Is there a role for rechallenge and reintroduction of anti-EGFR plus chemotherapy in later lines of therapy for metastatic colorectal carcinoma? A retrospective analysis
Amanda Karani1, Tiago Cordeiro Felismino1, Lara Diniz1, Mariana Petaccia Macedo2, Virgilio Souza e Silva1 and Celso Abdon Mello1
1Department of Medical Oncology, AC Camargo Cancer Center, Sao Paulo 01509-000, Brazil
2Department of Pathology, AC Camargo Cancer Center, Sao Paulo 01509-000, Brazil
ahttps://orcid.org/0000-0002-8315-1562
Abstract
Background: Mechanisms of resistance have been described during disease progression (PD) for patients under treatment with anti-EGFR plus chemotherapy (CT). The aim of our study was to evaluate efficacy of anti-EGFR rechallenge (ReCH) and reintroduction (ReIn) in metastatic colorectal cancer (mCRC).
Materials and methods: This is a retrospective analysis of patients with mCRC that previously received anti-EGFR CT and interrupted therapy due to PD in the ReCH group and other reasons in the ReIn group. We aimed to describe progression-free survival (PFS), overall survival (OS) and response rate (RR) after re-exposure and to evaluate prognostic factors associated with PFS.
Results: Sixty-eight patients met the inclusion criteria. The median follow-up after re-exposure was 39.3 months. ReCH was adopted in 25% and ReIn in 75%. The median anti-EGFR free interval was at 10.5 months. At re-exposure, the main CT regimen was FOLFIRI in 58.8%. Cetuximab and Panitumumab were used in 59 and 9 patients, respectively. mPFS for ReCH and ReIn was 3.3 × 8.4 months, respectively (p 0.001). The objective response rate for ReCH and ReIn was 18% and 52%, respectively. In univariate analysis, adverse prognostic factors related to PFS were: stable disease or PD at first anti-EGFR exposure (HR: 2.12, CI:1.20–3.74; p = 0.009); ReCH (HR: 3.44, CI:1.88–6.29, p < 0.0001); rechallenge at fourth or later lines (HR: 2.51, CI:1.49–4.23, p = 0.001); panitumumab use (HR: 2.26 CI:1.18–5.54, p = 0.017). In the multivariate model, only ReCH remained statistically significant (HR = 2.63, CI: 1.14–6.03, p = 0.022).
Conclusion: In our analysis, ReCH resulted in short PFS and low RR. However, reintroduction of anti-EGFR plus CT before complete resistance arose resulted in prolonged PFS. These data could be clinically useful to guide a treatment break due to side effects or patient decisions. Our data should be confirmed by larger and prospective trials.
Keywords: colorectal carcinoma, treatment, prognosis, anti-EGFR, rechallenge
Correspondence to: Celso Mello
Email: celso.mello@accamargo.org.br
Published: 13/07/2020
Received: 10/02/2020
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Colorectal cancer is a leading cause of cancer-related death [1]. Approximately 25% of all colon cancer patients will have advanced disease at diagnosis and almost 50% will eventually recur during the course of their disease [2]. Progress in systemic treatment seen in recent years has resulted in prolonged survival for patients with metastatic disease [2].
In the metastatic setting, EGFR pathway plays an important role driving cancer cell growth and survival [3]. Approximately 60% of metastatic colorectal cancer (mCRC) tumors develop mutations in the EGFR pathway (mainly KRAS/NRAS/BRAF) which will lead to a constitutively downstream activation and primary resistance to anti-EGFR agents [4]. On the other hand, the remaining 40% of all mCRC patients will derive benefit from these agents. Large phase 3 trials demonstrated that first-line anti-EGFR agents (cetuximab and panitumumab) plus standard chemotherapy (FOLFOX and FOLFIRI) induced objective responses in about 60% of patients. The median progression-free survival (PFS) was 10 months and the median overall survival (OS) surpassed 30 months [5–8].
However, two main issues will inexorably emerge with anti-EGFR agents: toxicity and acquired resistance. For instance, cutaneous toxicity might be an early side effect that significantly impacts quality of life and results in drug discontinuation [9]. Moreover, a series of acquired resistance mechanisms have been recently proposed that lead to progressive disease and consequently the need of alternative drugs [10].
