Disparities in the consensus for treatment of chemotherapy-induced thrombocytopenia
Liana Hambardzumyan1,2, Henrik Grigoryan1,3,4, Maria Badikyan1,5, Heghine Khachatryan1,3, Nelly Sargsyan1,3, Arliette Sulikhanyan6, Gevorg Tamamyan1,3,4,5 and Justin Stebbing2
1Hematology Center after Prof. R. H. Yeolyan, Yerevan 0014, Armenia
2Department of Surgery and Cancer, Imperial College, London SW7 2BX, UK
3Department of Pediatric Oncology and Hematology, Yerevan State Medical University, Yerevan 0025, Armenia
4Pediatric Cancer and Blood Disorders Center of Armenia, Hematology Center after Prof. R. H. Yeolyan, Yerevan 0014, Armenia
5Immune Oncology Research Institute, Yerevan 0014, Armenia
6California Department of Public Health, Los Angeles, CA 95899-7377, USA
Abstract
Introduction: Chemotherapy-induced thrombocytopenia (CIT) is an arduous complication of chemotherapy to be dealt with, and there are many unmet needs in this field to be addressed on the global front. We have conducted this study to contribute to the understanding of existing knowledge gaps of CIT management and highlight the direction to focus future investigations.
Methods: This was an academic single-institution report on a cross-sectional study evaluating CIT management practices using platelet (PLT) transfusions by haematologists and oncologists in Armenia.
Results: Physicians’ opinions differed significantly when it came to defining thrombocytopenia by PLT levels. 13.2% of those surveyed considered thrombocytopenia to be when PLT counts fall below 180 × 109/L, 42.1% defined thrombocytopenia to have a PLT threshold of 150 × 109/L, 15.8% and 21.0% specialists setting their thresholds at 140 × 109/L and 100 × 109/L, respectively.
All physicians managed CIT by performing PLT transfusions for prophylactic purposes (i.e., when PLT count falls below a certain threshold) with none of them transfusing PLTs only on-demand to address active bleeding. 73.3% haematologists (adult), 57.1% medical oncologists, and 50% paediatricians deemed 10 × 109/L as the threshold PLT count for transfusing afebrile patients with haematologic malignancies (besides acute promyelocytic leukaemia (APL)) and solid tumours.
PLT products availability varied among the respondents, with only 53% of them responding that they had 24/7 access.
Conclusion: CIT is a complication of interest to physicians worldwide and has not been resolved yet. This is the first conducted survey regarding CIT and the initial step for further research.
Keywords: platelets, thrombocytopenia, guidelines, chemotherapy
Correspondence to: Gevorg Tamamyan
Email: gevorgtamamyan@gmail.com
Published: 13/11/2023
Received: 06/07/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Chemotherapy-induced thrombocytopenia (CIT) usually refers to the decrease of platelet (PLT) counts in peripheral blood below 100 × 109/L for 3–4 weeks following the last chemotherapy, which may result in chemotherapy delays and/or dose reductions [1]. It is a frequent haematologic complication of myelosuppressive cancer therapy where incidence and prevalence vary greatly by cancer type and chemotherapy regimen. A review of published studies shows that CIT occurs in about 10%–40% of patients with solid tumours and 40%–70% of patients with haematologic malignancies [2–4]. To be considered clinically significant, PLT counts must fall below 50 × 109/L (grades 3 and 4 thrombocytopenia (The National Cancer Institute (NCI), common terminology criteria for adverse events, (version 5.0), shown in Figure 1a). This affects approximately 4% (grade 3) and 2% (grade 4) of patients with solid tumours and 16% and 12%, respectively, of patients with haematologic malignancies [4]. The highest prevalence of thrombocytopenia among solid tumour patients was observed in these cancer types in descending order: colorectal, non-small cell lung, and ovarian [5]. The main mechanism of thrombocytopenia is myelosuppression, but immune-mediated mechanisms, splenic sequestration and other issues also directly impact PLT release. Molecular mechanisms include inhibition of platelet-derived growth factor (PDGF), and apoptosis of megakaryocytes, or release of toxic mediators into the bone marrow milieu which may also play a role [6–9].
Currently, there are no standardized guidelines nor Food and Drug Administration-approved agent for the prevention or treatment of CIT. According to published literature, thrombopoietin receptor agonists (TPO-RA) improve PLT count in the majority of cases. Consistent maintenance of TPO-RA may allow for the resumption of chemotherapy without recurrence of CIT but it is not included in the guidelines for CIT management yet [2, 10, 11]. PLT transfusions can provide only temporary improvement and are not a rational treatment option during chemotherapy cycles for an extended period. Regardless, they are still the main treatment approach for CIT, including resource-poor settings. Chemotherapy dosage, cycle reduction and/or treatment delays also play a role in CIT management, reducing bleeding risk and the need for frequent PLT transfusions. These may, however, result in the reduction of relative dose intensity – a consequence that may impact the progression-free and overall survival [12–14].
