PDL1 expression and its correlation with outcomes in non-metastatic triple-negative breast cancer (TNBC)
Joydeep Ghosh1, Meheli Chatterjee1, Sandip Ganguly1, Anupurva Datta2, Bivas Biswas1, Geetashree Mukherjee2, Sanjit Agarwal3, Rosina Ahmed3, Sanjoy Chatterjee4 and Deepak Dabkara1
1Department of Medical Oncology, Tata Medical Center, 14 MAR (E-W), New Town, Rajarhat, Kolkata, West Bengal 700156, India
2Department of Pathology, Tata Medical Center, 14 MAR (E-W), New Town, Rajarhat, Kolkata, West Bengal 700156, India
3Department of Breast Oncology, Tata Medical Center, 14 MAR (E-W), New Town, Rajarhat, Kolkata, West Bengal 700156, India
4Department of Radiation Oncology, Tata Medical Center, 14 MAR (E-W), New Town, Rajarhat, Kolkata, West Bengal 700156, India
Abstract
Purpose: Triple-negative breast cancer (TNBC) has a poor outcome compared to other subtypes, even in those with early disease. Immune checkpoint inhibitors (ICIs) have been approved in metastatic diseases and are being tested as a neoadjuvant strategy also. The response to ICIs is largely determined by the programmed death ligand 1 (PDL1) score, which also acts as a prognostic marker for outcomes. Here, we report the proportion of PDL1 expression in non-metastatic TNBC and its correlation with response to chemotherapy and outcomes.
Methods: We included all patients who had non-metastatic TNBC treated with neoadjuvant chemotherapy, followed by surgery with/without adjuvant radiotherapy between September 2011 and November 2017. PDL1 testing was carried out on pre-treatment tumour cells with immunohistochemistry (Ventana SP142) and was correlated with pathological response, relapse-free survival (RFS) and overall survival (OS). PDL1 staining was interpreted as negative or positive (more than 1% staining).
Results: A total of 107 patients were included for analysis with a median age of 47 years (28–65 yrs). The PDL1 expression of more than 1% was seen in 31 (28.97%) patients. After a median follow-up of 55 months (range: 4–93 months), median RFS and OS were not reached. PDL1 expression did not affect the achievement of pathological complete response (pCR). However, PDL1 expression improved OS (p = 0.016) and trend towards RFS (p = 0.05). Patients who achieved pCR had better RFS and OC compared to those who did not.
Conclusion: Our study shows PDL1 expression in 29% of the cases. PDL1 expression leads to better RFS and OS. Also, pCR improves survival.
Keywords: PDL1, early breast cancer, triple negative
Correspondence to: Sandip Ganguly
Email: dr.babumashai@gmail.com
Published: 06/04/2021
Received: 20/11/2020
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abbreviations
PDL1: programmed death ligand 1, TNBC: triple-negative breast cancer, FEC-T: 5-fluorouracil, epirubicin, cyclophosphamide and docetaxel, EC: epirubicin and cyclophosphamide, AC: adriamycin and cyclophosphamide, FEC: 5-fluorouracil, epirubicin and cyclophosphamide, FAC: 5-fluorouracil, adriamycin and cyclophosphamide, NACT: neoadjuvant chemotherapy, pCR: pathological complete response , NIH: National Institute of Health, PARP: poly(ADP-ribose)polymerase, RFS: relapse-free survival, OS: overall survival, BCS: breast conservation surgery, AD: axillary dissection, NLR: neutrophil lymphocyte ratio, PLR: platelet lymphocyte ratio, HER2: human epidermal growth factor receptor 2, IQR: interquartile range.
