Peripheral T-cell lymphoma of the lip: a rare case unveiling key insights into diagnosis and management
Tooba Ali1, Laraib Khan1, Bilal Mazhar Qureshi1, Asim Hafiz1, Maria Tariq1, Khurram Minhas2, Nasir Ali1 and Ahmed Nadeem Abbasi1
1Department of Oncology, Section Radiation Oncology, Aga Khan University Hospital, Karachi 74800, Pakistan
2Department of Histopathology, Aga Khan University Hospital, Karachi 74800, Pakistan
Abstract
Peripheral T-cell lymphomas (PTCLs) represent a rare and heterogeneous group of lymphoproliferative disorders, accounting for about 10% of non-Hodgkin lymphomas. While PTCLs typically present at nodal sites, extra nodal involvement is uncommon, particularly in the oral cavity. This case report presents a rare instance of peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS), manifesting as a persistent lesion on the lower lip in a 70-year-old male patient. The patient underwent multiple biopsies, which required immunohistochemical staining to confirm the diagnosis. Initial histopathological examinations raised suspicion of a lymphoproliferative disorder, with further testing revealing a 4.5 × 1.5 × 2.8 cm Fluorodeoxyglucose (FDG)-avid lesion on positron emission tomography (PET)/CT. The lesion was confirmed to be PTCL-NOS, characterised by positive CD3 and CD56 markers and a high Ki-67 proliferative index. Treatment involved six cycles of CHOEP chemotherapy followed by consolidative radiation therapy, delivering a total dose of 36 Gy. The patient responded well to treatment, with an interim PET scan showing a complete metabolic response (Deauville score of 3). Follow-up visits confirmed the absence of residual or recurrent disease. A teleconsultation a 6-month post-radiotherapy, along with an examination by a plastic surgeon, also showed no signs of recurrence. This case highlights the diagnostic challenges associated with PTCL at rare non-nodal sites and underscores the importance of a multidisciplinary approach in managing such cases. The patient remains in remission, with ongoing surveillance recommended for up to 5 years to monitor for potential disease recurrence. Further studies and long-term follow-up of similar cases are warranted to better understand the behaviour and optimal treatment strategies for PTCLs in rare extra nodal locations.
Keywords: primary T-cell lymphoma P-TCL, radiotherapy, diagnostic challenge, extra nodal lymphoma, rare subsites, lip, multidisciplinary management
Correspondence to: Laraib Khan
Email: Laraib.khan@aku.edu
Published: 25/09/2025
Received: 06/03/2025
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Lymphomas, categorised by the World Health Organisation (WHO) classification into Hodgkin and non-Hodgkin lymphoma (NHL), represent a diverse group of malignancies that originate from lymphoepithelial cells [1]. NHL constitutes 86% of all lymphomas and is the second most common type of head and neck malignancy after squamous cell carcinoma. Extra nodal lymphomas account for 25%–40% of all NHL cases [2]. Based on the Revised European American Classification of Lymphoid Neoplasms, lymphomas are currently classified according to the WHO classification, which recognises over 20 different subtypes of NHL and considers their morphology, immunophenotype, genetic characteristics and clinical features, while stratifying it. Oral cavity lymphomas make up to 3.5% of all lymphomas with less commonly affected subsites including lips and uvula, as backed up by literature [3]. In our region, the diagnosis and management of peripheral T-cell lymphoma (P-TCL) is supported only by case reports and literature review, with less than 2% of P-TCL cases involving oral cavity subsites [2, 4]. The pathogenesis of this entity revolves around improper maturation of the T-cell lineage, often occurring in the context of immunodeficiency states or viral infections such as AIDS, autoimmune disorders and viruses like Human Herpes Virus and Epstein-Barr virus, as documented in a limited number of case reports in the literature [5].