CIT is considered an arduous complication of chemotherapy to be dealt with, and there are many unmet needs in this field to be addressed on the global front. We have conducted this study to contribute to the understanding of existing knowledge gaps of CIT management and highlight the direction to focus future investigations.
Materials and methods
This was an academic single-institution report on a cross-sectional study evaluating CIT management practices using PLT transfusions by haematologists and oncologists in Armenia. This study was conducted at the only haematology centre in Armenia, where adult and paediatric patients with haematological malignancies and paediatric cancer (both solid and liquid tumours) patients are managed. The centre also has a medical oncology department providing cancer care to adult patients with solid tumours.
The survey was conducted among all haematologists, oncologists and clinical residents providing care at departments of adult haematology (out- and inpatients), Pediatric Cancer and Blood Disorders Center of Armenia (situated at the Hematology Center), and the medical oncology department. The participants were asked to complete our self-administered cross-sectional survey and were informed that they could stop the survey at any moment or skip any questions they deemed to be uncomfortable (Appendix).
Questions used to generate the data for this study were organized into four sections. The first section enquired about their specialization and years of experience. The second section included questions assessing the evaluation of thrombocytopenia. The third section aimed to evaluate management approaches of CIT (depending on their specializations) by PLT transfusions, as well as PLT count thresholds for febrile and afebrile patients of various cancer types. The fourth section was dedicated to deducing accessibility of PLT products and details regarding transfusion costs.
Results
In total, 38 physicians completed the survey: 15 (39.5%) of them were haematologists (adult), 7 (18.4%) were medical oncologists, 6 (15.8%) paediatric haematologists, 3 (7.9%) paediatric oncologists and 7 (18.4%) were paediatric oncology/haematology residents. Twenty-five (65.8%) of the physicians had less than 6 years of professional experience, 6 (15.8%) physicians had more than 10 years and 7 (18.4%) physicians had more than 20 years.
Physicians’ opinions differed significantly when it came to defining thrombocytopenia by PLT levels. Five (13.2%) of those surveyed considered thrombocytopenia to be when PLT counts fall below 180 × 109/L. Of these, it was notable that 4 of the 5 had less than 6 years of professional experience, with one having 11 years. In contrast, 16 (42.1%) of those surveyed, defined thrombocytopenia to have a PLT threshold of 150 × 109/L, with another six (15.8%) and eight (21.0%) specialists setting their thresholds at 140 × 109/L and 100 × 109/L, respectively. One physician denoted a threshold of 130 × 109/L, and two participants did not provide an actual number – instead, noting that they consider thrombocytopenia when PLT counts fall below the ‘normal᾿ level (Figure 1a).
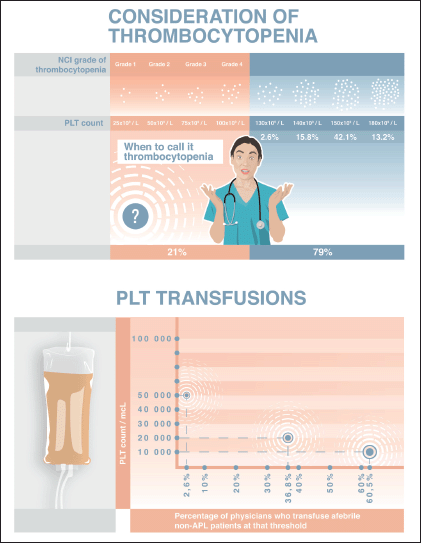
Figure 1. (a): According to NCI criteria there are 4 grades of thrombocytopenia based on PLT thresholds: 75 to <100 × 109/L (Grade 1), 50 to <75 × 109/L (Grade 2), 25 to <50 × 109/L (Grade 3); <25 × 109/L (Grade 4). However, only 21.0% of our respondents defined thrombocytopenia to have a PLT threshold of 100,000/mcL, while the majority (42.1%) denoted a threshold of 150,000/mcL. Also 15.8% and 13.2% of those surveyed considered thrombocytopenia to be when PLT counts fall below 140,000/mcL and 180,000/mcL, respectively. A threshold of 130,000/mcL was mentioned by 2.6% of the specialists. (b): All physicians preferred PLT transfusions for prophylactic purposes (not only on demand). Afebrile patients (non-APL) would be transfused when having PLT count below 10,000/mcL by 60.5% of physicians whereas 36.8% and 2.6% of specialists performed transfusions when PLT counts fell below 20,000/mcL and 50,000/mcL, respectively.
