Repurposing drugs in oncology (ReDO)—selective PDE5 inhibitors as anti-cancer agents
Pan Pantziarka1,2, Vidula Sukhatme3, Sergio Crispino1, Gauthier Bouche1, Lydie Meheus1 and Vikas P Sukhatme3,4
1Anticancer Fund, Brussels, Strombeek-Bever 1853, Belgium
2The George Pantziarka TP53 Trust, London KT1 2JP, UK
3GlobalCures Inc., Newton, MA 02459, USA
4Emory University School of Medicine, Atlanta, GA 30322, USA
Correspondence to: Pan Pantziarka. E mail: anticancer.org.uk@gmail.com
Abstract
Selective phosphodiesterase 5 inhibitors, including sildenafil, tadalafil and vardenafil, are widely-used in the treatment of erectile dysfunction and pulmonary arterial hypertension. They are also well-known as examples of successful drug repurposing in that they were initially developed for angina and only later developed for erectile dysfunction. However, these drugs may also be effective cancer treatments. A range of evidentiary sources are assessed in this paper and the case made that there is pre-clinical and clinical evidence that these drugs may offer clinical benefit in a range of cancers. In particular, evidence is presented that these drugs have potent immunomodulatory activity that warrants clinical study in combination with check-point inhibition.
Keywords: drug repurposing, PDE5 inhibitors, sildenafil, tadalafil, verdenafil, immunotherapy
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Published: 11/04/2018; Received: 22/01/2018
Introduction
Phosphodiesterase (PDE) inhibitors are drugs which block the activity of one or more of the 12 PDE isoforms, thereby modulating intracellular levels of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP). PDE5 acts principally on the nitric oxide (NO)/cGMP signalling pathway, clinically important in the treatment of pulmonary hypertension and erectile dysfunction [1, 2]. A range of partially selective PDE5 inhibitors have been developed, including sildenafil, tadalafil, vardenafil, avanafil and udenafil. There are also a number of drugs which have non-selective PDE inhibitory activity, of which some have a degree of PDE5 inhibition, for example, dipyridamole and cilostazol. This review focuses on the anti-cancer properties of the partially selective PDE5 inhibitors, particularly sildenafil, tadalafil and vardenafil.
Sildenafil was the first of the PDE5 inhibitors to be commercially developed, by Pfizer, in the late 1980s. It was originally intended as a treatment for angina pectoris, and entered into the first clinical trials in 1991 [3]. Among the side effects reported in these early studies were penile erections. At that time oral drug treatments did not exist for erectile dysfunction; therefore, as interest in using sildenafil for angina waned, the first trials in erectile dysfunction were initiated in 1993.These early trials were successful and eventually sildenafil, marketed as Viagra, was approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) in 1998. Subsequently sildenafil was repurposed as a treatment for pulmonary arterial hypertension [4], and has been investigated as a possible treatment for a number of other conditions, most notably Raynaud’s phenomenon [5].
Tadalafil, trade-marked as Cialis, was also developed as a treatment for erectile dysfunction [6]. In addition the drug is approved in multiple markets for pulmonary arterial hypertension and benign prostatic hyperplasia [7]. Vardenafil and avanafil have EMA and FDA approvals for erectile dysfunction. Udenafil is not FDA-approved, and has orphan drug designation by the EMA for the treatment of functional single ventricle congenital heart disease. It has approval in South Korea and Malaysia for the treatment of erectile dysfunction.
Sildenafil, tadalafil and vardenafil are generic medicines, with multiple manufacturers active in different markets.
Current usage
Dosage
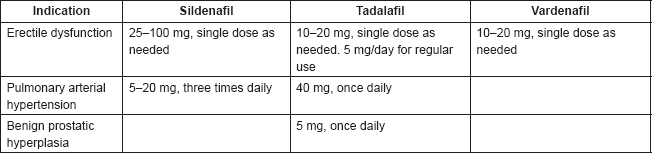
The posology of PDE5 inhibitors varies by indication and by drug, as shown in Table 1.
Toxicity
Common side-effects include dyspepsia, nausea, vomiting, headaches, dizziness, myalgia, back pain and visual disturbances. Less common side-effects can include red eyes, palpitations, tachycardia, hypotension, hypertension and nose bleeds. Rare adverse events have included severe cardiovascular events, sudden hearing loss and retinal vascular occlusion.
PDE5 inhibitors are contra-indicated in patients receiving nitrates or with a previous history of non-arteritic anterior ischemic optic neuropathy. Caution is also advised in patients with cardio-vascular disease, hypotension, recent stroke and left ventricular outflow obstruction.
Table 1. Posology of most commonly used PDE5 inhibitors.
Pharmacokinetics
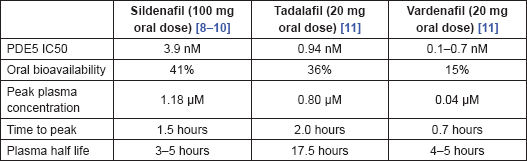
There is significant variation in the pharmacokinetic characteristics of the most widely used PDE5 inhibitors, summarised in Table 2. There are also differences in the degree of PDE5 selectivity. Sildenafil has a high selectivity for PDE5, >1000-fold compared to PDE2, PDE3 and PDE4, but has less so over PDE1 (>80-fold) and PDE6 (>10-fold). Tadalafil is also highly selective for PDE5, >700-fold relative to PDE6, >10,000-fold relative to PDE1–4 and PDE7–10, and >5-fold relative to PDE11. The figures for vardenafil for PDE5 are >15-fold relative to PDE6, >130-fold relative to PDE1, >300-fold relative to PDE11, and >1000-fold relative to PDE2–4 and PDE7–10.
Pre-clinical evidence in cancer—in vitro and in vivo
PDE5 inhibition was first shown to induce apoptosis in vitro in the SW480 colon tumour cell line using the drug exisulind (an active metabolite of the NSAID COX-inhibitor sulindac) by Thompson et al. in 2000 [12]. Exisulind analogues with no cyclo-oxygenase inhibitory activity but increased PDE isoform selectivity showed increased pro-apoptotic activity. PDE5 inhibition using exisulind, E4021 (a specific inhibitor of PDE5), sildenafil, dipyridamole and zaprinast was associated with a reduction in cGMP activity which correlated with apoptosis induction. The same group studied the effect of exisulind in chemically induced (N-butyl-N-(4-hydroxybutyl) nitrosamine) bladder cancer in rats [13]. Results showed that treatment reduced tumour size and multiplicity. Analysis using the human bladder cancer cell line HT1376 showed that the growth inhibitory effect was associated with a reduction in expression of PDE4 and PDE5.
Chronic lymphocytic leukaemia
In 2003 both sildenafil and vardenafil were shown to cause caspase-dependent apoptosis in patient-derived B-cell chronic lymphocytic leukaemia (B-CLL) cells [14]. The investigation was prompted by the case of a previously untreated man with B-CLL who showed clinically significant improvement following treatment with sildenafil (the patient was treated for erectile dysfunction at a dose of 50 mg/week for a period of 3 months). In vitro sildenafil, at a concentration of 50 μg/ml, induced apoptosis in 14 of 14 patient samples. The EC50 (effective concentration of drug that inhibited viability of treated B-CLL cells to 50% of untreated cells), was 4.1 μM for sildenafil and 1.5 μM for vardenafil.
Prostate
Qian et al. [15] investigated the effect of sildenafil in an orthotopic xenograft model of prostate cancer. Male athymic BALB/c nude mice were implanted with PC-3 human prostate cancer cells and treatment with sildenafil, at two different dose levels, or vehicle commencing on day 31 following tumour cell inoculation. Sildenafil was administered by oral gavage at doses of 50 mg/kg or 25 mg/kg, note that the upper dose was calculated to approximate a human sildenafil dose of 100 mg. Results showed no significant effect on primary tumour growth or on metastatic spread when compared to controls. Pernkopf et al. [16] later showed that sildenafil, vardenafil and tadalafil had no effect on the proliferation of prostate cancer cell lines in vitro, even at high concentrations (1 mg/ml).
Table 2. Pharmacokinetics of sildenafil, tadalafil and vardenafil.
A potentiation of therapeutic response to chemotherapy was reported by Das et al. [17]. The combination of sildenafil and doxorubicin were assessed in vitro. Co-treatment was found to have an additive effect in reducing proliferation and enhancing the rate of apoptosis in PC-3 and DU145 prostate cancer cells, in contrast treatment with sildenafil alone had no effect. In vivo, the combination of doxorubicin and sildenafil (at an oral dose of 10 mg/kg) in BALB/c mice bearing human PC-3 prostate cancer xenografts significantly (P < 0.05) reduced tumour growth compared to controls. The authors also noted an amelioration of the cardiotoxicity induced by doxorubicin by the addition of sildenafil. Later in vitro work by the same group showed that physiologically relevant concentrations of sildenafil, vardenafil and tadalafil enhanced the lethality of a range of chemotherapeutic drugs in a number of gastric cancer cell lines [18].
Colorectal
Serafini et al. [19] used a number of in vivo models to demonstrate an immune-mediated anti-tumour effect of sildenafil and tadalafil. BALB/c mice were challenged with CT26WT (colon carcinoma), C26GM (a more aggressive variant of CT26WT) or TS/A (mammary adenocarcinoma) and C57BL/6 with MCA203 (murine fibrosarcoma) cell lines and then treated with the PDE5 inhibitors, starting on the day of inoculation. Treatment reduced tumour growth by 50%–70% compared to controls. Sildenafil treatment commencing on day 7 following inoculation also showed sustained retardation of tumour growth. Experiments in immunodeficient mice showed no difference in tumour growth between mice treated with sildenafil and controls. Additional elucidation of the immune-related mechanisms, (discussed later), was later performed by some of the same authors in a B-cell lymphoma (A20) murine model [20] and by a different group in murine colon cancer and T-lymphoma models [21]. Rigamonti et al. [22] also investigated the immune-related effects of sildenafil in two murine models of prostate cancer.
Lin et al. [23] showed that PDE5 is over-expressed in human colorectal cancer samples and in azoxymethane/dextran sodium sulphate (AOM/DSS)-induced colon cancers in male BALB/c mice. Intraperitoneal injection of sildenafil (25 mg/kg) for 5 days significantly inhibited PDE5 over-expression, tumour multiplicity and volume in murine AOM/DSS-induced colon cancers compared to untreated controls. Further analysis suggested these positive findings were associated with a reduction in DSS-induced inflammation, specifically a reduction in myeloid-derived suppressor cells (MDSC) infiltration into colonic tissues—a finding also replicated in vitro. Islam et al. [24] also showed that oral sildenafil (in water, estimated daily dose 5.7 mg/kg) reduced chemically induced polyp formation and colonic inflammation in mice.
