Muscle invasive bladder cancer and radical cystectomy: a risk predictive model
Mohamad Ali Tfaily1,2, Hani Tamim2, Albert El Hajj3 and Deborah Mukherji2,4
1Department of Internal Medicine, Emory University, Atlanta, GA 30322, USA
2Department of Internal Medicine, American University of Beirut Medical Center, Beirut 1107 2020, Lebanon
3Division of Urology, Department of Surgery, American University of Beirut Medical Center, Beirut 1107 2020, Lebanon
4Division of Hematology Oncology, Department of Internal Medicine, American University of Beirut Medical Center, Beirut 1107 2020, Lebanon
Abstract
Background: Radical cystectomy (RC) for muscle invasive bladder cancer (MIBC) remains the historical gold standard for treatment despite significant perioperative morbidity and subsequent quality of life concerns. Trimodal therapy (TMT) is gaining acceptance as an alternative bladder preserving approach. We aim to identify patients for whom TMT may be the optimal approach by constructing risk calculators of morbidity and mortality associated with RC.
Methods: Using the American College of Surgeons National Surgical Quality Improvement Program database, we selected patients diagnosed with MIBC undergoing RC, with a total of 10,642 patients identified. The primary outcome was mortality and secondary outcome was morbidity within 30 days of the procedure. We conducted multivariate logistic regression to obtain the best fit model for each outcome on 70% of the sample. Validation of the models was then performed on the remaining 30% of the sample. Model performance was assessed using discrimination and calibration abilities and a risk calculator was constructed for pre-operative counselling.
Results: Of the full cohort, 199 patients (1.9%) died and 2,328 patients (21.9%) experienced morbidity. Variables selected for the model predicting mortality included age, frailty, the American Society of Anesthesiologists status and preoperative creatinine. For the mortality model, the area under the curve was 72% with a Hosmer–Lemeshow statistic of 0.722. For the morbidity model, the area under the curve was 60% with a Hosmer–Lemeshow statistic of 0.287. Variables significant in the model included continent diversion, smoking and frailty.
Conclusion: We have constructed statistically significant and clinically relevant models using readily available health indicators to be used in multi-disciplinary discussion to provide high-risk patients with individualised risks of morbidity and mortality from RC, allowing for counselling for alternative treatments such as TMT.
Keywords: muscle invasive bladder cancer, radical cystectomy, trimodal therapy, risk calculator
Correspondence to: Deborah Mukherji
Email: dm25@aub.edu.lb
Published: 18/10/2022
Received: 13/04/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Bladder cancer is a substantial public health burden causing significant mortality and morbidity globally. It is the tenth most common cause of cancer worldwide, with 573,278 incident cases in and over 200,000 deaths in 2020 [1].
The distinction between non-muscle invasive bladder cancer and muscle invasive bladder cancer (MIBC) on pathologic examination of tumour biopsies from the bladder has important prognostic and clinical significance. MIBC can be either confined to the bladder or metastasise distally. For patients with MIBCs that are organ-confined, the standard treatment is radical cystectomy (RC); however, this procedure has a high rate of peri-operative morbidity and post-operative quality of life concerns including sexual dysfunction [2]. For patients who are at high risk of peri-operative complications, alternative treatments with bladder preserving strategies have been investigated, the most common of which is trimodal therapy (TMT) [3]. TMT has historically been designated as the treatment strategy at diagnosis for patients unfit for RC and consists of maximal transurethral resection of bladder tumour, followed by radiotherapy with concurrent chemotherapy [4]. Observational studies have shown that a subset of patients can achieve long-term disease control with bladder preservation [3]. Retrospective data on the use of TMT is biased by patient selection and the preferential use of this strategy in patients with multiple medical co-morbidities. Unfortunately, there are no randomised controlled trials comparing RC to TMT since attempted studies were halted due to poor accrual [5]. Based on the recommendations of the European Association of Urology, the decision to recommend RC as opposed to bladder preservation approaches in elderly or frail patients with MIBC should be based on tumour stage and comorbidity scoring, such as the Charlson score [6]. With emerging data suggesting that with modern radiation therapy and the use of patient stratification and novel therapeutics, bladder preserving strategies could lead to comparable outcomes compared to RC [7–11], the genitourinary oncology community is revisiting the option of TMT for all patients with MIBC who wish to preserve their bladder [12].
