Progesterone receptor isoforms A and B: new insights into the mechanism of progesterone resistance for the treatment of endometrial carcinoma
Ruijin Shao
Department of Physiology/Endocrinology, Institute of Neuroscience and Physiology, The Sahlgrenska Academy, University of Gothenburg, Gothenburg 40530, Sweden
Correspondence to: Ruijin Shao. E-mail: ruijin.shao@fysiologi.gu.se
Abstract
Progesterone therapy is an effective treatment for atypical endometrial hyperplasia and early endometrial carcinoma (EC). However, progesterone resistance is the main obstacle to the success of conservative treatment in women with type I EC and remains a major clinical challenge. Studies indicate that progesterone and progesterone receptors (PRs) play a significant role in both normal and neoplastic endometria. Most EC arises in the epithelial cells of the endometrial glands, and a large body of in vitro evidence suggests that the absence or reduced expression of PR isoform B might result in the failure of progesterone treatment and lead to aberrant PRB-mediated signalling in EC cells. A recently developed in vivo knockout mouse model suggests that enhanced DNA methylation decreases the level of stromal PR isoform A and that this is also a main contributor to progesterone resistance in EC cells. The endometrial stroma within the EC might create a microenvironment that determines how epithelial-derived cancer cells respond to progesterone. This novel study opened a new avenue for research seeking to clarify the mechanisms that regulate the specific PR isoforms that are associated with the stromal cell responses to progesterone and has led to new understanding of both endometrial cell-specific and mechanical contributions of the stroma to EC development.
Keywords: progesterone receptor isoforms, progesterone resistance, cell—cell interaction, endometrial carcinoma, animal model
Introduction
Endometrial cancer (EC) remains the most common gynaecological malignancy in women across the globe [1, 2]. It has been reported that 7950 of the 43,470 women in the United States with diagnosed EC died in 2010 [3], and there are 1900 deaths per year from EC in the United Kingdom (http://www.cancerresearchuk.org). EC can be diagnosed early, but it remains difficult to manage with non-surgical interventions. Surgical procedures are still the first line and most effective treatments for the early stage of this disease [4]. There are numerous studies that describe human culture methods using several EC cell lines for the study of EC biology. However, a fundamental question remains as to how closely EC cell lines are able to recapitulate the biology of human EC in vivo. Co-culture experiments using EC cell line and human endometrial stromal cells designed for further understanding of the development of EC and interaction between normal tissue/stroma and cancer cells are lacking. Although our understanding of the pathophysiology of EC has improved, the results of potential preventive and therapeutic options, especially non-surgical treatments, have been disappointing.
Epidemiological studies have implicated steroid hormonal imbalance in the development of EC [5]. Continuous exposure of the endometrium to oestrogens can lead to endometrial overgrowth and hyperplasia [6], and progesterone acts as a protective factor against oestrogen-driven growth and proliferation in the endometrium [1]. Under pathological conditions, the survival and proliferation of EC cells can be suppressed by the actions of progesterone and its analogues, such as medroxyprogesterone acetate [7] and megestrol acetate [1]. Because of this dependence, treatment with progesterone and its analogues is the major non-surgical treatment for EC [8]. Such therapy is effective for atypical endometrial hyperplasia and early EC in many women who desire to maintain their fertility [9]. However, more than 30% of women with oestrogen-dependent, well-differentiated type I EC fail to respond to progesterone treatment due to progesterone resistance [10], and the underlying mechanisms behind this are still poorly understood.
Discussion
It is well accepted that progesterone responsiveness in the endometrium [11] is mediated by the coordinated actions of progesterone receptor (PR) isoforms A and B. PR is a member of the steroid receptor super family that regulates transcription of numerous target genes [12]. Both PRA and PRB are transcribed from two different promoters in a single gene, and PRA differs from PRB by the absence of the 164 amino acids at the amino terminus of the protein [13]. The PRA and PRB isoforms have different activities and functions. For example, in vitro experiments show that PRA functions as a transcriptional inhibitor of PRB when PRA and PRB are both present in the same cells [13]. In addition, selective ablation of PRA, but not PRB, results in mouse uteri that fail to display progesterone-mediated inhibition of oestrogen-induced epithelial cell proliferation [14]. These results suggest that the distinct expression of PRA and PRB in the endometrium is likely to have different functional consequences [15]. In normal human endometria, PRA and PRB are both expressed in the epithelial and stromal cells [16], and both isoforms appear to fluctuate in the cycling endometrium in an isoform-specific and cell-specific manner [17, 18].