All 38 physicians managed CIT by performing PLT transfusions for prophylactic purposes (i.e., when PLT count falls below a certain threshold) with none of them transfusing PLTs only on-demand to address active bleeding. Eleven (73.3%) haematologists (adult), four (57.1%) medical oncologists, and eight (50%) paediatricians deemed 10 × 109/L as the threshold PLT count for transfusing afebrile patients with haematologic malignancies (besides acute promyelocytic leukaemia (APL)) and solid tumours. Only one paediatrician preferred to transfuse when PLTs were less than 50 × 109/L. The other 14 (36.8%) of the 38 respondents used a threshold PLT count of 20 × 109/L (Figure 1b).
Febrile patients (non-APL) would be transfused when having PLT count below 20 × 109/L by 13 (81.3%) haematologists (adult), four (57.1%) medical oncologists, and 11 (73.3%) paediatricians. One haematologist (adult) and two paediatricians preferred to transfuse febrile patients when PLT count was below 30 × 109/L, whereas three medical oncologists and one paediatrician transfused when PLT count was less than 10 × 109/L. Three participants identified less than 50,000/mcL as their threshold; two of these individuals had less than 6 years of professional experience, and one individual had more than 30 years.
Afebrile and febrile adult APL patients would receive transfusions according to 12 (75%) and 11 (73.3%) haematologists (adult) when they have PLT count below 20 × 109/L, respectively. Another three (25%) and four (26.7%) haematologists (adult) consider a threshold PLT count less than 50 × 109/L to transfuse PLTs to afebrile and febrile APL patients. Nine (56.3%) paediatricians would transfuse both afebrile and febrile patients with APL when PLT falls below 50 × 109/L, with one specialist transfusing when PLT was less than 10 × 109/L for both groups. Three paediatricians denoted a threshold of 20 × 109/L as the transfusion threshold for afebrile patients, with one paediatrician noting that threshold as acceptable for febrile patients. Another two paediatricians set a transfusion threshold of 75 × 109/L for febrile APL patients.
PLT products availability varied among the respondents, with only 20 (53%) of them responding that they had 24/7 access.
67% of haematologists (adult) and medical oncologists mentioned that the cost of PLT products affects their decision on making a prophylactic transfusion as adult patients have to pay for these. Paediatricians, on the other hand, did not have that issue as charity foundations covered the cost of complete management for children with cancer, including providing needed blood products [15].
Discussion
Numerous clinical questions are raised when it comes to decision making such as whether to transfuse PLT prophylactically or therapeutically and which PLT count to use as a threshold for prophylactic transfusion. This especially applies to a low-income country such as ours, where drugs are often inaccessible. A review of randomized clinical trials reports that a therapeutic-only strategy is associated with an increased risk of low-to moderate-grade bleeding and reduced number of transfusions per patient. However, these associations occurred in patients with haematologic malignancies who had anamnesis of myelosuppressive therapy or haematopoietic stem cell transplantation (HSCT) [16, 17]. According to the 2017 American Society for Clinical Oncology guidelines prophylactic PLT transfusions are recommended if the bone marrow is suppressed (including cytotoxic chemotherapy) when PLT count falls below a certain threshold. The threshold varies according to the patient’s diagnosis, clinical condition, and treatment modality. A PLT count of 10 × 109/L is generally used as the threshold in patients receiving treatment for haematologic malignancies who are hospitalized, afebrile, and without active bleeding or infection. APL is considered an exception because of higher bleeding risk, so it is recommended to transfuse PLTs when the PLT count is below 30 × 109/L and up to 50 × 109/L. Higher thresholds may be needed under the following conditions: if fever, sepsis or coagulopathy is present, or if the patient is not hospitalized and/or cannot be closely monitored [18]. Randomized trials of PLT transfusion threshold in patients with solid tumours have not been performed but observational studies support 10 × 109/L as a threshold. For necrotic tumours, 20 × 109/L may be appropriate due to a higher risk of active bleeding or the need for invasive procedure [18].
One must take into account the costs and benefits of PLT transfusions because repeated transfusions can increase the risk of an array of potential adverse health effects [19]. These events can be immune-mediated such as febrile non-haemolytic transfusion reaction (FNHTR), allergic/anaphylaxis, TAGvHD, transfusion-related acute lung injury (TRALI), post-transfusion purpura, transfusion-related immunomodulation, and PLT refractoriness [19]. They can also be non-immune mediated events such as transfusion-associated circulatory overload, physical injury, sepsis, viral infection transmission and hypotensive reaction [19]. Although the majority of the aforementioned events are not dangerous, they are quite common. For example, febrile FNHTR and ‘allergic’ reactions are observed in up to 20%–30% of cases [20, 21]. According to Canadian health statistics, one in 50,000 PLT transfusions is associated with bacterial contamination, one in 153,000 with hepatitis B virus (HBV) infection, one in 5,000 can cause TRALI [22]. In contrast, 1 in ten PLT transfusions may cause febrile reactions [22]. Not only do increased transfusions result in a considerable potential risk to patients (transfusions reactions, infection and alloimmunization), but may also be associated with increased cancer-related mortality in adult studies [23–25].