Among NHL in the head and neck region, Burkitt’s lymphoma is the most common type, affecting the maxilla, mandible and premolar or molar areas, particularly in paediatric patients. It is endemic to the African region, with a key histopathological feature being the ‘starry sky’ appearance due to non-cleaved B-cell lymphocytes. Immunohistochemical (IHC) staining for CD20, CD43 and CD79 assisting in the diagnosis [6]. In diagnosing P-TCL of lip histopathological assessment of specimen and IHC staining was of prime importance. CD3 serves as a pan T-cell marker and is found on the majority of mature T and NK-cell lymphomas, except in cases of anaplastic large cell lymphoma (ALCL). Further IHC staining helps differentiate other T-Cell lymphomas from peripheral T-cell lymphoma, not otherwise specified (P-TCL NOS); these include CD4, CD5, CD8 and CD20 [7,8].
Case presentation
An elderly gentleman in his early 70s, with no significant past medical history or prior comorbidities, and from a low socioeconomic background, was referred to the radiation oncology clinic for a persistent left-sided lower lip lesion that had lasted for 5 months (Figure 1). An initial biopsy of the left lower lip lesion did not indicate invasive squamous cell carcinoma but raised suspicion of a lymphoproliferative disorder. Seeking a second opinion, a histopathological examination confirmed the presence of squamous mucosa with ulceration and necro-inflammatory debris. The primary physician subsequently recommended a contrast-enhanced CT scan of the head and neck, which revealed a 4.1 × 1.7 cm ulcerated lesion on the left buccal mucosa, closely abutting the body of the left hemimandible medially, along with multiple non-enhancing sub-centimetric neck nodes.
An incisional biopsy of the left lower lip was performed, which showed benign squamous epithelium alongside atypical lymphoid cells of concern. Given these findings, a lymph node biopsy was recommended. Further investigations, including a contrast-enhanced CT scan of the neck, chest, abdomen and pelvis, indicated interval progression of the left lower lip lesion and prominent bilateral axillary, portocaval, abdominal, iliac and inguinal lymph nodes, raising suspicion for a possible lymphoproliferative disorder. A biopsy of the left axillary lymph node, accompanied by IHC stains for CD20, CD3 and Ki-67 (Mib-1), displayed a reactive pattern, effectively ruling out granuloma or malignancy.
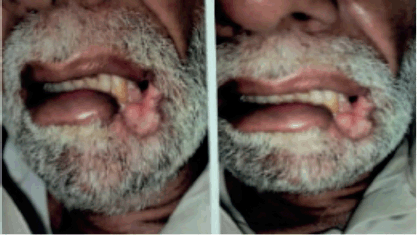
Figure 1. Left lower lip lesion at initial presentation.
To assess the extent of the condition, an 18F-fluorodeoxyglucose positron emission tomography (PET)/CT scan was performed, revealing an Fluorodeoxyglucose (FDG)-avid left lower lip lesion measuring 4.5 × 1.5 × 2.8 cm with a maximum standardised uptake value (max) of 15.66 (Figure 2). A repeat biopsy and IHC profiling of the left lower lip lesion demonstrated positivity for CD3 and CD56, a loss of CD5 staining in CD3-positive cells, a reserved CD4/CD8 ratio and negativity for CD20, TdT and EBV status. Additionally, a Ki-67 proliferative index of approximately 50% further supported the diagnosis of P-TCL NOS (Figure 3).
Although P-TCL is commonly associated with an immunosuppressive state, this patient had no underlying immunosuppressive conditions, making this case an atypical presentation.
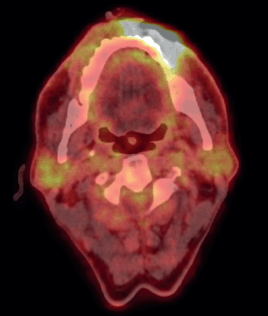
Figure 2. Pre-chemotherapy PET CT scan, showing FDG avid lesion involving left lower lip.
