Cost analysis of three-dimensional radiation therapy versus intensity-modulated chemoradiotherapy for locally advanced cervical cancer in Peruvian citizens
José Fernando Robles Díaz
Regional Institute for Neoplastic Diseases, Central Region, Concepción, Junín 12126, Peru and Los Andes Peruvian University, Huancayo 12002, Peru
Abstract
Background and objectives: The standard treatment for locally advanced cervical cancer (CC) is chemoradiotherapy (CTRT) followed by high-dose-rate brachytherapy (HDRBT). The ideal scenario would be under novel intensity-modulated radiation therapy (IMRT) volumetric-modulated arc therapy (VMAT) radiation techniques over three-dimensional (3D) radiation therapy. However, radiotherapy (RT) centres in low- and middle-income countries have limited equipment for teletherapy services like HDRBT. This is why the 3D modality is still in use. The objective of this study was to analyse costs in a comparison of 3D versus IMRT versus VMAT based on clinical staging.
Materials and methods: From 02/01/2022 to 05/01/2023 a prospective registry of the costs for oncological management was carried out for patients with locally advanced CC who received CTRT ± HDRBT. This included the administration of radiation with chemotherapy. The cost associated with patient and family transfers and hours in the hospital was also identified. These expenses were used to project the direct and indirect costs of 3D versus IMRT versus VMAT.
Results: The treatment regimens for stage IIIC2, including 3D and novel techniques, are those with the highest costs. The administration of 3D RT for IIIC2 and novel IMRT or VMAT techniques, is $3,881.69, $3,374.76, and $2,862.80, respectively. The indirect cost from stage IIB to IIIC1 in descending order is IMRT, 3D and VMAT, but in IIIC2 the novel technique regimens reduce by up to 33.99% compared to 3D.
Conclusion: In RT centres with an available supply of RT equipment, VMAT should be preferred over IMRT/3D since it reduces costs and toxicity. However, in RT centres where demand exceeds supply in the VMAT technique planning systems, the use of 3D teletherapy over IMRT/VMAT could continue to be used in patients with stage IIB to IIIC1.
Keywords: costs and cost analysis, cervical neoplasia, chemoradiotherapy, intensity-modulated radiation therapy, simultaneous integrated boost
Correspondence to: José Fernando Robles Díaz
Email: bayern014@hotmail.com
Published: 20/04/2023
Received: 20/01/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
According to GLOBOCAN 2020, cervical cancer (CC) ranks fourth with an incidence of 13.3 and second with an incidence of 22.2 in women throughout the world and Peru, respectively. It has an incidence-mortality rate of 14.9–7.6 and 22.5–11.5 per 100,000 people on a Latin American and national level, respectively [1], thus making it one of the most costly oncologic pathologies for the health system. Compared with countries with a high level of resources, like the United States or Western European countries, developing countries like Peru and Indonesia, which have limited monetary and educational resources in healthcare, have the highest CC incidence and mortality rates [2]. CC is the fourth most common type of cancer in women under 45 [3], yet the average at diagnosis with CC is 51 [2].
Treatment for invasive CC is stratified by the stage of the disease, which is determined by the International Federation of Gynaecology and Obstetrics (FIGO) as well as the Tumor, Nodal, Metastasis staging system of the American Joint Committee on Cancer/Union of International Cancer Control [4, 5]. These criteria were last updated in 2018 and 2021, respectively [6]. Chemoradiotherapy (CTRT), which involves platinum-based chemotherapy (CT) concurrent with external beam radiotherapy (RT) followed by high dose-rate brachytherapy (HDRBT), is the standard treatment as confirmed by Phase III trials and meta-analysis [7–9]. The use of intensity-modulated RT and three-dimensional (3D) image-guided adaptive HDRBT has improved the results and reduced lesions in the surrounding organs [10].
Up until now, there have been few economic evaluation reports available on 3D radiation versus novel techniques like intensity-modulated radiation therapy (IMRT) or volumetric-modulated arc therapy (VMAT) in countries with a low-to-high human development index (HDI). In a country where the health system has limited funding, it is thereby important to discuss on radiation in CC using cost analysis [11]. As such, the objective of this study was to conduct a cost analysis by comparing the 3D technique versus novel techniques in patients with advanced CC.
Materials and methods
From 02/01/2022 to 05/01/2023 the clinical features of patients with CC who received radical treatment with CTRT were prospectively recorded using medical histories. Transportation expenses were collected using patient questionnaires on their daily expenses for travelling to the institute. Medical costs were obtained from their medical records and pharmacy archives as well as those for facility-equipment stocks. The medications and supplies used in the preparation and administration of CT and RT were identified during this process. Also, the health professionals involved were interviewed to find out about the procedure times. This study was approved in advance by the Institutional Ethics Committee. Only patients who provided comprehensive data and signed the informed consent were analysed.
Estimated cost
A spreadsheet was made to project the cost of CC treatment based on FIGO clinical stages. The direct and indirect costs were included in Peruvian currency (sol, S/.) as well as in their American dollar equivalent ($). The direct medical costs were recorded with the monetary equivalent of the healthcare professionals’ working hours, equipment, facilities, medications and supplies used. The indirect costs associated with the time patients and relatives spent in the facility, including travel time from their homes were also included.
This data analysis was conducted on patients being treated who had stage IIB–IVB, had completed CTRT treatment and were thereby granted full costs access. The type of radiation technique was divided into two groups; those who began with 3D and those who began with the novel techniques (IMRT/VMAT).
Treatment regimen based on staging
All patients received CTRT as the initial treatment. The CT sensitiser was a weekly 40 mg/m2 dose of Cisplatin on an outpatient basis. There was a difference in the initial teletherapy modalities. This was either through 3D or novel techniques with the Synergy Platform® and Full de Elekta® linear accelerators (LINAC), respectively. HDRBT was administered at all stages except IVA using high dose-rate MicroSelectron equipment with iridium source by Elekta®. 3D HDRBT consisted of a dose of 7 Gy in 4 sessions over 2 weeks.