There is conflicting and contradictory clinical evidence regarding the use of PR isoform expression or the ratio of the two PR isoforms as a predictor of EC risk and prognosis [19–24]. However, the available data make it quite clear that the loss or downregulation of either one or both of the two PR isoforms in EC tissues is associated with higher clinical grade [24–26]. Regulation of PR expression is involved in several different processes including transcription, translation, and post-translational modification [1, 8]. In vivo studies with human EC tissues and in vitro studies with several EC cell lines have shown that epigenetic mechanisms such as DNA methylation and histone modification play crucial roles in regulating the total PRA and PRB expression [27–30].
There is no in vivo evidence for individual roles of the PR isoforms in the initiation and development of EC, but it has become progressively more evident from in vitro studies with human EC cell lines that activation of the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) pathway [31, 32], alteration of adhesion molecules [33], and activation of the cell cycle-regulatory proteins [34] required for cell proliferation and apoptosis are most likely a result of PRB activity. These studies are further supported by the fact that significant alterations of forkhead box O1 (FOXO1), an AKT downstream effector, and baculoviral IAP repeat containing 3 (BIRC3), a PRB-regulated protein, are induced by progesterone treatment in these cells [32, 34]. Furthermore, the altered response of EC cells to progesterone therapy is probably due to changes in the level of PRB between pre-treatment and post-treatment with medroxyprogesterone acetate [35]. These observations thus have led to the proposal that decreased PRB expression in EC cells could be responsible for progesterone treatment failure (Figure 1A). It should be noted, however, that the use of in vitro culture systems with the different EC cell lines to study the specific PR isoform-mediated effects on progesterone response might fail due to the absence of the in vivo conditions under which EC develops.
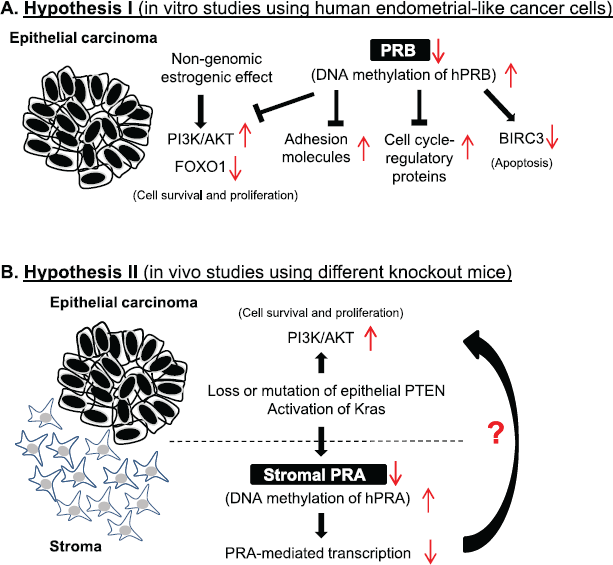
Figure 1. Two hypotheses have been developed to describe how endometrial cancer cells survive and proliferate by switching from progesterone sensitivity to progesterone resistance. Both of these hypotheses depend on the fact that transcription factors activated by progesterone receptor isoforms A and B play a central role in controlling cell proliferation, differentiation, and apoptosis in the endometrium under pathological conditions. In vitro studies using human endometrial cancer cells indicate that decreased PRB expression in endometrial cancer cells is likely responsible for progesterone treatment failure (A). Janzen et al used different knockout mouse models to show for the first time that the endometrial stromal component is also responsible for progesterone sensitivity and resistance, and that PRA is a critical factor mediating endometrial cellular response to progesterone treatment in endometrial cancer tissues in vivo (B).