Mei et al. [25] used a range of human colorectal cancer cell lines, (HT-29, SW480, SW620, HCT116 and SW1116), in vitro to assess the effect of sildenafil on proliferation and apoptosis. Results showed IC50 values in the range 190–270 μM. In vivo nude mice were implanted with SW480 or HCT116 human cancer cells and treated by oral gavage with sildenafil, either at 50 or 150 mg/kg every 2 days. Tumour volumes were reduced by 40.1% and 57.8% in the SW480 xenografts and by 13.3% and 61.4% in HCT116 xenografts, respectively (P < 0.05).
Brain
Using a rat gliosarcoma (9L) model, Black et al. [26] showed that a combination of vardenafil and doxorubicin increased survival compared to untreated controls or either drug alone. Treatment commenced four days after orthotopic implantation of 9L cells in female Fischer rats. Vardenafil was administered orally at a dose of 10 mg/kg, doxorubicin at a dose of 2 mg/kg IV and saline administered to controls. The combination treatment was superior to all three single drug treatments (mean 53 ± 4 days, P < 0.05), including doxorubicin alone (mean 42 ± 2 days) which significantly improved survival (P < 0.05) compared to control (mean 32 ± 2 days) or vardenafil alone (mean 35 ± 1 days). Subsequently the same group demonstrated improved survival in nude mice bearing cranially-implanted breast and lung cancer tumours, mimicking metastatic spread to the brain, and treated with trastuzumab and vardenafil [27].
Othman et al. [28] used primary and recurrent medulloblastoma cell cultures derived from pediatric patients to explore mechanisms of chemoresistance in vitro. Cell lines with increased expression of ABCB1 (also known as P-glycoprotein or MDR1) showed relative resistance to in vitro treatment with etoposide. However co-treatment with vardenafil (5 and 10 μM) or verapamil increased sensitivity to etoposide.
Roberts et al. [29] also determined that co-treatment of a number of medulloblastoma cell lines (D283, DAOY, HOSS 1 and VC312) with sildenafil (2 μM) or tadalafil (2 μM) increased the lethality of standard of care chemotherapy drugs (vincristine, cisplatin and etoposide). Similarly, they showed that sildenafil and tadalafil had additive effects when combined with a non-COX2-inhibitory derivative of celecoxib (OSU-03012) in vitro with parental glioma and stem-like glioma cells [30].
Breast
Di et al. [31] reported on the in vitro potentiation of doxorubicin cytotoxicity by sildenafil in a panel of breast cancer cell lines, and an in vivo reduction in tumour growth rate in a 4T1 breast cancer model (P ≤ 0.05), results also confirmed by Greish et al. [32].
Roberts et al. [33] showed that the combination of sildenafil (0.5 μM) with celecoxib (1 μM) was also cytotoxic in vitro in breast, hepatoma, colorectal cancer, glioblastoma and medulloblastoma cell lines. Furthermore, the addition of the multiple sclerosis drug FTY720 (fingolimod), fenretinide or all-trans retinoic acid (ATRA) increased the cytotoxicity of the sildenafil celecoxib combination. In vivo, athymic mice bearing BT474 breast cancer tumours were treated with vehicle, sildenafil (5 mg/kg/day), celecoxib (10 mg/kg/day) or combination for 5 days. The combination showed significantly (P < 0.05) lower tumour growth volume compared to single drug treatment. The addition of fingolimod (0.05 mg/kg) slowed tumour growth and increased survival compared to the sildenafil celecoxib combination (P < 0.01).
Sildenafil was also used as an adjuvant in an in vivo study of an experimental local tumour ablation modality DaRT (diffusing alpha-emitters radiation therapy) [34]. As with many local ablative therapies, there is some evidence that DaRT can initiate a systemic anti-tumour immune response (abscopal effects) via the release of tumour antigens during local tumour tissue destruction. Confino et al. treated BALB/c female mice bearing DA3 undifferentiated breast adenocarcinoma tumours were treated with DaRT, sildenafil, control or the combination (20 mg/kg/day in drinking water for 6 weeks). Combination treatment reduced tumour volume growth compared to single treatments or control (P < 0.05). The combination of DaRT, sildenafil and low-dose cyclophosphamide also slowed tumour growth, as did the further addition of CpG.
Melanoma
Meyer et al. [35] employed a ret transgenic mouse model of melanoma to investigate the impact of sildenafil on chronic inflammation and the immunosuppressive activity of MDSC. Tumour-bearing mice received sildenafil with drinking water (20 mg/kg/day) for 6 weeks and showed significant (P = 0.002) increase in survival compared to untreated controls. This improved survival was associated with inhibition of MDSC immunosuppressive functions and the restoration of T-cell function. The same group also showed that female C57BL/6 mice bearing syngeneic Panc02 pancreatic tumours and treated with sildenafil in drinking water (20 mg/kg/day) had increased survival compared to untreated mice (P < 0.01), however male mice showed a trend towards decreased survival [36].
Multiple myeloma
Kumazoe et al. [37] showed that a range of PDE5 inhibitors enhanced the apoptotic effects of the green tea polyphenol (–)-epigallocatechin-3-O-gallate (EGCG) in a panel of multiple myeloma (MM) cell lines. Sildenafil (10 μM) and vardenafil (5 μM) significantly reduced the viability of U266, ARH77 and RPMI8266 cell lines pre-treated with EGCG (5 μM) compared to EGCG alone (P < 0.001). Data from primary MM cells, (n = 10), showed that EGCG and vardenafil alone had little impact on viability, but that the combination reduced viability to a similar degree to the MM cell lines. Similar in vitro results were shown for MKN45 (gastric cancer), PANC-1 (pancreatic cancer), and PC3 (prostate cancer) cell lines. In vivo, female BALB/c mice injected with murine MM cells (MPC-11) were given treated with intraperitoneal injections of EGCG (15 mg/kg) and/or vardenafil (5 mg/kg) every 2 days data. Mice treated with the combination showed significantly reduced tumour volume (P = 0.019) and improved survival (P < 0.001). Subsequently the same group showed similar in vitro results, in primary patient samples and established cell lines, with the combination of EGCG and vardenafil in acute myeloid leukaemia and chronic lymphocytic leukaemia (CLL) [38, 39].
Lung
Sildenafil, vardenafil and dipyridamole were investigated by Li and Shu [40] as potential enhancers of response to cytotoxic chemotherapy drugs in the H1915 lung cancer cell line and in a murine model. In vitro results showed that all three drugs tested, (at concentrations: 20 μM vardenafil, 100 μM sildenafil and 20 μM dipyridamole), increased the uptake of doxorubicin and carboplatin. Vardenafil at 20 μM also reduced the viability of H1915 cells when treated with a range of doses of doxorubicin and carboplatin. For the in vivo experiments, using athymic nude mice, only vardenafil was used, at an oral dose of 10 mg/kg for 5 days per week. As with the in vitro results, vardenafil increased the tumour accumulation of dextran, trastuzumab and doxorubicin, although only the first two results were significant (P < 0.05). Furthermore, combination treatment of vardenafil and trastuzumab significantly slowed tumour growth compared to either drug alone or to untreated controls.
Booth et al. [41] showed the synthetic lethality of the combination of sildenafil, vardenafil or tadalafil with pemetrexed in non-small cell lung cancer (NSCLC) cell lines and in vivo of the combination of sildenafil (5 mg/kg) and pemetrexed. The work has also been extended to show that the combination of sildenafil, pemetrexed and sorafenib enhances the in vivo anti-tumour effects of the pemetrexed sorafenib combination in a H460 NSCLC model [42], the combination of celecoxib, sildenafil and sorafenib in a panel of ovarian cancer cells lines [43] and pemetrexed, sildenafil and sodium valproate in NSCLC and ovarian cell lines [44]. Domvri et al. [45] also showed in vitro that 100 μM sildenafil increased the response to carboplatin in the H1048 SCLC cell line and the A549 NSCLC cell line.
Lymphoma
Wang et al. [46] investigated whether tadalafil modulated rituximab activity in a murine model of brain lymphoma. Raji human lymphoma cells were implanted in the cranium of athymic mice and allowed to form and then mice were treated with saline, tadalafil at 1.5 mg/kg (oral gavage at twice per week), rituximab 30 mg/kg, (via tail vein injection twice per week) or combination of tadalafil and rituximab. Survival analysis showed that tadalafil alone was similar to untreated control, rituximab alone was significantly (P = 0.01) superior to tadalafil alone and that the combination of tadalafil and rituximab was significantly (P = 0.03) better than rituximab alone (rituximab group, 25.75 ± 1.892 days; and tadalafil rituximab group, 29 ± 1.414 days).
Liver
Sildenafil was also investigated pre-clinically by Tavallai et al. [47] as a potential synergist with sorafenib and regorafenib in liver and colorectal cancer. Sildenafil (2 μM) in combination with sorafenib and regorafenib increased cell death in hepatoma cell lines (HEP2G, HEP3B and HuH7) in vitro. In vivo athymic mice bearing HuH7 (hepatoma), HCT116 (colorectal) and BT474 (breast) tumours were treated with vehicle, sildenafil (5 mg/kg), regorafenib (25 mg/kg) or the combination. Combination treatment significantly reduced tumour growth (P < 0.05) compared to regorafenib alone. The combination of sildenafil and sorafenib reduced the increase in tumour volume compared to sorafenib alone in mice bearing HuH7 tumours. The addition of fingolimod to the combination of sildenafil and regorafenib in HT29 colon cancer models increased tumour regrowth after the cessation of drug treatment compared to the sildenafil and regorafenib combination.
Head and neck
In addition to showing that tadalafil and sildenafil reduced cell viability in a panel of head and neck squamous cell carcinoma (HNSCC) lines (UM1, UM6, UM47 and CAL27), Tuttle et al. [48] also explored the effect in an in vivo model. Athymic (nu/nu) mice inoculated with CAL27 cells were treated with tadalafil (via osmotic pumps delivering 1 mg/kg/day) or vehicle. Tumour weight and volume were significantly reduced compared to controls (P < 0.05).
Sponziello et al. [49] showed that PDE5A was overexpressed in a series of human papillary thyroid carcinomas compared to normal tissues. Expression was higher in samples with BRAF V600E mutation compared to wild-type BRAF. Furthermore it was shown that sildenafil and tadalafil reduced proliferation and migration in BCPAP, TPC-1 and 8505C thyroid cancer cell lines. The anti-proliferative effects could be enhanced through the use novel nano-formulations of the two drugs [50].