In the absence of clear-cut guidelines on how to choose between RC and TMT combined with emerging data suggesting comparable outcomes, predicting the risk of morbidity and mortality can aid clinical decision-making. In this study, we aimed at developing a new predictive model using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) to identify otherwise surgically fit patients who are at high risk of morbidity and mortality following RC and for whom counselling regarding bladder preserving strategies might be recommended.
Methods
Data source
The ACS-NSQIP database from years 2008 to 2017 is an externally validated database with patient information from more than 400 medical centres and maintains information for up to 30 days after undergoing surgical procedures [13]. This study retrospectively analysed deidentified patient data from the registry. This study was Institutional Review Board exempt as ACS-NSQIP does not provide protected health information from individuals.
Patient selection
Patients who underwent RC during the years 2008–2017 were identified using the current procedure terminology (CPT) codes: 51575, 51580, 51585, 51590, 51595, 51596 and 5157.
Patients with International Statistical Classification of Diseases and Related Health Problems (ICD) codes corresponding to nonmetastatic bladder cancer were selected. ICD 10 codes include: C66, C67, C68, C79.11, C79.19, D09.0, D30.3, D41.4 and D49.4. ICD 9 codes selected were: 188, 236.7 and 239.4. Furthermore, patients with disseminated cancer, a variable present in ACS-NSQIP, were excluded from the study. Patients with missing ICD codes (NULL) were excluded. A total of 10,642 individuals were included in the study.
Study covariates
Baseline demographic characteristics and baseline comorbidities and laboratory values provided by NSQIP such as age, gender, race, body mass index (BMI) and diabetes mellitus were included. Nutritional deficiency was defined as the presence of albumin <3.5 g/dL, >10% weight loss in the past 6 months or BMI < 18.5 kg/m2 [14]. Another covariate was the American Society of Anesthesiologist (ASA) class, which predicts patients’ risk for anaesthesia based on their physical status.
The modified frailty index (mFI) score (mFI5) was used to assess baseline morbidity. mFI5 was validated and used on ACS-NSQIP dataset [15]. It is a score that ranges from 0 to 1 calculated as follows:
mFI5 = (number of comorbidities)/5
Study outcomes
The study outcomes consisted of 30-day mortality and post-operative morbidity. Post-operative morbidity was assessed as a binary outcome: presence of two or more serious complications as defined by NSQIP [16]. Detailed description of each variable was extracted from the Participant Data Use file (2017).
Statistical analyses
All statistical analyses were conducted using IBM Statistical Package for Social Sciences version 26 (IBM Corp, Armonk, NY). We used a derivation cohort and a validation cohort: A sample of 70% was randomly selected from our total sample to derive the best fit model. The derived model was validated on the remaining 30%.
Clinically relevant variables including demographics, preoperative comorbidities and laboratory values with p < 0.2 from the bivariate analyses were considered in the multivariable logistic regression model building. Models were subsequently constructed using the forward likelihood ratio model in multivariate logistic regression. Clinically relevant variables with p-value < 0.2 in the bivariate analysis were inserted in the model using the Enter method. Each derivation and validation model’s performances were assessed by both the discriminatory ability and the calibration.
Developing the risk calculators
The risk calculators were developed on Excel, using the regressions mentioned above. A risk calculator was developed for each of the best-fit models (mortality and morbidity) based on the multivariate logistic regression.
Missing data
All variables in the model except for albumin had missing data <10% and were imputed based on the median or mode. Albumin had 33% missing data that was imputed using linear regression based on age, gender, body mass index and other laboratory values (Appendix). Sensitivity analysis was performed excluding missing cases.
Results
Patient characteristics
The ACS-NSQIP dataset from 2008 till 2017 yielded 10,642 patients who underwent RC due to bladder malignancy presented in Table 1. The mean patient age was 69, and 77% of the cohort were males. Twenty-four percent of the patients were current smokers and the most common ASA class was class III (70%). The most common pre-operative morbidity was hypertension requiring medication (60%) and elevated pre-operative creatinine (31.2%). The morbidities are presented in Table 2. The percentage of the main outcome, death, was 1.9% in the derivation, validation and full cohort. 13.9%, 13.4% and 13.76% of the patients had a prolonged length of stay in the derivation, validation and full cohorts, respectively.
