40 patients with PAs, one patient experienced visual deterioration in the absence of any tumour progression at 17 months follow-up that later resolved with the treatment in the next few months [14]. In a study of 50 patients undergoing CK SRS, 8% (n = 4) had post-SRS change in visual function [15]. In an extensive literature review by Kajiwara et al [16] there were no visual deficits reported in any study of PAs treated with CK SRS [16]. This has been further supported by Iwata et al [17] who studied the long-term effects of hypofractionated CK SRS in 52 patients with GH-secreting adenoma. He concluded that by using hypofractionation and low toxicity dose for the optic apparatus, one can avoid any worsened visual acuity in the extended post-SRS period [17]. Similarly, the visual outcome of 21 patients with residual or recurrent PAs reported no deterioration in vision post-CK SRS delivered in a multi-session fashion [6]. Our study is per the existing literature where only 1.1% of cases experienced a deteriorated vision in the post-CK SRS period; however, 80.4% of cases had visual improvement, whereas 18.5% of patients remained stable.
In one of the recent studies of GH-secreting PAs by Romero-Gameros et al [18] biochemical remission was achieved in 12.2% post-CK SRS, while 33.3% of the patients required additional medical treatment. Notably, 54.4% of the cohort exhibited persistent biochemical activity [18]. Fourteen (24.56%) patients developed panhypopituitarism at the end of the follow-up; however, there were no cases of optic pathway toxicity or any cerebrovascular accident [18]. Apaydin et al [19] determined that within a cohort of 38 cases undergoing either GK SRS or CK SRS, the median duration for the biochemical control rate was 52.6% whereas, the time to remission was 15 months. In a study by Ehret et al [15] a total of nine patients (18%) achieved biochemical remission, 24 patients (48%) maintained biochemical control and 17 patients (34%) exhibited uncontrolled biochemical profiles at the last follow-up. Moreover, three patients (6%) manifested new-onset hypopituitarism [15].
Similarly, within a retrospective analysis encompassing 53 patients, Plitt et al [4] determined that the hormonal status remained unchanged in 98.1% of cases following CK SRS. Notably, a singular patient exhibited a deteriorated hormonal profile attributed to pituitary apoplexy at 4 months post-SRS. Furthermore, there was a 75% rate of hormonal control for FA [4]. In a small study of 22 GH- secreting adenomas, 18.1% exhibited biochemical control, whereas 40.9% of patients achieved complete control. However, 6 new patients developed new onset pituitary deficiency at 31.6 ± 14.5 months [7]. Puataweepong et al [14, 20] investigated a cohort of 40 patients diagnosed with perioptic PAs who underwent hypofractionated CK SRS. Their findings, based on a prescribed dose of 25 Gy administered in 5 fractions over a 38.5-month follow-up period, revealed hormonal control in 54% of patients, with no occurrences of new-onset hypopituitarism. However, one patient with a prolactinoma experienced an increase in tumour size during the post-SRS follow-up period [20]. All the existing studies conclude hypopituitarism is the late toxicity of CK SRS as observed in 26 cases in our study cohort.
Conclusion
In summary, the results of our study support the efficacy and safety of CK SRS in managing PAs, emphasizing its role in achieving tumour control and preserving visual function. The utilisation of SRS is particularly beneficial for protecting critical optic pathways and providing maximum tumour control with a minimal toxicity profile. However, there is still a need for extended follow-up to evaluate further for post-SRS toxicity and hypopituitarism. We anticipate that our outcomes will contribute significantly to paving further way for establishing the standard treatment protocol for PAs.
Limitations
There are a few limitations to our study. This is a retrospective study and several cases were lost to follow-up in various instances. The follow-up period should be extended to evaluate further long-term complications.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Funding
The author(s) received no financial support for the research, authorship or publication of this article.
