The impact of armed conflict on cancer among civilian populations in low- and middle-income countries: a systematic review
Mohammed Jawad1, Christopher Millett1, Richard Sullivan2, Fadel Alturki3, Bayard Roberts4 and Eszter P Vamos1
1Public Health Policy Evaluation Unit, Imperial College London, Hammersmith, London W6 8RP, UK
2Institute of Cancer Policy, Cancer Epidemiology, Population and Global Health, King's College London and Guy's & St Thomas' NHS Trust, London, UK
3Faculty of Medicine, American University of Beirut, Lebanon
4Department of Health Services Research and Policy, London School of Hygiene and Tropical Medicine, London WC1H 9SH, UK
Abstract
Background: Armed conflicts are increasingly impacting countries with a high burden of cancer. The aim of this study is to systematically review the literature on the impact of armed conflict on cancer in low- and middle-income countries (LMICs).
Methods: In November 2019, we searched five medical databases (Embase, Medline, Global Health, PsychINFO and the Web of Science) without date, language or study design restrictions. We included studies assessing the association between armed conflict and any cancer among civilian populations in LMICs. We systematically re-analysed the data from original studies and assessed quality using the Newcastle-Ottawa Scale. Data were analysed descriptively by cancer site.
Results: Of 1,543 citations screened, we included 20 studies assessing 8 armed conflicts and 13 site-specific cancers (total study population: 70,172). Two-thirds of the studies were of low methodological quality (score <5) and their findings were often conflicting. However, among outcomes assessed by three or more studies, we found some evidence that armed conflict was associated with increases in the incidence and mortality of non-specific cancers, breast cancer and cervical cancer. Single studies reported a positive association between armed conflict and the incidence of stomach and testicular cancers, some as early as 3 years after the onset of conflict. Some studies reported a post-conflict impact on time to diagnosis.
Conclusion: Our findings support the need for more rigorous longitudinal and cohort studies of populations in and immediately post-conflict to inform the development of basic packages of cancer services, and post-conflict cancer control planning and development.
Keywords: cancer, conflict, war, systematic review, low-income countries, middle-income countries
Correspondence to: Mohammed Jawad
Email: mohammed.jawad06@imperial.ac.uk
Published: 08/05/2020
Received: 09/01/2020
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cancer caused 8.7 million deaths globally in 2015, making it the second leading cause of death after cardiovascular disease [1]. Although this figure is likely to be an underestimate [2], the burden of cancer is increasing in low- and middle-income countries (LMICs), where 80% of the world’s population live [3] and where about two-thirds of all cancer deaths occur [4]. This is due to increasing life expectancy coupled with changing patterns of behavioural risk factors associated with higher non-communicable disease risk, such as tobacco and alcohol use, obesity, physical inactivity and an unhealthy diet [5]. Occupational, environmental and dietary exposure to carcinogens also account for substantial numbers of cancer deaths [2]. Calls for better cancer prevention and early diagnosis and better treatment all form part of Target 3.4 of the Sustainable Development Goals (SDGs), which aims for a one-third reduction in premature mortality from non-communicable diseases by 2030 [6].
Efforts to meet SDG Target 3.4, and indeed other SDGs, are likely to be hampered by the presence of armed conflict. In 2018, there were 52 armed conflicts where at least one party was a government of state, and a record 82 active civil wars [7]. Although the number of armed conflicts has been increasing, the number of deaths occurring in armed conflicts has been markedly decreasing. Armed conflicts may increase cancer incidence, complications and mortality in the short term by disrupting patients seeking care and the delivery of all aspects of oncological care [9, 10]. Additional impacts on cancer services may result from sudden demographic shifts associated with armed conflict and forced migration (internally displaced persons or refugees). This may increase late diagnoses for potentially curable site-specific cancers, abandonment of treatment or sub-optimal treatment, all of which increase the burden of cancer on patients and health services.
Longer-term impacts of armed conflict on cancer incidence may also be a result of the toxic contamination of the environment. Examples include the Vietnam War, where 10% of south Vietnam was sprayed with the carcinogenic Agent Orange [11] and the Second World War where atomic bombs were dropped on the Japanese cities of Hiroshima and Nagasaki [12]. Furthermore, stress experienced during armed conflict may encourage unhealthy behaviours that increase the risk of cancer, such as tobacco and alcohol use [16–18]. Finally, mass population displacement increases the risk of communicable disease transmission, which can increase the infectious causes of cancer, such as human papillomavirus and chlamydia trachomatis (cervical cancer), Epstein–Barr virus (nasopharyngeal cancer and lymphomas), hepatitis B and C (liver cancer, non-Hodgkin lymphoma) and others.