Introduction
Triple-negative breast cancer (TNBC) is characterised by the absence of oestrogen receptors, progesterone receptors, and human epidermal growth factor receptor 2 (HER2) overexpression, usually in combination with a high proliferation index. It occurs more often in young women and patients with the BRCA1 mutation [1] (immune checkpoint blockade in patients with triple‑negative breast cancer) [2]. TNBC comprises 15%–20% of the total breast cancer cases globally [3]. Data from automated immunohistochemistry (IHC) analysis centres in India have reported a lower incidence (11%–12%) of TNBC subtype [4]. The outcomes of TNBC are far inferior to other subtypes [5, 6], despite having a higher response rate after neoadjuvant chemotherapy (NACT) [7, 8]. The response rate is further improved by the addition of carboplatin and recently poly(ADP-ribose)polymerase (PARP) inhibitors compared to standard anthracyclines and taxanes [9, 10]. Pathological complete responses (pCR) of TNBC correlate well with better relapse-free survival (RFS) and overall survival (OS) [11]. Moreover, tumours with a high degree of infiltrating lymphocytes (TILs) have a higher probability of achieving a pCR [12]. Immune checkpoint inhibitors (ICIs) such as atezolizumab have shown better outcomes when combined with chemotherapy in metastatic TNBC, more so in the programmed death ligand 1 (PDL1)-positive subset [13, 14]. Pembrolizumab, another ICI, has also been studied in the neoadjuvant setting and has showed significantly higher pCR [15]. All these studies have shown that ICIs’ benefit is more pronounced with higher PDL1 expression compared to those who had PDL1-negative tumours. Patients who did not have PDL1 expression also benefitted [13, 15]. PDL1 positivity in patients with TNBC is around 50% and is associated with better RFS [16]. The above-mentioned studies underline the importance of carrying out PDL1 for both prognostic and predictive factors. Studies in breast cancer have shown that there is a correlation between neutrophil lymphocyte ratio (NLR) and platelet lymphocyte ratio with long-term outcomes [17–20]. There is no data on the proportion of PDL1 expression in non-metastatic TNBC in the Indian subcontinent, and here we have analysed the PDL1 expression in those patients who received NACT, and also correlated it with pCR and survival outcomes.
Aims
The primary aim of this study was to look at the proportion of non-metastatic TNBCs which express PDL1 in their tumour cells. The secondary aim was to look at the correlation of PDL1 with response (pCR) to NACT and its effect on long-term outcomes, the effect of pCR, neutrophil lymphocyte ratio and platelet lymphocyte ratio on pathological response and outcomes. The endpoints for measuring response are the presence or absence of pCR and for long-term outcomes are RFS and OS.
Methods
Patient information
A consecutive series of patients was identified from retrospective analysis of pathology reports from electronic medical records (EMR). All those who had a diagnosis of non-metastatic TNBC who received NACT and then underwent breast surgery in our institution were included from June 2012 to June 2017. After initial histopathological confirmation and metastatic workup with contrast-enhanced computerised tomographic scan of thorax and whole abdomen and a bone scan, all of them received NACT containing anthracycline with/without taxane. After NACT, they underwent either mastectomy (MRM) or breast conservation surgery (BCS), and all of them had axillary dissection. All the cases were examined and reviewed by the breast pathology team. Pathological details including tumour size, nodal stage, grade, oestrogen receptor (OR), progesterone receptor (PR), and HER-2 score, tumour margin status and Ki-67 index were noted. The proportion of pCR, baseline neutrophil, lymphocyte count and platelet count was recorded. The median cut-off value for high NLR was taken as 3.0 and for platelet lymphocyte ratio (PLR) it was 185 based on a published meta-analysis
PDL1 testing
The PDL1 expression in the tissue was studied in tumour cells using immunohistochemistry. PDL1 testing was carried out in tumour cells and not the immune cells because at the time when this study was conducted there was no clear guideline on the role of immune cell PDL1 positivity or specific scoring methodology in breast cancer. Consecutive full-face 5-µm formalin-fixed paraffin-embedded sections were stained with PDL1 antibody (Ventana SP142) using the standard and validated protocol [21, 22]. Citrate low pH antigen retrieval was used for 20 minutes with the Bond III. PDL1 expression was assessed only in pre-therapeutic core needle biopsy specimens. All stained slides of pre-treatment biopsy were reviewed and scored by a single pathologist. PDL1 staining was evaluated in the tumour cells of the initial specimen of core biopsy before any chemotherapy, and not the surgical specimen. PDL1 staining 1% or more was considered positive. The PDL1 status was correlated with pCR, RFS and OS.
