Real-world evidence on first-line treatment for metastatic renal cell carcinoma with non-clear cell and sarcomatoid histologies: are sunitinib and pazopanib interchangeable?
Renata Colombo Bonadioa, Pedro Isaacsson Velho, Guilherme Nader Marta, Mirella Nardo, Manoel Carlos LA Souza, David QB Muniz, Regis OF Bezerra, Raisa KA Bispo, Sheila F Faraj, Diogo A Bastos and Carlos Dzik
Instituto do Cancer do Estado de São Paulo, Faculdade de Medicina da Universidade de São Paulo, Av Dr Arnaldo 251, São Paulo 01246-000, Brazil
ahttps://orcid.org/0000-0001-5818-922X
Abstract
Introduction: Non-clear cell renal cell carcinoma (nccRCC) and sarcomatoid renal cell carcinoma (sRCC) are underrepresented in clinical trials. Treatment approaches are frequently extrapolated from data of clear cell renal cell carcinoma, in which pazopanib is non-inferior to sunitinib. We aim to compare the effectiveness of first-line sunitinib and pazopanib for nccRCC and sRCC.
Methods: We evaluated a retrospective cohort of patients with metastatic nccRCC and sRCC treated with first-line sunitinib or pazopanib at an academic cancer centre. Overall survival (OS), progression-free survival (PFS) and response rate were measured. Kaplan–Meier and log-rank analyses were used for time-to-event data. Cox regression was used for prognostic factors.
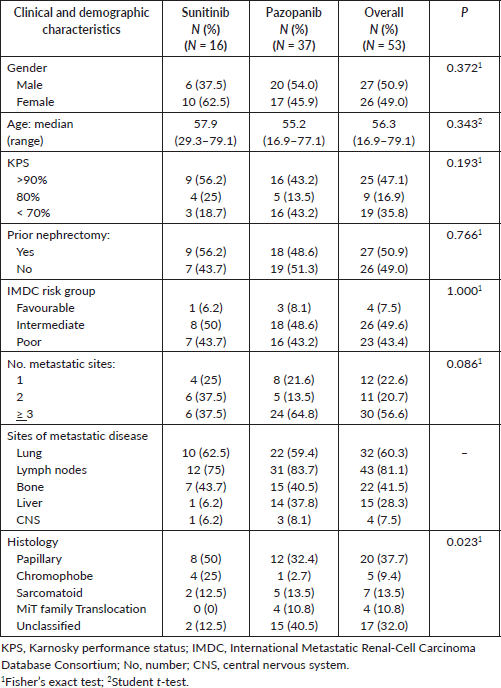
Results: Fifty-three patients were included; 16 (30.1%) treated with sunitinib and 37 (69.9%) with pazopanib. Forty-six (86.8%) patients had nccRCC and 7 (13.2%) had sRCC. The majority had intermediate or poor International Metastatic Renal-Cell Carcinoma Database Consortium risk (93%).
Median PFS was 6.6 months with sunitinib and 4.9 months with pazopanib (HR 1.75; P = 0.078). Treatment with pazopanib was associated with inferior OS in comparison with sunitinib (median OS: 30.4 months versus 8.7 months; HR 2.71, 95% CI 1.31–5.58, P = 0.007). These results were confirmed in subgroup analysis of patients with papillary, chromophobe and MiT family translocation histologies (median OS: 38.7 months versus 14.7 months; HR 3.16, 95% CI 1.20–8.29, P = 0.019). Unclassified and sarcomatoid histologies had inferior OS (median: 6.9 and 1.1 months, respectively) regardless of the treatment used.
Conclusion: In this patient cohort, pazopanib was associated with inferior OS in comparison with sunitinib for metastatic nccRCC. Larger trials are ideally warranted to confirm these results.
Keywords: non-clear cell, sarcomatoid, renal cell carcinoma, sunitinib, pazopanib
Correspondence to: Renata Colombo Bonadio
Email: rrccbonadio@gmail.com
Published: 04/11/2019
Received: 27/05/2019
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Renal cell carcinomas (RCCs) represent more than 80% of all primary renal neoplasms [1]. Clear cell RCC (ccRCC) comprises 85% of the RCC. Other RCC variants are classified as non-clear cell RCC (nccRCC), a heterogeneous group of neoplasms composed of several histological variants [2]. Although they are commonly grouped together, their morphological and molecular differences significantly impact clinical outcomes and prognosis [3, 4]. Sarcomatoid RCC (sRCC) also represents a small portion of RCC, having an aggressive phenotype and may be present in each different histologic epithelial subtypes [5].