In real-world care of patients with mCRC, toxicity and resistance are the main reasons for anti-EGFR discontinuation. The resection of all macroscopic diseases is also a common cause of suspension of these agents as clinical trials show a lack of benefit of these drugs in the “adjuvant” setting [11]. It could be argued that after an anti-EGFR free interval, toxicity is mitigated and resistant clones are reduced [12]. As there is still a paucity of effective treatment lines, re-challenging and reintroduction of previous drugs may become an important strategy (cancer treat review). We have previous shown that re-challenging patients with oxaliplatin containing regimen resulted in prolonged survival for a selected and heavily treated group of patients [13]. However, anti-EGFR rechallenge is currently under investigation. The aim of this study is to retrospectively evaluate the efficacy of anti-EGFR rechallenge and reintroduction after a previous exposure in real-word metastatic colon cancer patients.
Patients and methods
This is a single-centre, retrospective analysis that aimed to evaluate the efficacy of anti-EGFR rechallenge and reintroduction in real-world patients with metastatic colorectal cancer. The study was approved by the local ethics committee.
Inclusion criteria were: patients with histologically confirmed metastatic colorectal adenocarcinoma, KRAS or KRAS/NRAS wild-type (BRAF status was not mandatory), first treatment with anti-EGFR agent (cetuximab or panitumumab) plus chemotherapy for at least 3 months, re-exposure therapy after a minimum of 3 months since the last dose of anti-EGFR (a stop period), re-exposure to anti-EGFR plus chemotherapy duration for at least 2 months. Patients were grouped according to reason to discontinuation: group 1 due to progression disease or rechallenge (ReCH) and group 2 due to other reasons (toxicity, medical doctor preference or treatment holiday, metastasectomy) or reintroduction (ReIn)
Clinical data were retrospectively collected from medical records. Our primary endpoint was PFS, defined as the time from anti-EGFR re-exposure start until disease progression or death from any cause. Secondary endpoints were OS after anti-EGFR re-exposure, response rate by RECIST criteria [14] at the anti-EGFR re-exposure. We also aimed to evaluate clinical, pathological and treatment variables as prognostic factors during the anti-EGFR re-treatment.
Statistics
Survival curves were estimated using the Kaplan–Meier method and compared with log-rank test. Univariate and multivariate prognostic analysis for PFS were performed using the Cox regression method. Variables included in our model were: reason for first discontinuation (progressive disease × other reason); objective response at first anti-EGFR exposure (defined as complete response plus partial response); line of anti-EGFR re-exposure (second third × fourth later); antibody (cetuximab × panitubumab); metastasis sites (liver only x lung only × other) and anti-EGFR free interval (<6m × >6m). All variables considered statistically significant in the univariate analysis were included in the multivariate analysis. All tests were considered statistically significant with a two-sided p-value of <0.05. Statistical analysis was performed with IBM SPSS 20.
Results
From 2009 to 2017, we identified 68 patients who met our inclusion criteria. Clinical and pathologic characteristics are shown in Table 1. The median follow-up time from re-exposure was 39.3 months (95% CI: 26.7–51.9). The mean age was 56 years (29–85). Left-sided primary was found in 64 patients. Rechallenge was performed in 17 (25%); in the ReIn group, reasons for discontinuation anti-EGFR during first exposure was chemotherapy holiday in 23 (33.8%), metastasectomy in 12 (17.6%) and toxicity in 6 (8.8%). Anti-angiogenic therapy prior to re-exposure was employed in 40%. Median anti-EGFR free interval was 10.5 months (95% CI: 8.8–12.1) as shown in Table 2. Metastasectomy was frequent in this cohort of patients (61%). Liver, lung, peritoneum, combined liver lung other sites metastasectomies were performed in 76.1%, 11.9%, 4.7%, 7.1%, respectively (Table 1). As seen in Table 1, more patients were submitted to metastasectomy in the ReIn group as compared to Re-Ch (35 × 7 patients, respectively). Post re-exposure metastasectomy was not frequent and only 1 patient in the ReCH group and 2 patients in the ReIn group were submitted to liver metastasectomy after re-exposure. We found a relatively high number of second metastastasectomy in the ReIn as compared to the ReCH group (16 × 2 patients, respectively). However, only 1 patient in the ReIn group had second metastasectomy after re-exposure. All RAS status was known in 46% in the ReIn group and only KRAS wild type was seen in 3% in the ReCH group. During first treatment with anti-EGFR plus chemotherapy, 66.6% of patients in the ReIn group achieved PR as compared to only 31.3% of patients in the ReCH group.
