Radiation therapy for cervical cancer in Uganda: a practice guideline
Solomon Kibudde1,2, Awusi Kavuma1, Bonny Abal1, Moses Fredrick Katumba1, Cissy Bangidde Namutale1, Daniel Kanyike1 and Israel Luutu1
1Division of Radiation Oncology, Uganda Cancer Institute, PO Box 3935, Kampala, Uganda
2Department of Medicine, College of Health Sciences, Makerere University, PO Box 7072, Kampala, Uganda
Abstract
Introduction: Radiation therapy (RT) is crucial in the management of cervical cancer, particularly in resource-limited settings where most patients present with advanced-stage disease. Advances in external beam radiotherapy (EBRT) planning and delivery techniques, brachytherapy (BT) and systemic therapy necessitate context-adapted guidelines to standardise care. We developed a clinical practice guideline to improve and to harmonise the multidisciplinary management of cervical cancer in Uganda.
Methods: A multidisciplinary team of Radiation Oncologists, Medical Physicists and Radiation therapists developed the guideline using a modified Delphi process. The guideline was externally reviewed by experts from the International Gynaecological Radiation Oncology Consortium.
Results: All newly diagnosed patients should undergo multisciplinary evaluation prior to radiotherapy. For early-stage cervical cancer, adjuvant radiotherapy after hysterectomy is indicated in women with intermediate-risk factors, while concurrent cisplatin-based chemoradiation is indicated in women with high-risk factors. The standard EBRT dose is 45–50 Gy; women with vaginal and/or parametrial disease should receive adjuvant vaginal vault BT to achieve an equivalent dose in 2 Gy (EQD2) of 60 Gy. For locally advanced cervical cancer, the standard of care is pelvic EBRT (45–50 Gy in 25 fractions) with concurrent cisplatin (40 mg/m2 weekly for 5–6 cycles) followed by image-guided adaptive brachytherapy delivering 24–28 Gy in 3–4 fractions to achieve an EQD2 of 80–85 Gy for small tumours and 85–90 Gy for large tumours. The overall treatment time should not exceed 56 days. In recurrent disease, management depends on the location of the recurrence and the interval since the previous RT. In metastatic disease, palliative RT is directed to symptomatic sites.
Conclusion: This clinical practice guideline offers evidence-informed, context-specific recommendations for the use of EBRT and BT in cervical cancer management in Uganda. It aims to harmonise the role of RT within multidisciplinary care pathways.
Keywords: radiotherapy, cervical cancer, guidelines, Uganda
Correspondence to: Solomon Kibudde
Email: solomon.kibudde@uci.or.ug
Published: 25/09/2025
Received: 27/04/2025
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cervical cancer ranks as the fourth most prevalent cancer in women, contributing significantly to both incidence and mortality rates. In 2022, it was estimated that there were approximately 660,000 new cases and 350,000 deaths globally [1]. The burden of cervical cancer is highest in low-and middle-income countries (LMICs). In 2020, the highest age-standardised incidence rates for cervical cancer were observed in Eastern Africa, with 40 cases per 100,000 women [2]. In Uganda, cervical cancer is the leading cause of cancer-related incidence and mortality, with an estimated 6,900 new cases and 4,800 deaths reported in 2022 [3]. Squamous cell carcinomas account for approximately 80% of all cervical cancers, and most patients present with locally advanced stages at diagnosis, leading to poor treatment outcomes [4, 5].
Radiation therapy (RT) is crucial for the effective management of cervical cancer. Over the past two decades, there have been significant advancements in external beam radiation therapy (EBRT) treatment planning and delivery techniques, brachytherapy (BT), systemic therapy and automation with artificial intelligence (AI) [6–10]. However, access to advanced radiotherapy technologies remains challenging for most patients in resource-limited settings, where the burden of cervical cancer is highest. Several strategies to address access to radiotherapy for cervical cancer have been proposed, including the use of hypofractionated EBRT [11, 12] and the emerging role of induction chemotherapy [13].
To standardise the use of RT for cervical cancer in Uganda, we have developed a clinical practice guideline to improve and to harmonise the management of patients with cervical cancer within a multidisciplinary setting. This guideline provides an update to the current guideline published in 2005 [14], reflecting the transitions in radiotherapy techniques and technology while integrating recent evidence, in the era of modern radiotherapy practice [15]. Overall, the guideline is intended to enhance access to effective radiotherapy and promote evidence-based practices, while optimising the available resources.
Methods
Scope
This clinical practice guideline provides recommendations for the use of RT in the curative or palliative treatment of women diagnosed with all stages of invasive cervical cancer in Uganda. The recommendations are stratified into three broad categories, namely early cervical cancer, locally advanced cervical cancer (LACC), International Federation of Gynaecology and Obstetrics (FIGO stages IB3 – IVA) and metastatic and/or recurrent cervical cancer.
Population
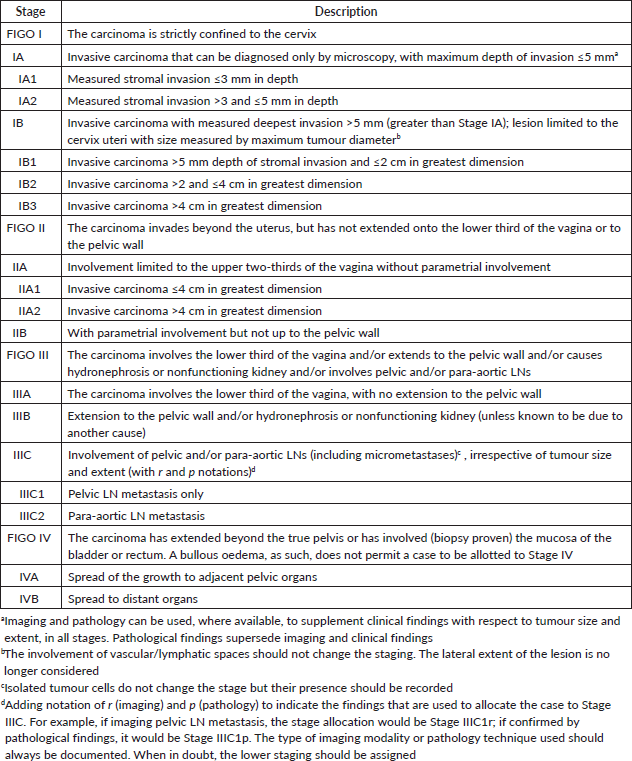
The target population comprises women aged 18 to 85 years with a histologically confirmed cervical squamous cell carcinoma or adenocarcinoma or adenosquamous carcinoma and FIGO stages IA to IVB, irrespective of human immunodeficiency virus (HIV) co-infection. Women with rare histological diagnoses such as small cell carcinoma, neuroendocrine tumour, lymphoma, sarcoma or melanoma, non-invasive and benign diseases of the cervix are excluded from the scope of this guideline.