Despite several advances that have been achieved in recent years in the treatment of metastatic RCC, clinical data derives predominantly from patients with ccRCC [6]. Most of the pivotal studies leading to the approval of the current standard therapies for RCC included a limited number of or completely excluded nccRCC and sRCC patients [7, 8]. The scarcity of clinical evidence regarding nccRCC and sRCC limits our knowledge on the efficacy and safety of therapies in these different variants. Thus, many management recommendations are extrapolated from existing evidence for ccRCC.
For patients with metastatic nccRCC, the standard first-line treatment options include angiogenesis inhibition with vascular endothelial growth factor (VEGF) pathway inhibitors and mammalian target of rapamycin (mTOR) inhibitors [9, 10]. Temsirolimus is an mTOR inhibitor that was compared with interferon-alpha for advanced renal-cell carcinoma in a phase III trial, which included patients with nccRCC. Results showed superior progression-free survival (PFS) and overall survival (OS) with temsirolimus, regardless of tumour histology [11, 12].
Additional evidence is available from phase II trials, such as the ASPEN trial, which randomly assigned patients with nccRCC to receive either sunitinib or everolimus in the first-line setting. Median PFS was longer in patients randomised to sunitinib (8.3 months versus 5.6 months, HR 1.41, 80% CI 1.03–1.92, P = 0.16) [13]. A second trial, the Everolimus versus Sunitinib Prospective Evaluation (ESPN), randomly assigned patients with nccRCC to treatment with everolimus or sunitinib, with crossover to the alternate agent at disease progression. No statistically significant differences in survival were seen between the two treatment arms (median OS: 16.2 months versus 14.9 months, 95% CI 8.0–23.4, stratified P = 0.18) [14]. Finally, the phase II RECORD-3 study evaluated the treatment sequencing with everolimus followed by sunitinib versus sunitinib followed by everolimus. A subset analysis of nccRCC patients failed to demonstrate a statistically significant difference in PFS between the two arms (median PFS: 7.2 months versus 5.1 months, HR 1.5, 95% CI 0.9–2.8) [15].
Results of a recent meta-analysis of randomised controlled trials have suggested a trend towards favouring sunitinib in terms of OS and PFS [16]. However, the results were not statistically significant. Thus, optimal therapy for nccRCC histologies remains unclear. Pazopanib is an anti-VEGF tyrosine kinase inhibitor (TKI) frequently used for RCC. Although pazopanib is non-inferior to sunitinib for ccRCC, evidence on its use for nccRCC and sRCC is scarce [17]. We sought to analyse our centre’s experience regarding the effectiveness of sunitinib or pazopanib as first-line therapy for metastatic nccRCC and sRCC.
Methods
Study design and participants
We retrospectively evaluated data from 222 consecutive patients who received sunitinib or pazopanib as initial systemic treatment for metastatic RCC from February 2009 to March 2017 at Instituto do Cancer do Estado de São Paulo (ICESP), Brazil. The use of sunitinib or pazopanib depended only on the time period. From February 2009 to September 2013, sunitinib was the TKI inhibitor available as first-line therapy for metastatic RCC at ICESP. From September 2013 until March 2017, pazopanib was the TKI inhibitor available.
Only patients with histological confirmation of nccRCC who received at least one dose of the VEGF inhibitor were included in the present analysis. One specialised pathologist centrally reviewed the tumour samples available to confirm the histopathological diagnosis.
Clinical records were evaluated in order to collect relevant data including age, gender, histology, Karnofsky performance status, prior nephrectomy, sites of metastatic disease and prognostic score according to the International Metastatic Renal-Cell Carcinoma Database Consortium (IMDC) system [18].
Two trained radiologists reviewed all radiologic imaging (computed tomography or magnetic resonance imaging) to assess tumour response, according to RECIST 1.1 [19].