Mortality
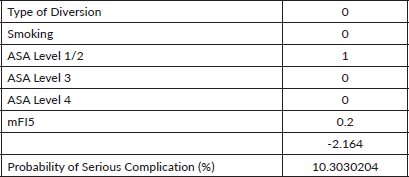
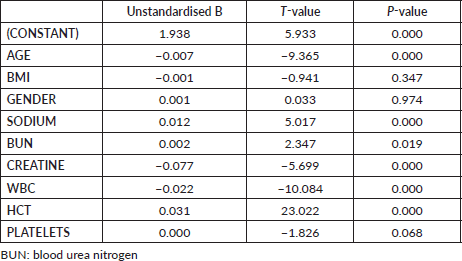
Every 1-year increase in age was significantly associated with a 6% increase in the odds of death, when adjusting for the other variables. Smoking conferred a clinically but not statistically significant odds ratio (OR) on the risk of death. The regression for mortality is presented in Table 3. The discriminative ability of the model was 72.2% (Figure 1). The calibrative ability of the model was shown by the Hosmer–Lemeshow statistic, which was 0.722 in the derivation model and 0.372, both greater than 0.05, indicating no significant difference between the predicted probabilities and observed probabilities of the outcome (Figure 2). An example of the mortality risk calculator is provided in Figure 3.
Table 1. Descriptive statistics of the derivation, validation and full cohorts.
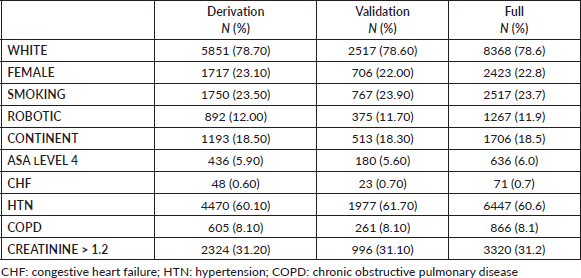
Table 2. Descriptive statistics of the peri-operative outcomes in the derivation, validation and full cohorts.
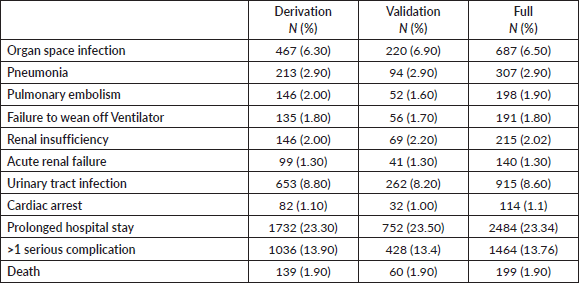
Table 3. Multivariable logistic regression of death within 30 days of the procedure.
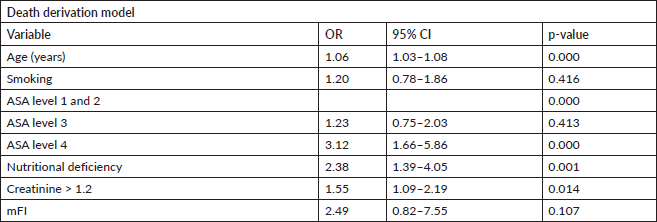
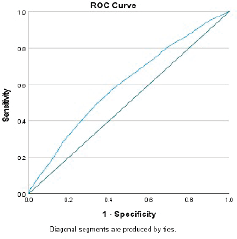
Figure 1. Receiver operator curve of the mortality model.
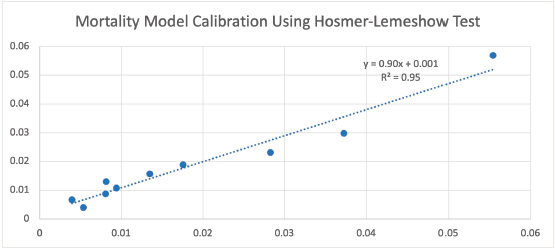
Figure 2. Observed versus expected probabilities of mortality using the Hosmer–Lemeshow test.