At re-exposure, cetuximab and panitumumab were used in 86.8% and 13.2% of cases, respectively. No patient in the ReCH group used panitumumab in the re-exposure (Table 2). The main chemotherapy backbone was FOLFIRI in 58.8%. The median re-exposure line was the fourth. However, 72.6% of patients in the ReIn group were re-exposed in the second and third line and 70.6% in ReCH received in the fourth line.
At the time of data analysis, 64 events occurred. The median OS since the first line for the entire group was 70 months. After re-exposure, the median PFS was 6.6 months (95% CI: 5.1–8.0) and the median OS was 24.4 months (95% CI: 12.4–36.8) (Figure 1). The median PFS was 3.3 and 8.4 months for ReCH and ReIn, respectively (p < 0.001), as shown in Figure 2, and the median OS was 7.5 and 33.4 months for ReCH and ReIn, respectively (p = 0.005). For the ReIn group, median PFS for CR/PR × SD during first exposure was 8.4 × 4,9 months, respectively (p = 0.083), previous bevacizumab versus no bevacizumabe was 6.1 × 10.4 months, respectively (p = 0.082), interval for reintroduction <6 months × >6 months was 7.2 × 8.4 months (p = 0.083). For the ReCH group, no significant difference was seen in PFS according to these variables (response to previous anti-EGFR (p = 0.06), previous bevacizumab (p = 0.07) and interval for reintroduction < 6 months (p = 0.16). Response evaluations (Table 1) were available in 67 cases and were as follows: complete response 3%, partial response 40.3%, SD 41.8% and progressive disease 14.9%.
We further performed a second analysis excluding three patients who underwent metastasectomy (two in the ReIn group and one in ReCH group), and the mOS was 6.86 months (95% CI: 4.77–8.96) for rechallenge versus 33.47 (95% CI: 23.08–43.86) for reintroduction (p = 0.004) and mPF was 2.92 (95% CI: 1.70–4.14) for rechallenge versus 8.18 (95% CI: 5.02–11.33) for reintroduction (p < 0.0001).
Univariate analysis for PFS
In our univariate model (shown in Table 3), main prognostic factors were: progressive disease as reason for first anti-EGFR discontinuation (HR: 3.44, 95% CI 1.88–6.29, p < 0.0001); rechallenge at the fourth line or later lines (HR: 2.51, 95% CI 1.49–4.23, p = 0.001); panitumumab use (HR: 2.56, 95% CI 1.18–5.54, p = 0.017) and absence of clinical benefit at first anti-EGFR exposure (HR: 2.12, 95% CI 1.20–3.74, p = 0.009). Anti-EGFR free interval (p = 0.67) and sites of metastasis were not related to prognosis (p = 0.16).
Table 1. Patients’ characteristics.
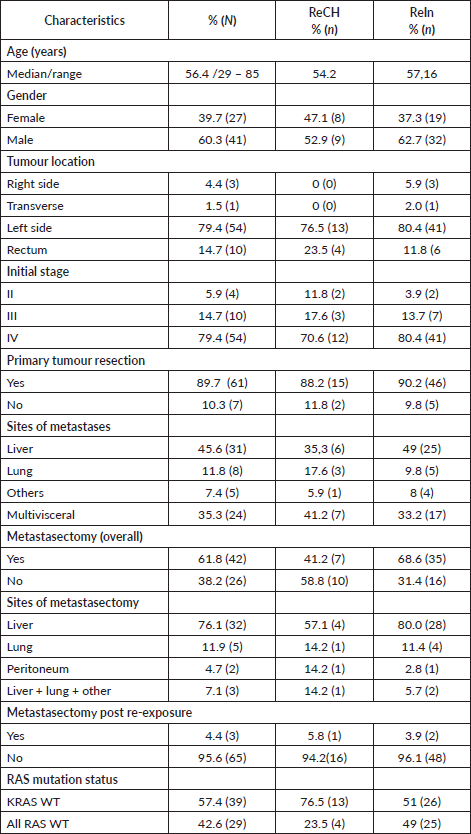
Table 2. Treatment characteristics for entire group and ReCH and ReIn group.