Rhabdomyosarcoma
Zenitani et al. [51] investigated the combination of sildenafil and C-type natriuretic peptide (CNP), an endogenous peptide secreted by vascular endothelial cells, pre-clinically in pediatric rhabdomyosarcoma (RMS) [51]. While CNP showed anti-proliferative effects on two RMS cell lines, sildenafil alone showed an anti-proliferative effect in the cell RMS-YM-GC-B cell line but not the RD-GC-B cell line (all P < 0.05). However, sildenafil enhanced the anti-proliferative effects of CNP in both cell lines compared to either single agent treatment (P < 0.05). In vivo the combination of CNP and sildenafil (20 mg/kg, intraperitoneally every second day) attenuated tumour weight and volume compared to vehicle control (P < 0.05).
Ehrlich ascites carcinoma
El-Naa et al. [52] used an in vivo ehrlich ascites carcinoma (EAC) model to demonstrate synergy between cisplatin and sildenafil. Sildenafil was administered in drinking water at a dose of 5 mg/kg/day for 15 days following inoculation with EAC cells. Single agent treatment with sildenafil and cisplatin showed a significant reduction in tumour volume growth compared to untreated controls (P < 0.05), and the combination of sildenafil and cisplatin showed significant reduction compared to both single agent treatments (P < 0.05).
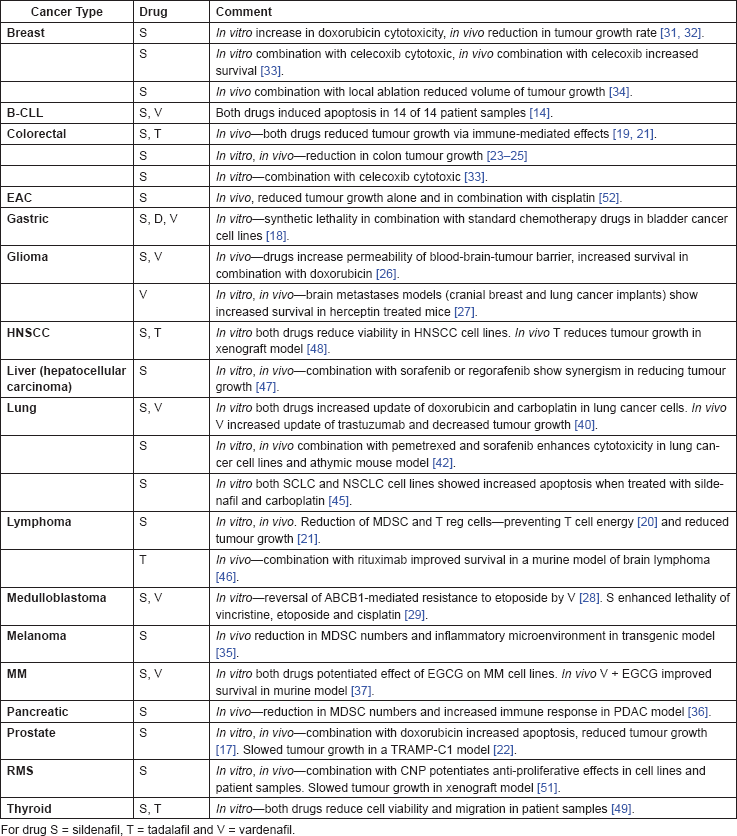
The pre-clinical evidence, both in vitro and in vivo, is summarised in Table 3.
Human evidence
The first report of anticancer activity of a PDE5 inhibitor in humans was published in 2004 [53]. Treon et al. published a report of five cases in which patients suffering from Waldenstrom’s macroglobulinemia, an uncommon and incurable B-cell malignancy, responded to treatment with sildenafil. The initial case was an 80-year old man who showed a complete response upon commencing sildenafil treatment for erectile dysfunction. On noting this unexpected outcome, four further cases were discovered in the same clinic, all four cases showing reductions in serum immunoglobulin M (IgM) levels—although none showed the complete response of the first patient. Additional ex vivo analysis showed evidence that sildenafil, at a concentration of 0.01 μg/mL, caused apoptosis in lymphoplasmacytic cells from the five patients.
Based on these incidental findings a small Phase II open-label clinical trial (NCT00165295) was initiated [54]. Patients, (n = 30, 18 of whom were treatment naive), with slowly progressive Waldenstrom’s macroglobulinemia and ineligible for active therapy were treated with 100 mg/day of sildenafil, with a starting dose of 25 mg/day and escalating to the target dose over a 4 week period. At a median of 3 months, serum IgM levels declined in 19/30 (63%) patients by around 18% and 5/30 patients (17%) demonstrated at least a minor response (≥25% IgM decrease).
An incidental finding that sildenafil has an effect on severe lymphatic malformations was also reported by Swetman et al. [55]. A 10-week old child suffering from pulmonary hypertension as a consequence of severe lymphatic malformations in the chest was treated with sildenafil to address the symptoms. After commencing treatment the malformation diminished and was subsequently confirmed by MRI. Based on this case two more children were treated and also showed reduction in the extent of the lesions and improvements in functioning. Gandhi et al also reported on two cases of pediatric orbital lymphangioma which responded to sildenafil treatment [56]. Although these are benign lesions, it is instructive to compare these results to the incidental finding that propranolol was effective in the treatment of infantile hemangioma and other highly vascularised benign tumours, subsequently leading to the investigation of propranolol in angiosarcoma [57].
Huilgol and Jain [58] reported on three patients with penile squamous cell carcinoma who were treated with sildenafil as a radiosensitiser. The authors hypothesised that the increased blood flow due to sildenafil when used for erectile dysfunction would be beneficial in reducing hypoxia and hence improving response to radiotherapy. The men were treated with sildenafil at 50 mg/day 5 days a week, administered 30 minutes prior to radiotherapy and all three completed planned treatment. One patient suffered local recurrence at 10 months and succumbed to disease at 23 months. The other two patients were disease free at 53 and 48 months respectively.
Noonan et al. [59] reported on a patient with end-stage MM treated with tadalafil in combination with lenalidomide, clarithromycin and dexamethasone. The 50-year old man had been treated with multiple regimens after recurrence following near complete remission with VAD (vincristine, doxorubicin and dexamethasone) induction therapy and autologous stem cell transplant. He had been treated with lenalidomide, dexamethasone and clarithromycin but had to stop due to intolerance. However, with the addition of tadalafil the patient was able to tolerate lenalidomide and dexamethasone, and with the addition of the antibiotic clarithromycin (which has evidence of anti-myeloma activity [60]), the patient achieved a very good partial response (near 90% reduction in disease burden). Analysis showed a reduction in MDSC numbers and a restoration of T cell function.
Table 3. Summary of in vitro and in vivo evidence.
Beneficial impacts of tadalafil were also shown in a small (n = 35) randomised clinical trial (NCT00843635) in HNSCC [61]. Patients were randomised to tadalafil at 10 mg/day, 20 mg/day or placebo for at least 20 days pre-operatively (drug was stopped 36 hours prior to surgery). Tadalafil significantly reduced MDSC and T reg numbers in the blood of patients, with optimal immunomodulatory response between a dose of 145 and 225 μg/kg. It was noted that the immunomodulatory effect was blunted at higher concentrations, possibly due to off-target inhibition of PDE11 by tadalafil. A separate randomised controlled trial in HNSCC patients (NCT00894413) at the same institution showed that tadalafil, at a dose of 20 mg/day, significantly decreased peripheral MDSC numbers and increased general immunity as measured by delayed type hypersensitivity response (P < 0.002) [62].
Building on prior work that showed that chronic inflammation, MDSCs and T reg cell numbers were associated with a worse prognosis in advanced melanoma [63], and in vivo studies in a transgenic mouse model [35], a small pilot trial (n = 12) of tadalafil was carried out in palliative care patients with metastatic melanoma [64]. The majority of patients (11/12) in the TaMe trial were heavily pre-treated with a range of interventions, including checkpoint inhibitors and chemotherapy; one patient had not been pre-treated due to a contra-indication to ipilimumab. A dose de-escalation design was used and four dose levels tested: 5, 10, 20 and 40 mg/day. Tumour and peripheral blood samples were taken before and 4 weeks after the start of treatment to assess immunological response, with change in CD8 tumour infiltrating lymphocytes (TILs) as primary end point. Stable disease was achieved in 3/12 patients (25%), with median progression-free survival (PFS) of 4.6 months (range 0.7–7.1) and overall survival (OS) 8.5 months (range 2.7–23.7). Patients with stable disease displayed significantly higher pre-treatment CD8 TILs in the centre of metastases and after therapy showed increased expression of ζ-chain in CD8 and CD4 TILs and CD8 T cells in the peripheral blood. Metastases of stable patients were also characterised by a significant reduction of FOXP3 T reg cells post-treatment as compared with baseline. There was no relationship between stable disease and tadalafil dose.
In addition to these examples of possible anticancer effects of PDE5 inhibitors, there have also been concerns that chronic use of sildenafil and related drugs may be associated with an increased risk of cancer incidence. Based on data that showed that low PDE5A expression increased the invasiveness of melanoma cells [65], Li et al. [66] conducted a large prospective cohort study in US men to assess the association between sildenafil use for erectile dysfunction and incidence of melanoma and other skin cancers. The authors reported an elevated risk of melanoma hazard ratio (HR), 1.92; 95% confidence interval (CI) 1.14—3.22] but no increased risk of squamous cell carcinoma or basal cell carcinoma. A subsequent Swedish analysis by Loeb et al. [67] also reported an association, but raised questions as to causality. A UK analysis also found a small association, (HR = 1.14, 95% CI 1.01–1.29, P = 0.04), but the data suggested that men with higher sun exposure were more likely to become PDE5 inhibitor users [68]. Other, more recent, datasets have also cast doubt on a causal effect of sildenafil and other PDE5 inhibitors on melanoma risk [69, 70].
A meta-analysis by Tang et al. [71] concluded that PDE5 inhibitor use was associated with a slightly elevated risk of melanoma (OR, 1.12; 95% CI 1.03–1.21) and basal cell carcinoma (OR, 1.14; 95% CI 1.09–1.19) but not squamous cell carcinoma, however they concluded that causality remained elusive. A meta-analysis by Loeb et al. [72] also concluded that there was an elevated risk, but that the data showed a lack of dose response suggesting the relationship was not causal.