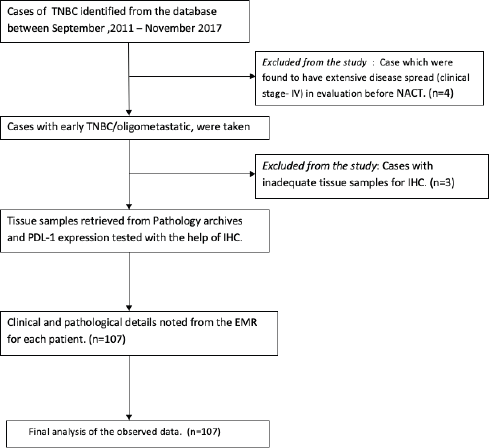
Figure 1. Consort diagram.
Statistical analysis
Descriptive analysis was used for baseline clinico-pathologic features. OS was defined as the length of the time interval from the date of surgery to the date of the last contact or death. Relapse-free survival (RFS) was defined as the time interval from the date of surgery to the date of diagnosis of local and/or distant recurrence. RFS included both distant recurrences (metastasis to other organs not including regional lymph nodes) and local recurrences. The correlation between PDL1 expression and pCR with baseline parameters was assessed by univariate regression. Those that were found to be significant were considered for multivariate analysis. The survival was estimated with the Kaplan–Meier method. The predictors of survival were analysed by the Cox proportional model. Log rank tests were used to calculate the significance between survival functions. STATA (version 14.1, StataCorp, 4905 Lakeway Dr College Station, TX 77845) statistical software was used for statistical analysis [23].
Results
Clinico-pathologic features
A total of 107 patients were enrolled with a median age of 47 years (range: 28–65 years). Baseline clinico-pathologic features are mentioned in Table 1. Postmenopausal were 59 (55.14%) patients. Clinically relevant family history was seen in seven (6.6%) patients. Clinical stage distribution as per AJCC was Stage I = 1 (0.9%), Stage II = 38 (35.51%) and Stage III = 68 (63.55%). The median NLR was 2.85 (interquartile range (IQR): 2.15–4.22) and median PLR was 128 (IQR: 79.84–165.61). All the six cycles of 5-flurouracil, epirubicin, cyclophosphamide and docetaxel (FEC-T) regimen was given to 98 (91.6%) patients. Breast conservation surgery (BCS) was carried out in 52 (48.6%) patients. All patients received adjuvant radiotherapy. The mean number of nodes dissected was 21 (range: 1–37), and the mean number of nodes which were positive were 2 (range: 0–31).
Pathological complete response was observed in 35 (32.71%) patients. PDL1 expression more than 1% was seen in 31 (28.97%) patients, negative in 68 (63.55%) patients and non-interpretable in 8 (7.48%) patients.
Response to chemotherapy
On univariate analysis, only the type of surgery (BCS versus MRM) correlated significantly with pCR (p < 0.001), with a greater number of patients in the BCS group who had pCR, as shown in Table 2. No other factors had a significant correlation with PDL1 expression, clinical stage, menopausal status, number of NACT cycles, NLR or PLR. On multivariate analysis, the statistical significance of the type of surgery (BCS versus MRM) persisted (p = 0.02), favouring BCS (Table 3).