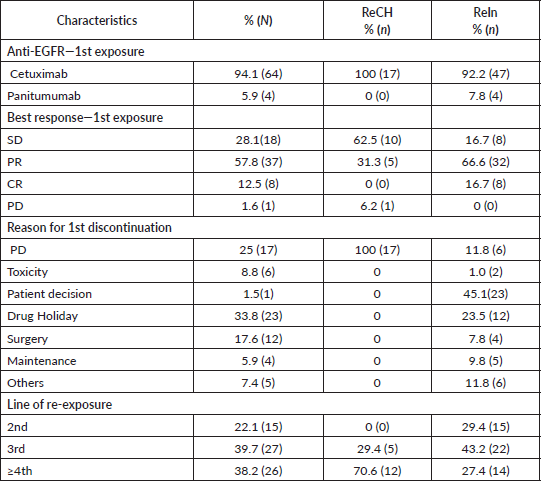
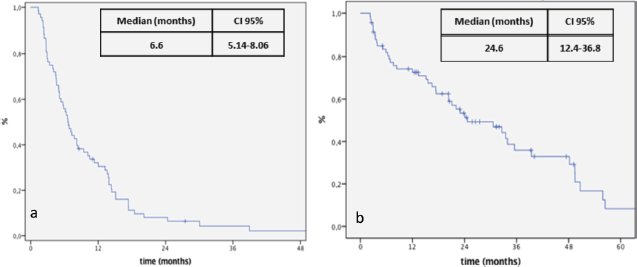
Figure 1. Kaplan-Meier curve for PFS (a) and OS (b) after re-exposure to anti-EGFR chemotherapy.
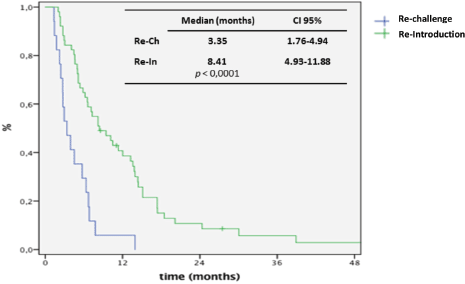
Figure 2. Kaplan-Meier curve for PFS for ReCH and ReIn.
Multivariate analysis for PSF
All statistically significant variables in the univariate analysis were included in the multivariate model (Table 3). PD as reason for first anti-EGFR discontinuation or ReCH remained statistically significant (HR: 2.63, 95% CI 1.14–6.03, p = 0.022). Rechallenge at the fourth line or later lines was marginally significant (HR: 1.18, 95% CI 0.99–3.32, p = 0.053).
Discussion
Rechallenge and reintroduction of previous agents is a relatively common practice in real-world oncology [15]. In the present study, we found that ReIN of anti-EGFR antibody plus chemotherapy resulted in prolonged survival. On the other hand, those patients that presented previous progression and were submitted to anti-EGFR plus chemotherapy did not seem to benefit from this strategy with a short PFS of only 3.3 months. By analysing the clinical and pathological variables of 68 re-treated patients, only the progression during previous anti-EGFR combination was associated with poor survival.
The OS of patients with mCRC has improved over the past years. The incorporation of anti-EGFR and anti-VEGF in the first and second line of treatment resulted in a median OS around 30 months [5–8; 16–20]. However, the median PFS time for the first line has been stable and is around 10 months in most of the trials. In the third line, prospective and randomised trials showed positive but modest benefit of regorafenib and tas-102 as compared to placebo [21, 22].