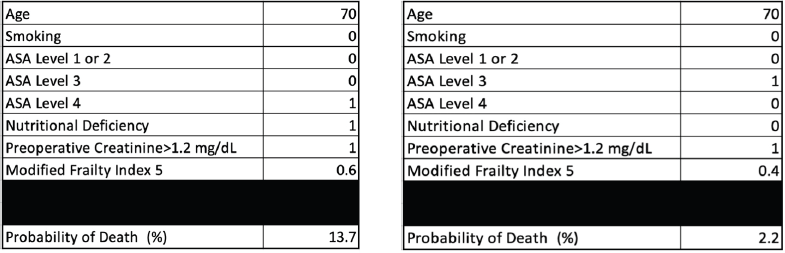
Figure 3. Risk calculator for death with an example of two patients with different characteristics.
Morbidity
Having a continent diversion was significantly associated with higher odds of developing serious morbidities when adjusting for the other variables. Similarly, mFI5 was associated with developing two or more serious morbidities (OR: 4.57; 95% CI: 2.87–7.29, p-value < 0.001). The results of the morbidity model are presented in Table 4. The discriminative ability of the model was 0.6 (Figure 4). Hosmer–Lemeshow statistic was 0.287 in the derivation model and 0.166 in the validation model.
In all the above, there was no significant difference in the discriminative ability of the models between the derivation and validation cohorts. An excel sheet of the morbidity calculator can be found in the Supplementary Material.
Discussion
The ACS-NSQIP dataset from 2008 to 2017 had 10,642 patients who had undergone RC due to bladder malignancy. We predicted post-operative mortality and morbidity by analysing several preoperative risk factors. For the mortality model, age, ASA class and nutritional deficiency were significantly associated with death. These variables can be easily assessed in the clinic setting prior to surgery, to provide the patient with individualised estimates of mortality and morbidity after RC. This is certainly in consensus with the literature where advanced age is an established risk factor for post-operative morbidity and mortality [17, 18]. Nutritional deficiency was also associated with higher mortality rate in our cohort and in other studies in the literature [14]. A meta-analysis involving ten studies on 4,692 geriatric patients with cancer found that malnutrition is significantly associated with all-cause mortality with a relative risk of 1.73. It is worth noting that this study included only observational studies and had a high level of heterogeneity (I2 = 73%; p < 0.01) [19]. Additionally, ASA was a strong predictor of postoperative mortality and morbidity in several studies [20, 21]. We used the mFI as an assessment of pre-operative comorbidity. mFI5 started being used after the mFI-11 was no longer applicable to NSQIP database after dropping certain variables crucial to this index. In a systematic review by Ornaghi et al [22], 8% of patients with urothelial bladder carcinoma (UBC) undergoing RC were frail while 31% were pre-frail. In our full cohort, 1.8% had four of the five mFI comorbidities, and around 20% had three.
Our risk calculator had fair predictive accuracy of 72% and was well calibrated with an R2 of 95% when comparing observed versus expected values. Our model’s discrimination is similar to that in the literature. A universal surgical risk calculator by the ACS-NSQIP is available; however, it was found not to be accurate enough for adaptation into clinical practice as the model was not procedure-specific [23]. Aziz et al [24] developed a nomogram for the prediction of 90-day mortality after RC for UBC, with a receiver operator curve of 68.8% using age, ASA class,
hospital volume and presence of preoperative nodal or distant metastasis. Our model excluded patients with metastasis, as these do not fall under the category of localised MIBC, on whom our study focused. Another model incorporating age, stage and histological subtype had a predictive accuracy of 70% [25]. However, this model included patients who underwent partial cystectomy and uses pre-operative and post-operative characteristics. Taylor et al [26] also developed a model using age and Charlson comorbidity score (CCI) with a discrimination area-under-the-curve of 70.2% [26]. Moreover, Morgan et al [27] developed a nomogram using age, CCI, clinical stage and preoperative albumin to predict time to death within 90 days of RC. The model’s adjusted c-statistic after internal validation was 0.71 [27]. While we had similar predictive ability, our model was restricted to 30 days post-operation given the dataset used. Another study by Mannas et al [28] developed a model with 62% accuracy in predicting death. We attribute this difference with the literature to our inclusion criteria whereby we selected patients by ICD codes indicating bladder malignancy and CPT codes referring to RC while excluding patients with metastatic disease.