Treatment
Pazopanib was administered orally at the dose of 800 mg or lower once daily (600 or 400 mg once daily). Sunitinib was administered orally at the dose of 50 mg or lower once daily, for treatment cycles of 4 weeks on treatment and 2 weeks off treatment, or at 37.5 mg once daily continuously. Treatment continued until disease progression, unacceptable toxicity, patient refusal or death.
Statistical analysis
The primary objective of the study was to compare the OS of patients who received pazopanib versus sunitinib. Secondary objectives were response rate, PFS and prognostic factors.
OS was defined as the time from initiation of systemic treatment to death. PFS was the time from treatment initiation until clinical or radiological progression, or death from any cause. Patients without the described events were censured at the time of the last follow-up.
Continuous variables were expressed as median and ranges, and compared using the unpaired t-test. Categorical variables were expressed as absolute and relative frequencies, and compared using the Chi-squared test or Fisher’s exact test. The response rate during treatment duration (complete response, partial response, stable disease or progressive disease) was compared between patients receiving sunitinib versus pazopanib with Fisher’s exact test.
Survival curves were estimated with the Kaplan–Meier method. Analyses of differences between survival curves were performed using the log-rank test. Prognostic factors were evaluated with univariable and multivariable analyses using the Cox proportional hazards model. Variables were included in the multivariable analyses if they presented a P value < 0.10 in the univariable analysis and were not associated with each other.
Statistical significance was defined by P values < 0.05. Statistical analyses were performed with Stata software, version 14 (StataCorp, Texas, USA).
Results
Patients’ characteristics
A total of 53 patients with metastatic nccRCC that received first-line sunitinib or pazopanib were identified. Sixteen (30.1%) patients received first-line treatment with sunitinib and 37 (69.9%), with pazopanib.
Median age was 56.3 years old (range 16.9–79.1). The predominant histologic subtypes were papillary (20 patients, 37.7%) and unclassified (17 patients, 32.1%). Other histologies that were present in smaller numbers were sarcomatoid differentiation (7 patients, 13.2%), chromophobe (5 patients, 9.4%) and MiT family translocation (4 patients, 7.6%). Central pathology review by a specialised urological pathologist resulted in the reclassification of two cases: one was reclassified as clear cell carcinoma (and, therefore, excluded from the present study), and another was reclassified from papillary to MiT family translocation nccRCC. The final histologic subtypes distribution is the one described here and presented in Figure 1.
All demographic, clinical and pathological characteristics of our patients are detailed in Table 1. The two treatment groups differed in terms of histologic subtypes, with a higher proportion of patients with unclassified and MiT family translocation histologies in the pazopanib group (P = 0.023). Other clinical and demographic characteristics were balanced between the two groups.
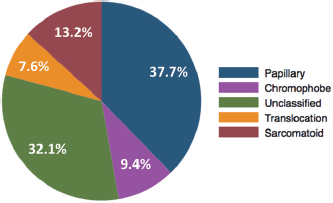
Figure 1. Pie chart of the distribution of histologic subtypes of nccRCC and sRCC in the study population.
Treatment efficacy
Thirty-five patients had adequate imaging available prior and after TKI treatment for evaluation of response according to RECIST 1.1. The response rate did not differ between the two groups (P = 1.000). Partial response occurred in 8.3% (N = 1) with sunitinib and 8.7% (N = 2) with pazopanib, while stable disease occurred in 66.6% (N = 8) and 60.8% (N = 14) and progressive disease in 25% (N = 3) and 30.4% (N = 7), respectively.
Forty-nine patients presented disease progression or death. Median PFS was 6.6 months with sunitinib and 4.9 months with pazopanib (HR 1.75, 95% CI 0.93–3.27, P = 0.078). Kaplan–Meier curves for PFS are presented in Figure 2.
At the time of the analysis, 46 patients had died. In the overall study population of nccRCC and sRCC, OS was inferior with pazopanib in comparison with sunitinib (median OS: 30.4 months with sunitinib versus 8.7 months with pazopanib, HR 2.71, 95% CI 1.31–5.58, P = 0.007). Three-year OS rates were 43.7% (95% CI 19.8–65.5%) with sunitinib versus 13.6% (95% CI 4.4–27.9%) with pazopanib. Kaplan–Meier curves for OS according to treatment group are presented in Figure 3.