The greater number and increasingly protracted nature of conflict globally warrants a better understanding of its relationship to cancer care and cancer mortality. Understanding the relationship between armed conflict and cancer is important as more conflicts occur in demographically and epidemiologically transitioned societies. It remains unclear which short- or long-term approaches are most important in mediating the impact of armed conflict on cancer burden, and whether any of these factors are feasibly modifiable during an active conflict or in the post-conflict setting. This study aimed to review the literature for the impact of armed conflict on cancer, in particular its incidence and mortality among civilians in LMICs.
Methods
This systematic review is registered on Prospero (ID: CRD42017065722) and follows the PRISMA reporting standards [20]. Our research questions is: ‘What is the association between armed conflict and cancer for civilians in LMICs, compared to civilians with less or no exposure to armed conflict?’
Search strategy and selection criteria
We searched five electronic databases (Embase, Medline, Global Health, PsychINFO and the Web of Science) in November 2019 without language or date restrictions, using synonyms for armed conflict, cancer and LMICs. The full search strategy can be found in Table S1. We also hand-searched citation lists of included studies to identify additionally relevant articles. In line with previous reviews, we did not search the grey literature given the limited information available [21].
The inclusion criteria comprised civilian populations (including children, internally displaced persons, and refugees) in LMICs exposed to author-defined armed conflict with a diagnosis of any type of cancer. We did not exclude studies by design but a component of comparison to a non- or less-conflict exposed group was required for eligibility. In the case of ecological studies collecting serial data points over time (e.g., hospital admission data pre-, during- and post-conflict), we excluded studies whose first post-conflict data point was greater than 3 years after the end of the conflict.
We excluded studies reporting on military veterans, combatants and studies from high-income countries (including where refugees had migrated to high-income countries). We also excluded studies whose exposure was weapons (often, nuclear) testing rather than armed conflict. Studies that mentioned armed conflict but did not attempt to measure it were further excluded.
Data analysis
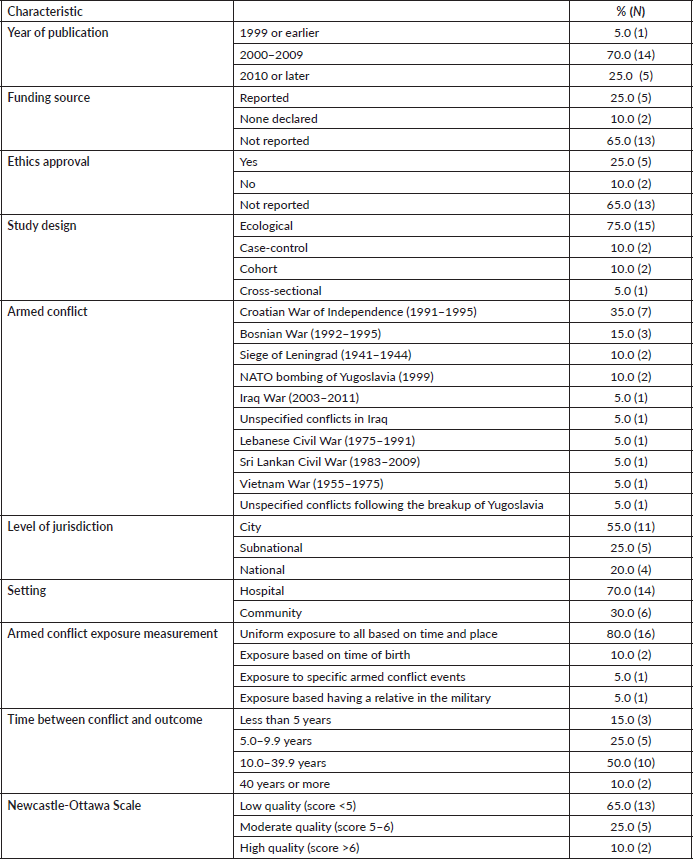
Two reviewers performed all citation screening and data abstraction in duplicate and independently using pilot-tested forms. Disagreements were resolved by discussion, and when needed with the help of a third reviewer. We retrieved full texts of citations considered eligible by at least one reviewer. Data extracted from eligible studies included study provenance (funding source, ethics approval and conflicts of interest), study features (design, timing, conflict, country and level of jurisdiction), population (sample size, mean age/age range and percentage of males) and results (outcome measure definition, outcome measure effect size and precision). We calculated the maximum number of years from the onset or end of conflict to the time of data collection, to give an indication of the length of armed conflict exposure. We used the Newcastle-Ottawa Scale (NOS) [22–24] to assess the quality of each study. The NOS has been recommended for use for non-randomised studies by the Cochrane Collaboration [25]. Although the NOS has no established threshold of quality, in line with previous reviews [26, 27], we defined studies as low quality (score <5), moderate quality (score 5–6) and high quality (score >6) to simplify the main analysis. Quality scores by NOS domains (selection, comparability and outcome) for each study are detailed in Table S2.