Concerns were also raised that long-term PDE5 inhibitor use might increase the risk of biochemical relapse after radical prostatectomy for localised prostate cancer [73]. Michl et al. reported that use of a PDE5 inhibitor after radical prostatectomy was associated with an increased risk of biochemical recurrence (HR 1.38, 95% CI 1.11–1.70, P = 0.0035). However, a more recent study has reported no such association [74] and a retrospective study indicated a trend towards PDE5-inhibitor mediated protective effect against primary prostate cancer [75].
Clinical trials
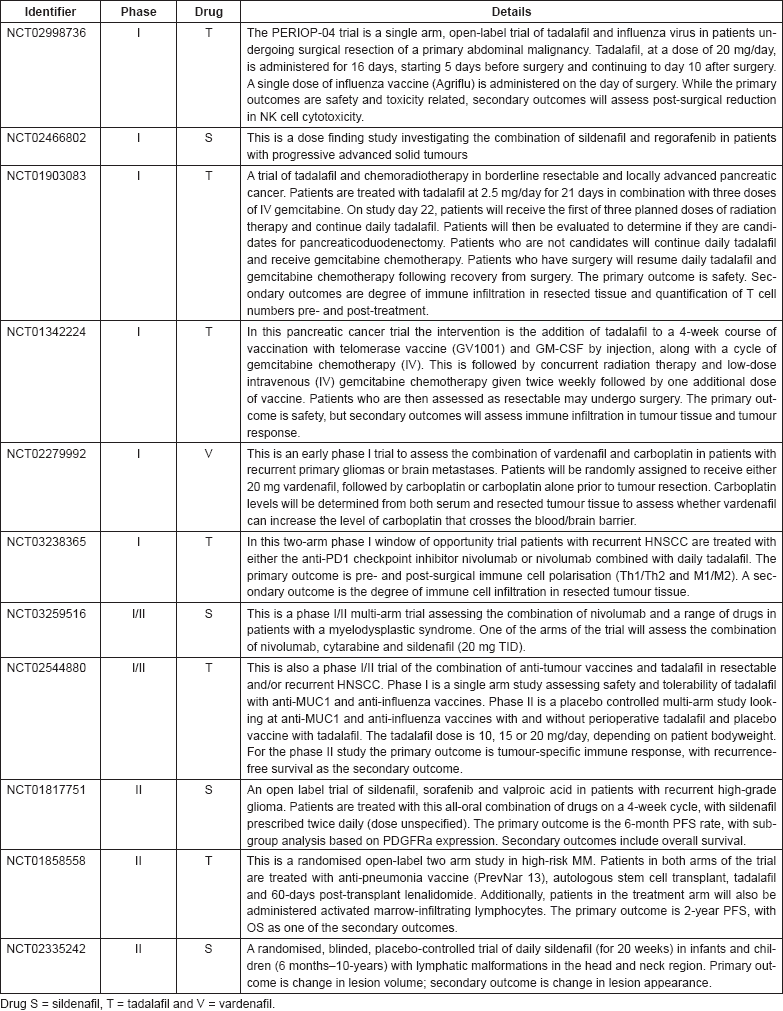
As of 24 August 2017, a number of clinical trials are investigating the anti-cancer uses of sildenafil, tadalafil and vardenafil, as shown in Table 4. Note that trials which are assessing non-cancer-related outcomes, for example trials such as NCT01375699 which is assessing the cardio-protective effect of sildenafil on doxorubicin-treated cancer patients are not included. Only trials which are currently open (recruiting or soon to commence recruitment) or on-going are included.
Table 4. Clinical trials in cancer using PDE5 inhibitors.
Mechanisms of action
The role of PDE5 in different tissues and diseases is reviewed by Kouvelas et al. [76], here we will focus on the different mechanisms of action which have been explored in relation to the anti-cancer effects of the PDE5 inhibitors discussed in this review.
Immunological
Serafini et al. [19] first outlined the immunomodulatory effects of PDE5 inhibitors in relation to cancer in 2006. In particular they showed that in a number of murine models tadalafil and sildenafil significantly delayed tumour growth in immune competent mice but not immunocompromised mice. Additionally they determined in vitro that these drugs had no impact on apoptosis in the relevant cell lines. Further experiments demonstrated that sildenafil enhanced the tumour-specific T cell response and increased intra-tumour CD8 T cell infiltration and activation. In vivo PDE5 inhibition reduced ARG1 and NOS2 and down-regulated IL-4Rα in tumour-associated MDSCs in BALB/c and C57BL/6 tumour-bearing mice. It was also shown that expansion of CD4 and CD8 T cells obtained from MM or head and neck cancer patients was significantly lower than that observed using healthy donors. However, in the presence of sildenafil, PBMCs from these patients expanded similarly to sildenafil treated PBMCs from healthy donors. Subsequently the same group explored the role of MDSCs in T cell anergy in the A20B-cell lymphoma model [20]. It was shown that MDSCs induce the proliferation of regulatory T cell (Tregs) and the establishment of T cell tolerance. Sildenafil treatment reduced the number of tumour-specific Tregs, and reverted tumour-induced T-cell anergy.
Capuano et al. [21] confirmed that sildenafil inhibited the development of an immunosuppressive phenotype in two murine models of colorectal cancer by downregulation of MDSC cell populations. Analysis showed the effects were mediated by inhibition of iNOS and arginine metabolism. Similar results were shown in C57BL/6 mice challenged with TRAMP-C1 cells where oral sildenafil retarded tumour growth compared to vehicle-treated controls [22]. However, while sildenafil reduced MDSCs numbers and affected arginine metabolism in spontaneous tumours in TRAMP mice but did not significantly retard tumour growth.
As discussed previously, Meyer et al. [35] showed that the inflammatory tumour microenvironment was associated with MDSC-mediated immunosuppression in melanoma-bearing mice. They showed that MDSC reduced both T cell proliferation and ζ-chain T cell receptor expression, thereby creating an immunosuppressive tumour microenvironment [77]. Treatment with sildenafil reduced MDSC numbers infiltrating primary tumours and metastatic lesions, increased CD8 T cells and induced a partial recovery of ζ-chain expression. Sildenafil treatment was also associated with improved survival in tumour-bearing mice. Lin et al. [23] also showed that sildenafil inhibited the development of colitis-associated colonic tumours in BALB/c mice by inhibiting MDSC infiltration into colon tissues.
Booth et al. [44] have also shown that the combination of sildenafil and pemetrexed enhanced immunogenic cell death in a syngeneic mouse Lewis Lung Carcinoma model. In vitro the combination treatment increased PD-L1 and ODC expression in tumour cells and enhanced the expression of MHCA and HMGB1. In vivo combination treatment with pemetrexed and sildenafil enhanced the anti-tumour efficacy of checkpoint inhibitory antibodies directed against programmed cell death protein 1 (PD-1) or cytotoxic T-lymphocyte-associated protein 4 (CTLA4).
A recent review has summarised the diverse effects of sildenafil in both the innate and adaptive immune systems [78].
Treatment sensitisation
Multidrug resistance protein 5 (MRP5/ABCC5) was shown to confer resistance to the commonly used cancer drug 5-FU in human embryonic kidney cells in vitro, increasing the EC50 value by a factor of ten in cells transfected with MRP5 compared to non-transfected cells [79]. It was further shown that a number of agents, including sildenafil, could reduce the ATP-dependent transport of active 5-FU metabolites.
Shi et al. [80, 81] showed that sildenafil increased the in vitro sensitivity of ABCB1-overexpressing drug-resistant cells to colchicine, vinblastine and paclitaxel but not cisplatin. Sildenafil was also shown to increase the accumulation of paclitaxel. Furthermore, in ABCG2-overexpressing cells, sildenafil inhibited resistance to ABCG2 substrate anticancer drugs, for example, mitoxantrone. It was noted also that sildenafil exhibited greater affinity to ABCB1 than to ABCG2. Some of the same authors also showed that vardenafil, and to a lesser extent tadalafil, also increased the sensitivity of ABCB1 over-expressing cells but did not alter the drug sensitivity in ABCC1 and ABCG2 overexpressing cells [82]. MRP7/ABCC10 is another of the MRP family is associated with resistance to a number of chemotherapeutic agents, including vinca alkaloids and taxanes, which has been shown to be amenable to PDE5 inhibitor-mediated sensitisation [83].
A study into the potential clinical use of sildenafil to address treatment sensitivity was published by Lin et al. [84] following the range of in vitro results described above. In a series of experiments using wild-type and ABC-transporter knock-out they showed that sildenafil (50 mg/kg) did not increase brain accumulation of topotecan or docetaxel. A second set of experiments using CT26 tumours in syngeneic BALB/c mice showed that sildenafil (10 and 50 mg/kg) increased tumour accumulation of doxorubicin, but also increased accumulation in plasma and heart tissue at the higher dose. However, this did not translate into a reduction in tumour volume compared to doxorubicin alone.
Das et al. [17, 85] also showed a potentiation of chemotherapeutic response via the increased generation of reactive oxygen species (ROS) using the combination of sildenafil and doxorubicin in mouse models of prostate cancer. In addition to showing that the drug combination additively increased apoptosis of PC-3 and DU145 prostate cancer cells, they also showed that in nude mice carrying PC-3 flank tumours the combination reduced tumour volume. Mechanistically the addition of sildenafil to doxorubicin increased ROS production in both PC-3 and DU145 cell lines though not in PrEC normal cells. A key role for CD95 was also identified [85]. Subsequent studies by the same authors assessed the combination of PDE5 inhibitors with standard chemotherapeutic drugs in a range of bladder and pancreatic cancer cells [18]. Bladder cancer cells (HT-1376, J82 and T24) showed increased levels of cell death when treated with the combination of sildenafil and mitomycin C, doxorubicin, cisplatin or gemcitabine compared to treatment without the addition of sildenafil. Similarly pancreatic cancer cells (PANC-1, Mia, Paca2 and AsPC-1) were more sensitive to combination of sildenafil and doxorubicin, gemcitabine or paclitaxel. The effect was mediated by the induction of ROS and an increase in DNA damage.
Sildenafil and vardenafil were also shown to enhance tumour permeability via effects on the blood brain barrier [26]. Specifically looking to address the blood–brain tumour barrier, which includes the microvessel supplying brain tumours, Black et al. showed that oral sildenafil (50 mg/kg) and vardenafil (10 mg/kg) increased tumour permeability of radioactively labelled sucrose. Furthermore, 9L gliosarcoma-bearing rats treated with the combination of vardenafil and doxorubicin had significantly (P < 0.05) longer survival than animals treated with either agent alone or vehicle control. Wang et al. [46] showed that tadalafil improved microvascular permeability leading to an improved survival in a murine brain lymphoma model treated with rituximab.