Survival outcome and prognostic features
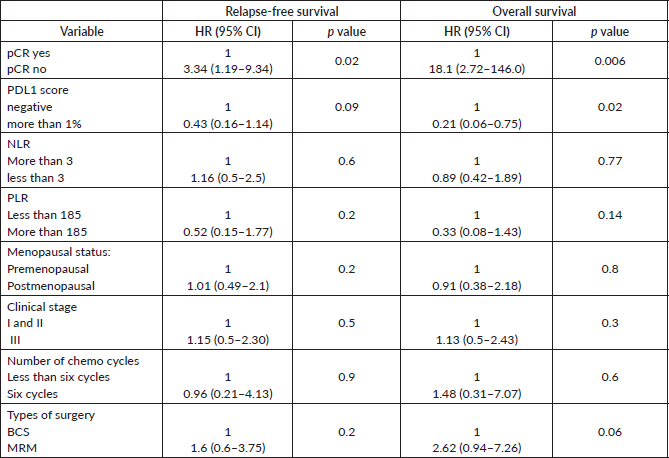
The median follow-up of patients included in this study was 55 months (range: 4–93 months). The median RFS and OS were not reached in this period (Figures 2 and 3). The achievement of pCR statistically and significantly improved the RFS (HR: 3.73, 95% CI: 1.44–9.63, p = 0.006) and the OS (HR = 16.46, 95% CI: 2.23–121.0, p = 0.006) compared to those who did not achieve pCR (Figures 4 and 5). The expression of PDL1 showed a trend towards improvement in RFS (HR: 0.41, 95% CI: 0.16–1.01, p = 0.055), but with OS, it was statistically significant (HR: 0.24, 95% CI: 0.07–0.82, p = 0.02) (Figures 6 and 7). Cox proportional-hazards for RFS and OS is shown in Table 4. None of the baseline parameters other than PDL1 and pCR had any significant impact on the RFS and OS. The differences between RFS and OS based on the NLR and PLR were non-significant (not reached in any group, HR: 0.78, 95% CI: 0.37–1.67, p = 0.527).
Table 1. Baseline characteristics.
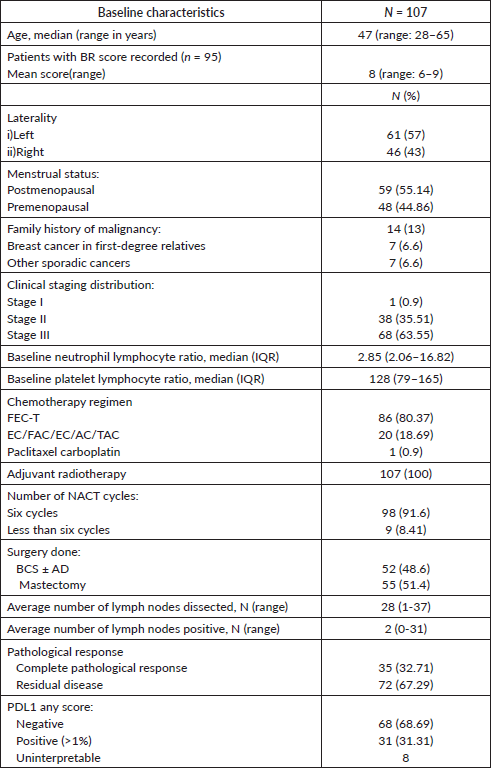
Table 2. Univariate logistic regression of pCR with baseline clinico-pathological parameters.
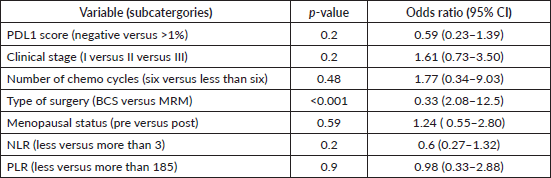
Table 3. Multivariate logistic regression of pCR with baseline clinico-pathological parameters.
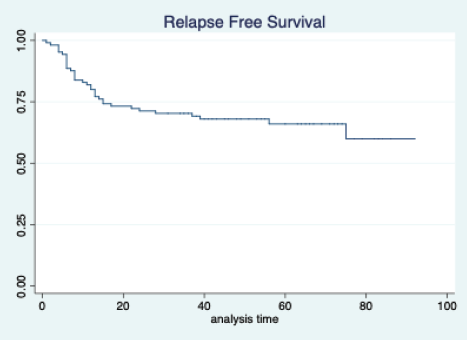
Figure 2. Relapse free survival of all patients.