• Stage IIB/IIIA/IIIB: Pelvis 3D CTRT at a dose of 5,000 cGy in 25 sessions over 5 weeks followed by HDRBT as the first option (3D CTRT25 + HDRBT). Novel technique pelvis QTRT25 CTRT at a dose of 5,000 cGy in 25 sessions over 5 weeks followed by HDRBT as the second option (IMRT CTRT25 + HDRBT/VMAT CTRT25 + HDRBT).
• Stage IIIC1: 3D pelvis CTRT at a dose of 5,600 cGy in 28 sessions over 6 weeks followed by HDRBT as the first option (3D CTRT28 + HDRBT). Novel technique pelvis CTRT at a dose of 5,880 cGy in 28 sessions over 6 weeks followed by HDRBT as the second option (IMRT CTRT28 + HDRBT/VMAT CTRT28 + HDRBT).
• Stage IIIC2: 3D pelvis CTRT at a dose of 5,600 cGy in 28 sessions over 6 weeks followed by HDRBT, before finishing with novel technique para-aortic (PA) at a dose of 5,500 cGy in 25 sessions over 5 weeks as the first option (3D CTRT28 + HDRBT + VMAT + CT25). Novel technique pelvic and para-aortic (PPA) CTRT at a dose of 5,880 cGy in 28 sessions over 6 weeks, followed by HDRBT as the second option (IMRT CTRT28 + HDRBT/VMAT CTRT28 + HDRBT).
• Stage IVA: 3D pelvis CTRT at a dose of 5,600 cGy in 28 sessions over 6 weeks, followed by a dose of 2,000 cGy 3D in 5 sessions over 1 week was the only option for the residual tumour. (3D CTRT28 + 3D RT5). No comparison was made with novel techniques and there are no HDRBT applicators for bladder and/or rectum involvement.
• Stage IVB: 3D pelvis and groin CTRT with a dose of 6,000 cGy in 30 sessions over 6 weeks and HDRBT (3D CTRT30 + HDRBT) was the only option. No comparison was made with the novel techniques, only patients who had inguinal node metastasis were entered. Those that had vesical or rectal involvement as well as PA or visceral metastases were excluded.
Direct cost
The calculation was based on the cost of medications and supplies for CTRT [12], the monetary equivalent for the equipment and facilities [13] usage time in consultation, simulation, treatment volume delimitation, planning, HDRBT preparation as well as CT administration procedures. It analysed healthcare professional costs proportional to the monthly salary for the time involved in procedures [14]. Toxic effects were not analysed as most patients do not have any significant adverse effects.
Indirect cost
This calculation was based on patients and companions. It included the monetary equivalent for the time of their admission into and discharge from the procedure facility. The average cost of transport from their home to the facility was added. Our analysis included costs associated with companions because the therapy that studies participants received often affects the patient’s independence. The productivity per hour worked reference value for the central macro-region [15] was used to calculate the loss of productivity due to being out of work for the patient and relatives alike.
Statistical analysis
All data and statistical analyses were conducted using SPSS version 25. Descriptive statistics are presented as means or proportions.
Results
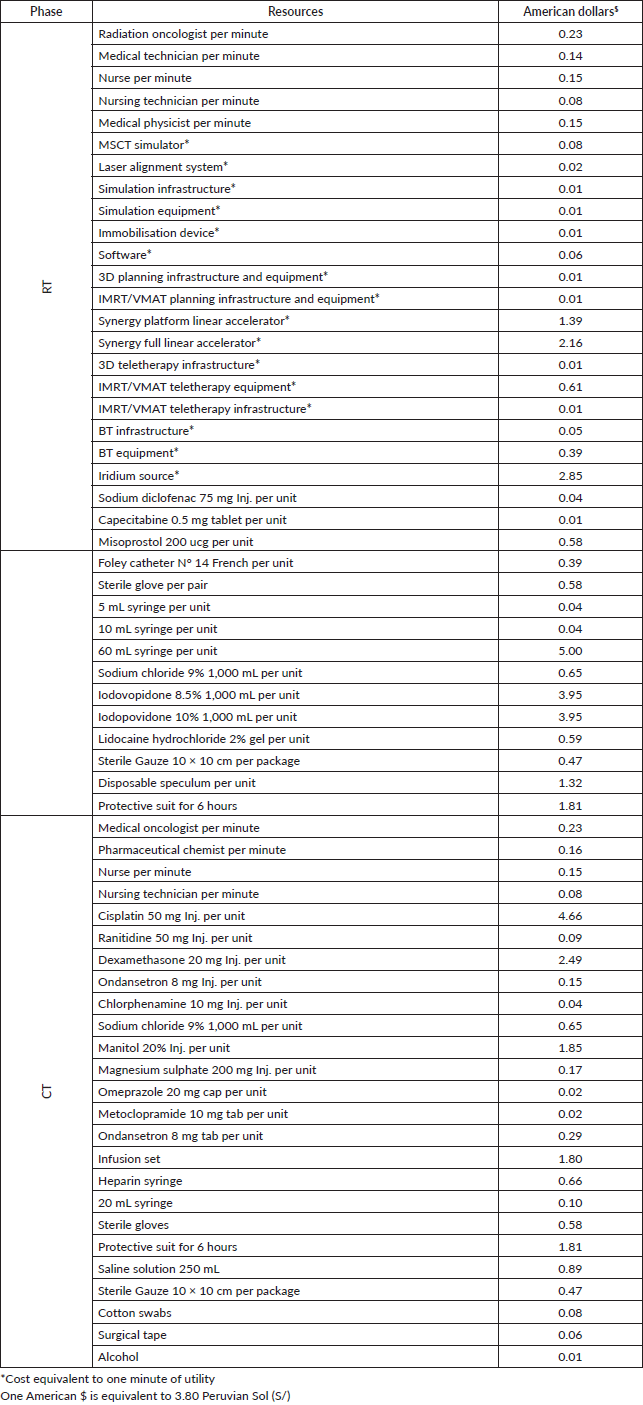
The research ended in on 05/01/2023 by filing 44 patients on CTRT, who had comprehensive data for the study. Details for patients and their companions are presented in Table 1. Moreover, the unit cost of the resources used for the comparative projections is presented in Table 2.