EC arises most commonly in the epithelial cells of endometrial glands [1, 2], but the human endometrium also includes other cell types such as stromal fibroblastic cells in the stroma [15]. A number of studies suggest that the stroma component is not only supportive of tumour growth but can also be a causative factor for the initiation and development of many human cancers [36]. Insulin-like growth factor binding protein-1 (IGFBP-1) in stromal cells is one such causative candidate. Several studies have shown that oestrogen-mediated insulin-like growth factor-1 (IGF-1) synthesis in endometrial epithelial cells [37] is inhibited by progesterone-stimulated IGFBP-1 expression in endometrial stromal cells [37, 38]. IGF-1 receptors are present in the endometrial epithelial cells [38], and activation of IGF-1 signalling is an important event in the development of endometrial hyperplasia and EC [39]. Previous tissue recombination experiments have demonstrated that stromal PR is required for the inhibition of oestrogen-induced epithelial cell proliferation in the mouse uterus [40]. These data suggest that aberrant paracrine regulators released from stromal cells might promote epithelial proliferation, endometrial hyperplasia, and the progression of EC.
Several molecules have been shown to have a temporal and/or cell-specific expression pattern in the endometrium under physiological and pathological conditions [2, 9]. Dominant loss-of-function alterations in phosphatase and tensin (PTEN) homolog, a well-defined tumour suppressing molecule, is associated with the development and onset of human EC [31], and experimental manipulation of endometrial PTEN levels in mice verifies this observation [41]. It has been shown that the phosphatase activity of PTEN is required for inhibition of the PI3K/AKT signalling pathway [42], and this suggests that decreased PTEN-dependent up-regulation of the PI3K/AKT pathway plays a role in tumour progression [43].
Although studies using PTEN knockout mice demonstrate uterine tumour growth [41], the interpretation of the loss of PTEN has been complicated by the inability to differentiate between epithelial and stromal cell defects. In the 1 August 2013 issue of Cancer Research, Janzen et al [44] revealed that mice in which PTEN was specifically deleted in epithelial cells exhibited uterine tumour growth similar to what is seen in women with EC [31], and that the anti-tumour effects of progesterone depend on uterine cell-specific PR expression and regulation.
The significance of the article by Janzen et al [44] is threefold. First, it provides the strongest evidence that time-dependent systemic treatment with progesterone in epithelial-PTEN knockout mice is capable of significant suppression of tumour cell proliferation and the induction of tumour resolution. This inhibitory effect is likely mediated by stromal PR expression because ablation of stromal PR, but not epithelial PR, makes the uterine tumours in epithelial-PTEN knockout mice resistant to progesterone therapy. Although it is still not clear how stromal cells create their microenvironment and promote PR signalling to reach the adjacent tumour cells, one can assume that stromal PR-mediated regulation of molecules such as IGFBP-1 [37, 38] contributes to the inhibitory effects of progesterone in uterine tumours via a paracrine mechanism.
Second, Janzen and colleagues found that the efficiency of progesterone treatment in mouse uterine tumours requires the presence of oestrogen. There is evidence that oestrogen can upregulate uterine PR expression in rats in vivo [45] and in most progesterone target cells in vitro [11]. These results suggest that oestrogen might maintain PR expression in the stromal cells of uterine tumours. As a result, progesterone action at the molecular level would be enhanced, and uterine tumour cell proliferation would be suppressed.
The third and most important finding of the Janzen study was the identification of a link between the occurrence of progesterone resistance and epigenetic regulation of stromal PR expression. They found that only mice in which PTEN had been specifically deleted in epithelial cells showed a shift from progesterone sensitivity to progesterone resistance when stromal PR expression was decreased. Mice lacking both epithelial PTEN and Kirsten rat sarcoma (KRAS) viral oncogene homolog, a genetic mutation detected in women with EC, exhibited uterine tumour growth but failed to respond to progesterone treatment due to the absence of stromal PR. The mechanism behind the loss of stromal PR expression – especially stromal PRA – is due to enhanced DNA methylation of the stromal PR gene. These results suggest that under normal circumstances, the activities of PTEN and KRAS coordinate the progesterone response of endometrial cells.