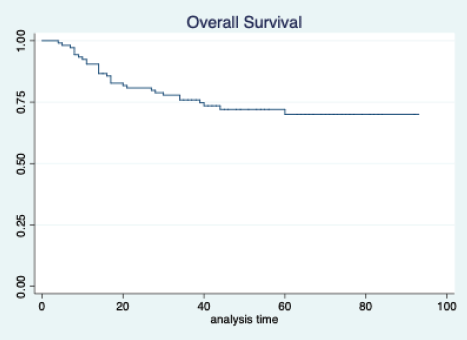
Figure 3. Overall survival of all patients.
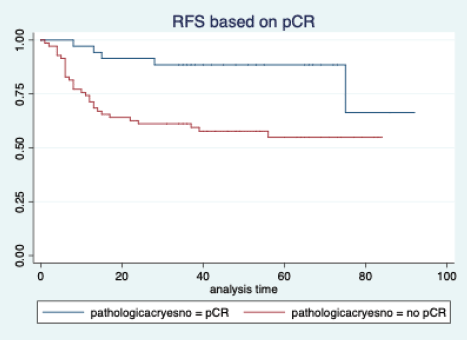
Figure 4. Relapse free survival based on pathological complete response.
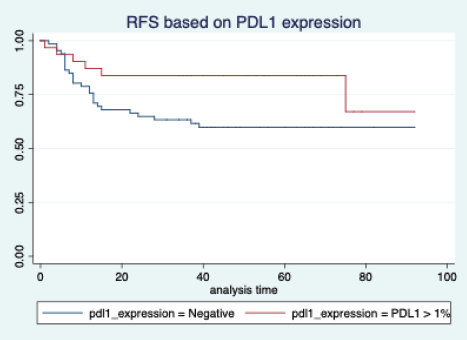
Figure 5. Relapse free survival based on PDL1 expression.
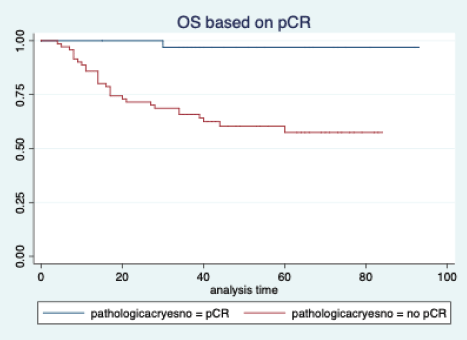
Figure 6. Overall survival based on pathological complete response.
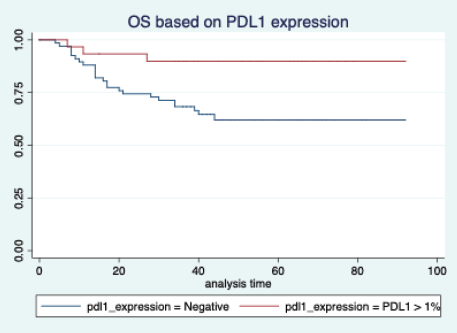
Figure 7. Overall survival based on PDL1 expression.
Table 4. Cox proportional-hazards for survival outcomes.
Discussion
Our study contributes to the ongoing knowledge about the role of PDL1 affecting the outcomes of TNBC when they are treated with chemotherapy. High PDL1 may allow room for chemo de-escalation studies with addition of immune-oncology agents.
The median age of our patient population was 47 years. This is in contrast with the median age for breast cancer diagnosis in the Western population, which is 61 years, as per NIH data [24]. The median age of Indian breast cancer patients has been reported to be 50–53 years in various population-based studies carried out in different parts of the country [25]. The patient population in this study closely represents our Indian population data. Moreover, TNBC tends to present at a younger age and has been shown to be more aggressive [26].
Around 60% of our patients were in stage III. This is because, for the purposes of this study, we selected only those patients who received NACT, followed by surgery, and also the fact that patients in India have a higher stage at presentation [27]. The finding of a higher number of pCR in those who underwent BCS is explained by selection bias, as patients who had a very good clinical response were considered for BCS. So, there is a higher chance of finding a pCR in BCS patients.