Hypoxia
Tumour hypoxia is associated with resistance to treatment in solid tumours, increased genomic instability and the development of a metastatic phenotype in multiple cancer types [86]. It has been shown that hypoxia-induced resistance to doxorubicin could be reversed with the use of NO mimetics such as nitroglycerin [87]. Bell et al. [88] showed that atrial natriuretic peptide, one of a class of polypeptides that cause diuresis/natriuresis and vasodilation, inhibited the hypoxia-induced resistance to doxorubicin in prostate cancer cell lines (DU-145 and PC-3). The same authors subsequently explored the potential of PDE5 inhibitors to address the same issue [89]. They showed that in DU-145 and PC-3 prostate cancer cell lines PDE5 and PDE11 were responsible for cGMP-specific PDE activity and that chemical inhibition of PDE5 with zaprinast was associated with reduced hypoxia-induced resistance to doxorubicin in vitro and reduced tumour growth in vivo.
Ikeda et al. [90] showed that hypoxia induced resistance to the novel anticancer agent Poly-SNO-HAS (poly-S-nitrosated human serum albumin) in murine colon 26 adenocarcinoma (C26) cells in vitro and in vivo. However, the combination of vardenafil and Poly-SNO-HAS showed increased tumour growth reduction compared to either drug alone or control under hypoxic conditions.
Ammirante et al. [91] showed that hypoxic conditions in the androgen-deprived prostate tumour microenvironment activates cancer-associated fibroblasts, induces TGF-β expression and stimulates CXCL13 production—a process associated with a more aggressive tumour phenotype. Treatment with sildenafil significantly delayed the emergence of castration-resistance in castrated Myc-CaP tumour-bearing mice by inhibiting the hypoxia-induced activation of fibroblasts. This result is notable given that tadalafil is already approved for the treatment of benign prostatic hyperplasia.
Other
In addition to immunological and chemo-sensitivity mechanisms, there is some evidence for a range of other effects that may be relevant in the context of cancer treatment—many of these effects are driven by the inhibition of PDE5 activity in cancer cells. Zhu and Strada [92] summarised the increased activity of PDE isoforms in a range of colon, breast, prostate, lung and other cancer cell lines and showed that PDE5 was the most commonly over-expressed isoform. They also showed that PDE5 inhibition with siRNA or exisulind in colon cancer cell lines was associated with mitotic arrest and the induction of apoptosis.
Karami-Tehrani et al. [93] compared PDE5 and PDE9 expression in normal breast tissue, benign and malignant breast tumour samples. The relative expression of both PDE isoforms in malignant tumours was significantly higher than those of respective normal breast tissues and benign tumours, with evidence of an association between overexpression and tumour grade, stage, and lymph node involvement. Catalano et al. [94] assessed PDE5 expression in breast cancer cell lines and found higher expression in HER2-overexpressing (SKBR3) and basal-like (BT-20/MDA-MB-468/MDA-MB-435) lines, suggesting a correlation between PDE5 expression and more aggressive disease. PDE5 over-expression in vitro was associated with an increase in cell motility and migration—which could be reversed by sildenafil. Retrospective analysis showed that high levels of PDE5 expression were associated with shorter overall survival in breast cancer patients (P = 0.014, HR = 1.2) [94].
The combination of CNP and sildenafil was shown to reduce growth rate in xenograft RMS tumours via sildenafil-mediated inhibition of degradation of CNP-induced accumulation of cGMP [51]. In vitro analysis suggested that the combination exerted anti-proliferative effects on RMS cells by inhibiting the Raf/MEK/ERK pathway.
Liu et al. [95] investigated the role of the Hippo pathway in PC3-derived prostate cancer stem cells (PCSC). The showed that PCSCs showed expressed 2.8-fold more PDE5 than non-stem prostate cancer cells and that PDE5/cGMP/PKG signalling was a key component of PCSC-related Hippo/TAZ pathway. In vitro inhibition of PDE5 signalling using vardenafil attenuated ‘stemness’ and dose dependently suppressed colony formation in both PC3 cells and PCSC. In vivo vardenafil reduced PSCS stemness and increased apoptosis. Similarly, Booth et al. [96] have shown that sildenafil synergised with celecoxib or the celecoxib derivative OSU-03012 to increase cell death of a range of glioblastoma stem-like cells.
Finally, a recent report by Baravalle et al. [97] used molecular and cell level analytics to show that PDE5 inhibition also acts to inhibit aromatase, an important target in breast cancer. Sildenafil was found to act as a partial inhibitor of human aromatase, with a maximal inhibition of around 35%.
Our take
The evidence from in vitro, in vivo and human studies, outlined above and summarised in Table 5, suggests that the licensed PDE5 inhibitors sildenafil, vardenafil and tadalafil have a number of distinct anti-cancer effects which may be of therapeutic value in different clinical settings. While a number of distinct mechanisms of action have been elucidated, the immunological and treatment sensitisation aspects of these drugs are especially intriguing. We note the encouraging number and range of on-going clinical trials exploring these aspects of the anti-cancer activity of PDE5 inhibition. In particular these drugs show potential as adjuncts to existing anticancer therapies via two distinct mechanisms.
Table 5. Summary of evidence by cancer type.
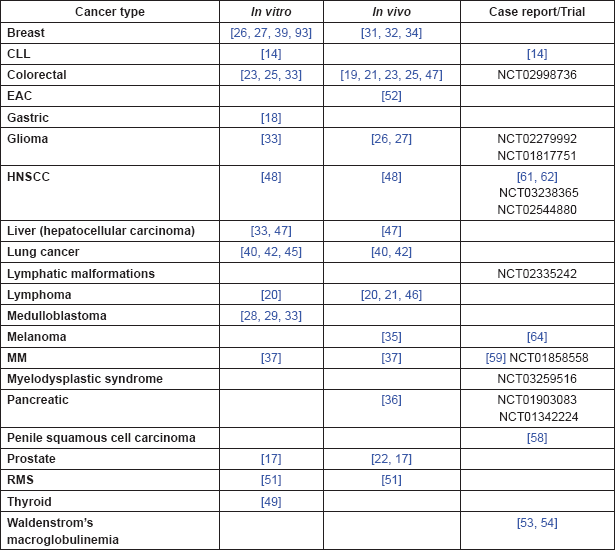
Firstly by synergising or potentiating the therapeutic effects of other agents, including a wide range of chemotherapeutics and other drugs. Reversing or reducing resistance to existing treatments—including radiotherapy, chemotherapy and endocrine therapy—is an important means of improving patient outcomes. This is particularly the case for brain tumours, both for primary tumours and metastases, where the blood-brain-barrier means there are few therapeutic agents that have proven efficacy. The use of drugs to increase permeability is an active area of research and one in which PDE5 inhibitors show great potential.
Secondly, by addressing the underlying mechanisms of immunosuppression through modulation of MDSCs and/or Treg cell populations. MDSCs play a key role in suppression of anti-cancer immune responses and are a high-value therapeutic target in oncology [98, 99]. Indeed, MDSCs are a key target in addressing resistance to immune checkpoint inhibitors [99]. PDE5 inhibitors are not the only repurposing candidates which have some evidence of activity related to MDSCs and Treg cells, others include histamine type-2 receptor antagonists such as cimetidine [100] and ranitidine [101], metronomic cyclophosphamide [102] or NSAIDs such as aspirin or celecoxib [103]. The potential synergism of these low-cost and low-toxicity treatments is intriguing and warrants both pre-clinical and clinical investigation. The inclusion of PDE5 inhibitors with checkpoint inhibition, as with the TONIC-trial of metronomic cyclophosphamide and nivolumab [104], and in the NCT03238365 trial of tadalafil and nivolumab is also an exciting prospect.
The treatment sensitisation and immunological effects are not mutually exclusive. PDE5 inhibitors therefore offer the attractive prospect of increasing immunogenic cell death while at the same time enhancing the immune response. The combination with immune checkpoint inhibitors is therefore particularly worthy of investigation.
Perioperative therapies are interventions designed to improve the outcomes from cancer surgery by reducing post-surgical recurrence rates [105, 106]. Interventions that have been explored include pre-operative ketorolac [107] or aspirin [108], depot progesterone [109], cimetidine [110] and immunonutrition with l-arginine [111]. There is some evidence, both pre-clinical and clinical, that perioperative vaccination with influenza vaccine may reduce post-surgical metastatic spread by enhancing natural killer cell number and cytotoxicity [112]. The PERIOP-04 (NCT02998736) trial is assessing the combination of pre-operative tadalafil and influenza vaccine in patients undergoing surgical resection for abdominal malignancies. Given the range of mechanisms which may be implicated in post-surgical locoregional and distant recurrence there is reason to believe that multiple interventions may be required to fully reduce the risks. Therefore it is proposed that multi-arm, multi-stage platform trials should be explored as a mechanism to explore the relative efficacy of different perioperative therapies and their combinations.
Next steps
As outlined previously, there are a number of active Phase I and Phase II clinical trials on-going at present. These trials are largely based on to the strong level of clinical evidence in a number of specific indications and it is to be hoped that positive reports from these trials will be forthcoming in the future. The data are strongest for clinical trials of PDE5 inhibitors, in combination with other agents, in the following cancer types:
(a) HNSCC
(b) Glioblastoma
(c) Pancreatic cancer
(d) Medulloblastoma
(e) Waldenstrom’s macroglobulinemia
(f) Melanoma
The perioperative use of PDE5 inhibitors in combination with other perioperative therapies is also of interest in the following cancers:
(a) Colorectal cancer
(b) Breast cancer
(c) HNSCC
The diagnostic and predictive significance of PDE5 expression in oncology is also an area that warrants additional attention. This is particularly the case in a precision oncology context where patient selection on the basis of PDE5 expression may be required.
Conclusions
A broad range of data, pre-clinical and clinical, has been summarised and presented to make the case that the commercially available and widely used PDE5 inhibitors sildenafil, vardenafil and tadalafil are very strong candidates for repurposing as anticancer agents. These low-cost, low-toxicity drugs show potential to be included with current and emerging standard of care treatments in oncology. The combination with immune checkpoint inhibitors or possible use as perioperative therapies are particularly compelling strategies with the potential to positively improve survival outcomes in a relatively short time-frame.
Author contributions
Primary author: Pan Pantziarka. Contributing authors: Vidula Sukhatme, Sergio Crispino, Gauthier Bouche, Lydie Meheus and Vikas P Sukhatme. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests. All of the authors are associated with not for profit organisations that aim to repurpose drugs for oncology treatments. VPS is also a scientific advisory board member of Berg Health and Mitra Biotech.