Meta-analysis was not feasible given the degree of between-study heterogeneity in design, armed conflict, population and outcome. We, therefore, analysed data descriptively. To standardise our analytical approach and to reduce bias, we systematically re-analysed reported data and presented a single effect estimate per outcome per study where possible. This included constructing 95% confidence intervals around all effect estimates and considering confidence intervals that did not overlap as statistically significant at an alpha level of 0.05. This also meant we combined outcomes stratified by population subgroups (e.g., by age and sex), and used the overall outcome in our analysis. We did not reanalyse data already presented as odds ratios, beta-coefficients or hazard ratios. Where data were available pre- during- and post-conflict, we used a single estimate for the differences between the pre- versus during-conflict data for each study. Furthermore, an analysis of post-conflict data was undertaken separately to understand better changes in trends throughout the conflict cycle. Each outcome from each study was assigned a qualitative effect direction (increase, decrease or no change) following exposure to armed conflict based on the statistical significance of effects. We stratified our analysis by cancer incidence and mortality, and outcomes with greater than three studies were described in more detail and displayed graphically using Harvest plots. Harvest plots take aspects of a forest plot to display data on a matrix of effect direction weighted by several variables [28]. Finally, we visually assessed publication bias by constructing an adapted funnel plot, using the sample size and the qualitative effect direction in place of the standard error and effect size, respectively.
Results
Study characteristics
Of 1,543 records identified through database searching, 38 were potentially eligible and 20 were included in the final analysis (Figure 1). The total study population was 70,172. Three-quarters of studies used an ecological design (75.0%) and over one-third analysed the Croatian War of Independence (1991–1995) (35.0%). Over half were conducted in cities (55.0%) and 70.0% utilised hospital-derived data. The average follow-up time was 16.8 years (range 3–64 years) and study quality was mostly rated as low (65.0%). Only four outcomes were assessed by three or more studies: the incidence of any, breast and cervical cancer, and mortality from any cancer.
Incidence of any cancer
Four studies, all low quality and ecological, assessed the incidence of any type of cancer (Figure 2, top left panel). One subnational cancer registry study analysed non-specific conflicts in Iraq over 30 years and showed an increase in the incidence rate ratio of cancers throughout the conflict and into the post-conflict period [29]. It did not compare incidence rate ratios in similar countries not at war during this period of time. Two hospital-based studies from the Balkans showed no change in cancer incidence during the conflict compared to the pre-conflict baseline [30, 31]. Another cancer registry study assessed the Lebanese Civil War and showed no change in cancer incidence during the conflict period (1983–1991, mean 786 cases/year) compared to the post-conflict period (1992 to 1994, mean 802.3 cases/year) [32].
Mortality from any cancer
Four studies assessed mortality from any cancer (Figure 2, bottom left panel). One moderate-to-high quality study assessed the 2003 US-led invasion of Iraq and reported an average 50% increase in the number of households reporting cancer deaths from the pre-conflict period (mean 9.9 cases/year in 2001–2002) to the conflict period (mean 14.8 cases/year in 2003–2010) [33]. We calculated this difference to be statistically significant (4.9 cases/year, 95% CI 0.4–9.4). Two survivor cohort studies from the Siege of Leningrad (1941–1944) reported no change in cancer mortality 41 to 64 years after the siege although both adjusted hazard ratios showed positive effect estimates (1.12 (95% CI 0.95 -1.31) and 1.11 (95% CI 0.97 -1.27)) [34, 35]. One modelling study (1973 to 1994) used data from the Federal Institute of Statistics to assess the impact of the breakup of Yugoslavia, and found that cancer mortality decreased during periods of war and sanctions [36].
Breast cancer incidence
Six studies, all assessing wars in the Balkans during the 1990s, reported on breast cancer incidence (Figure 2, top right panel). Both moderate-to-high quality studies showed an increase in breast cancer incidence [37, 38]. One of these was ecological in design, monitored trends 13 years before the 1999 NATO bombing of Yugoslavia, and reported an increase from an average of 67.2 cases/year before the conflict to 80.2 cases/year during the conflict [38]. We calculated this difference to be statistically significant (13.0 cases/year, 95% CI 4.1–21.9). The other study used a case-control design and reported increased odds of breast cancer among those with greater exposure to war-related events in Bosnia (pooled odds across all events: 1.55, 95% CI 1.37–1.73) [37]. The remaining four studies, all low quality and ecological in design, showed no change [39, 40] or a decrease [31, 41] in breast cancer incidence. The study with the shortest follow-up in this review (3 years) was one study that showed a decrease in breast cancer diagnosis during the Croatian War of Independence (32 cases in 2 years) compared to the pre-conflict baseline (86 cases in 2 years) [31]. We considered this decrease statistically significant (−54.0 cases/2 years, 95% CI–75.3 to −32.7).