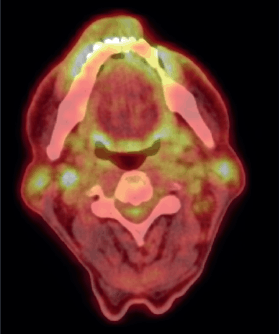
Figure 3. Post-chemotherapy PET CT scan, showing FDG avid residual left lower lip lesion.
Treatment
After a thorough discussion in the multidisciplinary lymphoma tumour board, the patient was started on a chemotherapy regimen consisting of Cyclophosphamide 750 mg/m² IV on day 1, Doxorubicin 50 mg/m² IV on day 1, Vincristine 1.4 mg/m² (max 2 mg) IV on day 1, Etoposide 100 mg/m² IV on days 1–3 and Prednisone 100 mg PO on days 1–5, administered over 6 cycles every 3 weeks. This regimen was well-tolerated by the patient. Interim PET scan was repeated after 4 cycles of chemotherapy, which showed complete metabolic response (Deauville 3). After the completion of six cycles, an end-of-treatment PET-CT was done, which revealed complete metabolic response (Deauville 3) with mild FDG-avidity over lip lesion and complete resolution of the FDG-avid lymph nodes (Figure 4).
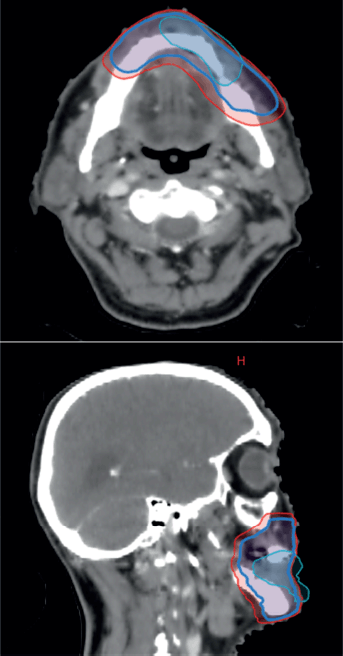
Figure 4. CT-based RT planning, representing pre-chemotherapy GTV, which has now regressed in cyan, CTV and PTV in red.
The case was revisited in the tumour board meeting, where the consensus was to proceed with consolidative radiotherapy (RT). Within 6 weeks of completing the sixth cycle of chemotherapy, the patient was scheduled for 3D conformal radiation therapy (3DCRT). Treatment planning done was involved site radiotherapy (ISRT) in which previously Pet avid site of primary lesion was contoured as pre chemo gross tumour volume (GTV) then a clinical target volume (CTV) was generated to include entire site (ISRT) in our radiation field followed by planning target volume (PTV) to incorporate set up error changes. A total dose of 3,600 cGy was delivered in 18 fractions, with 200 cGy per fraction, using 6 MeV energy and a 3 mm bolus to ensure adequate surface dose distribution (Figures 5–7).
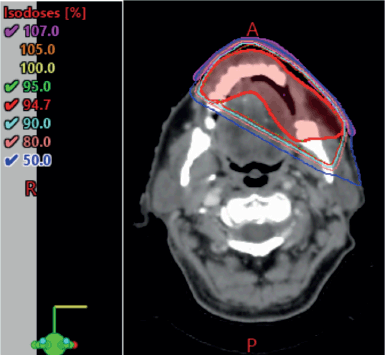
Figure 5. 3DCRT plan showing isodose curves.
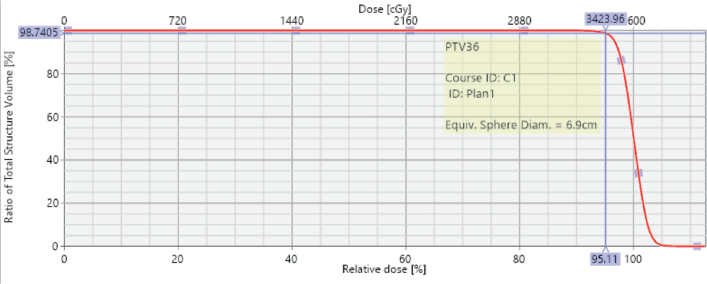
Figure 6. Dose volume histogram showing PTV coverage with 95% of the volume being covered with 95% isodose line.