Over the past decade, the number of PLT units issued in the United Kingdom by the NHS Blood and Transplant (NHSBT) service to hospitals has steadily increased; it has risen by just over 25% in the 9 years prior to April of 2016 [26]. PLTs are the most expensive commonly used component supplied by NHSBT, with one unit costing £240.90 (both apheresis and pooled, 1 ATD) [27]. This price has increased since 2016 when it was valued at £193.15. Additionally, there are added value service costs that must be accounted for (irradiation, etc.) [27]. There is evidence that the inappropriate use of PLTs is an ongoing problem [28, 29]. To illustrate this, it is estimated that, 28% of PLT transfusions could have been avoided in 2010 [30]. Moreover, according to the US National Blood Collection and Utilization Survey report, about 1.3 billion USD was spent for PLT transfusions in the USA only in 2008 and about 2/3 of those transfusions were performed for prophylactic purposes without enough proof of their clinical benefit [31].
The seriousness of CIT consequences is variable ranging from less significant issues such as petechiae and ecchymosis, all the way up to life-threatening bleeding [32]. Although the main danger of thrombocytopenia is associated with morbidity and mortality due to excessive bleeding, other potential costs that must be considered are side effects due to PLT transfusions, or decreased efficacy of treatment due to delays, dosage and cycle reductions in therapy [33–35].
Limitations
Several limitations of the current study must be noted. This was a questionnaire study conducted on a small number of physicians and clinical residents who were from the same region, thus reducing the generalizability of the findings, without a validation cohort elsewhere. However, the response rate was 100% eliminating certain biases and additionally, the questionnaire was pilot-tested. Barriers were measured using only a few questions, thus limiting our ability to generalize to a wide variety of issues. To address this, future studies should attempt to explore various realms of health and healthcare delivery by using more comprehensive measures. Despite these limitations, this study may serve as an initial step for further research, and for the introduction of several guidelines.
Conclusion
Although our centre is the only specialized haematology centre in Armenia, consensus for CIT management, as well as the definition and concept of thrombocytopenia, varies. There are also no accepted and definite guidelines for either. There is ample work to be done to address the knowledge gap regarding thrombocytopenia, and its management among our specialists. Compounding this, there is a need for advocacy and implementation of appropriate guidelines such that solutions can be found for this outstanding issue. Furthermore, access to PLT products is an important barrier to address as only half of the surveyed physicians responded having 24/7 access to such resources. The health system may benefit from further investigations into such discordance of resource-use, and the employment of strategies to reduce the number of inappropriate blood requests and transfusions.
CIT is a complication of interest to physicians worldwide and has not been resolved yet. CIT requires a global approach to whole system implementation such as social support networks and cooperation of specialists on the international scale. This is the first survey conducted regarding CIT and the initial step for further research, as we are going to do a larger survey soon for the upcoming Delphi study.
Declarations
The permission to access and use the data was received from the local board of Committee of Ethics of the Hematology Center after Prof. R. H. Yeolyan. Consent was obtained from all participants prior to data collection. All the selected respondents were given assurance of confidentiality that the information gathered will be used exclusively for research purposes.
Acknowledgments
We gratefully acknowledge the physicians and clinical residents who participated in this study and the administration who helped collect data for this study.
Conflicts of interest
Gevorg Tamamyan declares a research grant from Agenus Inc. and an advisory role at the Luzsana Biotechnology.
JS conflicts are listed here and none are relevant: https://www.nature.com/onc/editors
Funding
No funding was received for the current work.
Author contributions
LH: Conceptualization, methodology, validation, formal analysis, data curation, writing – original draft, writing – review and editing. HG: Conceptualization, validation, formal analysis, data curation, writing – original draft, writing – review and editing. MB: Validation, formal analysis, writing – original draft, writing – review and editing. HK: Validation, resources, writing – review and editing, supervision. NS: Validation, resources, writing – original draft, writing – review and editing. AS: Writing – reviewing and editing, writing – review and editing. JS: Supervision, validation, writing – review and editing. GT: Conceptualization, methodology, resources, writing – review and editing, supervision, validation.
Availability of data and materials
The data underlying this article are available in the article and in its accompanying questionnaires in the Appendix.