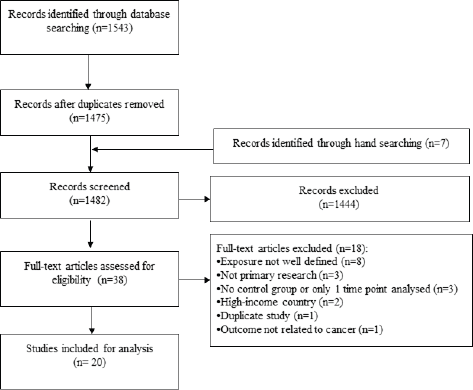
Figure 1. Study flow.
Cervical cancer incidence
Three studies assessed cervical cancer incidence (Figure 2, bottom right panel). One moderate-to-high quality case-control study of the Vietnam War showed that women with a husband in the army had higher odds of cervical cancer compared to those without (adjusted odds ratio (AOR) 1.32, 95% CI: 1.00–1.75) [42]. One low-quality ecological study in Greece assessed over 35,000 smear tests from hospitals with different proximity to the Yugoslav border, but showed no difference in either cervical cancer or cervical intraepithelial neoplasia incidence between the sites following the NATO bombing of Yugoslavia in 1999 [43]. Another low-quality hospital-based ecological study found a decrease in cervical cancer incidence, from 214 cases in 6 years before the Croatian war, to 142 in 6 years of the war [44]. We found this to be a statistically significant decrease (−72.0, 95% CI: −109.0 to −35.0).
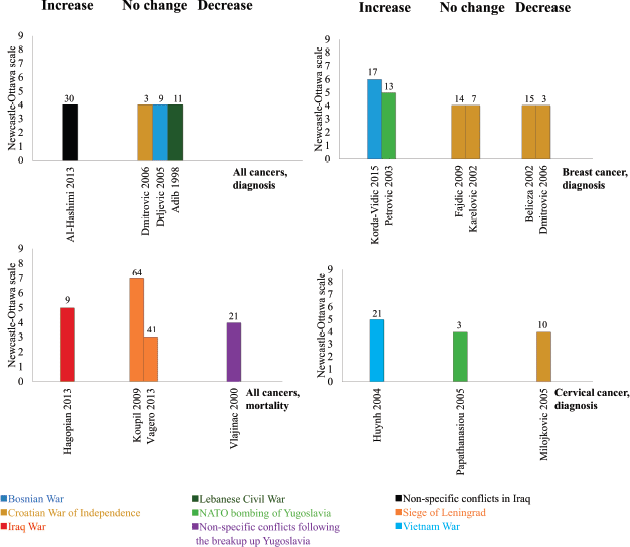
Figure 2. The impact of armed conflict on cancer incidence and mortality. Interpretation: Height refers to study quality, colour refers to armed conflict, number refers to length of follow-up between conflict exposure and outcome, bars grouped as showing either an increase, decrease, or no change following exposure to armed conflict.
Table 1. Study characteristics and methodological quality of 20 included studies.
Other cancers
Eight studies examined other site-specific cancers, but they were too few to display graphically and describe collectively. One hospital-based study from Croatia reported a rise in the incidence of malignant stomach and testicular cancers when comparing 2 years of conflict to 2 years prior [31]. Other studies of various study design and quality found no association between armed conflict and mortality from breast cancer [34, 35], colon cancer [34], lung cancer [34, 35] and stomach cancer [34], nor the incidence of corpus cancer [44], haematological cancers [45], lung cancer [31], pancreatic cancer [31] and prostate cancer [34]. One study reported a decrease in the incidence of colon cancer [31]. Finally, four studies reported mixed evidence for changes in the incidence of intracranial [46, 47], oropharyngeal [48] and ovarian [31, 44] cancers.
Post-conflict trends
All seven studies that assessed the conflict cycle (i.e., pre-conflict, conflict and post-conflict) were ecological, hospital-based studies analysing either the Croatian or Bosnian wars of the 1990s [30, 39, 41, 44–47]. The three studies that reported no change between the times before and during the conflict then showed an increase in incidence in the post-conflict period [30, 39, 44]. The one study that reported an increase in incidence between the pre- and during-conflict periods found that this increase was sustained into the post-conflict period [47]). In the three studies that reported a decrease in incidence between the pre- and during-conflict periods found that this either plateaued [41, 46] or returned to pre-conflict levels [44] during the post-conflict period. One ecological study showed mixed findings in the incidence of haematological cancers depending on the type of conflict exposure used (areas affected by depleted uranium, chemical damage or population mixing) and outcome (Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, lymphatic leukaemia and myeloid leukaemia), but generally found either no change or a decrease in incidence through the post-conflict period [45].
Publication bias
Figure 3 presents an adapted funnel plot to assess publication bias, which includes all 55 outcomes from the 20 included studies. While the absence of actual effect estimates limits interpretation, the plot does not present convincing evidence of asymmetry or the absence of small studies showing no effect, which are indicative of publication bias.