Subgroup analyses
Groups were unbalanced regarding histology, as shown above (Table 1). Since the poorer prognosis of unclassified and sarcomatoid histologies could influence the results, we repeated the analyses excluding these two histologies (unclassified and sarcomatoid). A total of 29 patients (papillary, chromophobe and MiT family translocation histologies) were included in the analysis.
In this subgroup analysis, treatment with sunitinib was associated with better PFS than pazopanib (HR 3.04, 95% CI 1.24–7.44, P = 0.015). Median PFS was 8.9 months with sunitinib and 5.1 months with pazopanib.
OS was also superior with sunitinib in comparison with pazopanib (HR 3.16, 95% CI 1.20–8.29, P = 0.019), with a median OS of 38.7 months with sunitinib and 14.7 months with pazopanib. Three-year OS rates were 58.3% (95% CI 27–80%) and 17.3% (95% CI 2.8–42.1%), respectively. Kaplan–Meier curves for PFS and OS of this subgroup analysis (papillary, chromophobe and MiT family translocation histologies) are presented in Figure 4A and B.
In an exploratory comparison, no difference in OS according to treatment group was seen in unclassified (P = 0.840) and sarcomatoid histologies (P = 0.701). Median OS was 6.9 months in the unclassified histology group and 1.1 months in the sarcomatoid histology group.
Factors associated with overall survival
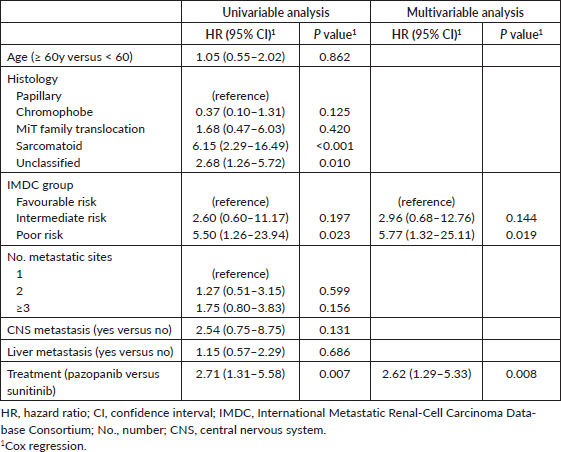
In the overall population, factors associated with inferior OS in the univariable analysis were unclassified histologic subtype (HR 2.68, 95% CI 1.26–5.72, P = 0.010), sarcomatoid histologic subtype (HR 6.15, 95% CI 2.29–16.40, P < 0.001), poor IMDC risk (HR 5.50, 95% CI 1.26–23.94, P = 0.023) and treatment with pazopanib (HR 2.71, 95% CI 1.31–5.58, P = 0.007).
Table 1. Patients’ characteristics.
The IMDC score and TKI treatment were included in the multivariable analysis. The histologic subtype was not included because it was associated with TKI treatment (P = 0.023). In the multivariable analysis, poor IMDC (HR 5.77, 95% CI 1.32–25.11, P = 0.019) and treatment with pazopanib (HR 2.62, 95% CI 1.29–5.33, P = 0.008) were associated with inferior OS. Table 2 summarises the results of factors associated with OS in the overall population.
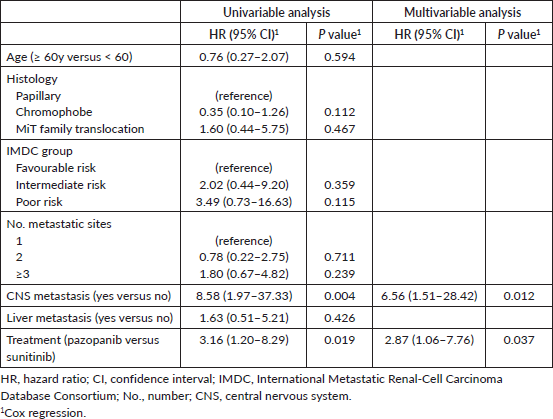
In the population excluding sarcomatoid and unclassified histologies, the presence of central nervous system (CNS) metastasis (HR 8.58, 95% CI 1.97–37.3, P = 0.004) and treatment with pazopanib (HR 3.16, 95% CI 1.20–8.29, P = 0.019) were associated with shorter OS. Both factors were also associated with inferior OS in the multivariable analysis (CNS: HR 6.56, 95% CI 1.51–28.42, P = 0.012; treatment with pazopanib: HR 2.87, 95% CI 1.06–7.76, P = 0.037). Table 3 presents the univariable and multivariable analyses of factors associated with OS in this population.