References
1. Ezzat S, Asa SL, and Couldwell WT, et al (2004) The prevalence of pituitary adenomas: a systematic review Cancer 101(3) 613–619 https://doi.org/10.1002/cncr.20412 PMID: 15274075
2. Randeva HS, Schoebel J, and Byrne J, et al (1999) Classical pituitary apoplexy: clinical features, management and outcome Clin Endocrinol (Oxf) 51(2) 181–188 https://doi.org/10.1046/j.1365-2265.1999.00754.x PMID: 10468988
3. Kong DS, Lee J, and Lim DH, et al (2007) The efficacy of fractionated radiotherapy and stereotactic radiosurgery for pituitary adenomas: long-term results of 125 consecutive patients treated in a single institution Cancer 110(4) 854–860 https://doi.org/10.1002/cncr.22860 PMID: 17599761
4. Plitt AR, El Ahmadieh TY, and Aoun SG, et al (2019) Fractionated CyberKnife stereotactic radiotherapy for perioptic pituitary adenomas World Neurosurg 126 e1359–e1364 https://doi.org/10.1016/j.wneu.2019.03.102 PMID: 30902774
5. Chang SD and Adler JR (2001) Robotics and radiosurgery – the Cyberknife Stereotact Funct Neurosurg 76(3–4) 204–208 https://doi.org/10.1159/000066719
6. Chen YH, Chang SD, and Ma HI, et al (2013) Multisession CyberKnife radiosurgery for post-surgical residual and recurrent pituitary adenoma: preliminary result from one center J Radiosurgery SBRT 2(2) 105–117
7. Sala E, Moore JM, and Amorin A, et al (2018) CyberKnife robotic radiosurgery in the multimodal management of acromegaly patients with invasive macroadenoma: a single center’s experience J Neurooncol [Internet] 138(2) 291–298 https://doi.org/10.1007/s11060-018-2793-9 PMID: 29429125
8. Iwata H, Sato K, and Tatewaki K, et al (2011) Hypofractionated stereotactic radiotherapy with CyberKnife for nonfunctioning pituitary adenoma: high local control with low toxicity Neuro Oncol 13(8) 916–922 https://doi.org/10.1093/neuonc/nor055 PMID: 21665918 PMCID: 3145469
9. Meral R, Selcukbiricik OS, and Uzum AK, et al (2023) Promising outcomes in acromegaly patients receiving CyberKnife stereotactic hypofractionated radiotherapy Cureus 15(10) https://doi.org/10.7759/cureus.47936 PMID: 37908695 PMCID: 10613787
10. Abdali A, Kalinin PL, and Trunin YY, et al (2021) CyberKnife for the management of Cushing’s disease: our institutional experience and review of literature Br J Neurosurg [Internet] 35(5) 578–583 https://doi.org/10.1080/02688697.2021.1921107 PMID: 33955316
11. Kajiwara K, Saito K, and Yoshikawa K, et al (2005) Image−guided stereotactic radiosurgery with the CyberKnife for pituitary adenomas Minim Invasive Neurosurg [Internet] 48(2) 91–96 [http://eprints.ncrm.ac.uk/2879/1/NCRM_workingpaper_0412.pdf] https://doi.org/10.1055/s-2004-830261 PMID: 15906203
12. Killory BD, Kresl JJ, and Wait SD, et al (2009) Hypofractionated CyberKnife radiosurgery for perichiasmatic pituitary adenomas: early results Neurosurgery 64(2 SUPPL.) 19–25 https://doi.org/10.1227/01.NEU.0000341630.42160.18
13. Moore JM, Sala E, and Amorin A, et al (2018) CyberKnife radiosurgery in the multimodal management of patients with cushing disease World Neurosurg [Internet] 112 e425–e430 https://doi.org/10.1016/j.wneu.2018.01.057 PMID: 29355797
14. Puataweepong P, Dhanachai M, and Hansasuta A, et al (2018) Clinical outcomes of perioptic tumors treated with hypofractionated stereotactic radiotherapy using CyberKnife® stereotactic radiosurgery J Neurooncol [Internet] 139(3) 679–688 https://doi.org/10.1007/s11060-018-2913-6 PMID: 29846895
15. Ehret F, Kufeld M, and Fürweger C, et al (2021) Robotic radiosurgery for persistent postoperative acromegaly in patients with cavernous sinus-invading pituitary adenomas- a multicenter experience Cancers (Basel) 13 537 https://doi.org/10.3390/cancers13030537
16. Kajiwara K, Saito K, and Yoshikawa K, et al (2010) Stereotactic radiosurgery/radiotherapy for pituitary adenomas: a review of recent literature Neurol Med Chir (Tokyo) 50(9) 749–755 https://doi.org/10.2176/nmc.50.749 PMID: 20885109
17. Iwata H, Sato K, and Nomura R, et al (2016) Long-term results of hypofractionated stereotactic radiotherapy with CyberKnife for growth hormone-secreting pituitary adenoma: evaluation by the Cortina consensus J Neurooncol 128 267–275 https://doi.org/10.1007/s11060-016-2105-1 PMID: 26961771
18. Romero-Gameros CA, González-Virla B, and Vargas-Ortega G, et al (2023) Efficiency and safety of CyberKnife robotic radiosurgery in the multimodal management of patients with acromegaly Cancers (Basel) 15(5) 1–11 https://doi.org/10.3390/cancers15051438
19. Apaydin T, Ozkaya HM, and Durmaz SM, et al (2021) Efficacy and safety of stereotactic radiotherapy in Cushing’s disease: a single center experience Exp Clin Endocrinol Diabetes 129(7) 482–491 https://doi.org/10.1055/a-1217-7365
20. Puataweepong P, Dhanachai M, and Hansasuta A, et al (2016) The clinical outcome of hypofractionated stereotactic radiotherapy with CyberKnife robotic radiosurgery for perioptic pituitary adenoma Technol Cancer Res Treat 15(6) NP10–NP15 https://doi.org/10.1177/1533034615607113