Guideline development
This guideline was compiled by radiation oncologists (ROs), medical physicists (MPs), radiation therapy technologists and radiation oncology nurses of the Division of Radiation Oncology at the Uganda Cancer Institute. The writing committee was led by an RO (Dr Solomon Kibudde) and met weekly for 2 months. Supplementary input was provided by experts from the International Gynaecological Radiation Oncology Consortium [16], led by a consultant clinical oncologist (Dr Alexandra Taylor), during weekly meetings from November 2024 to April 2025.
Target users
The primary intended users of this guideline are RO, MPs, radiation therapists, dosimetrists, radiation oncology nurses, gynaecological oncologists, pathologists, palliative care practitioners and general medical and allied health practitioners involved in the diagnosis, treatment and follow-up of women with confirmed or suspected cervical cancer. Secondary intended users include researchers, policymakers, students and administrators.
Literature search
A desk review was conducted on all search literature obtained using online electronic databases, including PubMed, Google Scholar, Scopus, Cochrane Library and Web of Science. The search was conducted in English, using MeSH terms generated from the guideline questions, such as 'Cervical Cancer' and ' RT' or 'Radiotherapy,' or ‘BT’ or ‘Treatment’ covering the period from 01 January 2000 to 30 April 2025. These data were reviewed and critically appraised by members of the writing committee.
Evidence review
The literature was appraised based on the study design, strengths and limitations (such as sampling, blinding, allocation concealment and analysis methods), comparison groups, outcome variables and consistency of results across other studies.
Formulation of recommendations
A modified Delphi technique [17] that follows a systematic and structured approach was employed to formulate the guideline recommendations. This process involved an extensive review of existing recommendations from other guidelines and adopting practices relevant to the Ugandan healthcare context. For each recommendation, we ensured alignment with the available supporting data regarding the benefits and harms to patients.
External review
The guideline was reviewed by experts in Radiation Oncology and Medical Physics from the International Gynaecological Radiation Oncology Consortium [16], representing three large leading academic institutions. This review aimed to enhance the quality of the guideline and obtain feedback on the recommendations.
Results
The guideline encompasses the use of EBRT and BT in the curative and palliative treatment of early-stage, locally-advanced and metastatic and/or recurrent cervical cancer in Uganda (Table 1).
General recommendations
All patients should be evaluated by a multisciplinary team and must provide informed consent before commencement of any radiotherapy-related procedures. The clinical stage, according to the FIGO staging system (Table 2), performance status (Eastern Cooperative Oncology Group, ECOG) score, clinical-radiological drawing of the initial disease, HIV status and intent of treatment should be well documented. The staging evaluation will be complemented by a review of the patient's complete blood count, renal function tests, liver function tests, HIV serology results, CD4 T-cell count and/or HIV viral load in HIV-positive patients and a computed tomography (CT) scan of the chest, abdomen and pelvis. When available, results from a pelvic magnetic resonance imaging (MRI) and positron-emission tomography (PET)/CT scan will be reviewed. Cystoscopy and proctoscopy are not routinely recommended.
Table 1. Radiotherapy treatment options based on cervical cancer FIGO staging.
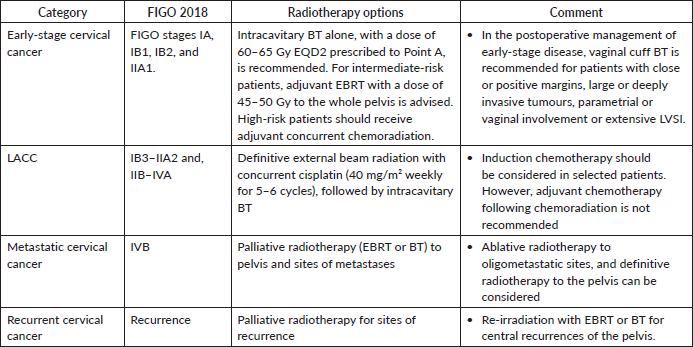
Table 2. 2018 FIGO staging for invasive cervical cancer [51].
Early-stage cervical cancer
For Stage IA1 disease without lymphovascular space invasion (LVSI), simple hysterectomy without lymphadenectomy is generally sufficient [18]. For stages IA2–IB2 and IIA1, hysterectomy with pelvic lymphadenectomy is recommended [19]. Patients with pathologically confirmed lymph node (LN) metastasis should be re-staged as FIGO IIIC1 per the 2018/2021 FIGO staging system. Post-operative radiotherapy alone is considered for patients with early cervical cancer (FIGO stages IA1–IB2 and IIA1) who have undergone hysterectomy and pathology findings reveals any of these intermediate-risk factors: LVSI plus deep one-third cervical stromal invasion and any tumour size or LVSI plus middle one-third cervical stromal invasion and tumour size ≥2 cm or LVSI plus superficial one-third cervical stromal invasion and tumour size <4 cm, noting that lesions ≥4 cm (IB3) represent locally advanced disease and should be managed accordingly or no LVSI plus middle or deep one-third cervical stromal invasion and tumour size ≥4 cm [20], referred to as the Sedlis criteria. Additionally, adenocarcinoma and adenosquamous histology have been considered an intermediate-risk factor [21]. The recommended EBRT dose is 45–50.4 Gy in 25–28 fractions (1.8 Gy per fraction), delivered 5 fractions per week over 5–5.5 weeks [20]. Advanced techniques such as image-guided radiotherapy utilising intensity-modulated radiotherapy (IMRT) and volumetric-modulated arc therapy (VMAT) are preferred over three-dimensional conformal radiotherapy (3DCRT) and two-dimensional radiotherapy (Figure 1). Patients with positive or close vaginal surgical margins should receive adjuvant vaginal vault BT dose of 10–15 Gy [22].
Adjuvant concurrent cisplatin-based chemoradiation improves overall survival and progression-free survival in women with high-risk factors referred to as Peter’s criteria, such as positive resection margins, LN involvement or parametrial invasion following radical hysterectomy [23]. The recommended EBRT dose is 50.4 Gy in 28 fractions (1.8 Gy per fraction), over 5.5 weeks [20] delivered using IMRT or VMAT using image-guidance with daily cone-beam CT or orthogonal kilovoltage images. Treatment with EBRT should commence within 6 to 8 weeks after surgery to maximise therapeutic efficacy and improve outcomes [24, 25]. The recommended BT dose scheme is 11 Gy in 2 fractions at 5.5 Gy per fraction to 5 mm below the vaginal surface or 18 Gy in 3 fractions at 6 Gy per fraction, to achieve an equivalent-dose in 2 Gy per fraction (EQD2) of 55 to 60 Gy and keeping the overall treatment time below 7 weeks [25] (Figure 2). However, for patients with central recurrence after hysterectomy, in the absence of interstitial BT, they should be offered a sequential EBRT boost to 66 Gy to the gross residual tumour by replanning to a smaller residual gross tumour after 4 weeks of the pelvic EBRT course.