However, after the second and third line of therapy, a great number of patients still have a good performance status and demand for active treatment instead of best supportive care alone. As a result, re-challenging and re-introducing previous agents is a relatively common practice in the real world despite the lack of robust evidence demonstrating the efficacy of these strategies. Reintroduction is defined as re-administration of a previous agent to which tumour has not already proved to be resistant and discontinuation was not due to progression of disease (chemotherapy holliday, toxicity, metastasectomy, for example) and rechallenge is the reintroduction exposure of an agent in patients that developed resistance during prior use with progression of disease [15]. Oxaliplatin is commonly re-introducted in the third line or further lines in a group of patients that interrupted treatment due to stop-and-go strategy or due to limiting neuropathy [23]. We have previously shown that even for patients that presented disease progression during oxaliplatin-containing regimen can benefit from rechallenge [13]. The use of anti-angiogenic agents such as bevacizumab, aflibercept, ramucirumab after previous exposure and progression is supported by many prospective trials [18–20].
On the other hand, there are limited data evaluating the maintenance of anti-EGFR after disease progression [24, 25]. In this scenario, only a small phase 2 study (CAPRI—GOIM) showed small benefit in PFS in the maintenance of cetuximab associated with chemotherapy with no statistically significant benefit in OS [24]. Most of the guidelines do not recommend the maintenance of anti-EGFR inpatients with PD [2]. In the scenario of partial resistance, our data showed that patients that interrupted therapy with anti-EGFR before progression presented a prolonged PFS and OS. The response rate after reintroduction was relatively high (52% CR PR) as compared to first- and second-line trials with the combination of anti-EGFR and chemotherapy [5, 16, 17]. It becomes more relevant if we consider that our patients were heavily pre-treated, since anti-EGFR re-exposure occurred during and after the fourth line on average. Moreover, our data could generate the hypothesis that stopping anti-EGFR is safe and could mitigate side effects and improve quality of life, since the median PFS was 8.4 months and OS was 33.4 months.
In our anaylsis, our patient population was heavily treated with systemic therapy and almost 70% had metastasectmy performed. Definitely it is responsible for a long survival time since the diagnosis of metastasis. However, it is important to note that only 3 patients (1 in the ReCH group and 2 in the ReIn group) were submitted to metastasectomy after re-exposure. Additionally, we reviewed the metastasectomy procedures and we found that the proportion of patients undergoing metastasectomy was higher in the reintroduction group. Moreover, second metastasectomy was performed in a relatively large group of patients (16 patients). It indicates that these patients have more favourable behaviour than those submitted to rechallenge. However, this finding does not invalidate our analysis, since we aim of the study is to evaluate PFS after re-exposure and after re-exposure there were insignificant number of metastasectomy and there were relevant differences in terms of OS and PFS according the re-expouse strategy (ReIn or ReCH).
Part of the failure to anti-EGFR agents is due to the emergence of acquired resistance [26–28]. Many mechanisms involved with secondary resistance have been described and most of them involve the RAS-MEK-ERK pathway [27]. The clonal selection hypothesis is suggested as the main mechanism of acquired resistance [27, 29]. It is based on tumour heterogeneity and drug induced clonal selection. Briefly, we could hypothesise that anti-EGFR sensitive clones would be controlled during cetuximab/panitumumab use. After an initial response, RASwt clones begin to be depleted and resistant clones overcome, with consequent progressive disease [26]. It has been shown that refractory patients have higher levels of mutant RAS and EGFR circulating tumour DNA [29].
Despite all of these preliminary data about anti-EGFR resistance, there is limited clinical evidence showing that the maintenance of these agents and a change in the backbone chemotherapy benefits a group of patients that experienced progression of disease. In one prospective, phase 2 study, Santini et al [30] evaluated 39 patients with mCRC in which rechallenge was offered to patients who developed progression during the first exposure to anti-EGFR therapy. The median re-exposure line was four, consistent with our study. The reported PFS was 6.6 months and the objective RR was 53.8% [30]. Additionally, Liu et al [31] conducted a retrospective analysis of 86 patients submitted to rechallenge therapy with cetuximabe and irinotecan and found a median PFS 4.3 months and response rate of 53% (CR, PR and SD). The authors found that previous response to anti-EGFR could predict benefit from rechallenge. Our data could not demonstrate that PR was associated with benefit from this strategy as shown in Table 2. The phase 2 study of reintroduction of cetuximab in patients previously treated with irinotecan and cetuximab-based regimens in the first line of therapy (CRICKET trial) is a recent Italian prospective trial that included 27 patients with documented disease progression during exposure to a regimen based on irinotecan and cetuximab in the first line of treatment [32]. The report ORR and DCR were 23% and 54%, respectively. These results were inferior to those of Santini et al [30] and are in accordance with our findings. In our study, patients submitted to rechallenge presented short median PFS of only 3.3 months as compared to previous studies and the response rate was only 19% (CR PR). No clinical variable was related to better PFS after rechallenge in the present study. However, those patients previously treated with bevacizumab presented a non-statistically significant difference in median PFS of 6.7 months as compared to 2.9 for those that did not receive the drug.