Table 1. Characteristics of patients and companions.
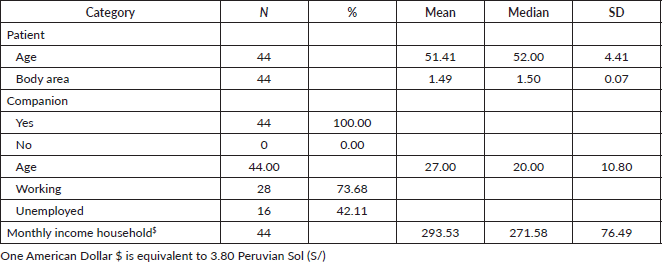
Table 2. RT and CT-related unit costs.
The regimens 3D CTRT25 + HDRBT, 3D CTRT28 + HDRBT, 3D CTRT28 + HDRBT + VMAT RT25, 3D CTRT28 + 3D RT5, 3D CTRT30 + HDRBT, IMRT CTRT25 + HDRBT, VMAT CTRT25 + HDRBT, IMRT CTRT28 + HDRBT, VMAT CTRT28 + HDRBT, IMRT CTRT28 + HDRBT (IIIC2) and VMAT CTRT28 + HDRBT (IIIC2), have a direct cost of $3 048.85, $3 399.33, $4 856.47, $2 790.87, $3 501.28, $3 574.12, $3 208.43, $4 001.62, $3 592.05, $4 349.55 and $3 837.58, respectively. The treatment regimens for stage IIIC2, including 3D and novel techniques, are those with the highest costs. The administration of 3D RT for IIIC2 and novel IMRT or VMAT techniques, is $3,881.69, $3,374.76 and $2,862.80, respectively. Planning increased in value from $25.3 to $58.83 when novel technique therapy began over 3D. Also, all first option sessions (28 3D pelvis sessions and 25 PA VMAT sessions) are almost double the cost of 28 PPA VMAT sessions yet much lower than PPA IMRT (Table 3).
The indirect cost from stage IIB to IIIC1 in descending order is IMRT, 3D and VMAT, but in IIIC2 the novel technique regimens reduce by up to 33.99% compared to 3D. The resulting costs of lost household productivity for 3D CTRT25 + HDRBT, 3D CTRT28 + HDRBT, 3D CTRT28 + HDRBT + VMAT RT25, 3D CTRT28 + 3D RT5, 3D CTRT30 + HDRBT, IMRT CTRT25 + HDRBT, VMAT CTRT25 + HDRBT, IMRT CTRT28 + HDRBT, VMAT CTRT28 + HDRBT, IMRT CTRT28 + HDRBT (IIIC2) and VMAT CTRT28 + HDRBT (IIIC2) are $218.47, $241.43, $346.63, $252.46, $249.99, $219.77, $217.17, $242.88, $239.98, $245.06 and $241.43, respectively (Table 3).
The schemes that comprise a higher number of sessions, due to the additional administration involved with using CT and the IMRT technique, result in the patient staying in the hospital for more than 96 hours; which is why the 3D CTRT28 + HDRBT + VMAT RT25 (IIIC2) and IMRT CTRT28 + HDRBT (IIIC2) schemes have the longest hospital stays – 131 and 97 hours, respectively (Figure 1).
Regarding procedure delivery, from stage IIB to IIIC1, the 3D and VMAT techniques are usually similar with values between 44 and 56 hours; however, the IMRT technique is usually longer than both techniques by at least 5 hours. The special techniques planning is usually longer, but this is offset by the reduced teletherapy time for VMAT, which is cut from 6.3 to 5.4 hours, 7.0 to 6.1 hours and 12.4 to 7 hours for stages IIB/IIIA/IIIB, IIIC1 and IIIC2, respectively (Figure 2).
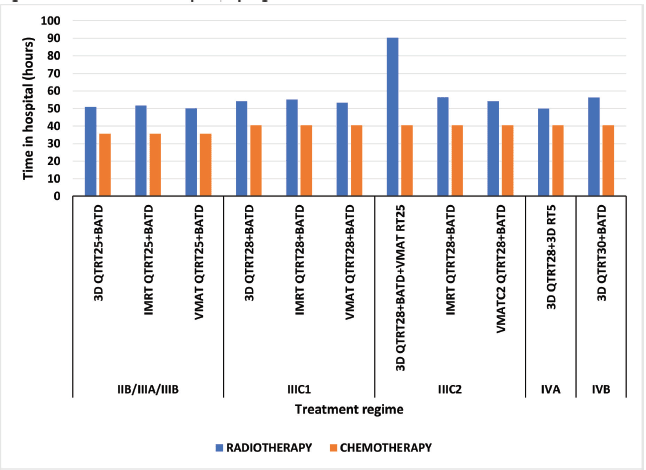
Figure 1. Patient time in hospital, by regimen.
Table 3. Comparison of direct and indirect costs by treatment regimens.