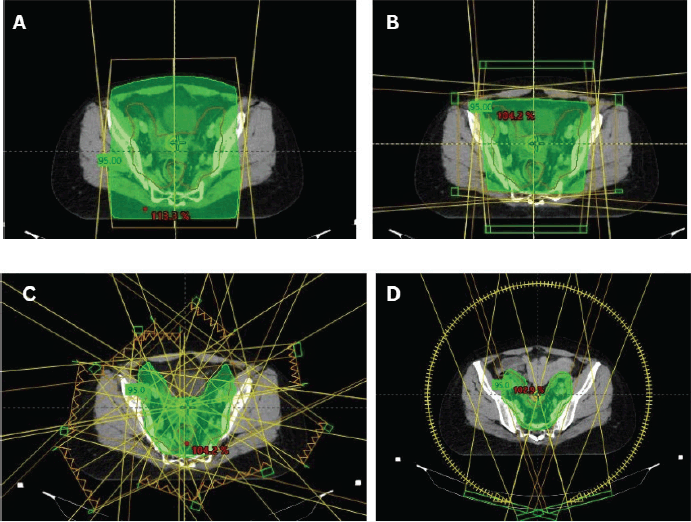
Figure 1. Cervical cancer EBRT techniques. (a): 2D radiotherapy beam arrangement with AP-PA opposing beams. (b): 3DCRT ‘box’ or 4-field beam arrangement. (c): 9-fields IMRT plan for cervical cancer. (d): VMAT plan for cervical cancer.
Locally advanced cervical cancer
The standard of care for LACC is definitive pelvic EBRT dose of 45–50 Gy in 25 fractions (1.8–2 Gy per fraction), delivered 5 fractions per week over 5 weeks with concurrent cisplatin (40 mg/m2) given weekly for 5–6 cycles, followed by Image-guided adaptive intracavitary/interstitial high-dose-rate (HDR) BT dose of 24–28 Gy in 3–4 fractions of 7–8 Gy, to achieve a total EBRT+BT dose of 80–85 Gy for small cervical tumours and 85–90 Gy for large cervical tumours or tumours with poor response to EBRT, adenocarcinoma and stage III at presentation [26]. The addition of concurrent chemotherapy results in a 6% improvement in 5-year survival compared with radiotherapy alone for women with LACC (HR 0·81, p < 0·001) [27]. While Cisplatin is the radiosensitiser of choice, carboplatin area under the curve 2 (AUC 2) weekly dose is an acceptable alternative to cisplatin among women with renal insufficiency (creatinine clearance ≤45 mL/min). Patients with pelvic and para-aortic LNs should receive EBRT boost to a dose of 55–60 Gy (EQD2) using simultaneous integrated boost or sequential boost technique, with consideration of contribution from BT (Figure 3).
Also, patients with involved para-aortic, common iliac LN and ≥3 involved pelvic LNs have a significant risk for para-aortic nodal recurrence and should receive extended-field EBRT. Although hypofractionated radiotherapy is not a standard of care, based on retrospective studies, it has demonstrated comparable safety with conventional fractionated radiotherapy when giving EBRT regimens of 45 Gy in 15 fractions [4] and 40 Gy in 16 fractions [28]. Several phase II prospective trials are currently underway to address this question.
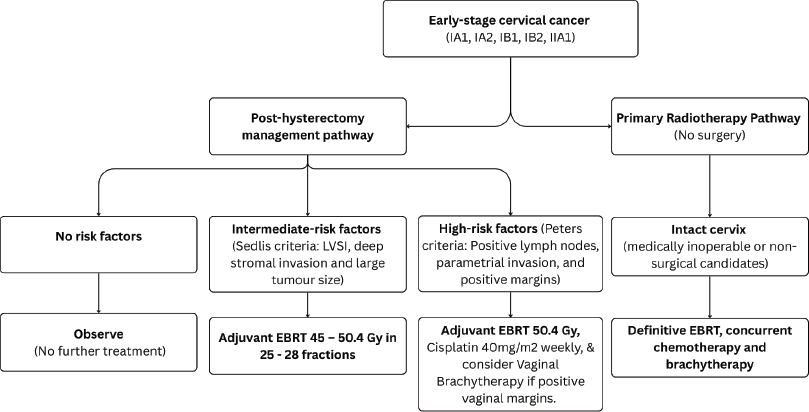
Figure 2. Use of RT in early-stage cervical cancer (IA1, IA2, IB1, IB2 and IIA1).
BT is a key component in the curative treatment of LACC. Image-guided adaptive intracavitary/interstitial HDR BT is the standard approach, with applicators selected based on tumour extent and patients’ anatomy. BT should commence in the third to fourth week of EBRT as 1–2 sessions per week, prescribed to the ICRU Point A or high-risk clinical target volume for two-dimension (2D) or three-dimension (3D) BT techniques, respectively (Figure 4). Alternative fractionation schedules include 9 Gy in a single fraction weekly for 2 weeks, 6.5 Gy in a single fraction twice weekly for four fractions and 7 Gy in a single fraction twice weekly for three to four fractions [29].
Based on data from the INTERLACE Trial, induction chemotherapy (once-a-week intravenous (IV) carboplatin area under the AUC 2 and paclitaxel 80 mg/m² for 6 weeks) followed by standard cisplatin-based chemoradiotherapy is acceptable in fit patients with stage IIB cervical cancer with granulocyte colony stimulating factor support and ability to bridge to concurrent chemoradiation within 7 days of completing induction chemotherapy [13]. The use of adjuvant chemotherapy following definitive chemoradiation is not recommended [30].
Metastatic and recurrent cervical cancer
For central pelvic recurrences after prior chemoradiation, salvage surgery—including pelvic exenteration or, less commonly, radical hysterectomy—can be considered, especially when the recurrent tumour is centrally located, ≤3 cm in size and in the absence of distant metastasis [31]. Palliative radiotherapy is administered to patients with metastatic or recurrent cervical cancer to alleviate symptoms that are not responsive to medical management. Common indications include pain, tumour haemorrhage, vaginal discharge and compression symptoms such as spinal cord compression, brain metastases or nodal and bone metastases. The prescribed total absorbed dose is typically 30 Gy, delivered in 10 fractions of 3 Gy per fraction, with five fractions per week over 2 weeks [32]. Alternative palliative radiotherapy regimens include 10 Gy in a single fraction to the whole pelvis, repeated monthly for 2–3 fractions [33]. Patients who attain a good partial response after the first two fractions of radiation may be considered for a single HDR intracavitary BT session, delivering 10 Gy to point A. Alternatively 20 Gy in 5 fractions to the pelvis [34]; and the 'QUAD Shot' regimen: 3.7 Gy twice daily (totalling 14.8 Gy per cycle) over 2 days, repeated monthly for up to 3 months [35] For localised central recurrences within the pelvis, re-irradiation with EBRT or BT is an option for selected patients depending on previous treatment, previous radiotherapy dose, time from prior radiotherapy and any persisting radiotherapy related toxicity (Figure 5).