In our study, tumour PDL1 expression was seen in 31% of the patients. In the published literature, the rate has varied from 40% to 50% [28, 29]. In another study, 50% of stromal cells had some PDL1 positivity
If we analyse the role of PDL1 expression with pCR, the relationship between PDL1 expression and pCR is not clearly defined in the literature. In one study, PDL1 expression was associated with an inferior response rate [33]. However, it was a heterogeneous population of all subtypes and not specific for TNBC. In our study, the achievement of pCR did not correlate with the PDL1 expression. One possible explanation for this may be the fact that we used a standard chemotherapy regimen and did not use ICIs. It is possible that the addition of ICIs with chemotherapy might have increased the pCR rate in those who have a higher PDL1 expression.
However, if we look at the survival outcomes, both the RFS and OS were significantly better in patients who achieved a pCR. This is consistent with the well-established fact that the achievement of pCR is a strong surrogate marker for improved survival in breast cancer [11, 32]. We do agree that this is a retrospective study and the groups may not be comparable, unlike a properly conducted prospective randomised trial.
The PDL1 expression also showed a correlation with survival. For RFS, the improvement was in the outcome of borderline significance, but for OS, it was highly significant. Many other studies have also shown that PDL1 expression is associated with favourable outcomes
Taking the standard cut-off for NLR and PLR, our study did not show any difference in the outcome. This is in contrast to other retrospective studies where a higher NLR and PLR predicted inferior outcomes [17, 19, 20]. So, we feel the interplay of PDL1 expression and outcome is an evolving area, and larger prospective data are required to reach a conclusion. Moreover, there can be regional differences in the median values of NLR and PLR [37].
There are few limitations of this study. Firstly, it has a small sample size, and larger numbers are needed to extrapolate the data to the population. Secondly, this is a single centre retrospective analysis. So, a selection bias is a possibility.
Conclusion
Overall, this study is one of the first to look at a very homogenous population of TNBC who have received standard NACT and evaluated the outcomes and response rates based on the PDL1 status. Our study proves that PDL1 expression may not correlate the response rate to NACT, but its expression may improve long-term outcomes. This study also reconfirms the fact that the achievement of pCR has a significant positive impact on the long-term survival for TNBC patients.
Authors’ contributions
Conceptualisation: JG, SC, SG and RA; Methodology: JG and AD; Design of the study: JG, SC, SG and RA; Data acquisition: MD, GM and JG; Validation: AD and GM; Data analysis: JG, BB and SG; Writing: JG, MC, BB and GM.
Compliance with ethical standards
Funding
This study was funded by the research grant from Indian Society of Medical and Paediatric Oncology (ISMPO) 2016 for best concept ward in ISMPOCON 2016, New Delhi, India
Conflicts of interest
Author JG has received a research grant from the Indian Society of Medical and Paediatric Oncology Conference 2016 as a prize for the best research concept award, for conducting this study. The rest of the authors have declared no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Waiver of consent was obtained from the IRB as this was a retrospective study on tissue blocks (vide: EC/TMC/86/17).