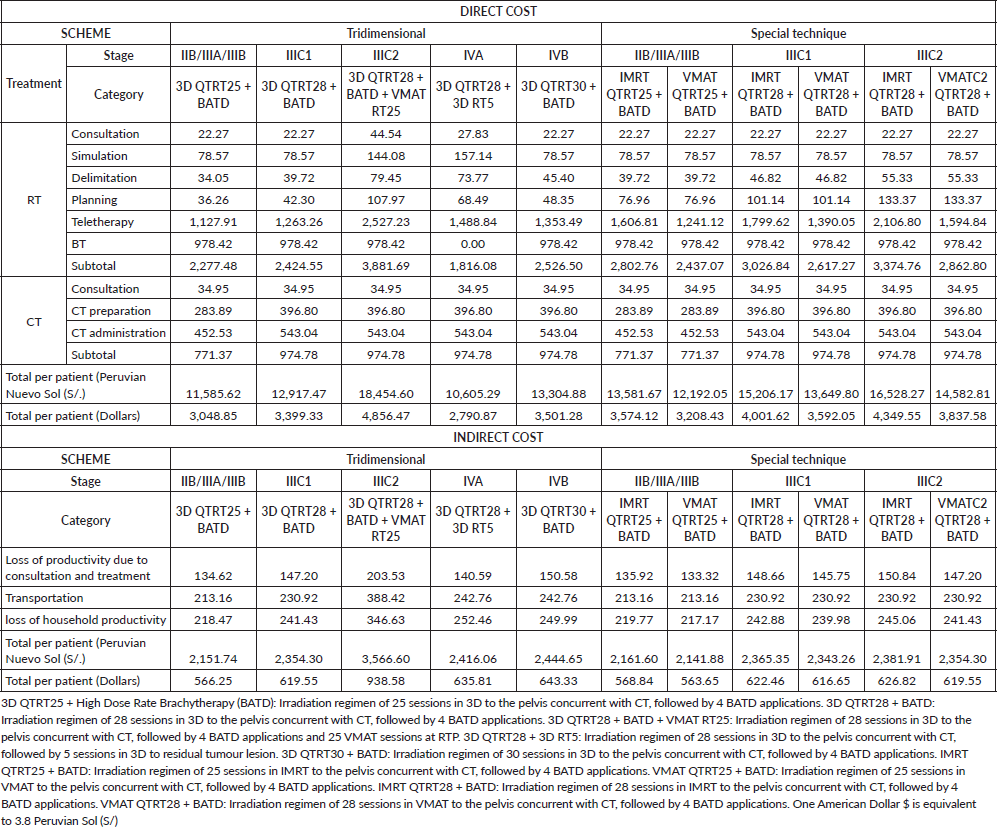
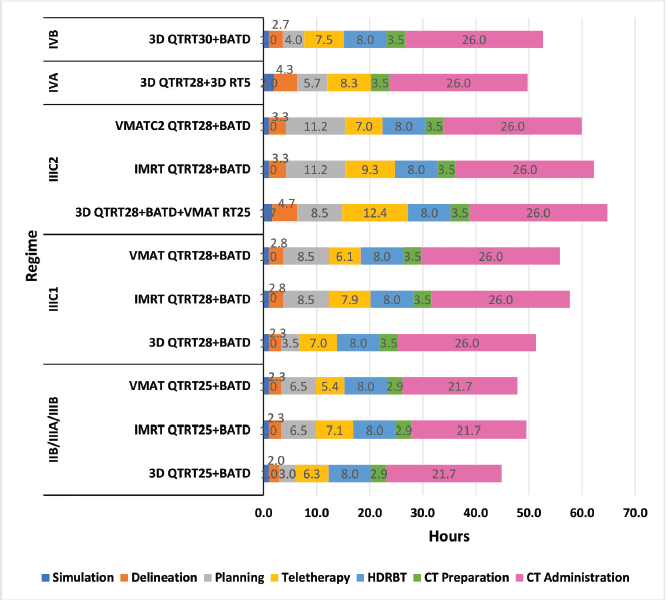
Figure 2. Comparison of procedure time, by regime.
Discussion
The institute is financed by the state, located 284 km from Lima and responsible for cancer treatment in the central part of the country. Patients with CC have comprehensive health insurance, which means that the state subsidises the direct costs of medical care. For CTRT and HDRBT, the hospital covers the cost of medication; associated supplies, equipment and infrastructure; and the work of healthcare professionals. However, finance and equipment are less than the demand, so it is pertinent to make managerial decisions, supported by efficiency and in addition to the associated costs, as this will allow more cancer treatments to be offered [16, 17].
It is expected that, by 2040, the increased incidence and mortality will be disproportionately higher in low (LIC) and middle-income countries (MIC), with an estimated 72% higher incidence and 76% higher mortality in countries with low-to-medium HDI (Peru or Indonesia). This imbalance in the cancer burden is compounded by current disparities in resources for combatting cancer, which must be addressed as a priority [18, 19].
CC is the fourth most common type of cancer and the fourth leading cause of cancer mortality in women worldwide [20]. Radical RT plays an important role in the definitive treatment of locally-advanced disease, with 8-year survival rates of up to 67% when combined with concurrent CT [21]. Even in retrospective analysis, the addition of local RT at stage IVB seems to confer better results in specific survival [22, 23], which is why at our institution the treatment is offered to those with inguinal metastasis. The therapy administered with an HDRBT boost is the standard of care [24]; IMRT or VMAT have become standard therapy methods, compared to 3D [25]. The dose is determined and administered via new technologies, which use inverse optimisation algorithms, as they are consistent with the planned target area and provide a more even distribution [26].
There are many published studies on CTRT regimens that have been widely used in clinical practice. However, there is limited research on economic analysis. This is the first Latin American study that analyses the costs separated by clinical stage, comparing irradiation techniques. Unlike other studies that are based on annual projections by incidence [27], and others that only focus on the cost of human resources and infrastructure [28], due to the nature of the study it has aimed to determine, in detail, both direct and indirect costs, not overlooking micro costs [29, 30].
The direct cost increases in line with the clinical stage from IIB to IIIC2 (Table 3). However, at stage IVA there is a decrease because HDRBT is not used, due to an absence of suitable applicators at the institution. This trend is comparable to the study by Nguyen et al [31], which shows that in hospitals in central Vietnam, the medical costs for the treatment of CC increase as the cancer progresses to the later stages. In addition, Granados-Garcia et al [32] report that the medical cost for stages I–IV was between $4,738 and $6,058. In the study, RT represented 77% of the direct cost; this data was consistent with the study by Santos et al [33] of Brazilian women with CC regardless of the stage, RT and CT represented a cost of $1,491 and $1,069, respectively.