Radiotherapy procedures
2D or conventional simulation
About 40%–50% of our cervical patients are planned with 2D conventional simulation procedure, which involves the use of bony anatomy and clinical judgement to define target volumes. The anterior – posterior (AP-PA) radiation field borders are superiorly: L4-L5 inter-disc space; inferiorly: lower border of the obturator foramen or 3 cm below the inferior extent of the vaginal disease; and laterally: 2 cm lateral to the true pelvis or pelvic brim to adequately cover the external iliac and obturator nodes. The lateral field borders maintain the same superior and inferior limits as for the AP-PA fields, with the anterior border at the anterior face (cortex) of the pubic symphysis and the posterior border at 1–1.5 cm beyond the sacrum hollow [36].
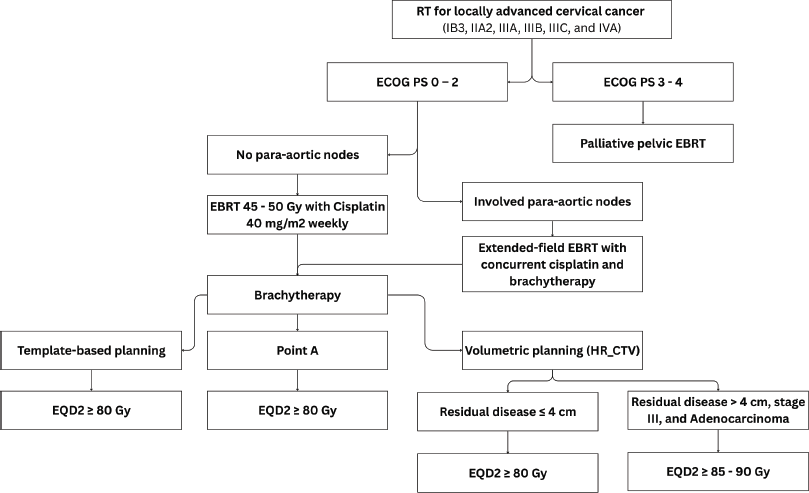
Figure 3. Use of RT in LACC (IB3, IIA2, IIIA, IIIB, IIIC and IVA).
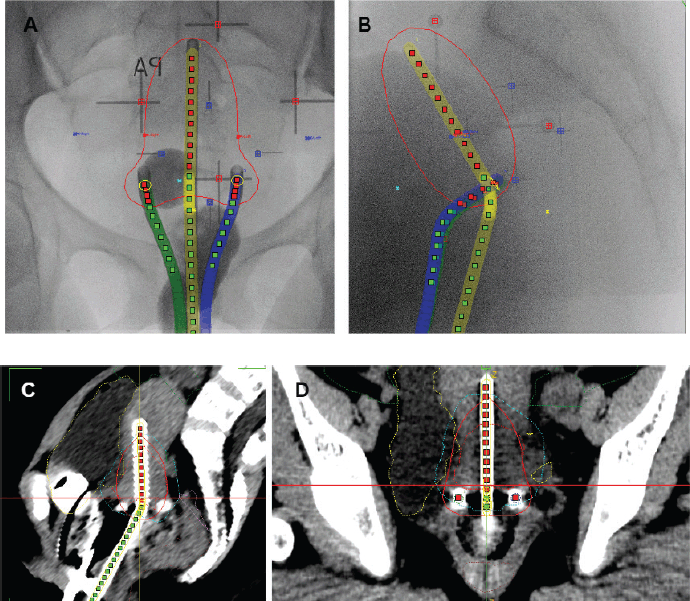
Figure 4. Cervical cancer BT techniques. (a): AP-PA view of X-ray image for 2D point A based BT planning. (b): Sagittal view of X-ray image for 2D point A based BT planning. (c): Sagittal view of CT-based 3D BT planning. (d): AP-PA view of CT-based 3D BT planning.
3D or CT simulation
The bladder preparation protocol requires the patient to empty their bladder and then drink 500 mL (1 cup) of water 30 minutes before imaging and each treatment session, maintaining optimal hydration throughout treatment. The rectum preparation protocol involves following a high-fibre diet and taking laxatives, e.g., bisacodyl tablets 10 mg taken orally at bedtime for 3 days before computerised tomography simulation and throughout treatment. The patient will be positioned supine on the couch of the CT or conventional simulator using orthogonal lasers and immobilised with an individualised ankle and knee fixator, a head sponge and arms positioned over the chest. The RO will place a radio-opaque marker to localise the inferior extent of vaginal disease. The CT scan will be acquired in 3 mm slices from the T10 vertebral body to mid-femur, 35 seconds after administration of IV contrast (1 mL/kg of body weight).
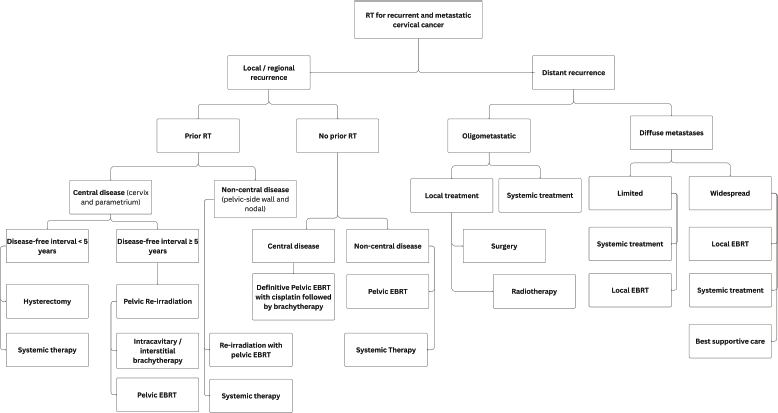
Figure 5. Use of RT for recurrent and metastatic cervical cancer.