Many advances have been achieved in the scenario of metastatic colorectal carcinoma. The notable and continuous increment in overall survival is possible by utilising active agents in the first and second line of therapy and best selection of patients based on molecular profile. Adequate care of patients leads to preserved performance status even after multiple therapies. As a result, it is becoming a very common scenario where patients have already used all available drugs and are still good candidates for treatment. To be enrolled in clinical trials would be the best option for these patients, but trials are scant in low- and middle-income countries, such as Brazil. Our cohort represents a very select group of patients with extremely high median OS. The median OS was 70 months for the 68 patients since the start of the first line and 24 months after re-treatment. It is noteworthy that 94% of our patients had a left-side primary tumour and it is known that patients with a left-sided tumour have prolonged survival when treated with anti-EGFR in the first line as demonstrated by many prospective trials [5, 6]. Therefore, re-exposure to previously used drugs becomes a strategy to be considered [15]. Anti-EGFR therapy re-exposure is a potential model to be successfully explored. Liquid biopsies over the course of the disease evolution could lead to the selection of better treatment options in the metastatic setting [33, 34]. Recently, a retrospective study of 135 patients with mCRC treated with anti-EGFR was carried out by sequencing of ctDNA with a very low allele frequency (Guardant360) platform [12]. The authors found that RAS and EGFR mutant alleles decrease exponentially over time since last anti-EGFR treatment explaining part of the benefits seen with rechallenge therapy with anti-EGFR. Moreover, the authors suggested that liquid biopsy could guide the best time of reintroduction of these agents. Anti-EGFR re-exposure is a relatively common practice in the real world. We demonstrated in this retrospective analysis that reintroduction of anti-EGFR before documented progression of disease can be beneficial for a group of patients. However, re-challenging patients that presented PD during previous treatment resulted in short PFS and few objective responses and this strategy could not be routinely advised with better patient selection with clinical and molecular tools. It should be mentioned that our patients were re-exposed after a median of four lines of therapy and most of them did not achieved partial response during first exposure. One hypothesis is that early rechallenge could have resulted in better outcomes.
Conclusion
In summary, our data may have clinical implications since reintroduction of anti-EGFR after a break could improve quality of life without impairing the outcome for a select group of patients. Our findings must be confirmed by prospective, randomised trials designed to evaluate the reintroduction strategy and better selection of patients, and rechallenge therapy with anti-EGFR for mCRC.
Funding
No funding was received for this study.
Conflicts of interest
Dr Celso Mello received a congress travel grant from Merck Serono and Amgen to ASCO and ESMO meetings. The other authors have no conflicts of interest to declare.
Ethical approval
Ethics approval for this study was approved by the local Ethic committee (CEP) at AC Camargo Cancer Center on 14/09/2018, under the number 2.894.896.
Consent to participate: NA.
Consent for publication: NA.