References
1. Griffiths EA, Roy V, and Alwan L, et al (2022) NCCN Guidelines® insights: hematopoietic growth factors, version 1.2022: featured updates to the NCCN guidelines J Natl Compr Cancer Netw 20(5) 436–442 https://doi.org/10.6004/jnccn.2022.0026
2. Soff GA, Miao Y, and Bendheim G, et al (2019) Romiplostim treatment of chemotherapy-induced thrombocytopenia J Clin Oncol 37(31) 2892 https://doi.org/10.1200/JCO.18.01931 PMID: 31545663 PMCID: 6823892
3. Al-Samkari H, Parnes AD, and Goodarzi K, et al (2021) A multicenter study of romiplostim for chemotherapy-induced thrombocytopenia in solid tumor s and hematologic malignancies Haematologica 106(4) 1148 https://doi.org/10.3324/haematol.2020.251900 PMCID: 8018116
4. Shaw Mph JL, Nielson CM, and Park JK, et al (2021) The incidence of thrombocytopenia in adult patients receiving chemotherapy for solid tumors or hematologic malignancies Eur J Haematol 106(5) 662–672 https://doi.org/10.1111/ejh.13595
5. Wu Y, Aravind S, and Ranganathan G, et al (2009) Anemia and thrombocytopenia in patients undergoing chemotherapy for solid tumors: a descriptive study of a large outpatient oncology practice database, 2000–2007 Clin Ther 31(PART. 2) 2416–2432
6. Ten Berg MJ, Van Den Bemt PMLA, and Shantakumar S, et al (2011) Thrombocytopenia in adult cancer patients receiving cytotoxic chemotherapy: results from a retrospective hospital-based cohort study Drug Saf 34(12) 1151–1160 https://doi.org/10.2165/11594310-000000000-00000 PMID: 22077503
7. Jardim DL, Rodrigues CA, and Novis YAS, et al (2012) Oxaliplatin-related thrombocytopenia Ann Oncol 23(8) 1937–1942 https://doi.org/10.1093/annonc/mds074 PMID: 22534771
8. Lonial S, Waller EK, and Richardson PG, et al (2005) Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma Blood 106(12) 3777–3784 https://doi.org/10.1182/blood-2005-03-1173 PMID: 16099887 PMCID: 1895114
9. Lambert MP, Rauova L, and Bailey M, et al (2007) Platelet factor 4 is a negative autocrine in vivo regulator of megakaryopoiesis: clinical and therapeutic implications Blood 110(4) 1153–1160 https://doi.org/10.1182/blood-2007-01-067116 PMID: 17495129 PMCID: 1976471
10. Al-Samkari H, Marshall AL, and Goodarzi K, et al (2018) The use of romiplostim in treating chemotherapy-induced thrombocytopenia in patients with solid tumors Haematologica 103(4) e169 https://doi.org/10.3324/haematol.2017.180166 PMCID: 5865422
11. Miao J, Leblebjian H, and Scullion B, et al (2018) A single center experience with romiplostim for the management of chemotherapy-induced thrombocytopenia Am J Hematol 93(4) E86–E88 https://doi.org/10.1002/ajh.25022
12. Hanna RK, Poniewierski MS, and Laskey RA, et al (2013) Predictors of reduced relative dose intensity and its relationship to mortality in women receiving multi-agent chemotherapy for epithelial ovarian cancer Gynecol Oncol 129(1) 74–80 https://doi.org/10.1016/j.ygyno.2012.12.017
13. Havrilesky LJ, Reiner M, and Morrow PK, et al (2015) A review of relative dose intensity and survival in patients with metastatic solid tumors Crit Rev Oncol Hematol 93(3) 203–210 https://doi.org/10.1016/j.critrevonc.2014.10.006
14. Denduluri N, Patt DA, and Wang Y, et al (2015) Dose delays, dose reductions, and relative dose intensity in patients with cancer who received adjuvant or neoadjuvant chemotherapy in community oncology practices J Natl Compr Cancer Netw 13(11) 1383–1393 https://doi.org/10.6004/jnccn.2015.0166
15. Medical expenses – city of SmileCity of smile [https://cityofsmile.org/medical-expenses/] Date accessed: 06/06/22
16. Crighton GL, Estcourt LJ, and Wood EM, et al (2015) A therapeutic-only versus prophylactic platelet transfusion strategy for preventing bleeding in patients with haematological disorders after myelosuppressive chemotherapy or stem cell transplantation Cochrane Database Syst Rev 2015(9) PMID: 26422767 PMCID: 4610062
17. Stanworth SJ, Estcourt L, and Powter G, et al (2012) The effect of a no-prophylactic versus prophylactic platelet transfusion strategy on bleeding in patients with hematological malignancies and severe thrombocytopenia (TOPPS trial). A randomized controlled, non-inferiority trial Blood 120(21) 1 https://doi.org/10.1182/blood.V120.21.1.1
18. Schiffer CA, Bohlke K, and Delaney M, et al (2018) Platelet transfusion for patients with cancer: American society of clinical oncology clinical practice guideline update J Clin Oncol 36(3) 283–299 https://doi.org/10.1200/JCO.2017.76.1734