Furthermore, our study shows that the direct cost is markedly reduced by starting irradiation with VMAT in clinical stage IIIC2, unlike the rest of the clinical stages, as starting the treatment with 3D only sequentially irradiates the pelvis and PA region, resulting in an increased cost, to meet the volumes of PPA nodes recommended by EMBRACE II [34]. Nevertheless, the inclusion of PA volumes has been shown to increase the risk of acute and delayed intestinal toxicity [35, 36]. This is why, at the institution at stage IIIC2, the pelvic segment is treated first and then the PA segment, increasing the number of sessions and, as a result the number of procedures [37]. Urban et al [38], based on patients at clinical stage IA1 to IIIC2, revealed that patients undergoing definitive teletherapy with an HDRBT boost reported less late and subacute gastrointestinal toxicity when treated with IMRT/VMAT, compared to 3D. So there is a clinical benefit, in addition to an economic benefit as demonstrated by the study, as there is a marked decrease in the cost of teletherapy when using VMAT rather than IMRT or 3D.
Regarding the intra-hospital procedure time, more time is generally required during the administration of CT, which is only considered concurrent, but not in the later stage of CTRT, given that for stage IIB to IVA, CTRT is more cost-effective than CTRT followed by adjuvant CT [39]. On the other hand, when the special technique is used, it requires a great deal more planning for the same complexity but shortens the teletherapy time for VMAT (Figure 2). This is due to advanced technology also carrying a corresponding computational burden, which considerably increases the total planning time. According to statistics, radiotherapists spend an average of 4 hours delineating the target volume plan and organs at risk, and it can also be delayed further by some complex illnesses. After this, the medical physicists formulate an RT plan which complies with treatment standards, which takes approximately 10 hours per patient [40, 41]. A large amount of time required for planning inevitably leads to a delay in treatment, which then affects the quality of treatment and the prognosis of the patients [42]. In the future, based on steep learning networks and optimisation algorithms, like the voxel dose restriction optimisation model or setting up predicted Dose Volume Histograms-aided targets, it will allow the development of an automated planning system that will ultimately serve the doctors and physicists, balancing the cost of time and precision [41].
Our study shows that indirect costs are lower for all stages using VMAT. The difference in the loss of productivity and the use of transportation is notable (Table 3). This is due to the reduction of hospital visits, and the shorter time involved in each teletherapy session; consequently, the accumulated time is less than with IMRT or 3D (Figure 1). These are the only categories evaluated for indirect cost, which represents at most 26.17% and 17.27% of the annual household income ($3,586.32) [15] with the 3D QTRT28 + BATD + VMAT RT25 and VMAT QTRT28 + BATD schemes, respectively (Table 3). This result is worrisome, due to the risk of incurring catastrophic costs and increasing barriers to compliance with treatment [43–45].
At present, the option exists to reduce the number of BATD sessions [46] but at the teletherapy level, it is not recommended to use hypofractionation in order to reduce the number of sessions as in other pathologies [8, 17]. However, the future option exists to offer hypofractionation of 15 sessions followed by 4 BATD applications in women with CT contraindications such as advanced illness with secondary renal dysfunction and hydronephrosis related to the tumour, borderline cardiac function; and fragile patients [47]. Similarly, by means of phase II trials NCT04070976 and NCT04583254, the efficacy and safety of hypofractionated doses are studied in CTRT. Currently, the recommended splits are between 25 and 28 sessions of special techniques with simultaneous integrated boost, which is impossible to accomplish this in a country with a marked deficit of RT equipment [48]. The international atomic energy agency ideally recommends four units of RT per million people, with a minimum of at least 1.5 units per million. However, currently, one unit of RT helps 0.12 million people in HIC, in contrast to 1 million in MIC and 5 million in LIC [19, 49]. The institute has an assigned population of approximately 3,681,400 with only two linear particle accelerators; therefore, it cannot provide special technical treatment to all of the patients due to the high demand. Replicate this scenario on a national level with a population of 33.72 million and only 59 sets of teletherapy equipment and 18 sets of brachytherapy (BT) equipment all of which are gathered in the capital city. This also occurs in China, India and Argentina where a disparity exists in the density of facilities within a country and only being able to receive treatment within a reasonable timeframe in modern cities [50–53].
In other studies of non-gynaecological pathology costs, they do simultaneous variation in the treatment time, capacity utilisation rates and the number of LINAC staff, obtaining a cost reduction [54]. The analysis was carried out with the minimum number of staff necessary for LINAC, two medical technologists. Staffing costs could thus not have been reduced further. Perhaps using another LINAC that optimises treatment speed through gantry mobility and MLC motion, such as the Varian® Halcyon [55, 56], could significantly lower teletherapy costs. Similarly, the limitation of the special techniques planning is that it only has two RT planning systems, running 12 hours a day, for the whole service. Consequently, the only way to increase the supply of special technical plans and LINAC services would be to recruit more professionals in order to increase the working hours and teletherapy supply capacity from 32 to 48 patients per day, but this involves more hours of doing radiation, which has a direct outcome of reducing the operational lifespan of LINAC [57, 58].
The limitations of the study are: First, we made some simplifying assumptions regarding the natural history and treatment of the disease. Second, the limited number of patients could have different costs due to social status. Third, it is possible that we did not account for differences in delayed toxic effects as follow-up data is limited. However, the total rate of these effects seems lower through special techniques according to clinical trials.
Conclusion
Our study is one of the few to evaluate the costs of 3D and special technique treatments that have incorporated patient costs, direct costs, added over time and the indirect cost to patients and their households. Moreover, this is the first report that includes clinical and economic oncologic data from a Peruvian public hospital.