References
1. Peak TC, Richman A, and Gur S, et al (2016) The Role of PDE5 Inhibitors and the NO/cGMP Pathway in Cancer Sex Med Rev 4(1) 74–84 https://doi.org/10.1016/j.sxmr.2015.10.004 PMID: 27872007
2. Lin C-S (2004) Tissue expression, distribution, and regulation of PDE5 Int J Impot Res 16(Suppl 1) S8–S10 https://doi.org/10.1038/sj.ijir.3901207 PMID: 15224128
3. Ghofrani HA, Osterloh IH, and Grimminger F (2006) Sildenafil: from angina to erectile dysfunction to pulmonary hypertension and beyond Nature Rev Drug Discov 5(8) 689–702 https://doi.org/10.1038/nrd2030
4. Galiè N, Ghofrani HA, and Torbicki A, et al (2005) Sildenafil citrate therapy for pulmonary arterial hypertension New Engl J Med 353(20) 2148–2157 https://doi.org/10.1056/NEJMoa050010 PMID: 16291984
5. Fries R, Shariat K, and von Wilmowsky H, et al (2005) Sildenafil in the treatment of Raynaud’s phenomenon resistant to vasodilatory therapy Circulation 112(19) 2980–2985 PMID: 16275885
6. Padma-Nathan H, McMurray JG, and Pullman WE, et al (2001) On-demand IC351 (Cialis) enhances erectile function in patients with erectile dysfunction Int J Impot Res 13(1) 2–9 https://doi.org/10.1038/sj.ijir.3900631 PMID: 11313831
7. Roehrborn CG, Kaminetsky JC, and Auerbach SM, et al (2010) Changes in peak urinary flow and voiding efficiency in men with signs and symptoms of benign prostatic hyperplasia during once daily tadalafil treatment BJU Int 105(4) 502–507 https://doi.org/10.1111/j.1464-410X.2009.08822.x
8. Nichols DJ, Muirhead GJ, and Harness JA (2002) Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality Br J Clini Pharmacol 53 (Suppl 1) 5S–12S https://doi.org/10.1046/j.0306-5251.2001.00027.x
9. Hong JH, Kwon YS, and Kim IY (2017) Pharmacodynamics, pharmacokinetics and clinical efficacy of phosphodiesterase-5 inhibitors Expert Opin Drug MetabToxicol 13(2) 183–192 https://doi.org/10.1080/17425255.2017.1244265
10. Boolell M, Allen MJ, and Ballard SA, et al (1996) Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction Int J Impot Res 8(2) 47–52 PMID: 8858389
11. Gupta M, Kovar A, and Meibohm B (2005) The Clinical Pharmacokinetics of Phosphodiesterase-5 Inhibitors for Erectile Dysfunction J Clin Pharmacol 45(9) 987–1003 https://doi.org/10.1177/0091270005276847 PMID: 16100293
12. Thompson WJ, Piazza GA, and Li H, et al (2000) Exisulind induction of apoptosis involves guanosine 3’,5’-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated beta-catenin Cancer Res 60(13) 3338–3342 PMID: 10910034
13. Piazza GA, Thompson WJ, and Pamukcu R, et al (2001) Exisulind, a novel proapoptotic drug, inhibits rat urinary bladder tumorigenesis Cancer Res 61(10) 3961–3968 PMID: 11358813
14. Sarfati M, Mateo V, and Baudet S, et al (2003) Sildenafil and vardenafil, types 5 and 6 phosphodiesterase inhibitors, induce caspase-dependent apoptosis of B-chronic lymphocytic leukemia cells Blood 101(1) 265–269 https://doi.org/10.1182/blood-2002-01-0075
15. Qian C-N, Takahashi M, and Kahnoski RJ, et al (2003) Effect of sildenafil citrate on an orthotopic prostate cancer growth and metastasis model J Urol 170(3) 994–997 https://doi.org/10.1097/01.ju.0000080321.99119.df PMID: 12913757
16. Pernkopf DG, Untergasser G, and Berger P, et al (2006) Phosphodiesterase (PDE) inhibitors reduce in vitro proliferation of prostate primary cells but do not interfere with growth of prostate carcinoma cell lines J Clin Oncol 24(18_suppl) 14604
17. Das A, Durrant D, and Mitchell C, et al (2010) Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction Proc Natl Acad Sci USA 107(42) 18202–18207 https://doi.org/10.1073/pnas.1006965107 PMID: 20884855 PMCID: 2964209
18. Booth L, Roberts JL, and Cruickshanks N, et al (2014) Phosphodiesterase 5 inhibitors enhance chemotherapy killing in gastrointestinal/genitourinary cancer cells Mol Pharmacol 85(3) 408–419 https://doi.org/10.1124/mol.113.090043 PMCID: 3935155
19. Serafini P, Meckel K, and Kelso M, et al (2006) Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function J Exp Med 203(12) 2691–2702 https://doi.org/10.1084/jem.20061104 PMID: 17101732 PMCID: 2118163
20. Serafini P, Mgebroff S, and Noonan K, et al (2008) Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells Cancer Res 68(13) 5439–5449 https://doi.org/10.1158/0008-5472.CAN-07-6621 PMID: 18593947 PMCID: 2887390
21. Capuano G, Rigamonti N, and Grioni M, et al (2009) Modulators of arginine metabolism support cancer immunosurveillance BMC Immunol 10(1) 1 https://doi.org/10.1186/1471-2172-10-1 PMID: 19134173 PMCID: 2648942
22. Rigamonti N, Capuano G, and Ricupito A, et al (2011) Modulators of arginine metabolism do not impact on peripheral T-cell tolerance and disease progression in a model of spontaneous prostate cancer Clin Cancer Res 17(5) 1012–1023 https://doi.org/10.1158/1078-0432.CCR-10-2547 PMID: 21248302
23. Lin S, Wang J, and Wang L, et al (2017) Phosphodiesterase-5 inhibition suppresses colonic inflammation-induced tumorigenesis via blocking the recruitment of MDSC Am J Cancer Res 7(1) 41–52 PMID: 28123846 PMCID: 5250679
24. Islam BN, Sharman SK, and Hou Y, et al (2017) Sildenafil Suppresses Inflammation-Driven Colorectal Cancer in Mice Cancer Prev Res 10(7) 377–388 https://doi.org/10.1158/1940-6207.CAPR-17-0015
25. Mei X, Yang Y, and Zhang Y-J, et al (2015) Sildenafil inhibits the growth of human colorectal cancer in vitro and in vivo American J Cancer Res 5(11) 3311–3324
26. Black KL, Yin D, and Ong JM, et al (2008) PDE5 inhibitors enhance tumor permeability and efficacy of chemotherapy in a rat brain tumor model Brain Res 1230 290–302 https://doi.org/10.1016/j.brainres.2008.06.122 PMID: 18674521 PMCID: 2632551
27. Hu J, Ljubimova JY, and Inoue S, et al (2010) Phosphodiesterase type 5 inhibitors increase Herceptin transport and treatment efficacy in mouse metastatic brain tumor models PLoS One 5(4) e10108 https://doi.org/10.1371/journal.pone.0010108 PMID: 20419092 PMCID: 2856671
28. Othman RT, Kimishi I, and Bradshaw TD, et al (2014) Overcoming multiple drug resistance mechanisms in medulloblastoma Acta Neuropathol Commun 2 18–22 https://doi.org/10.1186/2051-5960-2-57
29. Roberts JL, Booth L, and Conley A, et al (2014) PDE5 inhibitors enhance the lethality of standard of care chemotherapy in pediatric CNS tumor cells Cancer BiolTher 15(6) 758–767
30. Booth L, Roberts JL, and Cruickshanks N, et al (2014) Regulation of OSU-03012 toxicity by ER stress proteins and ER stress-inducing drugs Mol Cancer Ther 13(10) 2384–2398 https://doi.org/10.1158/1535-7163.MCT-14-0172 PMID: 25103559 PMCID: 4185238
31. Di X, Gennings C, and Bear HD, et al (2010) Influence of the phosphodiesterase-5 inhibitor, sildenafil, on sensitivity to chemotherapy in breast tumor cells Breast Cancer Res Treatment 124(2) 349–360 https://doi.org/10.1007/s10549-010-0765-7
32. Greish K, Fateel M, and Abdelghany S, et al (2017) Sildenafil citrate improves the delivery and anticancer activity of doxorubicin formulations in a mouse model of breast cancer J Drug Target 0(0) 1–6
33. Booth L, Roberts JL, and Cruickshanks N, et al (2015) PDE5 inhibitors enhance celecoxib killing in multiple tumor types J Cellular Physiol 230(5) 1115–1127 https://doi.org/10.1002/jcp.24843
34. Confino H, Schmidt M, and Efrati M, Hochman I, et al (2016) Inhibition of mouse breast adenocarcinoma growth by ablation with intratumoral alpha-irradiation combined with inhibitors of immunosuppression and CpG Cancer Immunol Immunother 65(10) 1149–1158 https://doi.org/10.1007/s00262-016-1878-6 PMID: 27495172
35. Meyer C, Sevko A, and Ramacher M, et al (2011) Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model Proc Natl Acad Sci USA 108(41) 17111–17116 https://doi.org/10.1073/pnas.1108121108 PMID: 21969559 PMCID: 3193202
36. Karakhanova S, Link J, and Heinrich M, et al (2015) Characterization of myeloid leukocytes and soluble mediators in pancreatic cancer: importance of myeloid-derived suppressor cells OncoImmunol 4(4) e998519 https://doi.org/10.1080/2162402X.2014.998519
37. Kumazoe M, Sugihara K, and Tsukamoto S, et al (2013) 67-kDa laminin receptor increases cGMP to induce cancer-selective apoptosis J Clin Invest 123(2) 787–799 PMID: 23348740 PMCID: 3561824
38. Kumazoe M, Kim Y, and Bae J, et al (2013) Phosphodiesterase 5 inhibitor acts as a potent agent sensitizing acute myeloid leukemia cells to 67-kDa laminin receptor-dependent apoptosis FEBS Letters 587(18) 3052–3057 https://doi.org/10.1016/j.febslet.2013.07.041 PMID: 23916810
39. Kumazoe M, Tsukamoto S, and Lesnick C, et al (2015) Vardenafil, a clinically available phosphodiesterase inhibitor, potentiates the killing effect of EGCG on CLL cells Br J Haematol 168(4) 610–613 https://doi.org/10.1111/bjh.13135