Target volume delineation
Target volume delineation will be performed by a RO using the ICRU Reports 50, 62 and 83 [37, 38] and approved within 15 days after CT simulation. The Clinical Target Volume-1 (CTV1) will include the gross tumour volume (GTV), entire cervix and uterus. The CTV2 will include the upper vagina, to at least 2 cm below the lowest aspect of the GTV or cervix, and parametrium, while CTV3 will include obturator, internal iliac, external iliac and common iliac nodes [39–41]. Inguinal nodes should be included if the lower third of the vagina is involved, while para-aortic nodes will be contoured up to the level of the renal hilum in patients with involved para-aortic nodes or multiple pelvic LNs, including the common iliac nodal station. Nodes are contoured as 7 mm around visible vessels, excluding bone and muscle, following guidelines for nodal contouring [41]. For post-operative cases, a reduced target volume for EBRT resulting in a small pelvic field not including the common iliac nodes may be considered in low - and intermediate- risk patients with negative LNs on imaging and no LVSI. The Planning Target Volume (PTV) margins are 0.7 cm for the nodes, designated as PTV3, 1 cm for CTV2 – designated as PTV2 and 1.0–1.5 cm for CTV1 – designated as PTV1. The EBRT dose will be prescribed to the total PTV, which is the structure summing PTV1, PTV2 and PTV3.
Organs at risk (OAR) delineation
The OAR structures will include the bladder, rectum, femoral heads, bowel bag, small bowel (particularly for post-operative cases) and bone marrow. For extended-field radiotherapy, additional OARs include the spinal cord, kidneys and small bowel loops. These structures will be contoured using international atlases for OAR delineation [42] or AI-manual assisted delineation [43].
Radiotherapy plan evaluation
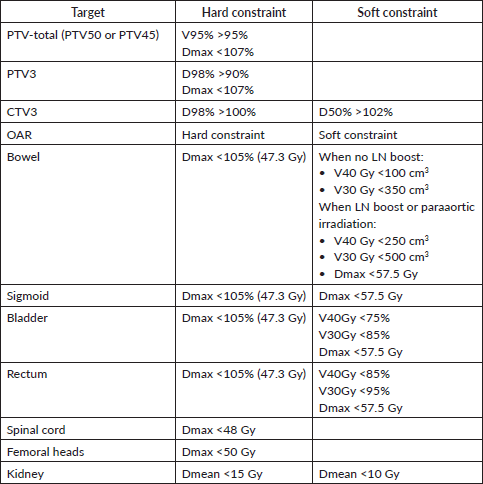
Plan configuration is performed by the planner (MP and/or Radiation therapist/dosimetrist) and approved by the RO. It involves several steps utilising the CT-CHOP criteria [44], reviewing the contours, targets, PTV coverage and conformity index, PTV dose heterogeneity and presence of hot spots, OAR constraints using the dose volume histogram (DVH) and clinical goal tool, and the prescription dose. The prescribed absorbed dose should be specified at the 100% isodose volume of the PTV or the ICRU reference point/isocentre. The planning goals will ensure that at least 98% of the PTV is covered by 95% of the prescription dose, without exceeding tolerances for OARs (Table 3).
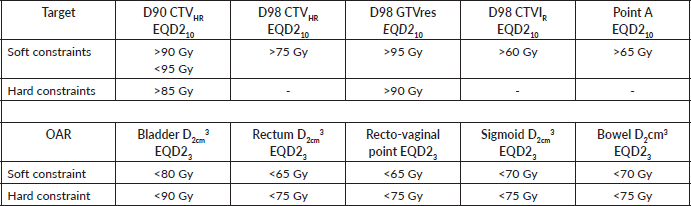
Table 3. Planning aims for targets and OAR constraints for EBRT planning (adopted from the EMBRACE II protocol) [52].
Dosimetric considerations
Dosimetric calculations shall be performed by the MP on CT images or water phantom for 2D radiotherapy technique, with the absorbed dose within the patient/phantom geometry calculated using either the AAA or Acuros algorithm, with a maximum grid calculation resolution of 2.5 mm. An independent quality check of the radiotherapy plan will be conducted by an experienced MP to ensure accuracy and compliance with treatment guidelines.
Patient-specific quality assurance (PSQA) and independent dose verification
All IMRT and VMAT radiotherapy plans for each new patient shall undergo PSQA checks before treatment commences using portal dosimetry. The monitor unit and absorbed dose calculations shall be independently verified, using Mobius3D ensuring that the independently calculated dose is within 5% of the planned dose in terms of target coverage, DVH limits (Table 3), 3D gamma pass/fail rates and deliverability.
Concurrent chemotherapy
The RO will ensure optimal performance status (ECOG performance status of 0–2), bone marrow and renal function before prescribing concurrent chemotherapy. Cisplatin 40 mg/m² (capped at 70 mg) is ideally administered IV in 200 mL of normal saline (NS) is administered over 30 minutes, preferably 30 minutes to 2 hours before radiotherapy, once a week on a Monday or Tuesday, for at least 5–6 cycles during EBRT. Supportive pre-chemotherapy medications include Ondansetron 8 mg IV, Dexamethasone 8 mg IV and pre-hydration with 1,000 mL NS with MgSO₄ 2 g over 1 hour. Post-chemotherapy, the patient is hydrated with 1,000 mL NS over 1 hour with KCl 40 mEq and oral antiemetics such as Ondansetron 8 mg PO twice daily (BD) for 5 days or Granisetron 1–2 mg PO twice daily (BD) for 5 days.
Brachytherapy
Applicators should be inserted with the aid of an ultrasound scan, and fluoroscopy images taken to confirm applicator position, and generate a 2D BT plan. For small tumours or patients with no residual disease, template-based planning with a standard loading pattern can be considered. For stage IB3 and IIB, consider the use of 3D imaging with CT scan for 3D-based image-guided adaptive brachytherapy (IGABT) planning using the ICRU report 38 and 89 for the first implant and ultrasound guidance for subsequent implants (Figure 3). The goal of IGABT is to deliver a BT dose of 40 to 45 Gy EQD2 to reach a total EBRT+BT dose of 80–85 Gy for small cervical tumours and >85 Gy for large cervical tumours or tumours with poor response to EBRT, adenocarcinoma and stage III/IVA at initial presentation[7, 45–47]. Doses to OAR, especially the bladder, rectum, sigmoid and bowel should be tabulated using EQD2 Excel calculators and kept within tolerance (Table 4).
EBRT boost
An EBRT boost is recommended for patients who are ineligible for BT due to factors such as tumour size, poor performance status, vesicovaginal or rectovaginal fistula or vaginal stenosis. While there is no standard fractionation, we routinely administer an EBRT dose to PTV1 only, ranging from 14 to 20 Gy in 4 to 10 fractions. Recommended techniques include 3D conformal RT or IMRT; however, 2D radiotherapy can be considered in selected patients. Several studies are exploring the use of stereotactic body radiotherapy in the delivery of EBRT boost, due to its physical and radiobiological benefits; however, this remains inferior to conventional BT [48, 49].
Overall treatment time
The overall treatment duration should not exceed 56 days (8 weeks) [48]. Any unintended treatment interruptions must be compensated within the intended overall treatment timeframe using hypofractionation, as directed by the RO. EBRT and BT are scheduled concurrently, starting from Week 1 to 5, once a significant tumour reduction (tumour size ≤4 cm, without parametrial involvement) is achieved. EBRT and/or chemotherapy should be omitted on days of BT implants to avoid excessive toxicity.