References
1. Turner N, Tutt A, and Ashworth A (2004) Hallmarks of “BRCAness” in sporadic cancers Nat Rev Cancer 4 814–819 https://doi.org/10.1038/nrc1457 PMID: 15510162
2. Michel LL, von Au A, and Mavratzas A, et al (2020) Immune checkpoint blockade in patients with triple-negative breast cancer Target Oncol 15 415–428 https://doi.org/10.1007/s11523-020-00730-0 PMID: 32514907
3. Bauer KR, Brown M, and Cress RD, et al (2007) Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype Cancer 109 1721–1728 https://doi.org/10.1002/cncr.22618 PMID: 17387718
4. Chatterjee S, Arun I, and Agrawal S, et al (2016) Immunohistochemistry heterogeneity in reported breast cancer demographics from India: triple-negative breast cancer rates could be lower than suggested in pooled meta-analysis J Glob Oncol 3 180–181 https://doi.org/10.1200/JGO.2016.006635
5. Dent R, Trudeau M, and Pritchard KI, et al (2007) Triple-negative breast cancer: clinical features and patterns of recurrence Clin Cancer Res 13 4429–4434 https://doi.org/10.1158/1078-0432.CCR-06-3045 PMID: 17671126
6. Wang J, Xie X, and Wang X, et al (2013) Locoregional and distant recurrences after breast conserving therapy in patients with triple-negative breast cancer: a meta-analysis Surg Oncol 22 247–255 https://doi.org/10.1016/j.suronc.2013.10.001 PMID: 24144808
7. Wu J, Li S, and Jia W, et al (2011) Response and prognosis of taxanes and anthracyclines neoadjuvant chemotherapy in patients with triple-negative breast cancer J Cancer Res Clin Oncol 137 1505 https://doi.org/10.1007/s00432-011-1029-6 PMID: 21830158
8. Liedtke C, Mazouni C, and Hess KR, et al (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer J Clin Oncol Off J Am Soc Clin Oncol 26 1275–1281 https://doi.org/10.1200/JCO.2007.14.4147
9. Loibl S, O’Shaughnessy J, and Untch M, et al (2018) Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial Lancet Oncol 19 497–509 https://doi.org/10.1016/S1470-2045(18)30111-6 PMID: 29501363
10. von Minckwitz G, Schneeweiss A, and Loibl S, et al (2014) Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial Lancet Oncol 15 747–756 https://doi.org/10.1016/S1470-2045(14)70160-3 PMID: 24794243
11. Cortazar P, Zhang L, and Untch M, et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis Lancet Lond Engl 384 164–172 https://doi.org/10.1016/S0140-6736(13)62422-8
12. Denkert C, Minckwitz G von, and Darb-Esfahani S, et al (2018) Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy Lancet Oncol 19 40–50 https://doi.org/10.1016/S1470-2045(17)30904-X
13. Schmid P, Rugo HS, and Adams S, et al (2020) Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial Lancet Oncol 21 44–59 https://doi.org/10.1016/S1470-2045(19)30689-8
14. Al-Jussani GN, Dabbagh TZ, and Al-Rimawi D, et al (2021) Expression of PD-L1 using SP142 CDx in triple negative breast cancer Ann Diagn Pathol 51 151703 https://doi.org/10.1016/j.anndiagpath.2021.151703 PMID: 33454500
15. Schmid P, Cortes J, and Pusztai L, et al (2020) Pembrolizumab for early triple-negative breast cancer N Engl J Med 382(9) 810-821 https://doi.org/10.1056/NEJMoa1910549 PMID: 32101663
16. Li X, Wetherilt CS, and Krishnamurti U, et al (2016) Stromal PD-L1 expression is associated with better disease-free survival in triple-negative breast cancer Am J Clin Pathol 146 496–502 https://doi.org/10.1093/ajcp/aqw134 PMID: 27686176
17. Bozkurt O, Karaca H, and Berk V, et al (2015) Predicting the role of the pretreatment neutrophil to lymphocyte ratio in the survival of early triple-negative breast cancer patients J BUON Off J Balk Union Oncol 20 1432–1439
18. Ethier JL, Desautels D, and Templeton A, et al (2017) Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis Breast Cancer Res 19 2 https://doi.org/10.1186/s13058-016-0794-1 PMID: 28057046 PMCID: 5217326
19. Patel DA, Xi J, and Luo J, et al. (2019) Neutrophil-to-lymphocyte ratio as a predictor of survival in patients with triple-negative breast cancer Breast Cancer Res Treat 174 443–452 https://doi.org/10.1007/s10549-018-05106-7 PMID: 30604000
20. Pistelli M, De Lisa M, and Ballatore Z, et al (2015) Pre-treatment neutrophil to lymphocyte ratio may be a useful tool in predicting survival in early triple negative breast cancer patients BMC Cancer 15 195 https://doi.org/10.1186/s12885-015-1204-2 PMID: 25884918 PMCID: 4428113
21. Adam J, Le Stang N, and Rouquette I, et al (2018) Multicenter harmonization study for PD-L1 IHC testing in non-small-cell lung cancer Ann Oncol Off J Eur Soc Med Oncol 29 953–958 https://doi.org/10.1093/annonc/mdy014
22. VENTANA PD-L1 (SP263) Assay (CE IVD). Diagnostics n.d. [https://diagnostics.roche.com/global/en/products/tests/ventana-pd-l1-_sp263-assay2.html] Data accessed 12/05/20