19. Kiefel V (2008) Reactions induced by platelet transfusions Transfus Med Hemother 35(5) 354–358 https://doi.org/10.1159/000151350 PMID: 21512624 PMCID: 3076327
20. Blumberg N, Gettings KF, and Turner C, et al (2006) An association of soluble CD40 ligand (CD154) with adverse reactions to platelet transfusions Transfusion 46(10) 1813–1821 https://doi.org/10.1111/j.1537-2995.2006.00979.x PMID: 17002639
21. Kelley DL, Mangini J, and Lopez-Plaza I, et al (2000) The utility of ≤3-day-old whole-blood platelets in reducing the incidence of febrile nonhemolytic transfusion reactions Transfusion 40(4) 439–442 https://doi.org/10.1046/j.1537-2995.2000.40040439.x PMID: 10773056
22. Ung Bướu -Huyết K (2010)Guideline for Platelet Transfusion Thresholds for Pediatric Hematology/Oncology Patients
23. Blumberg N, Heal JM, and Phillips GL, et al (2012) Platelets – to transfuse or not to transfuse Lancet (London, England) 380(9850) 1287–1289 https://doi.org/10.1016/S0140-6736(12)60983-0 PMID: 22877505
24. Vamvakas EC (2014) Allogeneic blood transfusion and cancer recurrence: 20 years later Transfusion 54(9) 2149–2153 https://doi.org/10.1111/trf.12689 PMID: 25212422
25. Blumberg N, Heal JM, and Liesveld JL, et al (2008) Platelet transfusion and survival in adults with acute leukemia Leukemia 22(3) 631–635 https://doi.org/10.1038/sj.leu.2404920
26. Estcourt LJ, Birchall J, and Allard S, et al (2017) Guidelines for the use of platelet transfusions Br J Haematol 176(3) 365–394 https://doi.org/10.1111/bjh.14423
27. Portfolio and prices – hospitals and science – NHSBT [https://hospital.blood.co.uk/components/portfolio-and-prices/] Date accessed: 09/07/22
28. Audit of the use of platelets in three UK transfusion Committee Regions NICE [https://www.nice.org.uk/sharedlearning/audit-of-the-use-of-platelets-in-three-uk-transfusion-committee-regions] Date accessed: 01/08/22
29. Information for hospitals served by NHS Blood and Transplant
30. 2010 re-audit of the use of platelets in haematology (2011)
31. Tamamyan G, Danielyan S, and Lambert MP (2016) Chemotherapy induced thrombocytopenia in pediatric oncology Crit Rev Oncol Hematol 99 299–307 https://doi.org/10.1016/j.critrevonc.2016.01.005 PMID: 26811139
32. Drozd-Sokolowska JE and Wiktor-Jedrzejczak W (2011) Factors determining the risk of severe (WHO grades 3 and 4) hemorrhage in hematologic patients Transfus Apher Sci 44(2) 129–134 https://doi.org/10.1016/j.transci.2011.01.004 PMID: 21334260
33. Economic burden of haematological adverse effects in cancer patients [https://www.medscape.com/viewarticle/557869_4] Date accessed: 09/07/22
34. Parker RI (2014) Transfusion in critically ill children: indications, risks, and challenges Crit Care Med 42(3) 675–690 https://doi.org/10.1097/CCM.0000000000000176 PMID: 24534955
35. Vadhan-Raj S (2009) Management of chemotherapy-induced thrombocytopenia: current status of thrombopoietic agents Semin Hematol 46(SUPPL. 2) S26–S32 https://doi.org/10.1053/j.seminhematol.2008.12.007 PMID: 19245931
Appendix. Survey questionnaires ‘CIT and transfusion of PLT components’.
For adult haematology specialists
1. Are you a:
• Haematologist
• Haematology resident
2. How long have you worked with blood disorders?
______________________ year (s)
3. When do you consider thrombocytopenia?
PLT level below ______________________
4. When do you perform PLT transfusions in patients >18 years of age with haematologic malignancies (except acute APL) ?
a) For prophylaxis, when PLT count is below from 75,000/mcL
• from 50,000/mcL
• from 20,000/mcL
• from 10,000/mcL
• from 5,000/mcL
• Other ______________________
b) Only on-demand (in case of bleeding)
• Minor (petechiae, epistaxis) bleeding
• Heavy bleeding
• In both of the above-mentioned cases
5. When do you perform PLT transfusions in patients >18 years of age with APL?
a) For prophylaxis, when PLT count is below
• from 75,000/mcL
• from 50,000/mcL
• from 20,000/ mcL
• from 10,000/mcL
• from 5,000/mcL
• Other ______________________
b) Only on-demand (in case of bleeding)
• Minor (petechiae, epistaxis) bleeding
• Heavy bleeding
• In both of the above-mentioned cases
6. When do you perform PLT transfusions in febrile patients >18 years of age with haematologic malignancies (except APL)?