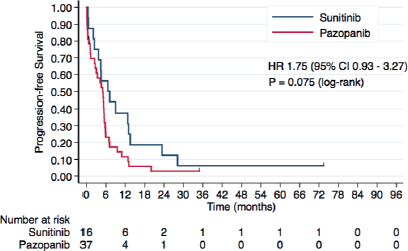
Figure 2. Kaplan–Meier curves for PFS according to the treatment group in the overall study population.
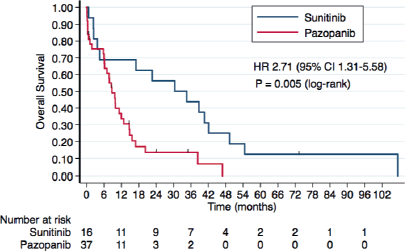
Figure 3. Kaplan–Meier curves for OS according to the treatment group in the overall study population.
Discussion
The present study suggests that patients with metastatic nccRCC have inferior OS when treated with pazopanib in comparison with sunitinib (median OS: 30.4 months versus 8.7 months; P = 0.005).
Since histologic subtypes were unbalanced between arms and unclassified and sarcomatoid histologies have poorer outcomes [20, 21], we repeated the analyses excluding these two histologies. This subgroup analysis showed that pazopanib was associated with inferior PFS (median: 8.9 months versus 5.1 months; P = 0.011) and OS (median: 38.7 months versus 14.7 months; P = 0.015) in patients with papillary, chromophobe and MiT family translocation subtypes. Multivariable analyses also supported the inferior outcomes with pazopanib. Unclassified and sarcomatoid histologies, otherwise, were associated with shorter survival, regardless of the TKI used.
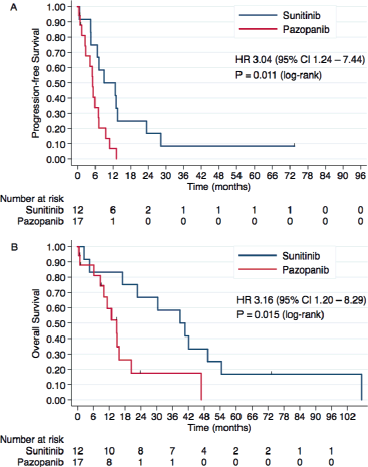
Figure 4. Kaplan–Meier curves for (A) PFS and (B) OS according to the treatment group in the population of nccRCC patients with papillary, chromophobe and MiT family translocation histologies.
For metastatic clear cell RCC, first-line therapy with pazopanib has shown to be non-inferior to sunitinib (17). Due to the extrapolation of this data to nccRCC, pazopanib has also been frequently used for these patients. As previously mentioned, sunitinib is the TKI agent with the most robust evidence for nccRCC [9, 13–16]. Prospective studies evaluating the efficacy of pazopanib are limited.
A previous retrospective cohort evaluated 37 patients with metastatic nccRCC treated with first-line pazopanib [22]. In this study, the median OS was 17.3 months, very similar to patients who received pazopanib in our study. On the other hand, a phase II single-arm trial with 29 patients with nccRCC treated with pazopanib showed more favourable outcomes, with median OS not reached and 1-year OS of 69% [23]. However, the majority of patients included had favourable Memorial Sloan-Kettering Cancer Center (MSKCC) risk, which may justify these better outcomes.
Data evaluating sRCC are scarce; however, some studies suggest poor outcomes with treatment with anti-VEGF TKIs [21, 24]. Phase III studies evaluating first-line immune-checkpoint inhibitors for metastatic RCC showed that the subgroup of patients with sRCC has high PD-L1 expression (47%–63%) [25, 26]. In the IMmotion151 trial, a study that compared the combination of atezolizumab and bevacizumab versus sunitinib, patients with sRCC had a benefit in PFS with the combination arm (HR 0.56, 95% CI 0.38–0.83) [27]. Moreover, the CheckMate214 trial showed that sRCC had encouraging response rate (57%) with nivolumab plus ipilimumab [28]. These results suggest that immune-checkpoint inhibitors might be a better therapeutic strategy for this subgroup of patients. Recently, two phase II trials have also shown some activity of immune checkpoint inhibitors for nccRCC. One evaluated atezolizumab plus bevacizumab and showed a response rate of 25% for nccRCC [29]. In the other, pembrolizumab was associated with response rates of 25.4% with papillary, 9.5% with chromophobe and 34.6% with unclassified nccRCC [30].