23. Stata|StataCorp LLC [https://www.stata.com/company/] Data accessed: 29/05/20.
24. Risk factors: age – national cancer institute 2015 [https://www.cancer.gov/about-cancer/causes-prevention/risk/age] Date accessed: 05/03/20
25. Consolidated report of the population based cancer registries 1990–1996 [http://ncdirindia.org/NCRP/Rep1/PBCR_1900_96.aspx] Data accessed: May 03/05/20
26. Hudis CA, and Gianni L. (2011) Triple-negative breast cancer: an unmet medical need Oncologist 16(Suppl 1) 1–11 https://doi.org/10.1634/theoncologist.2011-S1-01 PMID: 21278435
27. Ram Prabu MP, Raina V, and Shukla NK, et al (2011) A study of triple-negative breast cancer at a cancer institute in India J Clin Oncol 29 e11548 https://doi.org/10.1200/jco.2011.29.15_suppl.e11548
28. Beckers RK, Selinger CI, and Vilain R, et al (2016) Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome Histopathology 69 25–34 https://doi.org/10.1111/his.12904
29. Li X, Li M, and Lian Z, et al (2016) Prognostic role of programmed death ligand-1 expression in breast cancer: a systematic review and meta-analysis Target Oncol 11 753–761 https://doi.org/10.1007/s11523-016-0451-8 PMID: 27422273
30. Prat A, Fan C, and Fernández A, et al (2015) Response and survival of breast cancer intrinsic subtypes following multi-agent neoadjuvant chemotherapy BMC Med 13 303 https://doi.org/10.1186/s12916-015-0540-z PMID: 26684470 PMCID: 4683815
31. Masuda H, Baggerly KA, and Wang Y, et al (2013) Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes Clin Cancer Res Off J Am Assoc Cancer Res 19 5533–5540 https://doi.org/10.1158/1078-0432.CCR-13-0799
32. Houssami N, Macaskill P, and von Minckwitz G, et al (2012) Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy Eur J Cancer 48 3342–3354 https://doi.org/10.1016/j.ejca.2012.05.023 PMID: 22766518
33. Asano Y, Kashiwagi S, and Goto W, et al (2018) Prediction of treatment responses to neoadjuvant chemotherapy in triple-negative breast cancer by analysis of immune checkpoint protein expression J Transl Med 16(1) 87 https://doi.org/10.1186/s12967-018-1458-y PMID: 29615063 PMCID: 5883348
34. Emens LA, Cruz C, and Eder JP, et al (2019) Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study JAMA Oncol 5 74–82 https://doi.org/10.1001/jamaoncol.2018.4224 PMCID: 6439773
35. Mori H, Kubo M, and Yamaguchi R, et al (2017) The combination of PD-L1 expression and decreased tumor-infiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer Oncotarget 8 15584–15592 https://doi.org/10.18632/oncotarget.14698 PMID: 28107186 PMCID: 5362507
36. Wang K, Xu J, and Zhang T, et al (2016) Tumor-infiltrating lymphocytes in breast cancer predict the response to chemotherapy and survival outcome: ameta-analysis Oncotarget 7 44288–44298 https://doi.org/10.18632/oncotarget.9988 PMID: 27329588 PMCID: 5190096
37. Lin BD, Carnero-Montoro E, and Bell JT, et al (2017) 2SNP heritability and effects of genetic variants for neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio J Hum Genet 62 979–988 https://doi.org/10.1038/jhg.2017.76 PMID: 29066854 PMCID: 5669488