Janzen et al have provided new insights into the mechanism by which the stromal component of the endometrium is responsible for progesterone sensitivity and resistance. Their work has demonstrated that PRA is a critical factor mediating the endometrial cellular response to progesterone treatment in EC tissues (Figure 1B). It is well known that the two PR isoforms form dimers in vivo upon their activation [46], and PRA and PRB are approximately evenly expressed in non-malignant areas of EC [23, 26]. However, the PR-mediated responses depend on the coordinated, opposing, and compensatory functions of the two PR isoforms [13, 47], and it has been shown that the activation of individual PR isoforms results in differential regulation of progesterone target genes [48]. In this context, future studies should be directed toward deciphering the role that PR isoform-specific signalling plays in the tumour–stroma interaction in response to progesterone treatment.
Conclusion and future directions
Progesterone and PRs play a significant role in normal and neoplastic endometria [8–11], and specific PR isoforms in different endometrial cell populations might provide a molecular basis for the apparent relationship between progesterone sensitivity and resistance in women with early EC. In contrast to genetic mutations, DNA methylation is reversible. Therefore, increasing PRA or PRB expression by the epigenetic modulation might represent a promising therapeutic target for the treatment of EC in women with progesterone resistance.
Approximately 30% of women with polycystic ovary syndrome fail to respond to progesterone treatment and undergo progression to atypical hyperplasia and further transformation to EC [49]. The molecular mechanisms underlying endometrial progesterone resistance or sensitivity in these patients are not well understood, but the observations of Janzen et al help to explain why EC tissues respond the way they do to progesterone treatment in vivo. Because there are several morphological and physiological differences between rodent and human uteri, the challenge for future studies in this field is to determine whether the two PR isoform signalling intermediates play any role in progesterone resistance in the human endometrium under disease conditions. This is particularly important because for PRB signalling defects are not apparent [14]. We agree with the conclusion of Janzen et al that ‘defining mechanisms and site of origin for innate or acquired resistance to hormonal therapy in human EC trials will have immense translational application’ [44].
Conflicts of interest
The author has no conflicts of interest to declare.
Acknowledgments
This work was supported by the Swedish Medical Research Council (5859 and 10380), Jane and Dan Olsson’s Foundation, the Åke-Wiberg Foundation, the Hjalmar Svensson Foundation, Anna Cederberg’s Foundation, and Clas Groschinsky’s Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
1. Yang S, Thiel KW and Leslie KK (2011) Progesterone: the ultimate endometrial tumor suppressor Trends Endocrinol Metab 22(4) 145–52 DOI: 10.1016/j.tem.2011.01.005 PMID: 21353793
2. Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E and Vergote I (2005) Endometrial cancer Lancet 366(9484) 491–505 DOI: 10.1016/S0140-6736(05)67063-8 PMID: 16084259
3. Jemal A, Siegel R, Xu J and Ward E (2010) Cancer statistics, 2010 CA Cancer J Clin 60(5) 277–300 DOI: 10.3322/caac.20073 PMID: 20610543
4. Lee WL, Lee FK, Su WH, Tsui KH, Kuo CD, Hsieh SL, Wang PH (2012) Hormone therapy for younger patients with endometrial cancer Taiwanese J Obstet Gynecol 51(4) 495–505 DOI: 10.1016/j.tjog.2012.09.003
