Global initiative for childhood six-indexed cancers: how are we faring in Nigeria?
Motunrayo Oluwabukola Adekunle1,a, Aisha Musa2, Chioma Ginika3, Chisom Nri-Ezedii4, Uduak Offiong5, Hauwa Yusuf6, Peter Odion Ubuane1, Adewunmi Oyesakin7, Ijeoma Nnenna Diaku-Akinwumi1 and Adaorah Onyiaorah4
1Lagos State University Teaching Hospital, Ikeja, Lagos State, Nigeria
2Aminu Kano Teaching Hospital, Kano, Nigeria
3University of Port Harcourt, Rivers State, Nigeria
4Nnamdi Azikiwe University Teaching Hospital, Nnewi, Anambra, Nigeria
5University of Abuja Teaching Hospital, Gwagwalada, FCT, Abuja, Nigeria
6University Teaching Hospital, Maiduguri, Borno State, Nigeria
7National Hospital, Abuja, Nigeria
ahttps://orcid.org/0000-0003-2820-3948
Abstract
Background: WHO's Global Initiative for Childhood Cancer (GICC) aims to increase global survival of childhood cancers to 60% by the year 2030 with a focus on six index cancers. However, there is no nationally representative epidemiologic data on these index cancers in Nigeria.
Aim: To describe the distribution, outcomes and determinants of GICC six-indexed cancer in Nigeria.
Methodology: A multi-centre ambi-directional cohort study of children was done in children <19 years diagnosed with any of acute lymphoblastic leukaemia (ALL), Wilms tumour (WT), retinoblastoma (RB), Hodgkin lymphoma (HL), Burkitt lymphoma (BL) or low-grade glioma (LGG). Seven centres in the six geopolitical zones of the country participated. A 2-year study with 18 months of retrospective data collection (January 2022–June 2023) and follow up of subjects was done for 6 months (July–December 2023).
Results: A total number of 213 subjects were enrolled and ALL (n = 72;33.8%), WT (n = 57; 26.8%), RB (n = 52; 24.4%), BL (n = 17; 8.0%), HL (n = 13; 6.1%) and LGG (n = 2; 0.9%) accounted for the disease pattern. Median age at diagnosis was 5 years (51.6%). Mortality rate was 32.4% and treatment abandonment occurred in 37.6% of subjects. Treatment-related mortalities (TRMs) were 37.7% with infection and haemorrhage the commonest specific causes of TRM (36.1% and 42.5%). Only 7/95 (7%) of subjects with WT and RB stage III and IV benefited from RT. The most common reasons for non-RT were lack of funds (29%), lack of access to RT (54%) and lack of physicians’ referral (11%). Only 10 (4.3%) of subjects were enrolled in a health insurance scheme. Independent risk factor for mortality was advanced disease stage (p = <0.001). Amongst the mortalities, 36% died within the first 3 months of diagnosis.
Conclusion: Treatment abandonment, mortality and TRM are high in Nigeria. To attain the GICC goal, there is a need to educate physician on treatment protocol, ensure availability of supportive care, health insurance, RT and tackle late presentation.
Keywords: Global Initiative for Childhood Cancer, treatment abandonment, treatment-related-mortalities, Nigeria
Correspondence to: Motunrayo Adekunle
Email: motunbamm@yahoo.com
Published: 23/09/2025
Received: 15/01/2025
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Globally, about 400,000 cases of childhood and adolescent cancers are diagnosed yearly. Childhood cancers are on the increase globally, most especially in Africa, with projections that Africa will account for 50% of childhood cancer in the world by the year 2050. Nigeria is not left out in the upsurge in childhood cancer [1, 2].
Childhood cancer is the leading cause of death in children and adolescents globally [1]. Mortality in childhood cancer is high with a wide disparity between the high and low-income countries. Whilst an 80% survival rate occurs in the former, the mortality rate in Africa is as high as 80% [1]. To close this gap, the global initiative for childhood cancer was initiated by WHO in the year 2018 with the aim to increase survival of childhood cancer to 60% by the year 2030 [3].
The six core malignancies of focus are acute lymphoblastic leukaemia (ALL), Nephroblastoma, Hodgkin lymphoma, retinoblastoma, Burkitt lymphoma and low-grade glioma. These malignancies account for 50%–60% of cancers, have good prognosis and are highly curable. Some of the measures towards attaining the goal of GICC by the year 2030 include prompt diagnosis, availability of essential medicines, availability of cancer registries and advocacy [3, 4].
Nigeria’s population is 223.8 million, with 43% of this accounting for children below 15 years [5]. There is paucity of data on childhood cancer in Nigeria. The vast majority of problems in care of childhood cancer span from lack of supportive care to unavailability of tools for molecular diagnosis [6]. The contributory effect of improvement of outcome in children with cancer in Nigeria to the achievement of GICC year 2030 is crucial.