During RT, the patient was monitored weekly. By the second week, he developed grade 2 dermatitis and mucositis, which were managed conservatively with topical steroid ointments and mouthwashes containing pain killers and anti-inflammatory agents. This weekly review approach ensured that the patient could tolerate and complete the RT without any interruption, aligning with what has been observed in other cases where comprehensive planning and review, followed by complete radiation delivery radio biologically improves local control [9].
Outcome and follow-up
During the 1-month follow-up visit after RT, the patient’s dermatitis and mucositis had resolved. He was advised to return for a follow-up appointment in 2 months, at which time a PET-CT scan was performed. This scan demonstrated a sustained complete metabolic response (Figure 8), and clinical examination revealed no evidence of residual or recurrent disease.
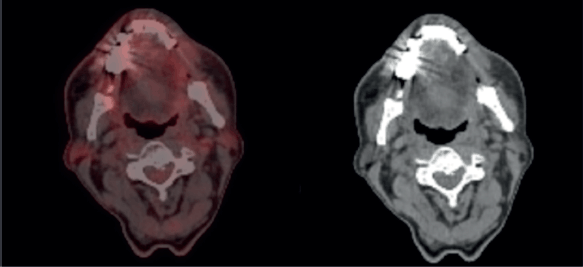
Figure 7. Post treatment follow-up PET CT scan, showing complete metabolic response.
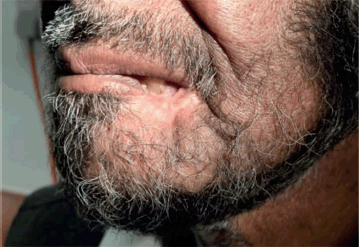
Figure 8. Complete resolution of left lower lip lesion on after treatment on follow up.
Following these encouraging results, the patient was referred to a plastic surgeon for surgical reconstruction of the lip. During a teleconsultation at 6 months post- RT, a local examination conducted by the plastic surgeon revealed no evidence of disease recurrence. However, the patient was advised to continue annual surveillance with evaluation by a primary surgeon along with PET/CT scans, as clinically indicated, for up to 5 years following the completion of treatment.
Discussion
Extra nodal involvement in Hodgkin lymphoma is rare, occurring in approximately 1% of cases, compared to 23%–30% in NHL. The Waldeyer’s ring is the most common site for NHL (36%), while NHL in the oral cavity is relatively uncommon, accounting for only 2% of cases. The hard palate and alveolus are the most frequently affected subsites, with other reported locations including the oral commissure, tongue, uvula, gingiva-buccal sulcus, palate and maxilla [10].
In a case series by Shah et al [10], the typical clinical presentation was painless, progressive swelling, without B symptoms such as fever, weight loss or night sweats. Among the 15 cases discussed, the alveolus and hard palate were the most common sites of presentation (n = 5 each). Only two patients had established immunosuppressive conditions (HIV). Most patients received a combination of chemotherapy and radiation therapy, with surgery reserved for biopsy in 10 out of 15 cases. Follow-up data were available for 11 out of 15 patients after 2 years of treatment with neoadjuvant chemotherapy and consolidative radiation therapy. Of these, five patients showed no evidence of local or distant disease [11].