In addition, the population in our cohort is assumed to have been cleared for surgery, meaning that they should have passed all prior pre-operative cardiology and anaesthesiology screenings. Although our model had fair to moderate accuracy, there are no other clinically relevant tools currently used, up to the authors’ knowledge, which aid in the clinical decision for this specific group of patients. Moreover, the model’s high calibration indicates a similarity between the expected and observed percentage probability of the outcome. This can provide the treating team with evidence upon which they, along with the patient, can base their decision.
Table 4. Multivariate logistic regression of developing more than one serious morbidity within 30 days of RC.
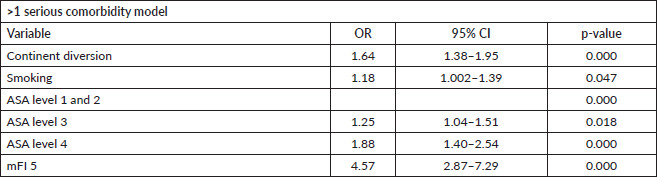
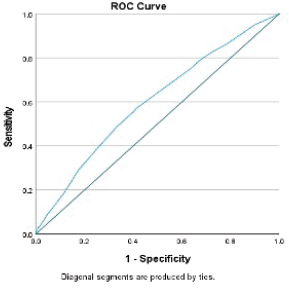
Figure 4. Receiver operator curve of the serious morbidity model.
Though our morbidity model’s predictive ability was poor, models in the literature using NSQIP for morbidity had poor predictive accuracy with an area under the curve between 60% and 65% [29–32]. However, we believe the high calibrative ability of our model, indicating a high correlation between observed versus expected probability of the outcome, can be useful in clinical practice.
Heavy emphasis has been placed on risk predictive models in the literature in the past decades [33–37]. These models aim at improving the decision-making process and involving the patients in their own management plan [38]. An increased risk of post-operative morbidity or mortality can aid the stakeholders involved in the patient’s care in preferring bladder preservation over RC. Information from risk predictors as ours can thus aid in: Informing patients of their personalised risk of morbidity and mortality, informed decision-making guides peri-operative management to reduce adverse outcomes [39], and most importantly, deferring RC and opting for TMT or vice versa.
As aforementioned, observational studies show nonsignificant difference between the two [5, 7–11]. However, in the absence of a randomised controlled trial comparing these two treatments, and with the rise of TMT as an alternative to RC, identifying patients at high risk of morbidity and mortality from RC can aid in the clinical decision-making process.
Strengths and limitations
While our study provides input for future studies and for clinical practice, several limitations exist. The large sample gives our study a high statistical power, and the variety of patients captured allows to better establish external validity. Unfortunately, the dataset lacks data relevant to RC in terms of variables specific to procedural details. This includes tumour characteristics and stage, which was shown to be associated with some of our outcomes in the literature. We overcame this limitation by including patients who only had a diagnosis of MIBC by ICD selection and excluding partial cystectomy candidates. Neoadjuvant chemotherapy’s effect on early postoperative morbidity and mortality as compared to placebo is still debated, and its absence is unlikely to be a major limitation [40]. Our analysis was limited to 30 days after surgery, which is below the standard follow-up period for RC which is 90 days.
Finally, we anticipate that our models can be of clinical use given the variables used that can be easily assessed prior to surgery. This risk predictor can aid in individualising patient care and in including patients in the decision-making process of their own healthcare management.
Conclusion
In this study, we developed a clinically relevant tool to risk-stratify patients with nonmetastatic MIBC based on pre-existing comorbidities for whom RC is being considered. For patients who are identified as being at high-risk of complications from RC, counselling regarding alternative bladder preserving strategies should be recommended.