Table 2. Univariable and multivariable analyses of factors associated with OS in the overall study population.
Certainly, in order to improve the outcomes in these subgroups of patients, the distinct behaviour of each histologic subtype needs to be recognised. To study each subtype separately, multicentre efforts are required. Hopefully, advances in the comprehension of molecular features of these rare histologies and their microenvironment will allow to better understand these different diseases and develop personalised treatments [31].
Our study has several limitations that should be considered when interpreting the results. First, this is a single-centre retrospective analysis with a small sample size. While several provocative associations have been demonstrated, causal relationships are difficult to assess. Possible bias should be considered while interpreting our results. The two treatment groups, for example, were unbalanced in terms of histologic subtype, which was an important prognostic factor. We considered this possibility and repeated the analyses after exclusion of unclassified and sarcomatoid histologies, which confirmed the results. However, other confusion factors, including unbalance for unknown factors, might have influenced the results. The intrinsic difficulties of studying nccRCC were also faced. The population included was heterogeneous, composed by histologic subtypes with diverse disease behaviour, treatment response and prognosis. Another limitation is that subsequent treatment lines for RCC are not available in the Brazilian public health system and most patients received no further treatment after progression on the first-line TKI.
On the other hand, the present study is valuable for providing new data on rare and difficult to study diseases. The new hypothesis generation is a major contribution. The large OS difference observed in our study, together with the previous literature available on sunitinib, suggests that this drug is the TKI inhibitor with the best available evidence for effectiveness in the treatment of nccRCC so far.
Table 3. Univariable and multivariable analyses of factors associated with OS in the population of patients with papillary, chromophobe and MiT family translocation histologies.
Conclusions
In conclusion, in this single-centre retrospective cohort, pazopanib was associated with inferior OS in comparison with sunitinib as first-line treatment for metastatic nccRCC. However, the effectiveness of TKI treatment may differ importantly depending on the histologic subtype of nccRCC and sRCC. Ideally, this study’s findings should be confirmed prospectively by multicentre collaborative efforts with a larger number of patients.
Authors’ contributions
RC Bonadio, PI Velho, GN Marta, M Nardo, MCLA Souza, DQB Muniz and C Dzik participated in the concept and design of the study. RC Bonadio, PI Velho, GN Marta, M Nardo, MCLA Souza, ROF Bezerra, RKA Bispo and SF Faraj participated in the data collection. ROF Bezerra, PI Velho, GN Marta, M Nardo, MCLA Souza, David QB Muniz, ROF Bezerra, RKA Bispo, SF Faraj, DA Bastos and C Dzik participated in the data analysis, drafted, reviewed and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Ethical approval
The study was approved by the Local Research Ethics Committees.
Authors’ disclosures of potential conflicts of interest
Renata Colombo Bonadio
Travel, Accomodation, Expenses: Roche.
Pedro Isaacsson Velho
Honoraria: Bayer
Speakers’ Bureau: AstraZeneca, Pfizer, Bristol-Myers Squibb
Research Funding: Bristol-Myers Squibb, Pfizer (Inst)
Expert Testimony: Bayer
Travel, Accomodations, Expenses: AstraZeneca, Astellas Pharma, Pfizer, Merck Serono, Merck
Guilherme Nader Marta
Travel, Accomodation, Expenses: Bayer Schering Pharma and Roche.
Manoel Carlos Leonardi de Azevedo Souza
Travel, Accommodations, Expenses: Zodiac Pharma
Speakers’ Bureau: MSD, BMS, Amgen.
David QB Muniz
Speakers’ Bureau: Janssen and Pfizer.
Research funding: Pfizer
Travel, Accomodation, Expenses: Janssen.
Diogo Assed Bastos
Honoraria: Janssen, Bayer, Astellas, MSD, and BMS.