Kaplan et al [11], in a recent literature review of 23 cases (7 new and 16 from the literature), found that the lower lip was the most affected site (16 cases, 69.56%). Fourteen cases (60.87%) were limited to the lips, while 8 (34.78%) were multifocal. Nine cases (39.13%) were associated with Sjögren’s syndrome, with one patient also having Hashimoto’s thyroiditis. IgG4-related disease and HIV were each reported in one case. In most cases (19, 82.6%), the mucosal lip or salivary glands were involved, with only three cases (13.6%) showing cutaneous involvement. The typical presentation was single or multiple nodules (15 cases, 65.21%), with surface ulceration seen in only two cases (8.69%). No constitutional symptoms were reported, though paraesthesia was noted in one case (4.34%). Most cases (18, 78.26%) were extra nodal marginal zone B-cell lymphoma (MALT lymphoma), with one case each of mantle cell lymphoma, NK-T cell lymphoma, CD30-positive lymphoma and plasmablastic lymphoma. Notably, only one case of P-TCL was reported in this review [11].
PTCLs are a diverse group of lymphoproliferative disorders derived from mature T cells, representing approximately 10% of NHLs. The most common subtype is PTCL-NOS; 26%, followed by angioimmunoblastic T-cell lymphoma (19%), ALCL with anaplastic lymphoma kinase (ALK)-positive (7%) and ALK-negative (6%) forms, and enteropathy-associated T-cell lymphoma (<5%). However, P-TCL typically presents at nodal sites, our case involved a presentation at one of the rarest non-nodal subsites [12].
PTCL NOS, includes all PTCLs that do not fit into other specific categories. PTCL NOS tumour cells are typically CD3-positive, and CD3 staining is more effective than H&E staining in highlighting cytologic atypia in lymphocytes. It is critical to assess for cytologic atypia when reviewing CD3-stained slides, and institutions should ensure quality control in immunostaining techniques, tumour cells generally express either CD4 or CD8, although they may sometimes lack both markers. While both CD4- and CD8-positive cells may appear together under low magnification, it is rare for a single cell to express both markers, requiring careful interpretation. CD56 expression can vary across PTCL subtypes, and β-F1 and T-cell receptor delta staining can help distinguish between αβ and γδ T cells. CD30 staining is increasingly used in T-cell lymphomas, particularly with the development of new treatments targeting CD30-positive tumour cells [7].
Anthracycline-based chemotherapy regimens, such as cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP), CHOP plus etoposide (CHOEP) or dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin, are the most used first-line treatments, as they have shown a tendency toward reducing mortality. In a retrospective analysis of 289 patients with PTCL treated in the DSHNHL trials, CHOEP was found to be associated with improved event-free survival [13].
In a recent case report, Bianco et al [14] emphasised the important role of radiation therapy in treating localised NHL of the lip that was unresponsive to induction chemotherapy. The patient achieved a complete response after receiving conventional radiation therapy (4 Gy in 2 fractions via 3DCRT with bolus placement), and this response was sustained for 1 year during follow-up. According to the NHL Classification Project, PTCL accounts for 8%–10% of all NHLs, with clinical presentations ranging from indolent to aggressive and potentially fatal [14].
In the era of PET scan imaging for lymphomas response criteria known as the Lugano criteria has been established since 2014 for assessing response using PET/CT scans, following the 5-point (PS) system. The 5-PS relies on the visual evaluation of FDG uptake in the affected areas compared to that of the mediastinum and liver. A score of 1 indicates no abnormal FDG avidity, while a score of 2 signifies uptake less than that of the mediastinum. A score of 3 represents uptake greater than the mediastinum but less than the liver, whereas scores of 4 and 5 indicate uptake greater than the liver, and greater than the liver with new sites of disease, respectively. With our patient showing Deauville 3 response after completion of chemotherapy [15].
As per the NHL Classification Project, PTCL constitutes a total of 8%–10% of all non-Hodgkin with varied clinical patterns ranging from indolent to aggressive to potentially fatal [10].
Additionally, certain sites have demonstrated a poor response to chemotherapy and radiation therapy, as observed in cases of uvular and nasal PTCL [11, 16]. For example, a case of PTCL of the uvula in a 54-year-old patient initially showed disease remission after three cycles of CHOP chemotherapy but experienced disease progression and ultimately succumbed after the fourth cycle of treatment [17]. Another rare manifestation seen in the case of Nasal T Cell Lymphoma, despite the absence of systemic disease, a delay in the diagnosis occurred due to atypical disease manifestation with aggressive disease showing poor response to chemotherapy [18].