Acknowledgments and funding
Research reported in this publication was supported by the Fogarty International Center and Office of Dietary Supplements of the National Institutes of Health under Award Number D43 TW009118. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The author Mohamad Ali Tfaily would like to acknowledge the training received under the Scholars in HeAlth Research Program (SHARP) that set the required foundations for a career in clinical and translational research. This study has been part of the thesis project of the first author’s degree in SHARP, submitted to the American University of Beirut’s libraries. The authors would also like to acknowledge Dr Martine Elbejjani at the Clinical Research Institute of the American University of Beirut for her thoughtful comments and input in this project.
Conflicts of interest
The authors declare no conflicts of interest.
References
1. Globocan (2020) Bladder Cancer Report (France: Institutional Agency for Research on Cancer)
2. Zippe CD, Raina R, and Massanyi EZ, et al (2004) Sexual function after male radical cystectomy in a sexually active population Urology 64(4) 682–685 https://doi.org/10.1016/j.urology.2004.05.056 PMID: 15491700
3. Park JC, Citrin DE, and Agarwal PK, et al (2014) Multimodal management of muscle-invasive bladder cancer Curr Probl Cancer 38(3) 80–108 https://doi.org/10.1016/j.currproblcancer.2014.06.001 PMID: 25087173 PMCID: 4190161
4. Chang SS, Bochner BH, and Chou R, et al (2017) Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO Guideline J Urol 198(3) 552–559 https://doi.org/10.1016/j.juro.2017.04.086 PMID: 28456635 PMCID: 5626446
5. Huddart RA, Hall E, and Lewis R, et al (2010) Life and death of spare (selective bladder preservation against radical excision): reflections on why the spare trial closed BJU Int 106(6) 753–755 https://doi.org/10.1111/j.1464-410X.2010.09537.x PMID: 20707796
6. Abdollah F, Sun M, and Schmitges J, et al (2012) Development and validation of a reference table for prediction of postoperative mortality rate in patients treated with radical cystectomy: a population-based study Ann Surg Oncol 19(1) 309–317 https://doi.org/10.1245/s10434-011-1852-7
7. Giacalone NJ, Shipley WU, and Clayman RH, et al (2017) Long-term outcomes after bladder-preserving tri-modality therapy for patients with muscle-invasive bladder cancer: an updated analysis of the Massachusetts general hospital experience Eur Urol 71(6) 952–960 https://doi.org/10.1016/j.eururo.2016.12.020 PMID: 28081860
8. Halabi IE, Husseini ZE, and Haibe Y, et al (2020) Cystectomy vs. bladder preservation after neoadjuvant chemotherapy in muscle-invasive bladder cancer: a tertiary medical center experience Cancer Treat Res Commun 25 100222. https://doi.org/10.1016/j.ctarc.2020.100222 PMID: 33080450
9. Royce TJ, Feldman AS, and Mossanen M, et al (2019) Comparative effectiveness of bladder-preserving tri-modality therapy versus radical cystectomy for muscle-invasive bladder cancer Clin Genitourin Cancer 17(1) 23–31 https://doi.org/10.1016/j.clgc.2018.09.023
10. Williams SB, Shan Y, and Ray-Zack MD, et al (2019) Comparison of costs of radical cystectomy vs trimodal therapy for patients with localized muscle-invasive bladder cancer JAMA Surg 154(8) e191629 https://doi.org/10.1001/jamasurg.2019.1629 PMID: 31166593 PMCID: 6551585
11. Fahmy O, Khairul-Asri MG, and Schubert T, et al (2018) A systematic review and meta-analysis on the oncological long-term outcomes after trimodality therapy and radical cystectomy with or without neoadjuvant chemotherapy for muscle-invasive bladder cancer Urol Oncol Semin Orig Investig 36(2) 43–53
12. Winquist E and Booth CM (2020) Trimodality therapy for muscle-invasive bladder cancer: concurrent chemotherapy is not enough J Clin Oncol 38(24) 2709–2711 https://doi.org/10.1200/JCO.19.02959 PMID: 32459596