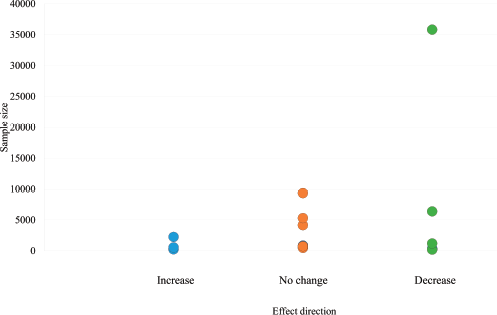
Figure 3. Adapted funnel plot assessing publication bias.
Discussion
The literature on the impact of armed conflict on cancer incidence and mortality is very sparse, methodologically poor, and often contradictory. This is despite the fact that some have extensive follow-up periods, which averaged 18 years. The main limitations to many studies were their design, namely, ecological, and thus subject to ecological fallacies; nearly all failed to acknowledge this, in addition to failing to account for sudden population demographic changes following forced migration. There was also limited adjustment for confounding variables in risk factor exposure and behaviour changes. The lack of data on factors, which may mediate the impact of armed conflict on cancer, is an additional serious limitation in the extant literature.
The one cancer (breast) that did have several studies showing an increase in incidence following armed conflict did not have, however, sufficient data to advance understanding of plausible aetiological factors. Armed conflict has been shown to change reproductive strategies in populations affected with greater parity and lower maternal age, both of which are protective of breast cancer [49]. Thus it is unclear, whether the increased incidence of breast cancer is real or an artefact.
The factors that affect cancer incidence and mortality in armed conflict are multifactorial and multilevel; these includes changes to risk factor exposure, behavioural changes, delays to presentation, the availability of timely and affordable complex care (depending on the site-specific cancer), the ability to access care, etc. Furthermore, the ability to collect reliable data from registries, hospitals or camps can be substantially hampered during periods of conflict. In some cases, this is because systems are destroyed, data are not collected (too costly or to protect patients identities) or because care data are fragmented across multiple disconnected places of care [50, 51]. Reported data may be inaccurate due to limited diagnostic facilities and available pathologists, so any statistical inference should provide a contextual interrogation to the quality of the data. Reduced case ascertainment featured prominently as a serious lacunae in data collected during the Lebanese Civil War (1975–1991), when the American University of Beirut Medical Center (AUBMC) was the only functioning cancer referral site in the entire county and it was estimated at least two-thirds of the cancer burden during this period went either undiagnosed or unreported [32]. AUBMC and other cancer centres only become accessible after the end of the conflict [32], so any increase in incidence during the post-conflict period may simply reflect a return of the status quo. A similar conclusion was reached in analysing the cancer incidence data collected during the Croatian War of Independence; road blockades across the country and the removal of free care services such as breast cancer check-ups radically reduced health service accessibility [40]. In another analysis of the same conflict, an observed post-conflict increase in cancer incidence was also attributed to the introduction of a new cancer screening programme, better organisation of cancer care services and the introduction of more accurate and up-to-date diagnostic equipment in hospitals [39].
In armed conflict, there is an expected rise in cancer-related mortality due to the loss of skilled personnel, the shift of such personnel into acute care, shortage or failure of key equipment—diagnostic imaging, surgical instruments, radiotherapy and cancer drugs, for example—and the inability of patients to access what care remains due to security or affordability barriers, all factors that led to the rise in cancer mortality during the armed conflict in Serbia in 1999 [38]. Yet it is possible that the same factors that worsen cancer mortality are the same that inhibit the timely and accurate reporting of such mortality, which may explain why many of the studies included in this review reported no change in the incidence or mortality of cancer during or after armed conflict.
Better quality research to study cancer in armed conflict is essential, and our review findings have several research implications. Although resources are often scarce in conflict settings, making use of hospital-based registries or other sources of routinely collected data have excellent potential for robust inquiry. In instances where control groups are not feasible, data could be subject to interrupted time series or difference-in-difference analyses with adjustment for confounders or with age-/sex-standardised rates of cancer incidence. Importantly, researchers should outline the status of screening programmes and other mediators in the relationship between armed conflict and cancer, so that these can be appropriately accounted for in the study design. This will make a more informative contribution to the current literature which is lacking in methodological rigour and often reports crude numbers over time. One notable absence from the literature was studies from humanitarian organisations. Although often unable to collect pre-conflict data, they are in a strong position to assess the degree of conflict exposure among their patients using tools such as the Harvard Trauma Questionnaire [52]. Future research could assess the impact of armed conflict on stage of diagnosis, in addition to inequalities by socioeconomic groups (e.g. age, sex, residence and deprivation). Most studies with very long follow-up times (>30 years) hypothesised that in utero, infant or adolescent exposure to armed conflict would have a greater impact on cancer risk to those exposed at older ages [34, 35, 53]. However, the failure to properly control for the many confounders has seriously hampered research to examine the link between toxic contamination of the environment due to armed conflict and long-term health impacts such as cancer.