5. Setiawan VW, Yang HP, Pike MC, McCann SE, Yu H, Xiang YB, Wolk A, Wentzensen N, Weiss NS, Webb PM, et al (2013) Type I and II endometrial cancers: have they different risk factors? J Clin Oncol: Official Journal of the American Society of Clinical Oncology 31(20) 2607–18 DOI: 10.1200/JCO.2012.48.2596
6. Horn LC, Meinel A, Handzel R and Einenkel J (2007) Histopathology of endometrial hyperplasia and endometrial carcinoma: an update Ann Diagn Pathol 11(4) 297–311 DOI: 10.1016/j.anndiagpath.2007.05.002 PMID: 17630117
7. Ushijima K, Yahata H, Yoshikawa H, Konishi I, Yasugi T, Saito T, Nakanishi T, Sasaki H, Saji F, Iwasaka T, et al (2007) Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women J Clin Oncol: Official Journal of the American Society of Clinical Oncology 25(19) 2798–803 DOI: 10.1200/JCO.2006.08.8344
8. Yang S, Thiel KW, De Geest K and Leslie KK (2011) Endometrial cancer: reviving progesterone therapy in the molecular age Discov Med 12(64) 205–12 PMID: 21955848
9. Kim JJ, Kurita T and Bulun SE (2013) Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer Endocr Rev 34(1) 130–62 DOI: 10.1210/er.2012-1043 PMID: 23303565 PMCID: 3565104
10. Kim JJ and Chapman-Davis E (2010) Role of progesterone in endometrial cancer Semin Reprod Med 28(1) 81–90 DOI: 10.1055/s-0029-1242998 PMID: 20104432
11. Graham JD and Clarke CL (1997) Physiological action of progesterone in target tissues Endocr Rev 18(4) 502–19 DOI: 10.1210/er.18.4.502 PMID: 9267762
12. Wetendorf M and DeMayo FJ (2012) The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network Mol Cell Endocrinol 357(1–2) 108–18 DOI: 10.1016/j.mce.2011.10.028 PMCID: 3443857
13. Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H and Chambon P (1990) Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B EMBO J 9(5) 1603–14 PMID: 2328727 PMCID: 551856
14. Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP and O’Malley BW (2002) Reproductive functions of progesterone receptors Recent Prog Horm Res 57 339–55 DOI: 10.1210/rp.57.1.339 PMID: 12017551
15. Shao R, Weijdegard B, Ljungstrom K, Friberg A, Zhu C, Wang X, Zhu Y, Fernandez-Rodriguez J, Egecioglu E, Rung E, et al (2006) Nuclear progesterone receptor A and B isoforms in mouse fallopian tube and uterus: implications for expression, regulation, and cellular function Am J Physiol Endocrinol Metab 291(1) E59–72 DOI: 10.1152/ajpendo.00582.2005 PMID: 16449295
16. Critchley HO and Saunders PT (2009) Hormone receptor dynamics in a receptive human endometrium. Reprod Sci 16(2) 191–9 DOI: 10.1177/1933719108331121 PMID: 19208787
17. Mote PA, Balleine RL, McGowan EM and Clarke CL (1999) Colocalization of progesterone receptors A and B by dual immunofluorescent histochemistry in human endometrium during the menstrual cycle J Clin Endocrinol Metab 84(8) 2963–71 DOI: 10.1210/jc.84.8.2963 PMID: 10443705
18. Shao R, Wang X, Weijdegard B, Norstrom A, Fernandez-Rodriguez J, Brannstrom M and Billig H (2012) Coordinate regulation of heterogeneous nuclear ribonucleoprotein dynamics by steroid hormones in the human Fallopian tube and endometrium in vivo and in vitro Am J Physiol Endocrinol Metab 302(10) E1269–82 DOI: 10.1152/ajpendo.00673.2011 PMID: 22436695
19. Sakaguchi H, Fujimoto J, Hong BL, Nakagawa Y and Tamaya T (2004) Drastic decrease of progesterone receptor form B but not A mRNA reflects poor patient prognosis in endometrial cancers Gynecol Oncol 93(2) 394–99 DOI: 10.1016/j.ygyno.2004.01.042 PMID: 15099952