Post-treatment follow-up
The RO shall conduct an end-of-treatment evaluation during the final week of radiotherapy. Post-treatment follow-up to assess quality of life and disease recurrence will occur at 4–6 weeks, then every 3–4 months for the first 2 years, followed by every 6 months up to 5 years, and then annually thereafter. In patients with uncertainty of clinical remission after 3 months post chemoradiation, a follow-up assessment in 2–3 months is recommended with consideration of biopsy to exclude residual disease. Clinical follow-up will be based on physical examination and symptom-guided imaging as appropriate and coordinated with the gynaecological oncology team. Routine laboratory tests and vaginal vault or cervical cytology tests are not recommended. Consideration of hysterectomy after definitive chemoradiation is not recommended, particularly when the patient has received IGABT.
Table 4. Planning aims for targets and OAR for BT [52].
Management of treatment-related morbidity
RT may result in acute toxicities such as gastrointestinal symptoms (e.g., diarrhoea and nausea), genitourinary irritation (e.g., frequency and urgency) and hematologic suppression during concurrent chemotherapy [50]. Long-term effects may include vaginal stenosis, fibrosis, chronic proctitis and radiation cystitis [50]. To mitigate these risks, patients should receive supportive care, counselling and be monitored closely during and after treatment. Vaginal dilator use and pelvic floor physiotherapy are encouraged post-treatment. Severe or persistent late effects should be referred to appropriate specialists.
Discussion
This clinical practice guideline provides a comprehensive and pragmatic framework for the use of RT in the management of cervical cancer in Uganda. Developed in the context of resource-constrained settings, the guideline integrates contemporary evidence with practical adaptations that reflect the realities of radiotherapy delivery in Uganda and other LMICs. It emphasises evidence-informed decision-making, efficient resource utilisation and context-appropriate innovations to address Uganda’s significant burden of cervical cancer.
The recommendations align with core principles endorsed by leading professional bodies such as the American Society for Radiation Oncology, European Society for Therapeutic Radiation Oncology, American Brachytherapy Society and the National Comprehensive Cancer Network [51–53], namely prioritisation of tumour control, patient-centred care and toxicity mitigation. However, the guideline departs in its pragmatic approach to technology and infrastructure, such as the use of 3D simulation with the aid of pelvic MRI and PET/CT, and MRI-guided adaptive BT, as suggested remaining challenge for resource-constrained settings. Therefore, we adopted CT simulation for both EBRT and BT planning, which is cost-effective, consistent with guideline recommendations for resource-limited settings [7, 54].
One of the key strengths of this guideline is the incorporation of hypofractionated EBRT regimens, particularly in the management of LACC. These schedules reduce treatment duration, machine burden and patient costs—factors that are critically important in high-volume clinics where treatment delays compromise clinical outcomes. Similarly, the use of 2D or template-based BT planning, when 3D image-guidance is unavailable, demonstrates how high-impact treatment can still be delivered safely using simplified clinical workflows.
Despite these advances, implementation will require proactive engagement from institutional and national stakeholders. Frequent machine breakdowns, limited BT capacity, insufficient trained personnel and an unreliable power supply remain significant barriers. To mitigate these, deliberate investments in preventive maintenance, staff capacity building, stable power systems and procurement of essential planning and quality assurance tools must accompany clinical guideline rollout.
Importantly, the guideline serves as a foundation for integrating radiotherapy into Uganda’s broader cancer control plan. It is structured to inform policy development, resource allocation and workforce planning and guide the scaling up of regional radiotherapy services. Additionally, further research is needed to evaluate the clinical outcomes of these context-adapted strategies, particularly hypofractionation and CT-based BT, in Ugandan populations.
Conclusion
This Uganda-specific clinical practice guideline offers a pragmatic yet evidence-based framework for improving radiotherapy access and quality in cervical cancer care. By leveraging hypofractionation, simplified BT and CT-based planning, it enables rational technology use without compromising outcomes. This guideline represents the prevailing standard of care regarding cervical cancer management in Uganda and manifests tremendous progress in techniques and potential outcomes, which is a result of continued partnerships, particularly with the International Gynaecological Radiation Oncology Consortium, which have provided continued technical guidance and mentorship.
List of abbreviations
2D, 2-dimensional; 3D, 3-dimensional; CT, computed tomography; D2cc, is the minimal dose to the 2 cm3 (2 mL) of the organ at risk; EBRT, external beam radiation therapy; EQD210, dose calculation to an equivalent dose of 2 Gy with an α-to-β ratio of 10; EQD23, dose calculation to an equivalent dose of 2 Gy with an α-to-β ratio of 3; HDR, high-dose-rate; HR-CTV, high-risk clinical target volume; ICRU, International Commission of Radiation Units and Measurements; IMRT, Intensity modulated radiation therapy; LVSI, Lymphovascular space involvement; MRI, Magnetic resonance imaging; OARs, Organs at risk; PET, Positron emission tomography; RT, Radiation therapy; VMAT, Volumetric modulated arc therapy.
Acknowledgments
We extend our gratitude to colleagues from the Division of Radiation Oncology at the Uganda Cancer Institute who actively participated in drafting these guidelines. We also acknowledge the invaluable input from experts within the International Gynaecological Radiation Oncology (IGRO) consortium, namely Surendra Prajapati, Taran Paulsen Hellebust, Anuja Jhingran and Alexandra Taylor, supported by the International Gynaecological Cancer Society (IGCS).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Funding
There was no funding for this study.