40. Li Q and Shu Y (2014) Pharmacological modulation of cytotoxicity and cellular uptake of anti-cancer drugs by PDE5 inhibitors in lung cancer cells Pharm Res 31(1) 86–96 https://doi.org/10.1007/s11095-013-1134-0
41. Booth L, Roberts JL, and Poklepovic A, et al (2017) PDE5 inhibitors enhance the lethality of pemetrexed through inhibition of multiple chaperone proteins and via the actions of cyclic GMP and nitric oxide Oncotarget 8(1) 1449–1468 https://doi.org/10.18632/oncotarget.13640 PMCID: 5352068
42. Booth L, Roberts JL, and Poklepovic A, et al (2017) PDE5 inhibitors enhance the lethality of [pemetrexed sorafenib] Oncotarget 8(8) 13464–13475 https://doi.org/10.18632/oncotarget.14562 PMID: 28088782 PMCID: 5355112
43. Webb T, Carter J, and Roberts JL, et al (2015) Celecoxib enhances [sorafenib sildenafil] lethality in cancer cells and reverts platinum chemotherapy resistance Cancer Biol Ther 16(11) 1660–1670 https://doi.org/10.1080/15384047.2015.1099769 PMID: 26417912 PMCID: 4846137
44. Booth L, Roberts JL, and Poklepovic A, et al (2017) [pemetrexed sildenafil], via autophagy-dependent HDAC downregulation, enhances the immunotherapy response of NSCLC cells Cancer Biol Ther 18(9) 705–714 https://doi.org/10.1080/15384047.2017.1362511 PMID: 28812434 PMCID: 5663410
45. Domvri K, Zarogoulidis K, and Zogas N, et al (2017) Potential synergistic effect of phosphodiesterase inhibitors with chemotherapy in lung cancer J Cancer 8(18) 3648–3656 https://doi.org/10.7150/jca.21783 PMID: 29151951 PMCID: 5688917
46. Wang R, Chen W, and Zhang Q, et al (2015) Phosphodiesterase type 5 inhibitor Tadalafil increases Rituximab treatment efficacy in a mouse brain lymphoma model J Neuro-Oncol 122(1) 35–42 https://doi.org/10.1007/s11060-014-1690-0
47. Tavallai M, Hamed HA, and Roberts JL, et al (2015) Nexavar/Stivarga and viagra interact to kill tumor cells J Cellular Physiol 230(9) 2281–2298 https://doi.org/10.1002/jcp.24961
48. Tuttle TR, Mierzwa ML, and Wells SI, et al (2016) The cyclic GMP/protein kinase G pathway as a therapeutic target in head and neck squamous cell carcinoma Cancer Lett 370(2) 279–285 https://doi.org/10.1016/j.canlet.2015.10.024 PMCID: 4711273
49. Sponziello M, Verrienti A, and Rosignolo F, et al (2015) PDE5 expression in human thyroid tumors and effects of PDE5 inhibitors on growth and migration of cancer cells Endocrine 50(2) 434–441 https://doi.org/10.1007/s12020-015-0586-x PMID: 25837309
50. De Rose RF, Cristiano MC, and Celano M, et al (2016) PDE5 Inhibitors-Loaded Nanovesicles: Physico-Chemical Properties and In Vitro Antiproliferative Activity Nanomaterials 6(5) 92 https://doi.org/10.3390/nano6050092
51. Zenitani M, Nojiri T, and Uehara S, et al (2016) C-type natriuretic peptide in combination with sildenafil attenuates proliferation of rhabdomyosarcoma cells Cancer Med 5(5) 795–805 https://doi.org/10.1002/cam4.642 PMID: 26816265 PMCID: 4864809
52. El-Naa MM, Othman M, and Younes S (2016) Sildenafil potentiates the antitumor activity of cisplatin by induction of apoptosis and inhibition of proliferation and angiogenesis Drug Des Devel Ther 10 3661–3672
53. Treon SP, Tournilhac O, and Branagan AR, et al (2004) Clinical responses to sildenafil in Waldenstrom’s macroglobulinemia Clin Lymphoma, 5(3) 205–207 https://doi.org/10.3816/CLM.2004.n.029
54. Patterson CJ, Soumerai J, and Hunter Z, et al (2006) Sildenafil citrate suppresses disease progression in patients with Waldenstrom’s macroglobulinemia J Clin Oncol 24(18_suppl) 7556
55. Swetman GL, Berk DR, and Vasanawala SS, et al (2012) Sildenafil for severe lymphatic malformations New Engl J Med 366(4) 384–386 https://doi.org/10.1056/NEJMc1112482 PMID: 22276841
56. Gandhi NG, Lin LK, and O’Hara M (2013) Sildenafil for pediatric orbital lymphangioma JAMA Ophthalmol 131(9) 1228–1230 https://doi.org/10.1001/jamaophthalmol.2013.4201 PMID: 23828510
57. Pantziarka P, Bouche G, and Sukhatme V, et al (2016) Repurposing Drugs in Oncology (ReDO)-Propranolol as an anti-cancer agent Ecancermedicalscience 10 680 PMID: 27899953 PMCID: 5102691
58. Huilgol NG and Jain A (2006) A new indication of sildenafil in medicine: hypoxic cell sensitizer for penile cancer J Cancer Res Ther 2(3) 132–135 https://doi.org/10.4103/0973-1482.27589
59. Noonan KA, Ghosh N, and Rudraraju L, et al (2014) Targeting Immune Suppression with PDE5 Inhibition in End-Stage Multiple Myeloma Cancer Immunol Res 2(8) 725–731 https://doi.org/10.1158/2326-6066.CIR-13-0213 PMID: 24878583 PMCID: 4152913
60. Van Nuffel AM, Sukhatme V, and Pantziarka P, et al (2015) Repurposing Drugs in Oncology (ReDO)-clarithromycin as an anti-cancer agent Ecancermedicalscience 9 513 https://doi.org/10.3332/ecancer.2015.513 PMID: 25729426 PMCID: 4341996
61. Weed DT, Vella JL, and Reis IM, et al (2015) Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma Clin Cancer Res 21(1) 39–48 https://doi.org/10.1158/1078-0432.CCR-14-1711 PMCID: 4322895
62. Califano JA, Khan Z, and Noonan KA, et al (2015) Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma Clin Cancer Res 21(1) 30–38 https://doi.org/10.1158/1078-0432.CCR-14-1716 PMID: 25564570 PMCID: 4329916
63. Jiang H, Gebhardt C, and Umansky L, et al (2015) Elevated chronic inflammatory factors and myeloid-derived suppressor cells indicate poor prognosis in advanced melanoma patients Int J Cancer 136(10) 2352–2360 https://doi.org/10.1002/ijc.29297
64. Hassel JC, Jiang H, and Bender C, et al (2017) Tadalafil has biologic activity in human melanoma. Results of a pilot trial with Tadalafil in patients with metastatic Melanoma (TaMe) Oncoimmunology 6(9) e1326440 https://doi.org/10.1080/2162402X.2017.1326440 PMID: 28932631 PMCID: 5599085
65. Arozarena I, Sanchez-Laorden B, and Packer L, et al (2011) Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A Cancer Cell 19(1) 45–57 https://doi.org/10.1016/j.ccr.2010.10.029 PMID: 21215707
66. Li W-Q, Qureshi AA, and Robinson KC, et al (2014) Sildenafil use and increased risk of incident melanoma in US men: a prospective cohort study JAMA Internal Med 174(6) 964–970 https://doi.org/10.1001/jamainternmed.2014.594
67. Loeb S, Folkvaljon Y, and Lambe M, et al (2015) Use of Phosphodiesterase Type 5 Inhibitors for Erectile Dysfunction and Risk of Malignant Melanoma JAMA 313(24) 2449–2455 https://doi.org/10.1001/jama.2015.6604 PMID: 26103029
68. Matthews A, Langan SM, and Douglas IJ, et al (2016) Phosphodiesterase Type 5 Inhibitors and Risk of Malignant Melanoma: Matched Cohort Study Using Primary Care Data from the UK Clinical Practice Research Datalink PLoS Med 13(6) e1002037 https://doi.org/10.1371/journal.pmed.1002037 PMID: 27299522 PMCID: 4907438
69. Pottegård A, Schmidt SAJ, and Olesen AB, et al (2016) Use of sildenafil or other phosphodiesterase inhibitors and risk of melanoma Br J Cancer 115(7) 895–900 https://doi.org/10.1038/bjc.2016.248 PMID: 27529513 PMCID: 5046205
70. Lian Y, Yin H, and Pollak MN, et al (2016) Phosphodiesterase Type 5 Inhibitors and the Risk of Melanoma Skin Cancer Eur Urol 70(5) 808–815 https://doi.org/10.1016/j.eururo.2016.04.035 PMID: 27178449
71. Tang H, Wu W, and Fu S, et al (2017) Phosphodiesterase type 5 inhibitors and risk of melanoma: a meta-analysis J Am Acad Dermatol 77(3) 480.e9–488.e9 https://doi.org/10.1016/j.jaad.2017.04.1129
72. Loeb S, Ventimiglia E, and Salonia A, et al (2017) Meta-Analysis of the Association Between Phosphodiesterase Inhibitors (PDE5Is) and Risk of Melanoma J Natl Cancer Inst 109(8) 1–3 https://doi.org/10.1093/jnci/djx086
73. Michl U, Molfenter F, and Graefen M, et al (2015) Use of phosphodiesterase type 5 inhibitors may adversely impact biochemical recurrence after radical prostatectomy J Urol 193(2) 479–483 https://doi.org/10.1016/j.juro.2014.08.111
74. Gallina A, Bianchi M, and Gandaglia G, et al (2015) A Detailed Analysis of the Association Between Postoperative Phosphodiesterase Type 5 Inhibitor Use and the Risk of Biochemical Recurrence After Radical Prostatectomy Eur Urol 68(5) 750–753 https://doi.org/10.1016/j.eururo.2015.02.002 PMID: 25700565
75. Jamnagerwalla J, Howard LE, and Vidal AC, et al (2016) The Association between Phosphodiesterase Type 5 Inhibitors and Prostate Cancer: Results from the REDUCE Study J Urol 196(3) 715–720 https://doi.org/10.1016/j.juro.2016.03.172 PMID: 27060053 PMCID: 5014695
76. Kouvelas D, Goulas A, and Papazisis G, et al (2009) PDE5 inhibitors: in vitro and in vivo pharmacological profile Curr Pharm Des 15(30) 3464–3475 https://doi.org/10.2174/138161209789206971 PMID: 19860692