a) For prophylaxis, when PLT count is below
• from 75,000/mcL
• from 50,000/mcL
• from 20,000/ mcL
• from 10,000/mcL
• from 5,000/mcL
• Other ______________________
b) Only on-demand (in case of bleeding)
• Minor (petechiae, epistaxis) bleeding
• Heavy bleeding
• In both of the above-mentioned cases
7. When do you perform PLT transfusions in febrile patients >18 years of age with APL?
a) For prophylaxis, when PLT count is below
• from 75,000/mcL
• from 50,000/mcL
• from 20,000/mcL
• from 10,000/mcL
• from 5,000/mcL
• Other ______________________
b) Only on-demand (in case of bleeding)
• Minor (petechiae, epistaxis) bleeding
• Heavy bleeding
• In both of the above-mentioned cases
8. Is there round-the-clock availability of PLT components (if not, how do you manage thrombocytopenia, and what measures do you take in case of unavailability?
• Yes
• No ______________________
9. Do patients pay for PLT components out-of-pocket?
• Yes
• No
10. Does your selection of the PLT component (i.e., pooled or apheresis PLTs ) depend on the difficulty of the patient's solvency?
• Yes
• No
• Other ______________________
For adult oncology specialists
1. Are you an:
• Oncologist
• Oncology resident
2. How long have you worked with solid tumour patients?
______________________ year (s)
3. When do you consider thrombocytopenia?
PLT level below ______________________
4. When do you perform PLT transfusions in patients with solid tumours >18 years of age?
a) For prophylaxis, when PLT count is below
• from 75,000/mcL
• from 50,000/mcL
• from 20,000/mcL
• from 10,000/mcL
• from 5,000/mcL
• Other ______________________
a) Only on-demand (in case of bleeding)
• Minor (petechiae, epistaxis) bleeding
• Heavy bleeding
• In both of the above-mentioned cases
5. When do you perform PLT transfusions in febrile patients with solid tumours >18 years of age?
a) For prophylaxis, when PLT count is below
• from 75,000/mcL
• from 50,000/mcL
• from 20,000/mcL
• from 10,000/mcL
• from 5,000/mcL
• Other _______________
b) Only on-demand (in case of bleeding)
• Minor (petechiae, epistaxis) bleeding
• Heavy bleeding
• In both of the above-mentioned cases
6. Is there round-the-clock availability of PLT components (if not, how do you manage thrombocytopenia, and what measures do you take in case of unavailability?
• Yes
• No ______________________
7. Do patients pay for PLT components out-of-pocket?
• Yes
• No
8. Does your selection of the PLT component (i.e., pooled or apheresis PLTs) depend on the difficulty of the patient's solvency?
• Yes
• No
• Other ________________
For paediatric haematology specialists
1. How long have you worked with blood disorders among patients under 18 years of age? _______ year (s)
2. When do you consider thrombocytopenia?
PLT level below __________________
3. When do you perform PLT transfusions in patients <18 years of age with haematologic malignancies (except APL)?
a) For prophylaxis, when PLT count is below
• from 75,000/mcL
• from 50,000/mcL
• from 20,000/mcL
• from 10,000/mcL
• from 5,000/mcL
• Other ______________________
b) Only on-demand (in case of bleeding)
• Minor (petechiae, epistaxis) bleeding
• Heavy bleeding
vIn both of the above-mentioned cases
4. When do you perform PLT transfusions in patients <18 years of age with APL?
a) For prophylaxis, when PLT count is below
• from 75,000/mcL
• from 50,000/mcL
• from 20,000/mcL
• from 10,000/mcL
• from 5,000/mcL
• Other ______________________
b) Only on-demand (in case of bleeding)
• Minor (petechiae, epistaxis) bleeding
• Heavy bleeding
• In both of the above-mentioned cases
5. When do you perform PLT transfusions in febrile patients <18 years of age with haematologic malignancies (except APL)?
a) For prophylaxis, when PLT count is below
• from 75,000/mcL
• from 50,000/mcL
• from 20,000/mcL
• from 10,000/mcL
• from 5,000/mcL
• Other ______________________
b) Only on-demand (in case of bleeding)
• Minor ( petechiae, epistaxis) bleeding
• Heavy bleeding
• In both of the above-mentioned cases
6. When do you perform PLT transfusions in febrile patients <18 years of age with APL?
a) For prophylaxis, when PLT count is below
• from 75,000/mcL
• from 50,000/mcL
• from 20,000/mcL
• from 10,000/mcL
• from 5,000/mcL
• Other ______________________
b) Only on-demand (in case of bleeding)
• Minor ( petechiae, epistaxis) bleeding
• Heavy bleeding
• In both of the above-mentioned cases
7. Is there round-the-clock availability of PLT components (if not, how do you manage thrombocytopenia, and what measures do you take in case of unavailability?
• Yes
• No ______________________
8. Do patients pay for PLT components out-of-pocket?
• Yes
• No
9. Does your selection of the PLT component (i.e., pooled or apheresis PLTs) depend on the difficulty of the patient's solvency?