References
1. Bray F, Laversanne M, and Sung H, et al (2024) Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 74(3) 229–263 PMID: 38572751
2. Singh D, Vignat J, and Lorenzoni V, et al (2023) Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative Lancet Glob Health 11(2) e197–e206 https://doi.org/10.1016/S2214-109X(22)00501-0 PMCID: 9848409
3. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 PMID: 33538338
4. Kavuma A, Luutu I, and Kibudde S, et al (2021) A retrospective review of conventional versus hypo-fractionated pelvic radiotherapy for locally advanced cervical cancer, in limited-resource countries: the Uganda experience S Afr J Oncol 5 https://doi.org/10.4102/sajo.v5i0.186
5. Wabinga H, Ramanakumar AV, and Banura C, et al (2003) Survival of cervix cancer patients in Kampala, Uganda: 1995-1997 Br J Cancer 89(1) 65–69 https://doi.org/10.1038/sj.bjc.6601034 PMID: 12838301 PMCID: 2394214
6. Potter R, Tanderup K, and Schmid MP, et al (2021) MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): a multicentre prospective cohort study Lancet Oncol 22(4) 538–547 https://doi.org/10.1016/S1470-2045(20)30753-1 PMID: 33794207
7. Mahantshetty U, Poetter R, and Beriwal S, et al (2021) IBS-GEC ESTRO-ABS recommendations for CT based contouring in image guided adaptive brachytherapy for cervical cancer Radiother Oncol 160 273–284 https://doi.org/10.1016/j.radonc.2021.05.010 PMID: 34019918 PMCID: 8675891
8. Lin AJ, Kidd E, and Dehdashti F, et al (2019) Intensity modulated radiation therapy and image-guided adapted brachytherapy for cervix cancer Int J Radiat Oncol Biol Phys 103(5) 1088–1097 https://doi.org/10.1016/j.ijrobp.2018.11.012
9. Sagae S, Toita T, and Matsuura M, et al (2023) Improvement in radiation techniques for locally advanced cervical cancer during the last two decades Int J Gynecol Cancer https://doi.org/10.1136/ijgc-2022-004230 PMCID: 10423558
10. Adam JA, Loft A, and Chargari C, et al (2021) EANM/SNMMI practice guideline for [(18)F]FDG PET/CT external beam radiotherapy treatment planning in uterine cervical cancer v1.0 Eur J Nucl Med Mol Imaging 48(4) 1188–1199 https://doi.org/10.1007/s00259-020-05112-2 PMCID: 8041686
11. Mendez LC, Raziee H, and Davidson M, et al (2020) Should we embrace hypofractionated radiotherapy for cervical cancer? A technical note on management during the COVID-19 pandemic Radiotherapy and Oncology vol 148 (Elsevier Ireland Ltd) pp 270–273 https://doi.org/10.1016/j.radonc.2020.05.032
12. Ngwa W, Addai BW, and Adewole I, et al (2022) Cancer in sub-Saharan Africa: a Lancet Oncology Commission Lancet Oncol 23(6) e251–e312 https://doi.org/10.1016/S1470-2045(21)00720-8 PMID: 35550267 PMCID: 9393090
13. McCormack M, Eminowicz G, and Gallardo D, et al (2024) Induction chemotherapy followed by standard chemoradiotherapy versus standard chemoradiotherapy alone in patients with locally advanced cervical cancer (GCIG INTERLACE): an international, multicentre, randomised phase 3 trial Lancet 404(10462) 1525–1535 https://doi.org/10.1016/S0140-6736(24)01438-7 PMID: 39419054
14. Kiguli-Malwadde E, Gakwaya A, and Robinson A, et al (2005) Cancer of the cervix: the Uganda guidelines East Cent Afr J Surg 10(2) 93–99
15. Kavuma A, Kibudde S, and Kanyike D, et al (2025) Evolution and recent radiation therapy advancement in Uganda: a precedent on how to increase access to quality radiotherapy services in low- and middle-income countries JCO Glob Oncol 11 e2400339 https://doi.org/10.1200/GO-24-00339 PMID: 39883898
16. The International Gynecologic Radiation Oncology Consortium [https://igcs.org/international-gynecologic-radiation-oncology-consortium/]
17. Hasson F, Keeney S, and McKenna H (2000) Research guidelines for the Delphi survey technique J Adv Nurs 32(4) 1008–1015 https://doi.org/10.1046/j.1365-2648.2000.t01-1-01567.x PMID: 11095242
18. Guimaraes YM, Godoy LR, and Longatto-Filho A, et al (2022) Management of early-stage cervical cancer: a literature review Cancers (Basel) 14(3) https://doi.org/10.3390/cancers14030575
19. Togami S, Kamio M, and Yanazume S, et al (2014) Can pelvic lymphadenectomy be omitted in stage IA2 to IIB uterine cervical cancer? Int J Gynecol Cancer 24(6) 1072–1076 https://doi.org/10.1097/IGC.0000000000000163 PMID: 24905616
20. Sedlis A, Bundy BN, and Rotman MZ, et al (1999) A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a gynecologic oncology group study Gynecol Oncol 73(2) 177–183 https://doi.org/10.1006/gyno.1999.5387 PMID: 10329031
21. Levinson K, Beavis A, and Purdy C, et al (2020) Beyond sedlis: a novel, histology-based nomogram for predicting recurrence risk and need for adjuvant radiation in cervical cancer – a NRG/GOG ancillary analysis J Clin Oncol 38(15_suppl) 6019–6019 https://doi.org/10.1200/JCO.2020.38.15_suppl.6019
22. Small Jr W, Beriwal S, and Demanes DJ, et al (2012) American Brachytherapy Society consensus guidelines for adjuvant vaginal cuff brachytherapy after hysterectomy Brachytherapy 11(1) 58–67
23. Peters Iii WA, Liu PY, and Barrett RJ, et al (2000) Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix Obst Gynecol Survey 55(8) 491–492 https://doi.org/10.1097/00006254-200008000-00017
24. Kim SI, Kim TH, and Lee M, et al (2020) Impact of adjuvant radiotherapy on survival outcomes in intermediate-risk, early-stage cervical cancer: analyses regarding surgical approach of radical hysterectomy J Clin Med 9(11) 3545 https://doi.org/10.3390/jcm9113545 PMID: 33153125 PMCID: 7692216
25. Jhawar S, Hathout L, and Elshaikh MA, et al (2017) Adjuvant chemoradiation therapy for cervical cancer and effect of timing and duration on treatment outcome Int J Radiat Oncol* Biol* Phys 98(5) 1132–1141 https://doi.org/10.1016/j.ijrobp.2017.03.045 PMID: 28721897
26. Datta NR, Stutz E, and Liu M, et al (2017) Concurrent chemoradiotherapy vs. radiotherapy alone in locally advanced cervix cancer: a systematic review and meta-analysis Gynecol Oncol 145(2) 374–385 https://doi.org/10.1016/j.ygyno.2017.01.033 PMID: 28188016
27. L VC (2010) Chemoradiotherapy for cervical cancer meta-analysis collaboration (CCCMAC). Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: individual patient data meta-analysis Cochrane Database Syst Rev 2010(1)
28. Komen AA (2014) A Retrospective Study of Advanced Carcinoma of the Cervix Treated With a Hypofractionated Radiation Therapy Protocol at the Department of Radiation Oncology, University of the Witwatersrand, Johannesburg, South Africa (Johannesburg: University of the Witwatersrand)