20. Saito S, Ito K, Nagase S, Suzuki T, Akahira J, Okamura K, Yaegashi N and Sasano H (2006) Progesterone receptor isoforms as a prognostic marker in human endometrial carcinoma Cancer Sci 97(12) 1308–14 DOI: 10.1111/j.1349-7006.2006.00332.x PMID: 16999816
21. Jongen V, Briet J, de Jong R, ten Hoor K, Boezen M, van der Zee A, Nijman H and Hollema H (2009) Expression of estrogen receptor-alpha and -beta and progesterone receptor-A and -B in a large cohort of patients with endometrioid endometrial cancer Gynecol Oncol 112(3) 537–42 DOI: 10.1016/j.ygyno.2008.10.032
22. Kumar NS, Richer J, Owen G, Litman E, Horwitz KB and Leslie KK (1998) Selective down-regulation of progesterone receptor isoform B in poorly differentiated human endometrial cancer cells: implications for unopposed estrogen action Cancer Res 58(9) 1860–5 PMID: 9581825
23. Arnett-Mansfield RL, DeFazio A, Mote PA and Clarke CL (2004) Subnuclear distribution of progesterone receptors A and B in normal and malignant endometrium J Clin Endocrinol Metab 89(3) 1429–1442. DOI: 10.1210/jc.2003-031111 PMID: 15001645
24. Miyamoto T, Watanabe J, Hata H, Jobo T, Kawaguchi M, Hattori M, Saito M and Kuramoto H (2004) Significance of progesterone receptor-A and -B expressions in endometrial adenocarcinoma J Steroid Biochem Mol Biol 92(3) 111–8 DOI: 10.1016/j.jsbmb.2004.07.007 PMID: 15555905
25. Mortel R, Zaino R and Satyaswaroop PG (1984) Heterogeneity and progesterone-receptor distribution in endometrial adenocarcinoma Cancer 53(1) 1136
26. Arnett-Mansfield RL, deFazio A, Wain GV, Jaworski RC, Byth K, Mote PA and Clarke CL (2001) Relative expression of progesterone receptors A and B in endometrioid cancers of the endometrium Cancer Res 61(11) 4576–82 PMID: 11389093
27. Xiong Y, Dowdy SC, Gonzalez Bosquet J, Zhao Y, Eberhardt NL, Podratz KC and Jiang SW (2005) Epigenetic-mediated upregulation of progesterone receptor B gene in endometrial cancer cell lines Gynecologic oncology 99(1) 135–41 DOI: 10.1016/j.ygyno.2005.05.035 PMID: 16024066
28. Sasaki M, Dharia A, Oh BR, Tanaka Y, Fujimoto S and Dahiya R (2001) Progesterone receptor B gene inactivation and CpG hypermethylation in human uterine endometrial cancer Cancer Res 61(1) 97–102 PMID: 11196205
29. Ren Y, Liu X, Ma D, Feng Y and Zhong N (2007) Down-regulation of the progesterone receptor by the methylation of progesterone receptor gene in endometrial cancer cells Cancer Genet Cytogenet 175(2) 107–16 DOI: 10.1016/j.cancergencyto.2007.02.002 PMID: 17556066
30. Yang S, Xiao X, Jia Y, Liu X, Zhang Y, Devor EJ, Meng X, Thiel KW and Leslie KK (2013) Epigenetic modification restores functional PR expression in endometrial cancer cells Curr Pharm Des in press
31. Westin SN and Broaddus RR (2012) Personalized therapy in endometrial cancer: challenges and opportunities Cancer Biology Therapy 13(1) 1–13 DOI: 10.4161/cbt.13.1.18438 PMCID: 3335980
32. Neubauer NL, Ward EC, Patel P, Lu Z, Lee I, Blok LJ, Hanifi-Moghaddam P, Schink J and Kim JJ (2011) Progesterone receptor-B induction of BIRC3 protects endometrial cancer cells from AP1-59-mediated apoptosis Hormones Cancer 2(3) 170–81 DOI: 10.1007/s12672-011-0065-7 PMID: 21760855 PMCID: 3133609
33. Dai D, Wolf DM, Litman ES, White MJ and Leslie KK (2002) Progesterone inhibits human endometrial cancer cell growth and invasiveness: down-regulation of cellular adhesion molecules through progesterone B receptors Cancer Res 62(3) 881–6 PMID: 11830547
34. Ward EC, Hoekstra AV, Blok LJ, Hanifi-Moghaddam P, Lurain JR, Singh DK, Buttin BM, Schink JC and Kim JJ (2008) The regulation and function of the forkhead transcription factor, Forkhead box O1, is dependent on the progesterone receptor in endometrial carcinoma Endocrinology 149(4) 1942–50 DOI: 10.1210/en.2007-0756