• Yes
• No
• Other ______________________
For paediatric oncology specialists
1. How long have you worked with solid tumour patients under 18 years of age? ______________________ year (s)
2. When do you consider thrombocytopenia?
PLT level below ______________________
3. When do you perform PLT transfusions in patients with solid tumours <18 years of age?
a) For prophylaxis, when PLT count is below
• from 5,000/mcL
• from 75,000/mcL
• from 50,000/mcL
• from 20,000/mcL
• from 10,000/mcL
• from 5,000/mcL
• Other ______________________
b) Only on-demand (in case of bleeding)
• Minor (petechiae, epistaxis) bleeding
• Heavy bleeding
• In both of the above-mentioned cases
4. When do you perform PLT transfusions in febrile patients with solid tumours <18 years of age?
a) For prophylaxis, when PLT count is below
• from 75,000/mcL
• from 50,000/mcL
• from 20,000/mcL
• from 10,000/mcL
• from 5,000/mcL
• Other ______________________
b) Only on-demand (in case of bleeding)
• Minor (petechiae, epistaxis) bleeding
• Heavy bleeding
• In both of the above-mentioned cases
5. Is there round-the-clock availability of PLT components (if not, how do you manage thrombocytopenia, and what measures do you take in case of unavailability?
• Yes
• No ______________________
6. Do patients pay for PLT components out-of-pocket?
• Yes
• No
7. Does your selection of the PLT component (i.e., pooled or apheresis PLTs) depend on the difficulty of the patient's solvency?
• Yes
• No
• Other ______________________
For paediatric oncology/haematology residents
1. How long have you worked with blood disorders among patients under 18 years of age? ______________________ year (s)
2. When do you consider thrombocytopenia?
PLT level below ______________________
3. When do you perform PLT transfusions in patients <18 years of age with haematologic malignancies (except APL) ?
a) For prophylaxis, when PLT count is below
• from 75,000/mcL
• from 50,000/mcL
• from 20,000/mcL
• from 10,000/mcL
• from 5,000/mcL
• Other ______________________
b) Only on-demand (in case of bleeding)
• Minor (petechiae, epistaxis) bleeding
• Heavy bleeding
• In both of the above-mentioned cases
4. When do you perform PLT transfusions in patients <18 years of age with APL?
a) For prophylaxis, when PLT count is below
• from 75,000/mcL
• from 50,000/mcL
• from 20,000/mcL
• from 10,000/mcL
• from 5,000/mcL
• Other ______________________
b) Only on-demand (in case of bleeding)
• Minor (petechiae, epistaxis) bleeding
• Heavy bleeding
• In both of the above-mentioned cases
5. When do you perform PLT transfusions in patients with solid tumours <18 years of age?
a) For prophylaxis, when PLT count is below
• from 75,000/mcL
• from 50,000/mcL
• from 20,000/mcL
• from 10,000/mcL
• from 5,000/mcL
• Other ______________________
a) Only on-demand (in case of bleeding)
• Minor (petechiae, epistaxis) bleeding
• Heavy bleeding
• In both of the above-mentioned cases
6. When do you perform PLT transfusions in febrile patients <18 years of age with haematologic malignancies (except APL) ?
a) For prophylaxis, when PLT count is below
• from 75,000/mcL
• from 50,000/mcL
• from 20,000/mcL
• from 10,000/mcL
• from 5,000/mcL
• Other ______________________
b) Only on-demand (in case of bleeding)
• Minor (petechiae, epistaxis) bleeding
• Heavy bleeding
• In both of the above-mentioned cases
7. When do you perform PLT transfusions in febrile patients <18 years of age with APL?
a) For prophylaxis, when PLT count is below
• from 75,000/mcL
• from 50,000/mcL
• from 20,000/mcL
• from 10,000/mcL
• from 5,000/mcL
• Other ______________________
b) Only on-demand (in case of bleeding)
• Minor (petechiae, epistaxis) bleeding
• Heavy bleeding
• In both of the above-mentioned cases
8. When do you perform PLT transfusions in febrile patients with solid tumours <18 years of age?
a) For prophylaxis, when PLT count is below
• from 75,000/mcL
• from 50,000/mcL
• from 20,000/mcL
• from 10,000/mcL
• from 5,000/mcL
• Other ______________________
b) Only on-demand (in case of bleeding)
• Minor (petechiae, epistaxis) bleeding
• Heavy bleeding
• In both of the above-mentioned cases
9. Is there round-the-clock availability of PLT components (if not, how do you manage thrombocytopenia, and what measures do you take in case of unavailability?
• Yes
• No ______________________
10. Do patients pay for PLT components out-of-pocket?
• Yes
• No
11. Does your selection of the PLT component (i.e., pooled or apheresis PLTs) depend on the difficulty of the patient's solvency?
• Yes
• No
• Other ______________________