Research funding: Astellas, Janssen and Pfizer.
Mirella Nardo, Regis OF Bezerra, Raisa KA Bispo, Sheila F Faraj and Carlos Dzik have no relationship to disclose.
References
1. Cohen HT and McGovern FJ (2005) Renal-cell carcinoma N Engl J Med 353(23) 2477–2490 https://doi.org/10.1056/NEJMra043172 PMID: 16339096
2. Tsimafeyeu I (2017) Management of non-clear cell renal cell carcinoma: current approaches Urol Oncol Semin Orig Investig 35(1) 5–13 https://doi.org/10.1016/j.urolonc.2016.07.011
3. Cancer Genome Atlas Research Network (2016) Comprehensive molecular characterization of papillary renal-cell carcinoma N Engl J Med 374(2) 135–145 https://doi.org/10.1056/NEJMoa1505917
4. Durinck S, Stawiski E, and Pavía-Jiménez A, et al (2015) Spectrum of diverse genomic alterations define non-clear cell renal carcinoma subtypes Nat Genet 47(1) 13 https://doi.org/10.1038/ng.3146 PMCID: 4489427
5. Cheville J, Lohse C, and Zincke H, et al (2004) Sarcomatoid renal cell carcinoma: An examination of underlying histologic subtype and an analysis of associations with patient outcome Am J Surg Pathol 28 435–441 https://doi.org/10.1097/00000478-200404000-00002 PMID: 15087662
6. Hsieh JJ, Purdue MP, and Signoretti S, et al (2017) Renal cell carcinoma Nat Rev Dis Prim 3 17009 https://doi.org/10.1038/nrdp.2017.9 PMID: 28276433 PMCID: 5936048
7. Sternberg CN, Davis ID, and Mardiak J, et al (2010) Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III Trial J Clin Oncol 28(6) 1061–1068 https://doi.org/10.1200/JCO.2009.23.9764 PMID: 20100962
8. Motzer RJ, Hutson TE, and Tomczak P, et al (2007) Sunitinib versus Interferon Alfa in Metastatic Renal-Cell Carcinoma N Engl J Med 356(2) 115–124 https://doi.org/10.1056/NEJMoa065044 PMID: 17215529
9. Escudier B, Porta C, and Schmidinger M, et al Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up
10. Network. NCC. Kidney Cancer (Version 4.2019) (2019) [Internet] [https://www.nccn.org/professionals/physician_gls/pdf/kidney_blocks.pdf]
11. Hudes G, Carducci M, and Tomczak P, et al (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma N Engl J Med 356(22) 2271–2281 https://doi.org/10.1056/NEJMoa066838 PMID: 17538086
12. Dutcher J, de Souza P, and McDermott D, et al (2009) Effect of temsirolimus versus interferon-alpha on outcome of patients with advanced renal cell carcinoma of different tumor histologies Med Oncol 26 202–209 https://doi.org/10.1007/s12032-009-9177-0 PMID: 19229667
13. Armstrong AJ, Halabi S, and Eisen T, et al (2016) Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): A multicentre, open-label, randomised phase 2 trial Lancet Oncol 17(3) 378–388 https://doi.org/10.1016/S1470-2045(15)00515-X PMID: 26794930
14. Tannir NM, Jonasch E, and Albiges L, et al (2016) Everolimus versus sunitinib prospective evaluation in metastatic non-clear cell renal cell carcinoma (ESPN): A randomized multicenter phase 2 trial Eur Urol 69(5) 866–874 https://doi.org/10.1016/j.eururo.2015.10.049 PMCID: 4879109
15. Motzer RJ, Barrios CH, and Kim TM, et al (2014) Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma J Clin Oncol 32(25) 2765–2772 https://doi.org/10.1200/JCO.2013.54.6911 PMID: 25049330 PMCID: 5569681
16. Fernández-Pello S, Hofmann F, and Tahbaz R, et al (2017) A systematic review and meta-analysis comparing the effectiveness and adverse effects of different systemic treatments for non-clear cell renal cell carcinoma Eur Urol 71(3) 426–436 https://doi.org/10.1016/j.eururo.2016.11.020
17. Motzer RJ, Hutson TE, and Cella D, et al (2013) Pazopanib versus sunitinib in metastatic renal-cell carcinoma N Engl J Med 369(8) 722–731 https://doi.org/10.1056/NEJMoa1303989 PMID: 23964934