13. Khuri SF, Henderson WG, and Daley J, et al (2007) The patient safety in surgery study: background, study design, and patient populations J Am Coll Surg 204(6) 1089–1102 https://doi.org/10.1016/j.jamcollsurg.2007.03.028 PMID: 17544068
14. Gregg JR, Cookson MS, and Phillips S, et al (2011) Effect of preoperative nutritional deficiency on mortality after radical cystectomy for bladder cancer J Urol 185(1) 90–96 https://doi.org/10.1016/j.juro.2010.09.021 PMCID: 3049248
15. Subramaniam S, Aalberg JJ, and Soriano RP, et al (2018) New 5-factor modified frailty index using American college of surgeons NSQIP data J Am Coll Surg 226(2) 173–181 https://doi.org/10.1016/j.jamcollsurg.2017.11.005
16. Xia L, Taylor BL, and Newton AD, et al (2018) Early discharge and post-discharge outcomes in patients undergoing radical cystectomy for bladder cancer BJU Int 121(4) 583–591 https://doi.org/10.1111/bju.14058
17. Massarweh NN, Legner VJ, and Symons RG, et al (2009) Impact of advancing age on abdominal surgical outcomes Arch Surg 144(12) 1108–1114 https://doi.org/10.1001/archsurg.2009.204 PMID: 20026827
18. Djaladat H, Bruins HM, and Miranda G, et al (2014) The association of preoperative serum albumin level and American society of anesthesiologists (ASA) score on early complications and survival of patients undergoing radical cystectomy for urothelial bladder cancer BJU Int 113(6) 887–893 https://doi.org/10.1111/bju.12240
19. Zhang X, Tang T, and Pang L, et al (2019) Malnutrition and overall survival in older adults with cancer: a systematic review and meta-analysis J Geriatr Oncol 10(6) 874–883 https://doi.org/10.1016/j.jgo.2019.03.002 PMID: 30917937
20. Hackett NJ, De Oliveira GS, and Jain UK, et al (2015) ASA class is a reliable independent predictor of medical complications and mortality following surgery Int J Surg 18 184–190 https://doi.org/10.1016/j.ijsu.2015.04.079 PMID: 25937154
21. Davenport DL, Bowe EA, and Henderson WG, et al (2006) National surgical quality improvement program (NSQIP) risk factors can be used to validate American society of anesthesiologists physical status classification (ASA PS) levels Ann Surg 243(5) 636–644 https://doi.org/10.1097/01.sla.0000216508.95556.cc PMID: 16632998 PMCID: 1570549
22. Ornaghi PI, Afferi L, and Antonelli A, et al (2020) Frailty impact on postoperative complications and early mortality rates in patients undergoing radical cystectomy for bladder cancer: a systematic review Arab J Urol 19(1) 9–23 https://doi.org/10.1080/2090598X.2020.1841538
23. Mannas MP, Lee T, and Forbes CM, et al (2020) Predicting complications following radical cystectomy with the ACS NSQIP universal surgical risk calculator World J Urol 38(5) 1215–1220 https://doi.org/10.1007/s00345-019-02915-3
24. Aziz A, May M, and Burger M, et al (2014) Prediction of 90-day mortality after radical cystectomy for bladder cancer in a prospective European multicenter cohort Eur Urol 66(1) 156–163 https://doi.org/10.1016/j.eururo.2013.12.018 PMID: 24388438
25. Isbarn H, Jeldres C, and Zini L, et al (2009) A population based assessment of perioperative mortality after cystectomy for bladder cancer J Urol 182(1) 70–77 https://doi.org/10.1016/j.juro.2009.02.120 PMID: 19447427
26. Taylor JM, Feifer A, and Savage CJ, et al (2012) Evaluating the utility of a preoperative nomogram for predicting 90-day mortality following radical cystectomy for bladder cancer BJU Int 109(6) 855–889 https://doi.org/10.1111/j.1464-410X.2011.10391.x
27. Morgan TM, Keegan KA, and Barocas DA, et al (2011) Predicting the probability of 90-day survival of elderly patients with bladder cancer treated with radical cystectomy J Urol 186(3) 829–834 https://doi.org/10.1016/j.juro.2011.04.089 PMID: 21788035 PMCID: 6472701