77. Umansky V and Sevko A (2012) Overcoming immunosuppression in the melanoma microenvironment induced by chronic inflammation Cancer Immunol Immunother 61(2) 275–282 https://doi.org/10.1007/s00262-011-1164-6
78. Kniotek M and Boguska A (2017) Sildenafil Can Affect Innate and Adaptive Immune System in Both Experimental Animals and Patients J Immunol Res 2017 4541958 https://doi.org/10.1155/2017/4541958 PMID: 28316997 PMCID: 5337856
79. Pratt S, Shepard RL, and Kandasamy RA, et al (2005) The multidrug resistance protein 5 ( ABCC5 ) confers resistance to 5-fluorouracil and transports its monophosphorylated metabolites Mol Cancer Ther 5 855–864 https://doi.org/10.1158/1535-7163.MCT-04-0291
80. Shi Z, Tiwari AK, and Shukla S, et al (2011) Sildenafil Reverses ABCB1- and ABCG2-Mediated Chemotherapeutic Drug Resistance Cancer Res 710(8) 3029–3042 https://doi.org/10.1158/0008-5472.CAN-10-3820
81. Shi Z, Tiwari AK, and Patel AS, et al (2011) Roles of sildenafil in enhancing drug sensitivity in cancer Cancer Res 71(11) 3735–3738 https://doi.org/10.1158/0008-5472.CAN-11-0375 PMID: 21610107 PMCID: 3107342
82. Ding PR, Tiwari AK, and Ohnuma S, et al (2011) The Phosphodiesterase-5 Inhibitor Vardenafil Is a Potent Inhibitor of ABCB1 / P-Glycoprotein Transporter PLoS ONE 6(4) e19329 https://doi.org/10.1371/journal.pone.0019329 PMID: 21552528 PMCID: 3084276
83. Chen JJJ-JJ, Sun YLYL, and Tiwari AKK, et al (2012) PDE5 inhibitors, sildenafil and vardenafil, reverse multidrug resistance by inhibiting the efflux function of multidrug resistance protein 7 (ATP-binding Cassette C10) transporter Cancer Sci 103(8) 1531–1537 https://doi.org/10.1111/j.1349-7006.2012.02328.x PMID: 22578167 PMCID: 3407321
84. Lin F, Hoogendijk L, and Buil L, et al (2013) Sildenafil is not a useful modulator of ABCB1 and ABCG2 mediated drug resistance in vivo Eur J Cancer 49(8) 2059–2064 https://doi.org/10.1016/j.ejca.2012.12.028 PMID: 23422148
85. Das A, Durrant D, and Mitchell C, et al (2016) Sildenafil (Viagra) sensitizes prostate cancer cells to doxorubicin-mediated apoptosis through CD95 Oncotarget 7(4) 4399–4413 https://doi.org/10.18632/oncotarget.6749 PMCID: 4826214
86. Wilson WR and Hay MP (2011) Targeting hypoxia in cancer therapy Nature Rev Cancer 11(6) 393–410 https://doi.org/10.1038/nrc3064
87. Matthews NE, Adams MA, and Maxwell LR, et al (2001) Nitric oxide-mediated regulation of chemosensitivity in cancer cells J Natl Cancer Inst 93(24) 1879–1885 https://doi.org/10.1093/jnci/93.24.1879 PMID: 11752013
88. Bell EN, Tse MY, and Frederiksen LJ, et al (2007) Atrial Natriuretic Peptide Attenuates Hypoxia Induced Chemoresistance in Prostate Cancer Cells J Urol 177(2) 751–756 https://doi.org/10.1016/j.juro.2006.09.075 PMID: 17222675
89. Hamilton TK, Hu N, and Kolomitro K, et al (2013) Potential therapeutic applications of phosphodiesterase inhibition in prostate cancer World J Urol 31(2) 325–330 https://doi.org/10.1007/s00345-012-0848-7
90. Ikeda M, Ishima Y, and Chuang VTG, et al (2017) Apoptosis induction of poly-S-nitrosated human serum albumin in resistant solid tumor under hypoxia can be restored by phosphodiesterase 5 inhibition Nitric Oxide 69 28–34 https://doi.org/10.1016/j.niox.2017.04.005 PMID: 28414103
91. Ammirante M, Shalapour S, and Kang Y, et al (2014) Tissue injury and hypoxia promote malignant progression of prostate cancer by inducing CXCL13 expression in tumor myofibroblasts Proc Natl Acad Sci USA 111(41) 14776–14781 https://doi.org/10.1073/pnas.1416498111 PMID: 25267627 PMCID: 4205637
92. Zhu B and Strada SJ (2007) The novel functions of cGMP-specific phosphodiesterase 5 and its inhibitors in carcinoma cells and pulmonary/cardiovascular vessels Curr Top Medi Chem 7(4) 437–454 https://doi.org/10.2174/156802607779941198
93. Karami-tehrani F, Moeinifard M, and Aghaei M, et al (2012) Evaluation of PDE5 and PDE9 Expression in Benign and Malignant Breast Tumors Arch Med Res 43(6) 470–475 https://doi.org/10.1016/j.arcmed.2012.08.006 PMID: 22960860
94. Catalano S, Campana A, and Giordano C, et al (2016) Expression and Function of Phosphodiesterase Type 5 in Human Breast Cancer Cell Lines and Tissues: Implications for Targeted Therapy Clin Cancer Res 22(9) 2271–2282 https://doi.org/10.1158/1078-0432.CCR-15-1900
95. Liu N, Mei L, and Fan X, et al (2016) Phosphodiesterase 5/protein kinase G signal governs stemness of prostate cancer stem cells through Hippo pathway Cancer Lett 378(1) 38–50 https://doi.org/10.1016/j.canlet.2016.05.010 PMID: 27179930
96. Booth L, Roberts JL, and Tavallai M, et al (2015) OSU-03012 and Viagra Treatment Inhibits the Activity of Multiple Chaperone Proteins and Disrupts the Blood-Brain Barrier: Implications for Anti-Cancer Therapies J Cell Physiol 230(8) 1982–1998 https://doi.org/10.1002/jcp.24977 PMID: 25736380 PMCID: 4835175
97. Baravalle R, Valetti F, and Catucci G, et al (2017) Effect of sildenafil on human aromatase activity: From in vitro structural analysis to catalysis and inhibition in cells J Steroid Biochem Mol Biol 165 438–447 https://doi.org/10.1016/j.jsbmb.2016.09.003
98. Wesolowski R, Markowitz J, and Carson WE (2013) Myeloid derived suppressor cells - a new therapeutic target in the treatment of cancer J Immunotherapy Cancer 1(1) 10 https://doi.org/10.1186/2051-1426-1-10
99. Chesney JA, Mitchell RA, and Yaddanapudi K (2017) Myeloid-derived suppressor cells-a new therapeutic target to overcome resistance to cancer immunotherapy J Leukoc Biol 102(3) 727–740 https://doi.org/10.1189/jlb.5VMR1116-458RRR PMID: 28546500
100. Pantziarka P, Bouche G, and Meheus L, et al (2014) Repurposing drugs in oncology (ReDO)-cimetidine as an anti-cancer agent Ecancermedicalscience 8 485 https://doi.org/10.3332/ecancer.2014.485 PMID: 25525463 PMCID: 4268104
101. Vila-Leahey A, Oldford SA, and Marignani PA, et al (2016) Ranitidine modifies myeloid cell populations and inhibits breast tumor development and spread in mice OncoImmunology 5(7) 1–13 https://doi.org/10.1080/2162402X.2016.1151591
102. Wu J and Waxman DJ (2015) Metronomic cyclophosphamide eradicates large implanted GL261 gliomas by activating antitumor Cd8( ) T-cell responses and immune memory Oncoimmunology 4(4) e1005521 https://doi.org/10.1080/2162402X.2015.1005521 PMID: 26137402 PMCID: 4485826
103. Fujita M, Kohanbash G, and Fellows-Mayle W, et al (2011) COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells Cancer Res 71(7) 2664–2674 https://doi.org/10.1158/0008-5472.CAN-10-3055 PMID: 21324923 PMCID: 3075086
104. Kok M, Horlings HM, and van de Vijver K, et al (2017) LBA14Adaptive phase II randomized non-comparative trial of nivolumab after induction treatment in triple negative breast cancer: TONIC-trial Annals of Oncol 28(suppl_5) 608 https://doi.org/10.1093/annonc/mdx440.006
105. Pantziarka P, Bouche G, and Sullivan R, et al (2017) Perioperative therapies - Enhancing the impact of cancer surgery with repurposed drugs Eur J Surg Oncol 43(11) 1985–1988 https://doi.org/10.1016/j.ejso.2017.08.010 PMID: 28928011
106. Horowitz M, Neeman E, and Sharon E, et al (2015) Exploiting the critical perioperative period to improve long-term cancer outcomes Nature Rev Clinical oncology 12(4) 213–226 https://doi.org/10.1038/nrclinonc.2014.224
107. Forget P, Berlière M, and van Maanen A, et al (2013) Perioperative ketorolac in high risk breast cancer patients. Rationale, feasibility and methodology of a prospective randomized placebo-controlled trial Med Hypotheses 81(4) 707–712 https://doi.org/10.1016/j.mehy.2013.07.033 PMID: 23937996
108. Restivo A, Cocco IMF, and Casula G, et al (2015) Aspirin as a neoadjuvant agent during preoperative chemoradiation for rectal cancer Br J Cancer 1–7
109. Badwe R, Hawaldar R, and Parmar V, et al (2011) Single-injection depot progesterone before surgery and survival in women with operable breast cancer: a randomized controlled trial J Clin Oncol 29(21) 2845–2851 https://doi.org/10.1200/JCO.2010.33.0738 PMID: 21670457
110. Deva S and Jameson M (2012) Histamine type 2 receptor antagonists as adjuvant treatment for resected colorectal cancer Cochrane Database Syst Rev 8(8) CD007814
111. Buijs N, van Bokhorst-de van der Schueren MA, and Langius JA, et al (2010) Perioperative arginine-supplemented nutrition in malnourished patients with head and neck cancer improves long-term survival AmJ Clini Nutr 92(5) 1151–1156 https://doi.org/10.3945/ajcn.2010.29532
112. Tai L-H, Zhang J, and Scott KJ, et al (2013) Perioperative influenza vaccination reduces postoperative metastatic disease by reversing surgery-induced dysfunction in natural killer cells Clin Cancer Res 19(18) 5104–5115 https://doi.org/10.1158/1078-0432.CCR-13-0246 PMID: 23881927