Our findings also have important policy implications. Despite a number of guidance documents on cancer care in complex emergencies and post disaster, e.g., post typhoon Haiyan issued by WHO [54, 55] the literature is silent on what might constitute basic packages of cancer care, for UN and international NGOs for example and on approaches to post-conflict cancer systems reconstruction, or in supporting host countries absorb and provide care to refugees in both formal and informal (sans papier) settings. Although, it is to be recognised that the latter is intimately linked to post-conflict health systems reconstruction per se. More research is needed to urgently inform cancer policies and planning in the context of armed conflicts, particularly now that so many are occurring in high-burden countries with populations that have gone through the demographic and epidemiological transitions.
Conflicts of interest
The authors have declared no conflicts of interest.
Funding
This work was supported by the Medical Research Council Doctoral Training Partnership. The Public Health Policy Evaluation Unit, Imperial College London is supported by the NIHR School of Public Health Research. RS is funded through the UK Research and Innovation GCRF RESEARCH FOR HEALTH IN CONFLICT (R4HC-MENA); developing capability, partnerships and research in the Middle and Near East (MENA) ES/P010962/1. The funders had no role in the design, analysis or writing of this manuscript, nor the decision to submit for publication. The corresponding author (MJ) has full access to all the data in the study and had final responsibility for the decision to submit for publication.
References
1. Wang H, Naghavi M, and Allen C, et al (2016) Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015 Lancet 388(10053) 1459–1544 https://doi.org/10.1016/S0140-6736(16)31012-1
2. GBD 2016 Occupational Carcinogens Collaborators (2020) Global and regional burden of cancer in 2016 arising from occupational exposure to selected carcinogens: a systematic analysis for the Global Burden of Disease Study 2016 Occup Environ Med 77(3) 151–159 https://doi.org/10.1136/oemed-2019-106012
3. World Bank World Bank Open Data [online] [https://bit.ly/2yUZmdS] Date accessed: 12/04/19
4. World Health Organization Global status report on non-communicable diseases 2010 [online] [https://bit.ly/2QVq6UH] Date accessed: 24/09/18
5. Vineis P and Wild CP (2014) Global cancer patterns: causes and prevention Lancet 383(9916) 549–557 https://doi.org/10.1016/S0140-6736(13)62224-2
6. Sustainable Development Knowledge Platform. Sustainable Development Goal 3 [online] [https://bit.ly/1Yndp0n] Date accessed: 24/09/18
7. Pettersson T, Högbladh S, and Öberg M (2019) Organized violence, 1989–2018 and peace agreements J Peace Res 56(4) 589–603 https://doi.org/10.1177/0022343319856046
8. Uppsala Data Conflict Program (2017) [online] [https://bit.ly/2QODHwZ] Date accessed: 24/09/18
9. Burnham GM, Lafta R, and Doocy S (2009) Doctors leaving 12 tertiary hospitals in Iraq, 2004–2007 Soc Sci Med 69(2) 172–177 https://doi.org/10.1016/j.socscimed.2009.05.021 PMID: 19501443
10. Fouad FM, Sparrow A, and Tarakji A, et al (2017) Health workers and the weaponisation of health care in Syria: a preliminary inquiry for The Lancet–American University of Beirut Commission on Syria Lancet 390(10111) 2516–2526 https://doi.org/10.1016/S0140-6736(17)30741-9 PMID: 28314568
11. Kramárová E, Kogevinas M, and Anh CT, et al (1998) Exposure to Agent Orange and occurrence of soft-tissue sarcomas or non-Hodgkin lymphomas: an ongoing study in Vietnam Environ Health Perspect 106(suppl 2) 671–678 PMID: 9599715 PMCID: 1533419
12. Furukawa K, Preston D, and Funamoto S, et al (2013) Long-term trend of thyroid cancer risk among Japanese atomic-bomb survivors: 60 years after exposure Int J Cancer 132(5) 1222–1226 https://doi.org/10.1002/ijc.27749
13. NATO (2001) Data concerning the locations of depleted uranium ordnance expended during Operation Allied Force (grid co-ordinates) [online] [https://bit.ly/2zpHhqH] Date accessed: 24/09/18
14. LeShan L (1959) Psychological states as factors in the development of malignant disease: a critical review J Natl Cancer Inst 22(1) 1–18 PMID: 13621196