28. Mannas MP, Lee T, and Forbes CM, et al (2020) Predicting complications following radical cystectomy with the ACS NSQIP universal surgical risk calculator World J Urol 38(5) 1215–1220 https://doi.org/10.1007/s00345-019-02915-3
29. Meng X, Press B, and Renson A, et al (2018) Discriminative ability of commonly used indexes to predict adverse outcomes after radical cystectomy: comparison of demographic data, American society of anesthesiologists, modified charlson comorbidity index, and modified frailty index Clin Genitourin Cancer 16(4) e843–e850 https://doi.org/10.1016/j.clgc.2018.02.009 PMID: 29550199
30. Sathianathen NJ, Jarosek S, and Lawrentschuk N, et al (2019) A simplified frailty index to predict outcomes after radical cystectomy Eur Urol Focus 5(4) 658–663 https://doi.org/10.1016/j.euf.2017.12.011
31. Golan S, Adamsky MA, and Johnson SC, et al (2018) National surgical quality improvement program surgical risk calculator poorly predicts complications in patients undergoing radical cystectomy with urinary diversion Urol Oncol Sem Orig Investig 36(2) 77
32. Taylor J, Meng X, and Renson A, et al (2019) Different models for prediction of radical cystectomy postoperative complications and care pathways Ther Adv Urol 11 1756287219875587 https://doi.org/10.1177/1756287219875587 PMID: 31565072 PMCID: 6755632
33. Parikh NI, Pencina MJ, and Wang TJ, et al (2008) A risk score for predicting near-term incidence of hypertension: the Framingham heart study Ann Int Med 148(2) 102–110 https://doi.org/10.7326/0003-4819-148-2-200801150-00005 PMID: 18195335
34. Tice JA, Cummings SR, and Smith-Bindman R, et al (2008) Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model Ann Int Med 148(5) 337–347 https://doi.org/10.7326/0003-4819-148-5-200803040-00004 PMID: 18316752 PMCID: 2674327
35. Lauer MS, Pothier CE, and Magid DJ, et al (2007) An externally validated model for predicting long-term survival after exercise treadmill testing in patients with suspected coronary artery disease and a normal electrocardiogram Ann Int Med 147(12) 821–828 https://doi.org/10.7326/0003-4819-147-12-200712180-00001 PMID: 18087052
36. Cook NR, Buring JE, and Ridker PM (2006) The effect of including C-reactive protein in cardiovascular risk prediction models for women Ann Int Med 145(1) 21–29 https://doi.org/10.7326/0003-4819-145-1-200607040-00128 PMID: 16818925
37. van der Steeg WA, Boekholdt SM, and Stein EA, et al (2007) Role of the apolipoprotein B–apolipoprotein AI ratio in cardiovascular risk assessment: a case–control analysis in EPIC-Norfolk Ann Int Med 146(9) 640–648 https://doi.org/10.7326/0003-4819-146-9-200705010-00007 PMID: 17470832
38. Janes H, Pepe MS, and Gu W (2008) Assessing the value of risk predictions by using risk stratification tables Ann Int Med 149(10) 751–760 https://doi.org/10.7326/0003-4819-149-10-200811180-00009 PMID: 19017593 PMCID: 3091826
39. Meguid RA, Bronsert MR, and Juarez-Colunga E, et al (2016) Surgical risk preoperative assessment system (SURPAS): III. accurate preoperative prediction of 8 adverse outcomes using 8 predictor variables Ann Surg 264(1) 23–31 https://doi.org/10.1097/SLA.0000000000001678 PMID: 26928465
40. Gandaglia G, Popa I, and Abdollah F, et al (2014) The effect of neoadjuvant chemotherapy on perioperative outcomes in patients who have bladder cancer treated with radical cystectomy: a population-based study Eur Urol 66(3) 561–568 https://doi.org/10.1016/j.eururo.2014.01.014 PMID: 24486024
Appendix
Appendix Table 1. Linear regression model used for imputation of missing values for albumin.
Appendix Table 2. Table of the risk calculator for mortality.
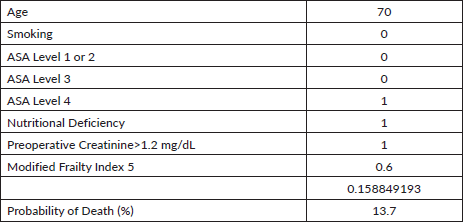
Appendix Table 3. Table of the risk calculator for morbidity.