References
1. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries
2. Van Cutsem E, Cervantes A, and Nordlinger B, et al (2014) ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up Ann Oncol 25(Suppl 3) iii1–iii9 https://doi.org/10.1093/annonc/mdu260
3. Mendelsohn J and Baselga J (2000) The EGF receptor family as targets for cancer therapy Oncogene 19 6550–6565 https://doi.org/10.1038/sj.onc.1204082
4. Peeters M, Kafatos G, and Taylor A, et al (2015) Prevalence of RAS mutations and individual variation patterns among patients with metastatic colorectal cancer: a pooled analysis of randomised controlled trials Eur J Cancer 51 1704–1713 https://doi.org/10.1016/j.ejca.2015.05.017 PMID: 26049686
5. Stintzing S, Modest DP, and Rossius L, et al (2016) FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial Lancet Oncol 17 1426–1434 https://doi.org/10.1016/S1470-2045(16)30269-8 PMID: 27575024
6. Venook AP, Niedzwiecki D, and Lenz H-J, et al (2017) Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial JAMA 317 2392–2401 https://doi.org/10.1001/jama.2017.7105 PMID: 28632865 PMCID: 5545896
7. Douillard JY, Siena S, and Cassidy J, et al (2014) Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer Ann Oncol 25 1346–1355 https://doi.org/10.1093/annonc/mdu141 PMID: 24718886
8. Van Cutsem E, Lenz H-J, and Köhne C-H, et al (2015) Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer J Clin Oncol 33 692–700 https://doi.org/10.1200/JCO.2014.59.4812 PMID: 25605843
9. Pérez-Soler R, Delord JP, and Halpern A, et al (2005) HER1/EGFR inhibitor-associated rash: future directions for management and investigation outcomes from the HER1/EGFR inhibitor rash management forum Oncologist 10 345–356 https://doi.org/10.1634/theoncologist.10-5-345 PMID: 15851793
10. Misale S, Di Nicolantonio F, and Sartore-Bianchi A, et al (2014) Resistance to anti-EGFR therapy in colorectal cancer: from heterogeneity to convergent evolution Cancer Discov 4 1269–1280 https://doi.org/10.1158/2159-8290.CD-14-0462 PMID: 25293556
11. Primrose J, Falk S, and Finch-Jones M, et al (2014) Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial Lancet Oncol 15 601–611 https://doi.org/10.1016/S1470-2045(14)70105-6 PMID: 24717919
12. Parseghian CM, Loree JM, and Morris VK, et al (2018) Anti-EGFR resistant clones decay exponentially after progression: Implications for anti-EGFR rechallenge J Clin Oncol 36(15_suppl) 3511 https://doi.org/10.1200/JCO.2018.36.15_suppl.3511
13. Costa T, Nuñez J, and Felismino T, et al (2017) REOX: evaluation of the efficacy of retreatment with an oxaliplatin-containing regimen in metastatic colorectal cancer: a retrospective single-center study Clin Colorectal Cancer 16 316–323 https://doi.org/10.1016/j.clcc.2017.03.002 PMID: 28392022
14. Eisenhauer EA, Therasse P, and Bogaerts J, et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer 45 228–247 https://doi.org/10.1016/j.ejca.2008.10.026
15. Vogel A, Hofheinz RD, and Kubicka S, et al (2017) Treatment decisions in metastatic colorectal cancer—beyond first and second line combination therapies Cancer Treat Rev 59 54–60 https://doi.org/10.1016/j.ctrv.2017.04.007 PMID: 28738235
16. Seymour MT, Brown SR, and Middleton G, et al (2013) Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial Lancet Oncol 14 749–759 https://doi.org/10.1016/S1470-2045(13)70163-3 PMID: 23725851 PMCID: 3699713
17. Passardi A, Scarpi E, and Gelsomino F, et al (2017) Impact of second-line cetuximab-containing therapy in patients with KRAS wild-type metastatic colorectal cancer: results from the ITACa randomized clinical trial Sci Rep 7 10426 https://doi.org/10.1038/s41598-017-11048-9 PMID: 28874797 PMCID: 5585399
18. Bennouna J, Sastre J, and Arnold D, et al (2013) Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial Lancet Oncol 14 29–37 https://doi.org/10.1016/S1470-2045(12)70477-1
19. Tabernero J, Yoshino T, and Cohn AL, et al (2015) Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study Lancet Oncol 16 499–508 https://doi.org/10.1016/S1470-2045(15)70127-0 PMID: 25877855