35. Utsunomiya H, Suzuki T, Ito K, Moriya T, Konno R, Sato S, Yaegashi N, Okamura K and Sasano H (2003) The correlation between the response to progestogen treatment and the expression of progesterone receptor B and 17beta-hydroxysteroid dehydrogenase type 2 in human endometrial carcinoma Clin Endocrinol (Oxf) 58(6) 696–703 DOI: 10.1046/j.1365-2265.2003.01766.x
36. Tlsty TD and Coussens LM (2006) Tumor stroma and regulation of cancer development Annu Rev Pathol 1 119–50 DOI: 10.1146/annurev.pathol.1.110304.100224
37. Lin J, Li R and Zhou J (2003) The influence of insulin on secretion of IGF-I and IGFBP-I in cultures of human endometrial stromal cells Chin Med J 116(2) 301–304 PMID: 12775252
38. Wang HS and Chard T (1999) IGFs and IGF-binding proteins in the regulation of human ovarian and endometrial function J Endocrinol 161(1) 1–13 DOI: 10.1677/joe.0.1610001 PMID: 10194523
39. Kaaks R, Lukanova A and Kurzer MS (2002) Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review Cancer Epidemiol Biomarkers Prev 11(12) 1531–43 PMID: 12496040
40. Cunha GR, Cooke PS and Kurita T (2004) Role of stromal-epithelial interactions in hormonal responses Arch Histol Cytol 67(5) 417–34 DOI: 10.1679/aohc.67.417
41. Daikoku T, Hirota Y, Tranguch S, Joshi AR, DeMayo FJ, Lydon JP, Ellenson LH and Dey SK (2008) Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice Cancer Res 68(14) 5619–27 DOI: 10.1158/0008-5472.CAN-08-1274 PMID: 18632614 PMCID: 2824329
42. Carracedo A and Pandolfi PP (2008) The PTEN-PI3K pathway: of feedbacks and cross-talks Oncogene 27(41) 5527–41 DOI: 10.1038/onc.2008.247 PMID: 18794886
43. Carracedo A, Alimonti A and Pandolfi PP (2011) PTEN level in tumor suppression: how much is too little? Cancer Res 71(3) 629–33 DOI: 10.1158/0008-5472.CAN-10-2488 PMID: 21266353 PMCID: 3249925
44. Janzen DM, Rosales MA, Paik DY, Lee DS, Smith DA, Witte ON, Iruela-Arispe ML and Memarzadeh S (2013) Progesterone receptor signaling in the microenvironment of endometrial cancer influences its response to hormonal therapy Cancer Res 73(15) 4697–710 DOI: 10.1158/0008-5472.CAN-13-0930 PMID: 23744837 PMCID: 3752407
45. Kraus WL and Katzenellenbogen BS (1993) Regulation of progesterone receptor gene expression and growth in the rat uterus: modulation of estrogen actions by progesterone and sex steroid hormone antagonists. Endocrinology 132(6) 2371–9 DOI: 10.1210/en.132.6.2371 PMID: 8504742
46. Leonhardt SA, Altmann M and Edwards DP (1998) Agonist and antagonists induce homodimerization and mixed ligand heterodimerization of human progesterone receptors in vivo by a mammalian two-hybrid assay Mol Endocrinol 12(12) 1914–30 DOI: 10.1210/me.12.12.1914 PMID: 9849965
47. Li X and O’Malley BW (2003) Unfolding the action of progesterone receptors J Biol Chem 278(41) 39261–4 DOI: 10.1074/jbc.R300024200 PMID: 12893816
48. Smid-Koopman E, Kuhne LC, Hanekamp EE, Gielen SC, De Ruiter PE, Grootegoed JA, Helmerhorst TJ, Burger CW, Brinkmann AO, Huikeshoven FJ, et al (2005) Progesterone-induced inhibition of growth and differential regulation of gene expression in PRA- and/or PRB-expressing endometrial cancer cell lines J Soc Gynecol Investig 12(4) 285–92 DOI: 10.1016/j.jsgi.2005.01.003 PMID: 15866122
49. Aghajanova L, Velarde MC and Giudice LC (2010) Altered gene expression profiling in endometrium: evidence for progesterone resistance Semin Reprod Med 28(1) 51–8 DOI: 10.1055/s-0029-1242994 PMID: 20104428