15. Reiche EMV, Nunes SOV, and Morimoto HK (2004) Stress, depression, the immune system, and cancer Lancet Oncol 5(10) 617–625 https://doi.org/10.1016/S1470-2045(04)01597-9 PMID: 15465465
16. Jawad M, Vamos EP, and Najim M, et al (2019) The impact of armed conflict on cardiovascular disease risk among civilian populations in low- and middle-income countries: a systematic review Heart [Epub ahead of print] https://doi.org/10.1136/heartjnl-2018-314459
17. Lo J, Patel P, and Shultz JM, et al (2017) A systematic review on harmful alcohol use among civilian populations affected by armed conflict in low-and middle-income countries Subst Use Misuse 52(11) 1494–1510 https://doi.org/10.1080/10826084.2017.1289411 PMID: 28471305
18. Lo J, Patel P, and Roberts B (2016) A systematic review on tobacco use among civilian populations affected by armed conflict Tob Control 25(2) 129–140 https://doi.org/10.1136/tobaccocontrol-2014-052054
19. American Cancer Society (2020) Can infections cause cancer? [online] [https://bit.ly/34l5CvF] Date accessed: 09/04/20
20. Moher D, Liberati A, and Tetzlaff J, et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement PLoS Med 6(7) e1000097 https://doi.org/10.1371/journal.pmed.1000097 PMID: 19621072 PMCID: 2707599
21. Ruby A, Knight A, and Perel P, et al (2015) The effectiveness of interventions for non-communicable diseases in humanitarian crises: a systematic review PLoS One 10(9) e0138303 https://doi.org/10.1371/journal.pone.0138303 PMID: 26406317 PMCID: 4583445
22. Wells G, Shea B, and O’Connell D, et al The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [online] [http://bit.ly/1SkJ3w3] Date accessed: 21/01/18
23. Alshabanat A, Zafari Z, and Albanyan O, et al (2015) Asthma and COPD overlap syndrome (ACOS): a systematic review and meta analysis PLoS One 10(9) e0136065 https://doi.org/10.1371/journal.pone.0136065 PMID: 26336076 PMCID: 4559416
24. The Ottawa Hospital. Our Research: Clinical Epidemiology Program. Newcastle-Ottawa Quality Assessment Scale Case Control Studies [online] [http://bit.ly/2rrR2me] Date accessed: 21/01/18
25. Higgins J (2011) Cochrane handbook for systematic reviews of interventions Version 5.1. 0 [updated March 2011] The Cochrane Collaboration [www.cochrane-handbook.org]
26. Simunovic N, Devereaux P, and Sprague S, et al (2010) Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis CMAJ 182(15) 1609–1616 https://doi.org/10.1503/cmaj.092220 PMID: 20837683 PMCID: 2952007
27. Roy A, Eisenhut M, and Harris R, et al (2014) Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis BMJ 349 g4643 https://doi.org/10.1136/bmj.g4643 PMID: 25097193 PMCID: 4122754
28. Ogilvie D, Fayter D, and Petticrew M, et al (2008) The harvest plot: A method for synthesising evidence about the differential effects of interventions BMC Med Res Methodol 8(1) 8 https://doi.org/10.1186/1471-2288-8-8 PMID: 18298827 PMCID: 2270283
29. Al-Hashimi MM and Wang X (2013) Comparing the cancer in Ninawa during three periods (1980–1990, 1991–2000, 2001–2010) using Poisson regression J Res Med Sci 18(12) 1026–1039
30. Drljevic K and Mehmedbasic S (2005) [The frequency of female genital cancer at Gynecological Department in Cantonal Hospital Zenica--before, during and postwar time in Bosnia-Herzegovina] Med Arh 59(3) 183–187 PMID: 15997680
31. Dmitrović B, Kurbel S, and Margaretić D, et al (2006) Utjecaj ratnih zbivanja na pobol od zloćudnih tumora Med Glas (Zenica) 3(1) 26–29
32. Adib SM, Mufarrij AA, and Shamseddine AI, et al (1998) Cancer in Lebanon: an epidemiological review of the American University of Beirut Medical Center Tumor Registry (1983–1994) Ann Epidemiol 8(1) 46–51 https://doi.org/10.1016/S1047-2797(97)00109-9 PMID: 9465993
33. Hagopian A, Flaxman AD, and Takaro TK, et al (2013) Mortality in Iraq associated with the 2003–2011 war and occupation: findings from a national cluster sample survey by the university collaborative Iraq Mortality Study PLoS Med 10(10) e1001533 https://doi.org/10.1371/journal.pmed.1001533 PMID: 24143140 PMCID: 3797136
34. Koupil I, Plavinskaja S, and Parfenova N, et al (2009) Cancer mortality in women and men who survived the siege of Leningrad (1941–1944) Int J Cancer 124(6) 1416–1421 https://doi.org/10.1002/ijc.24093
35. Vågerö D, Koupil I, and Parfenova N, et al (2013) Long term health consequences following the Siege of Leningrad 225 p