This study aims to evaluate the six-core cancers of focus by GICC, their incidence, outcomes and determinants of the outcomes. The findings from this study will help to identify areas that require urgent attention and need to develop strategies that will enhance improvement of any of these malignancies lagging in contributing to the GICC goal 2030.
Aim and objectives
The general aim of the study is to assess the incidence of six core malignancies in children in Nigeria, identify the outcomes, as well as factors that determine the outcomes.
The specific objectives of the study were to determine the incidence of ALL, Nephroblastoma, Hodgkin lymphoma, retinoblastoma, Burkitt lymphoma and low-grade glioma in study centres, the case fatality rates in the disease of interest over the study period as well as identify the contributor to mortality in the disease of interest over the duration of study (Age, sex, disease type, disease stage, time of presentation and treatment abandonment).
Subjects and methods
We conducted this multicentre collaborative national study at seven paediatric haemato-oncology units of seven teaching hospitals which provide treatment for children and adolescents with cancer across all six geopolitical zones of Nigeria (Figure 1):
- Lagos State University Teaching Hospital, Ikeja, Lagos (South-West);
- University of Port Harcourt, Rivers State (South-South);
- Nnamdi Azikiwe Teaching Hospital, Nnewi, Anambra (South-East);
- University of Abuja Teaching Hospital, Gwagwalada, FCT, Abuja (North-Central) and National Hospital, Abuja (North-Central);
- University Teaching Hospital, Maiduguri, Borno State (North-East);
- Aminu Kano Teaching Hospital, Kano (North-West).
Figure 1. Map of Nigeria highlighting the study centres.
Study design
We employed an ambi-directional cohort design involving both a retrospective and prospective aspect. At each site, the site investigator used a standardised data collection form to extract relevant data of children below 19 years of age who were diagnosed and managed for any of the six GICC malignancies (acute lymphoblastic leukaemia, Hodgkin lymphoma, retinoblastoma, nephroblastoma, low grade glioma and Burkitt lymphoma) from January 1, 2022 till June 30, 2023 from hospital medical records. Enrolled patients who were still alive at the commencement of the study were thereafter prospectively followed up till December 31, 2023.
Data obtained include demographic (age and sex), clinical data (diagnosis and age at diagnosis), treatment resources (access to blood transfusion, radiotherapy, chemotherapy, surgery and supportive care) and outcome data (treatment abandonment, loss to follow-up, outcome at end of study – death versus survival). We defined treatment-related mortality as death occurring in the absence of disease progression [7]; treatment abandonment as failure to start or complete curative therapy (except when such treatment is contraindicated for medical reasons) and is defined by missed therapy for 4 or more consecutive weeks [8]; curative treatment is defined as treatment that is meant to cure the malignancy with the goal of a full recovery that includes an acceptable quality of life [9]. Loss to follow-up is defined as patients who have stopped follow-up appointment after completion of treatment for at least 3 consecutive months from the last appointment [10]; events are defined as any of death, relapse, progression, treatment abandonment and loss to follow-up. Overall survival is defined as the proportion of participants who were alive by the end of the study period [11]. All centres use International Society for Paediatric Oncology protocol for the treatment of Wilms tumour. A combination of vincristine, etoposide and carboplatin is used unanimously for the treatment of retinoblastoma. A combination of L-asparaginase, vincristine, 6-mecarpotopurine and prednisolone is used at different courses in the treatment of ALL. Five centres use cyclophosphamide, vincristine, Adriamycin and prednisolone in the treatment of BL, others use methotrexate-based regimen. A combination of Adriamycin, bleomycin, vinblastine and dacarbazine is the treatment protocol for HL in 5 centres, while 2 centres adopted cyclophosphamide, methotrexate, vincristine and prednisolone.
Ethical approval was obtained from the National Health Research Ethics Committee of Nigeria (NHREC) with approval number: NHREC/01/01/2007-12/10/2023.
Data analysis
Data analysis was done using IBM SPSS Statistics version 20.0. Test of normality was assessed using Kolmogorov–Smirnov test. Patients’ demographics are represented as frequency and percentages. Tables and figures are used to represent those variables as appropriate. Continuous variables were summarised using mean and standard deviation, while non-parametric data were summarised using median. Comparison between parametric data was done using independent t-test and ANOVA test, while comparison of non-parametric data was done using Mann–Whitney test and Kruskal–Wallis. Comparison between qualitative data was done using chi square. Probability value less than 5% (0.05) was considered statistically significant. A survival analysis was done to determine the overall survival using Kaplan–Meier analysis.