20. Van Cutsem E, Joulain F, and Hoff PM, et al (2016) Aflibercept plus FOLFIRI vs. placebo plus FOLFIRI in second-line metastatic colorectal cancer: a post hoc analysis of survival from the phase III VELOUR study subsequent to exclusion of patients who had recurrence during or within 6 months of completing adjuvant oxaliplatin-based therapy Target Oncol 11 383–400 https://doi.org/10.1007/s11523-015-0402-9
21. Grothey A, Van Cutsem E, and Sobrero A, et al (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial Lancet 381 303–312 https://doi.org/10.1016/S0140-6736(12)61900-X
22. Mayer RJ, Van Cutsem E, and Falcone A, et al (2015) Randomized trial of TAS-102 for refractory metastatic colorectal cancer N Engl J Med 372 1909–1919 https://doi.org/10.1056/NEJMoa1414325 PMID: 25970050
23. Maindrault-Goebel F, Tournigand C, and André T, et al (2004) Oxaliplatin reintroduction in patients previously treated with leucovorin, fluorouracil and oxaliplatin for metastatic colorectal cancer Ann Oncol 15 1210–1214 https://doi.org/10.1093/annonc/mdh305 PMID: 15277260
24. Ciardiello F, Normanno N, and Martinelli E, et al (2016) Cetuximab continuation after first progression in metastatic colorectal cancer (CAPRI-GOIM): a randomized phase II trial of FOLFOX plus cetuximab versus FOLFOX Ann Oncol 27 1055–1061 https://doi.org/10.1093/annonc/mdw136 PMID: 27002107
25. Feng Q, Wei Y, and Ren L, et al (2016) Efficacy of continued cetuximab for unresectable metastatic colorectal cancer after disease progression during first-line cetuximab-based chemotherapy: a retrospective cohort study Oncotarget 7 11380–11396 https://doi.org/10.18632/oncotarget.7193 PMID: 26863631 PMCID: 4905480
26. Pietrantonio F, Vernieri C, and Siravegna G, et al (2017) Heterogeneity of acquired resistance to anti-egfr monoclonal antibodies in patients with metastatic colorectal cancer Clin Cancer Res 23 2414–2422 https://doi.org/10.1158/1078-0432.CCR-16-1863
27. Sartore-Bianchi A, Valtorta E, and Amatu A, et al (2016) Clonal evolution and KRAS-MET coamplification during secondary resistance to EGFR-targeted therapy in metastatic colorectal cancer ESMO Open 1 e000079 https://doi.org/10.1136/esmoopen-2016-000079 PMCID: 5070243
28. Van Emburgh BO, Arena S, and Siravegna G, et al (2016) Acquired RAS or EGFR mutations and duration of response to EGFR blockade in colorectal cancer Nat Commun 7 13665 https://doi.org/10.1038/ncomms13665 PMID: 27929064 PMCID: 5155160
29. Morelli MP, Overman MJ, and Dasari A, et al (2015) Characterizing the patterns of clonal selection in circulating tumor DNA from patients with colorectal cancer refractory to anti-EGFR treatment Ann Oncol 26 731–736
30. Santini D, Vincenzi B, and Addeo R, et al (2012) Cetuximab rechallenge in metastatic colorectal cancer patients: how to come away from acquired resistance? Ann Oncol 23 2313–2318 https://doi.org/10.1093/annonc/mdr623 PMID: 22396447
31. Liu X, George GC, and Tsimberidou AM, et al (2015) Retreatment with anti-EGFR based therapies in metastatic colorectal cancer: impact of intervening time interval and prior anti-EGFR response BMC Cancer 15 713 https://doi.org/10.1186/s12885-015-1701-3 PMID: 26474549 PMCID: 4609167
32. Rossini D, Cremolini C, and Salvatore L, et al (2017) PD-026 rechallenge with cetuximab irinotecan in 3rd-line in RAS and BRAF wild-type metastatic colorectal cancer (mCRC) patients with acquired resistance to 1st-line cetuximab irinotecan: the phase II CRICKET study by GONO Ann Oncol 28(Issue suppl_3) mdx263.025 https://doi.org/10.1093/annonc/mdx263.025
33. Arena S, Bellosillo B, and Siravegna G, et al (2015) Emergence of multiple EGFR extracellular mutations during cetuximab treatment in colorectal cancer Clin Cancer Res 21 2157–2166 https://doi.org/10.1158/1078-0432.CCR-14-2821 PMID: 25623215
34. Strickler JH, Loree JM, and Ahronian LG, et al (2018) Genomic landscape of cell-free DNA in patients with colorectal cancer Cancer Discov 8 164–173 https://doi.org/10.1158/2159-8290.CD-17-1009 PMCID: 5809260