36. Vlajinac H, Marinkovic J, and Kocev N, et al (2000) [Trends in mortality in Serbia, excluding the provinces, 1973–1994] Srp Arh Celok Lek 128(9–10) 309–315
37. Korda-Vidic V and Vasilj I, Babic D (2015) The stress of war and breast cancer incidence Psychiatr Danub 27(Suppl 2) 571–577 PMID: 26657984
38. Petrovic B, Kocic B, and Filipovic S, et al (2003) Epidemiology of breast cancer in the city of Nis, Serbia J BUON 8(2) 147–150
39. Fajdic J, Gotovac N, and Hrgovic Z, et al (2009) Influence of stress related to war on biological and morphological characteristics of breast cancer in a defined population Adv Med Sci 54(2) 283 https://doi.org/10.2478/v10039-009-0040-5 PMID: 20022862
40. Karelovic D, Bukovic D, and Strinic T, et al (2002) Influence of war circumstances on tumor morphological characteristics in patients with breast cancer Coll Antropol 26(1) 99–106 PMID: 12137329
41. Belicza M, Lenicek T, and Glasnovic M, et al (2002) [Change in the occurrence of breast cancer in hospital registries (1980–2000)] Lijec Vjesn 124(11–12) 347–353
42. Huynh ML, Raab SS, and Suba EJ (2004) Association between war and cervical cancer among Vietnamese women Int J Cancer 110(5) 775–777 https://doi.org/10.1002/ijc.20164 PMID: 15146569
43. Papathanasiou K, Gianoulis C, and Tolikas A, et al (2005) Effect of depleted uranium weapons used in the Balkan war on the incidence of cervical intraepithelial neoplasia (CIN) and invasive cancer of the cervix in Greece Clin Exp Obstet Gynecol 32(1) 58–60 PMID: 15864941
44. Milojković M, Pajtler M, and Rubin M (2005) Influence of the war in Croatia on the frequency of gynecological cancer in the University Hospital Osijek in the period from 1985 to 2002 Coll Antropol 29(2) 573–578
45. Labar B, Rudan I, and Ivankovic D, et al (2004) Haematological malignancies in childhood in Croatia: investigating the theories of depleted uranium, chemical plant damage and ‘population mixing’ Eur J Epidemiol 19(1) 55–60 https://doi.org/10.1023/B:EJEP.0000013400.65418.60 PMID: 15012023
46. Alajbegovic A, Hrnjica M, and Dimitrijevic J, et al (2002) [Central nervous system neoplasms in clinical data from the Neurology Clinic KCU in Sarajevo 1990–1999] Med Arh 56(1) 15–19
47. Telarović S, Relja M, and Franinović-Marković J (2006) Impact of war on central nervous system tumors incidence–a 15-year retrospective study in Istria County, Croatia Coll Antropol 30(1) 149–155
48. Ariyawardana A and Warnakulasuriya S (2011) Declining oral cancer rates in Sri Lanka: are we winning the war after being at the top of the cancer league table? Oral Dis 17(7) 636–641 https://doi.org/10.1111/j.1601-0825.2011.01809.x PMID: 21762396
49. Urdal H and Che CP (2013) War and gender inequalities in health: the impact of armed conflict on fertility and maternal mortality Int Interact 39(4) 489–510 https://doi.org/10.1080/03050629.2013.805133
50. Ahmad K (2006) Conflict puts pressure on cancer-care resources in Lebanon Lancet Oncol 7(9) 709 https://doi.org/10.1016/S1470-2045(06)70844-0 PMID: 16977728
51. Alwan N and Kerr D (2018) Cancer control in war-torn Iraq Lancet Oncol 19(3) 291–292 https://doi.org/10.1016/S1470-2045(18)30135-9 PMID: 29508747
52. Berthold SM, Mollica RF, and Silove D, et al (2018) The HTQ-5: revision of the Harvard Trauma Questionnaire for measuring torture, trauma and DSM-5 PTSD symptoms in refugee populations Eur J Public Health 29(3) 468–474 https://doi.org/10.1093/eurpub/cky256 PMID: 30561573
53. Stanner SA, Bulmer K, and Andres C, et al (1997) Does malnutrition in utero determine diabetes and coronary heart disease in adulthood? Results from the Leningrad siege study, a cross sectional study BMJ 315(7119) 1342–1348 https://doi.org/10.1136/bmj.315.7119.1342 PMID: 9402775 PMCID: 2127836
54. World Health Organization Noncomunicable diseases and their risk factors. Tools for implementing WHO PEN (Package of essential noncommunicable disease interventions) [online] [https://bit.ly/2xNsFiH] Date accessed: 24/09/18
55. Martinez RE, Quintana R, and Go JJ, et al (2015) Use of the WHO package of essential noncommunicable disease interventions after typhoon Haiyan Western Pac Surveill Response J 6(Suppl 1) 18–20 https://doi.org/10.5365/wpsar.2015.6.3.HYN_024
Supplementary information
Table S1. Search strategy: Medline, Embase, PsychInfo, Global Health.
Table S2. Characteristics of individual studies.