29. Sushant S, Sharma D, and Pandey R, et al (2023) Multiple sessions vs. single session image-based intracavitary brachytherapy for locally advanced cervical cancer: a randomized control trial Int J Radiat Oncol Biol Phys 117(2S) S41–S42 https://doi.org/10.1016/j.ijrobp.2023.06.314
30. Mileshkin LR, Moore KN, and Barnes EH, et al (2023) Adjuvant chemotherapy following chemoradiotherapy as primary treatment for locally advanced cervical cancer versus chemoradiotherapy alone (OUTBACK): an international, open-label, randomised, phase 3 trial Lancet Oncol 24(5) 468–482 https://doi.org/10.1016/S1470-2045(23)00147-X PMID: 37080223 PMCID: 11075114
31. Rema P, Mathew AP, and Suchetha S, et al (2017) Salvage surgery for cervical cancer recurrences Indian J Surg Oncol 8(2) 146–149 https://doi.org/10.1007/s13193-015-0472-2 PMID: 28546709 PMCID: 5427013
32. Friedlander M and Grogan M (2002) Guidelines for the treatment of recurrent and metastatic cervical cancer Oncologist 7(4) 342–347 https://doi.org/10.1634/theoncologist.2002-0342 PMID: 12185296
33. Sanjib KM, Siddhartha L, and Mary Ann M, et al (2005) Monthly palliative pelvic radiotherapy in advanced carcinoma of uterine cervix J Cancer Res Ther 1(4) 208–212 https://doi.org/10.4103/0973-1482.19588
34. Kim DH, Lee JH, and Ki YK, et al (2013) Short-course palliative radiotherapy for uterine cervical cancer Radiat Oncol J 31(4) 216–221 https://doi.org/10.3857/roj.2013.31.4.216
35. Kiattikul CL, Narayan K, and Bernshaw D, et al (2022) Tumor control after palliative hypofractionated, “Quad-shot,” external beam radiotherapy followed by brachytherapy: an effective approach in medically compromised and/or elderly patients with cervix cancer J Cancer Res Ther 18(1) 173–179 https://doi.org/10.4103/jcrt.JCRT_1346_20 PMID: 35381780
36. Gulia A, Patel F, and Rai B, et al (2013) Conventional four field radiotherapy versus computed tomography-based treatment planning in cancer cervix: a dosimetric study S Asian J Cancer 2(3) 132 https://doi.org/10.4103/2278-330X.114116
37. Chavaudra J and Bridier A (2001) Definition of volumes in external radiotherapy: ICRU reports 50 and 62 Cancer Radiother 5(5) 472–478 https://doi.org/10.1016/S1278-3218(01)00117-2 PMID: 11715299
38. Grégoire V and Mackie TR (2011) State of the art on dose prescription, reporting and recording in intensity-modulated radiation therapy (ICRU report No. 83) Cancer/Radiothérapie 15(6-7) 555–559 https://doi.org/10.1016/j.canrad.2011.04.003
39. Lim K, Small W Jr, and Portelance L, et al (2011) Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy for the definitive treatment of cervix cancer Int J Radiat Oncol Biol Phys 79(2) 348–355 https://doi.org/10.1016/j.ijrobp.2009.10.075
40. Small W Jr, Mell LK, and Anderson P, et al (2008) Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancer Int J Radiat Oncol Biol Phys 71(2) 428–434 https://doi.org/10.1016/j.ijrobp.2007.09.042
41. Taylor A, Rockall AG, and Reznek RH, et al (2005) Mapping pelvic lymph nodes: guidelines for delineation in intensity-modulated radiotherapy Int J Radiat Oncol Biol Phys 63(5) 1604–1612 https://doi.org/10.1016/j.ijrobp.2005.05.062 PMID: 16198509
42. Gay HA, Barthold HJ, and O’Meara E, et al (2012) Pelvic normal tissue contouring guidelines for radiation therapy: a radiation therapy oncology group consensus panel atlas Int J Radiat Oncol* Biol* Phys 83(3) e353–e362 https://doi.org/10.1016/j.ijrobp.2012.01.023 PMID: 22483697 PMCID: 3904368
43. Kibudde S, Kavuma A, and Hao Y, et al (2024) Impact of artificial intelligence-based autosegmentation of organs at risk in low-and middle-income countries Adv Radiat Oncol 9(11) 101638 https://doi.org/10.1016/j.adro.2024.101638 PMID: 39435039 PMCID: 11491949
44. Dean M, Jimenez R, and Mellon E, et al (2017) CB-CHOP: a simple acronym for evaluating a radiation treatment plan Appl Rad Oncol 6(4) 28–30 https://doi.org/10.37549/ARO1136
45. Haie-Meder C, Potter R, and Van Limbergen E, et al (2005) Recommendations from gynaecological (GYN) GEC-ESTRO working group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV Radiother Oncol 74(3) 235–245 https://doi.org/10.1016/j.radonc.2004.12.015 PMID: 15763303
46. Potter R, Haie-Meder C, and Van Limbergen E, et al (2006) Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology Radiother Oncol 78(1) 67–77 https://doi.org/10.1016/j.radonc.2005.11.014 PMID: 16403584
47. Viswanathan AN, Thomadsen B, and American Brachytherapy Society Cervical Cancer Recommendations C, et al (2012) American brachytherapy society consensus guidelines for locally advanced carcinoma of the cervix. Part I: general principles Brachytherapy 11(1) 33–46 https://doi.org/10.1016/j.brachy.2011.07.003 PMID: 22265436
48. Holschneider CH, Petereit DG, and Chu C, et al (2019) Brachytherapy: a critical component of primary radiation therapy for cervical cancer: from the Society of Gynecologic Oncology (SGO) and the American Brachytherapy Society (ABS) Brachytherapy 18(2) 123–132 https://doi.org/10.1016/j.brachy.2018.11.009 PMID: 30665713
49. Lee TH, Song C, and Kim IA, et al (2021) Stereotactic ablative body radiotherapy boost for cervical cancer when brachytherapy boost is not feasible Radiat Oncol 16 1–11 https://doi.org/10.1186/s13014-021-01877-4
50. Tsepov D, Guth U, and Hadwin R (2011) Treatment related morbidity in cervical cancer Curr Women's Health Rev 7(1) 87–93 https://doi.org/10.2174/157340411794474775
51. Shaili Aggarwal N, McMillian N, and Peter Frederick MS, et al (2024) NCCN guidelines version 3.2024 cervical cancer continue NCCN guidelines
52. Cibula D, Raspollini MR, and Planchamp F, et al (2023) ESGO/ESTRO/ESP guidelines for the management of patients with cervical cancer - update 2023 Int J Gynecol Cancer 33(5) 649–666 https://doi.org/10.1136/ijgc-2023-004429 PMID: 37127326 PMCID: 10176411
53. Chino J, Annunziata CM, and Beriwal S, et al (2020) Radiation therapy for cervical cancer: executive summary of an ASTRO clinical practice guideline Pract Radiat Oncol 10(4) 220–234 https://doi.org/10.1016/j.prro.2020.04.002 PMID: 32473857 PMCID: 8802172
54. Suneja G, Brown D, and Chang A, et al (2017) American brachytherapy society: brachytherapy treatment recommendations for locally advanced cervix cancer for low-income and middle-income countries Brachytherapy 16(1) 85–94 https://doi.org/10.1016/j.brachy.2016.10.007