18. Heng D, Xie W, and Regan M, et al (2013) External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study Lancet Oncol 14(2) 141–148 https://doi.org/10.1016/S1470-2045(12)70559-4 PMID: 23312463 PMCID: 4144042
19. Eisenhauer E, Therasse P, and Bogaerts J, et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer 45(2) 228–247 https://doi.org/10.1016/j.ejca.2008.10.026
20. Zisman A, Chao D, and Pantuck A, et al (2002) Unclassified renal cell carcinoma: clinical features and prognostic impact of a new histological subtype J Urol 168(3) 950–955 https://doi.org/10.1016/S0022-5347(05)64549-1 PMID: 12187197
21. El Mouallem N and Smith SC PA (2018) Sarcomatoid renal cell carcinoma: Biology and treatment advances Urol Oncol Semin Orig Investig 36(6) 265–271 https://doi.org/10.1016/j.urolonc.2017.12.012
22. Buti S, Bersanelli M, and Maines F, et al (2017) First-line pazopanib in non-clear-cell renal carcinoma: the Italian retrospective multicenter PANORAMA study Clin Genitourin Cancer 15(4) e609–e614 https://doi.org/10.1016/j.clgc.2016.12.024 PMID: 28108284
23. Jung K, Lee S, and Park S, et al (2018) Pazopanib for the treatment of non-clear cell renal cell carcinoma: a single-arm, open-label, multicenter, phase II study Cancer Res Treat Off J Korean Cancer Assoc 50(2) 488
24. Beuselinck B, Lerut E, and Wolter P, et al (2014) Sarcomatoid dedifferentiation in metastatic clear cell renal cell carcinoma and outcome on treatment with anti–vascular endothelial growth factor receptor tyrosine kinase inhibitors: a retrospective analysis Clin Genitourin Cancer 12(5) e205–e214 https://doi.org/10.1016/j.clgc.2014.04.004 PMID: 24861951
25. Motzer R, Tannir N, and McDermott D, et al (2018) Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma N Engl J Med 378(14) 1277–1290 https://doi.org/10.1056/NEJMoa1712126 PMID: 29562145 PMCID: 5972549
26. Rini B, Huseni M, and Atkins M, et al (2018) Molecular correlates differentiate response to atezolizumab (atezo) bevacizumab (bev) vs sunitinib (sun): results from a Phase III study (IMmotion151) in untreated metastatic renal cell carcinoma (mRCC) ESMO Annual Meeting, Genitourinary Cancers/Cancer Immunology and Immunotherapy/Anticancer agents & Biologic Therapy 20
27. Motzer R, Powles T, and Atkins M, et al (2018) IMmotion151: A randomized phase III study of atezolizumab plus bevacizumab vs sunitinib in untreated metastatic renal cell carcinoma (mRCC) J Clin Oncol 36(suppl 578) https://doi.org/10.1200/JCO.2018.36.6_suppl.578
28. McDermott D, Motzer R, and Rini B, et al (2018) ChechMate214 retrospective analyses of nivolumab plus ipilimumab or sunitinib in IMDC intermediate/poor-risk patients with previously untreated advanced renal cell carcinoma with sarcomatoid features Seventeenth International Kidney Cancer Symposium
29. McKay R, McGregor B, and Gray K, et al (2019) Results of a phase II study of atezolizumab and bevacizumab in non-clear cell renal cell carcinoma (nccRCC) and clear cell renal cell carcinoma with sarcomatoid differentiation (sccRCC) J Clin Oncol 37(suppl 548) https://doi.org/10.1200/JCO.2019.37.7_suppl.548
30. McDermott D, Lee J, and Ziobro M, et al (2019) First-line pembrolizumab (pembro) monotherapy for advanced non-clear cell renal cell carcinoma (nccRCC): Results from KEYNOTE-427 cohort B J Clin Oncol 37(suppl 546) https://doi.org/10.1200/JCO.2019.37.7_suppl.546
31. Kotecha RR and Motzer RJ VM (2019) Towards individualized therapy for metastatic renal cell carcinoma Nat Rev Clin Oncol 1 epub