In RT centres with a positive supply over demand of modern LINAC equipment, VMAT should be preferred over IMRT/3D as it lowers costs and toxicity, but in RT centres where demand exceeds the supply of planning systems with VMAT techniques, patients with stages IIB to IIIC1 cancer could continue making use of 3D teletherapy over IMRT/VMAT for the short period of time during the planning process, but VMAT is preferable for stage IIIC2 patients, as it saves hospital resources and indirect costs.
Acknowledgments
The authors would like to thank the Junín Regional Health Authority and the Regional Institute for Neoplastic Diseases (Central Region).
Conflicts of interest declaration
There are no conflicts of interest in this research.
Project funding
No funding has been received.
References
1. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality Worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
2. Mayadev JS, Ke G, and Mahantshetty U, et al (2022) Global challenges of radiotherapy for the treatment of locally advanced cervical cancer Int J Gynecol Cancer 32(3) 436–445 https://doi.org/10.1136/ijgc-2021-003001 PMID: 35256434 PMCID: 8921593
3. Sturdza A, Pötter R, and Fokdal LU, et al (2016) Image guided brachytherapy in locally advanced cervical cancer: improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study Radiother Oncol 120(3) 428–433 https://doi.org/10.1016/j.radonc.2016.03.011 PMID: 27134181
4. Schernberg A, Bockel S, and Annede P, et al (2018) Tumor shrinkage during chemoradiation in locally advanced cervical cancer patients: prognostic significance, and impact for image-guided adaptive brachytherapy Int J Radiat Oncol Biol Phys 102(2) 362–372 https://doi.org/10.1016/j.ijrobp.2018.06.014 PMID: 29920324
5. Bhatla N, Aoki D, and Sharma DN, et al (2018) Cancer of the cervix uteri Int J Gynaecol Obstet 143(Suppl 2) 22–36 https://doi.org/10.1002/ijgo.12611 PMID: 30306584
6. Olawaiye AB, Baker TP, and Washington MK, et al (2021) The new (Version 9) American joint committee on cancer tumor, node, metastasis staging for cervical cancer CA Cancer J Clin 71(4) 287–298 https://doi.org/10.3322/caac.21663 PMID: 33784415
7. Chargari C, Peignaux K, and Escande A, et al (2022) Radiotherapy of cervical cancer Cancer Radiother 26(1–2) 298–308 https://doi.org/10.1016/j.canrad.2021.11.009
8. Datta NR, Stutz E, and Liu M, et al (2017) Concurrent chemoradiotherapy vs. radiotherapy alone in locally advanced cervix cancer: a systematic review and meta-analysis Gynecol Oncol 145(2) 374–385 https://doi.org/10.1016/j.ygyno.2017.01.033 PMID: 28188016
9. Shrivastava S, Mahantshetty U, and Engineer R, et al (2018) Cisplatin chemoradiotherapy vs radiotherapy in FIGO stage IIIB squamous cell carcinoma of the uterine cervix: a randomized clinical trial JAMA Oncol 4(4) 506–513 https://doi.org/10.1001/jamaoncol.2017.5179 PMID: 29423520 PMCID: 5885185
10. Tan Mbbs Mrcp Frcr LT, Tanderup K, and Kirisits C, et al (2019) Image-guided adaptive radiotherapy in cervical cancer Semin Radiat Oncol 29(3) 284–298 https://doi.org/10.1016/j.semradonc.2019.02.010
11. Olivera Changra H and Robles Díaz JF (2022) Costos de la administración intravenosa vs. subcutánea del trastuzumab en pacientes peruanas con cáncer de mama HER2 positivo. Un análisis observacional de los costos directos e indirectos [Costs of intravenous vs. subcutaneous administration of trastuzumab in peruvian patients with HER2-positive breast cancer - An observational analysis of direct and indirect costs] J Healthc Qual Res 37(3) 147–154 https://doi.org/10.1016/j.jhqr.2021.10.008
12. Consulta de Medicamentos – HNSEB [Internet] (2020) Hospital Nacional Sergio E. Bernales - Portal Institucional. Hospital Nacional Sergio E. Bernales - Portal Web https://portal.hnseb.gob.pe/medicamentos-hnseb/ Date accessed: 19/01/23
13. La Contraloría (2019) Contrato N° 112-2014-GRJ/ORAF “Implementación del Instituto Regional de Enfermedades Neoplásicas de la Macro Región del Centro de Perú” [Internet] (Lima: LACONTRALORÍA) https://s3.amazonaws.com/spic-informes-publicados/informes/2019/12/2019CPOL3340133_ADJUNTO.pdf Date accessed: 19/01/23
14. Instituto Regional de Enfermedades Neoplásicas del Centro (2022) Bases del Concurso Público 2022 [Internet] (Junín: IRENCENTRO) https://portal.irencentro.gob.pe/wp-content/uploads/2022/07/rrhhcas01final.pdf] Date accessed: 19/01/23
15. Instituto Nacional de Estadística e Informática (INEI) (2018) Evolución de los Indicadores de Empleo e Ingresos Por Departamento, 2007-2017 [Internet] (Lima: INEI) https://www.inei.gob.pe/media/MenuRecursivo/publicaciones_digitales/Est/Lib1870/libro.pdf Date accessed: 19/01/23
16. Cañizares FW (2017) Evolución del sistema de salud de Perú: buenas prácticas y desafíos en su construcción. Década 2005-2014 An Fac Med 78(4) 445–451 https://doi.org/10.15381/anales.v78i4.14269
17. Díaz JFR and Changra HO (2022) Cost analysis of total neoadjuvant therapy with 5 × 5 Gy radiation therapy versus conventional chemoradiotherapy for locally advanced rectal cancer among Peruvians Ecancermedicalscience 16 1406 https://doi.org/10.3332/ecancer.2022.1406