Literature on complete remission in rare sites of PTCL is limited; however, case reports support a complete response in PTCL of the oral commissure, with disease remission and no local recurrence after a 3-year follow-up when treated initially with RT alone [19]. Similarly, in our case, the patient's disease, located at the lip commissure, was treated with six cycles of R-CHOP chemotherapy followed by consolidative radiation therapy. A PET scan performed 3 months post-treatment completion showed a complete metabolic response.
Evidence supporting the role of radiation in T-cell lymphoma of the lip underscores the importance of maintaining quality with daily peer reviews of radiation therapy treatment contours and plans. Qureshi et al [20] emphasised the value of daily peer review meetings before commencing radiation therapy, which resulted in 12.9% minor adjustments and 8.6% major changes to treatment plans. Notably, more changes were made in radical treatment contours compared to those for palliative patients (92.3% versus 7.7%) [20]. Overall, for patients with early-stage PTCL, the role of consolidative radiation therapy following combination chemotherapy remains debated. Retrospective studies offer conflicting evidence regarding its benefit, with some suggesting improved local control and survival, while others find no significant advantage. This reflects the variability in treatment outcomes and the need for individualised approaches based on disease characteristics and patient factors [21, 22].
Conclusion
In conclusion, this case report poses a rare presentation of PTCL holding a diagnostic challenge warranting multiple biopsies and IHC staining followed by extensive systemic treatment with consolidative radiation therapy, implying the need to extensively study such cases with long-term follow-up required to understand disease behaviour and adequate treatment options in a multidisciplinary setting.
Acknowledgments
None.
Conflicts of interest
The author(s) declare that they have no conflicts of interest.
Funding
None.
Informed consent
Written consent for publication was obtained from the patient.
Author contributions
Tooba Ali: Manuscript writing, Laraib Khan: Drafting, Bilal Mazhar Qureshi: Review, Asim Hafiz: Review, Maria Tariq: Review, Khurram Minhas: Pathology Imaging and Manuscript Review, Nasir Ali: Proof reading and Ahmed Nadeem Abbasi: Conceptualisation.
References
1. Vose J, Armitage J, and Weisenburger D (2008) International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes J Clin Oncol 26 4124–4130 https://doi.org/10.1200/JCO.2008.16.4558 PMID: 18626005
2. Sharma P, Gawande M, and Chaudhary M, et al (2018) T-cell lymphoma of oral cavity: a rare entity J Oral Maxillofac Pathol 22(1) 104 https://doi.org/10.4103/jomfp.JOMFP_153_16 PMID: 29731565 PMCID: 5917515
3. Epstein JB, Epstein JD, and Le ND, et al (2001) Characteristics of oral and paraoral malignant lymphoma: a population-based review of 361 cases Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 92(5) 519–525 https://doi.org/10.1067/moe.2001.116062
4. Asadi AA, Mahmoudi H, and Mofidi A, et al (2023) Peripheral T‐cell lymphoma of the oral cavity: a case report Cancer Rep 6(1) e1751
5. Vigouroux A, Lillo F, and Hofer FD (2020) Peripheral T-cell lymphoma not otherwise specified with oral manifestation: a case report J Oral Res 9(6) 516–521 https://doi.org/10.17126/joralres.2020.098
6. Rasheed RH, Al-Delaimi TN, and Khalil AA (2010) Immunohistochemical expression of CD20, CD43, and CD79 in Burkitt’s lymphoma N Iraqi J Med 6(2) 66–69