18. Lievens Y, Gospodarowicz M, and Grover S, et al (2017) Global impact of radiotherapy in oncology: saving one million lives by 2035 Radiother Oncol 125(2) 175–177 https://doi.org/10.1016/j.radonc.2017.10.027 PMID: 29173397
19. Maitre P, Krishnatry R, and Chopra S, et al (2022) Modern radiotherapy technology: obstacles and opportunities to access in low- and middle-income countries JCO Glob Oncol 8 e2100376 https://doi.org/10.1200/GO.21.00376 PMID: 35839434 PMCID: 9812473
20. Bray F, Ferlay J, and Soerjomataram I, et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 68(6) 394–424 https://doi.org/10.3322/caac.21492 PMID: 30207593
21. Eifel PJ, Winter K, and Morris M, et al (2004) Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01 J Clin Oncol 22(5) 872–880 https://doi.org/10.1200/JCO.2004.07.197 PMID: 14990643
22. Huang K, Jia M, and Li P, et al (2018) Radiotherapy improves the survival of patients with metastatic cervical cancer: a propensity-matched analysis of SEER database Int J Gynecol Cancer 28(7) 1360–1368 https://doi.org/10.1097/IGC.0000000000001313 PMID: 30036221
23. Xu JY, Chen JN, and Lei J, et al (2022) Local treatment improves survival in patients with stage IVB cervical cancer Gynecol Oncol 165(3) 538–545 https://doi.org/10.1016/j.ygyno.2022.04.013 PMID: 35490033
24. Chino J, Annunziata CM, and Beriwal S, et al (2020) Radiation therapy for cervical cancer: executive summary of an ASTRO clinical practice guideline Pract Radiat Oncol 10(4) 220–234 https://doi.org/10.1016/j.prro.2020.04.002 PMID: 32473857 PMCID: 8802172
25. Chen VE, Gillespie EF, and Manger RP, et al (2019) The impact of daily bladder filling on small bowel dose for intensity modulated radiation therapy for cervical cancer Med Dosim 44(2) 102–106 https://doi.org/10.1016/j.meddos.2018.02.010
26. Cibula D, Pötter R, and Planchamp F, et al (2018) The European society of gynaecological oncology/European society for radiotherapy and oncology/European society of pathology guidelines for the management of patients with cervical cancer Virchows Arch 472(6) 919–936 https://doi.org/10.1007/s00428-018-2362-9 PMID: 29725757
27. Katanyoo K, Riewpaiboon A, and Chaikledkaew U, et al (2021) The cost of locally advanced cervical cancer in Thailand: an empirical study for economic analysis Asian Pac J Cancer Prev 22(10) 3171–3179 https://doi.org/10.31557/APJCP.2021.22.10.3171 PMID: 34710993 PMCID: 8858260
28. Yaremko HL, Locke GE, and Chow R, et al (2021) Cost minimization analysis of hypofractionated radiotherapy Curr Oncol 28(1) 716–725 https://doi.org/10.3390/curroncol28010070 PMID: 33573158 PMCID: 7985773
29. Husereau D, Drummond M, and Petrou S, et al (2013) Consolidated health economic evaluation reporting standards (CHEERS)--explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force Value Health 16(2) 231–250 https://doi.org/10.1016/j.jval.2013.02.002 PMID: 23538175
30. Defourny N, Dunscombe P, and Perrier L, et al (2016) Cost evaluations of radiotherapy: what do we know? An ESTRO-HERO analysis Radiother Oncol 121(3) 468–474 https://doi.org/10.1016/j.radonc.2016.12.002 PMID: 28007378
31. Nguyen AD, Hoang MV, and Nguyen CC (2018) Medical costs for the treatment of cervical cancer at central hospitals in Vietnam. Health Care Women Int 39(4) 442–449 https://doi.org/10.1080/07399332.2017.1402912
32. Granados-García V, Piña-Sánchez P, and Reynoso-Noveron N, et al (2019) Medical cost to treat cervical cancer patients at a social security third level oncology hospital in Mexico city Asian Pac J Cancer Prev 20(5) 1547–1554 https://doi.org/10.31557/APJCP.2019.20.5.1547 PMID: 31128061 PMCID: 6857888
33. Santos CL, Souza AI, and Figueiroa JN, et al (2019) Estimation of the costs of invasive cervical cancer treatment in Brazil: a micro-costing study Rev Bras Ginecol Obstet 41(6) 387–393 https://doi.org/10.1055/s-0039-1692412 PMID: 31247667
34. Pötter R, Tanderup K, and Kirisits C, et al (2018) The EMBRACE II study: the outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies Clin Transl Radiat Oncol 9 48–60 https://doi.org/10.1016/j.ctro.2018.01.001 PMID: 29594251 PMCID: 5862686
35. Mundt AJ, Lujan AE, and Rotmensch J, et al (2002) Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies Int J Radiat Oncol Biol Phys 52(5) 1330–1337 https://doi.org/10.1016/S0360-3016(01)02785-7 PMID: 11955746
36. Klopp AH, Yeung AR, and Deshmukh S, et al (2018) Patient-reported toxicity during pelvic intensity-modulated radiation therapy: NRG oncology-RTOG 1203 J Clin Oncol 36(24) 2538–2544 https://doi.org/10.1200/JCO.2017.77.4273 PMID: 29989857 PMCID: 6097832
37. Rotman M, Choi K, and Guse C, et al (1990) Prophylactic irradiation of the para-aortic lymph node chain in stage IIB and bulky stage IB carcinoma of the cervix, initial treatment results of RTOG 7920 Int J Radiat Oncol Biol Phys 19(3) 513–521 https://doi.org/10.1016/0360-3016(90)90475-Y PMID: 2211198
38. Urban R, Wong J, and Lim P, et al (2022) Cervical cancer patient reported gastrointestinal outcomes: intensity/volumetric modulated vs. 3D conformal radiation therapy J Gynecol Oncol 33(5) e70 https://doi.org/10.3802/jgo.2022.33.e70 PMID: 35882607 PMCID: 9428301