7. Cho J (2022) Basic immunohistochemistry for lymphoma diagnosis Blood Res 57(S1) 55–61 https://doi.org/10.5045/br.2022.2022037 PMID: 35483927 PMCID: 9057666
8. National Comprehensive Cancer N NCCN Guidelines Version 1.2024, T-cell Lymphomas [https://www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf] Date accessed: 25/01/24
9. Ali T, Hina M, and Khan L, et al (2024) In regard to Kim et al Clin Transl Radiat Oncol 46 100775 PMID: 38596817 PMCID: 11001760
10. Shah GH, Panwar SK, and Chaturvedi PP, et al (2011) Isolated primary extranodal lymphoma of the oral cavity: a series of 15 cases and review of literature from a tertiary care cancer centre in India Indian J Med Paediatr Oncol 32(2) 76–81 https://doi.org/10.4103/0971-5851.89776 PMID: 22174494 PMCID: 3237184
11. Kaplan I, Shuster A, and Frenkel G, et al (2019) Non-Hodgkin lymphoma of the lips: a rare entity Acta Histochem 121(8) 151449 https://doi.org/10.1016/j.acthis.2019.151449 PMID: 31570207
12. Weisenburger DD, Savage KJ, and Harris NL, et al (2011) Peripheral T-cell lymphoma, not otherwise specified: a report of 340 cases from the international peripheral T-cell lymphoma project Blood 117(12) 3402–3408 https://doi.org/10.1182/blood-2010-09-310342 PMID: 21270441
13. Schmitz N, Trümper L, and Ziepert M, et al (2010) Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group Blood 116(18) 3418–3425 https://doi.org/10.1182/blood-2010-02-270785 PMID: 20660290
14. Bianco L, Solla SD, and Parvis G, et al (2021) Low-dose radiotherapy for extranodal marginal zone B lymphoma of the lip: case report and literature review Hematol/Oncol Stem Cell Ther 14(1) 76–81 https://doi.org/10.1016/j.hemonc.2018.12.002
15. Meignan M, Gallamini A, and Itti E, et al (2012) Report on the third international workshop on interim positron emission tomography in lymphoma held in Menton, France, 26–27 September 2011 and Menton 2011 consensus Leuk Lymphoma 53(10) 1876–1881 https://doi.org/10.3109/10428194.2012.677535 PMID: 22432519
16. Rüdiger T, Weisenburger DD, and Anderson JR, et al (2002) Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the Non-Hodgkin's Lymphoma Classification Project Ann Oncol 13(1) 140–149 https://doi.org/10.1093/annonc/mdf033 PMID: 11863096
17. Lai WS, Wang CH, and Shih CP (2013) T-cell lymphoma manifesting as a uvular mass Rheumatology (Oxford) 52(4) 608 https://doi.org/10.1093/rheumatology/kes372
18. Pontes HA, Pontes FS, and Silva BS, et al (2013). Extranodal nasal NK/T-cell lymphoma: a rare oral presentation and FASN, CD-44 and GLUT-1 expression Braz Dent J 24(3) 244–248 https://doi.org/10.1590/0103-6440201302202
19. Villa A, Mariani U, and Villa F (2010) T‐cell lymphoma of the oral cavity: a case report Aust Dent J 55(2) 203–206 https://doi.org/10.1111/j.1834-7819.2010.01212.x PMID: 20604765
20. Qureshi BM, Mansha MA, and Karim MU, et al (2019) Impact of peer review in the radiation treatment planning process: experience of a tertiary care university hospital in Pakistan J Glob Oncol 5 1–7 PMID: 31393752 PMCID: 6733206
21. Briski R, Feldman AL, and Bailey NG, et al (2015) Survival in patients with limited-stage peripheral T-cell lymphomas Leuk Lymphoma 56(6) 1665–1670 https://doi.org/10.3109/10428194.2014.963078 PMCID: 4456336
22. Khan L, Abbasi AN, and Rasool S (2023) Importance of development of rare cancers national tumor board J Coll Physicians Surg Pak 33(3) 364 https://doi.org/10.29271/jcpsp.2023.03.364 PMID: 36945174