39. Rongsriyam K, Tangjitgamol S, and Leelahavarong P, et al (2020) Cost-utility analysis of adjuvant chemotherapy after concurrent chemoradiation in patients with locally advanced cervical cancer J Med Imaging Radiat Oncol 64(6) 873–881 https://doi.org/10.1111/1754-9485.13103 PMID: 32978901
40. Boon IS, Au Yong TPT, and Boon CS (2018) Assessing the role of artificial intelligence (AI) in clinical oncology: utility of machine learning in radiotherapy target volume delineation Medicines (Basel) 5(4) 131 https://doi.org/10.3390/medicines5040131 PMID: 30544901 PMCID: 6313566
41. Zhang G, Jiang Z, and Zhu J, et al (2022) Dose prediction for cervical cancer VMAT patients with a full-scale 3D-cGAN-based model and the comparison of different input data on the prediction results Radiat Oncol 17(1) 179 https://doi.org/10.1186/s13014-022-02155-7 PMID: 36372897 PMCID: 9655866
42. Delahaut G, Témam S, and Ambroise J, et al (2019) Prognostic impact of tumor growth velocity in head and neck squamous cell carcinoma treated by radiotherapy: a pilot study Head Neck 41(9) 3041–3048 https://doi.org/10.1002/hed.25789 PMID: 31087727
43. Binka C, Nyarko SH, and Awusabo-Asare K, et al (2019) Barriers to the uptake of cervical cancer screening and treatment among rural women in Ghana Biomed Res Int 2019 6320938 https://doi.org/10.1155/2019/6320938 PMID: 31781631 PMCID: 6874950
44. Zamorano AS, Barnoya J, and Gharzouzi E, et al (2019) Treatment compliance as a major barrier to optimal cervical cancer treatment in Guatemala J Glob Oncol 5 1–5 https://doi.org/10.1200/JGO.18.00243 PMID: 31067142 PMCID: 6550059
45. Nwankwo C, Shah R, and Shah A, et al (2022) Treatment patterns and economic burden among newly diagnosed cervical and endometrial cancer patients Future Oncol 18(8) 965–977 https://doi.org/10.2217/fon-2021-0727 PMID: 35105169
46. Kirchheiner K, Czajka-Pepl A, and Ponocny-Seliger E, et al (2014) Posttraumatic stress disorder after high-dose-rate brachytherapy for cervical cancer with 2 fractions in 1 application under spinal/epidural anesthesia: incidence and risk factors Int J Radiat Oncol Biol Phys 89(2) 260–267 https://doi.org/10.1016/j.ijrobp.2014.02.018 PMID: 24721589
47. Bacorro W, Baldivia K, and Dumago M, et al (2022) Phase 1/2 trial evaluating the effectiveness and safety of dose-adapted hypofractionated pelvic radiotherapy for advanced cervical cancers INeligible for chemotherapy (HYACINCT) Acta Oncol 61(6) 688–697 https://doi.org/10.1080/0284186X.2022.2048070 PMID: 35285405
48. Ministerio de Salud (MINSA) (2020) Análisis de la situación del Cáncer en el Perú, 2018 [Internet] (Lima: MINSA) http://bvs.minsa.gob.pe/local/MINSA/5158.pdf Date accessed: 19/01/23
49. Ndlovu N (2019) Radiotherapy treatment in cancer control and its important role in Africa Ecancermedicalscience13 942 https://doi.org/10.3332/ecancer.2019.942 PMID: 31552115 PMCID: 6722105
50. Datta NR, Samiei M, and Bodis S (2014) Radiation therapy infrastructure and human resources in low- and middle-income countries: present status and projections for 2020 Int J Radiat Oncol Biol Phys 89(3) 448–457 https://doi.org/10.1016/j.ijrobp.2014.03.002 PMID: 24751411
51. Datta NR, Rogers S, and Bodis S (2019) Challenges and opportunities to realize “The 2030 agenda for sustainable development” by the United Nations: implications for radiation therapy infrastructure in low- and middle-income countries Int J Radiat Oncol Biol Phys 105(5) 918–933 https://doi.org/10.1016/j.ijrobp.2019.04.033 PMID: 31451317
52. Atun R, Jaffray DA, and Barton MB, et al (2015) Expanding global access to radiotherapy Lancet Oncol 16(10) 1153–1186 https://doi.org/10.1016/S1470-2045(15)00222-3 PMID: 26419354
53. Apás Pérez De Nucci A, Minig L, and Perrotta M (2017) Patterns of cervical cancer care in Argentina: applying ASCO recommendations adjusted by local resources Gynecol Oncol Rep 21 61–66 https://doi.org/10.1016/j.gore.2017.06.012 PMID: 28702501 PMCID: 5496480
54. Hanly P, Céilleachair AÓ, and Skally M, et al (2015) Direct costs of radiotherapy for rectal cancer: a microcosting study BMC Health Serv Res 15 184 https://doi.org/10.1186/s12913-015-0845-9 PMID: 25934169 PMCID: 4494796
55. Li C, Chen J, and Zhu J, et al (2019) Plan quality comparison for cervical carcinoma treated with Halcyon and Trilogy intensity-modulated radiotherapy J Cancer 10(24) 6135–6141 https://doi.org/10.7150/jca.32500 PMID: 31762823 PMCID: 6856582
56. Pawlicki T, Atwood T, and McConnell K, et al (2019) Clinical safety assessment of the Halcyon system Med Phys 46(10) 4340–4345 https://doi.org/10.1002/mp.13736 PMID: 31350914
57. Healy BJ, van der Merwe D, and Christaki KE, et al (2017) Cobalt-60 machines and medical linear accelerators: competing technologies for external beam radiotherapy Clin Oncol (R Coll Radiol) 29(2) 110–115 https://doi.org/10.1016/j.clon.2016.11.002
58. Cetnar AJ and DiCostanzo DJ (2021) The lifetime of a linac monitor unit ion chamber J Appl Clin Med Phys 22(12) 108–114 https://doi.org/10.1002/acm2.13463 PMID: 34762336 PMCID: 8664141