Results
A total of 213 patients were recruited with a slightly higher female proportion (0.9:1.0) in all the diseases. The median age at time of diagnosis is 5 years (IQR 11 months, 17 years). Adolescents aged 18 and 19 years were not seen in in the paediatric section of any institution. Patients with ALL, WT, RB and LGG presented at a significantly earlier years compared to those with BL and HL (χ2 211.685; p = 0.020). The most are ALL (n = 72; 34%), WT (n = 57; 27%) and RB (n = 52; 24%). There was no case of bilateral Wilms tumour (stage V).
The median time to presentation to the hospital in all subjects from onset of symptoms is 90 days (IQR 6 days, 1095 days). The majority of the subjects presented with advanced disease. There was no relationship between disease stage and the type of malignancies (χ2 = 4.661, p = 0.550) as shown in Table 1.
A higher proportion of ALL was diagnosed in the southern part of the country compared to the northern part (52/72; 72% versus 20/72; 28%,) while Burkitt lymphoma (10/17 (58.8% versus 7/17, 41.1%) and HL 8/13 (61.5^versus 5/13 (38.4%); were more common in the north than the south; χ2 14.804, p = 0.010, as shown in Figure 2.
Table 1. Characteristics of the disease spectrum in the study participants.
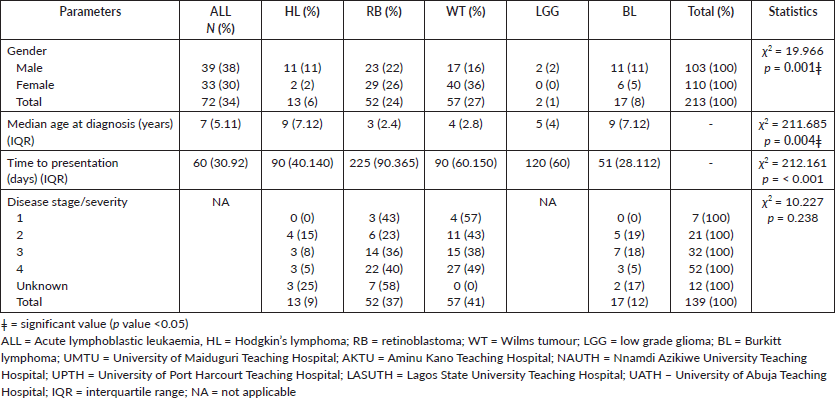
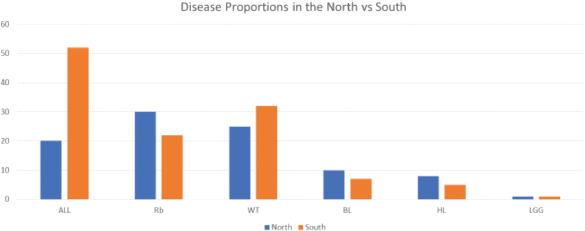
Figure 2. Proportion of diseases in the regions. ALL: acute lymphoblastic leukaemia; Rb: retinoblastoma; WT: Wilms tumour; BL: Burkitt lymphoma; HL: Hodgkin’s lymphoma; LGG: low grade glioma.
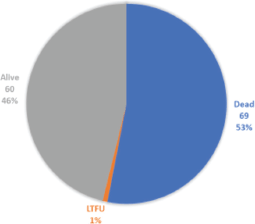
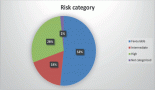
Figure 3. Disease outcomes in study subjects.
Out of 130 subjects, 53% died whilst 60 (46%) were still alive at the completion of the study, as shown in Figure 3. Early deaths within the first 3 months of diagnosis occurred in 25/69 (36%), and 44/66 (64%) deaths were recorded after the first 3 months of diagnosis.
Case fatality was highest in subjects with ALL while treatment abandonment was more common in those with WT and RB, as shown in Table 2.
Treatment-related mortalities occurred in 38% and the commonest cause is febrile neutropenia, as shown in Figure 4.
Availability of services
All centres have access to blood for transfusion. Four centres have equipment for pooled platelet and none for apheresis. Two centres have RT machines, while 1 has access to this service within the same city. All surgeons operate on patients; different treatment protocol exists in the study centres. A total of 109 patients had WT and RB; 95 of these required RT, i.e., WT (at least stage III) and RB (at least stage 1). Only 7 of these (7.4%) had the RT. None of the 5 patients with WT that had RT did so within 4 weeks post-surgery. No access to RT and delayed histology report were the commonest reasons for failure to irradiate when required and delayed irradiation, respectively, as shown in Figure 5.
Table 2. Treatment outcomes in study participants.
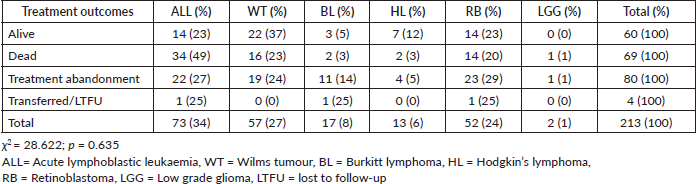
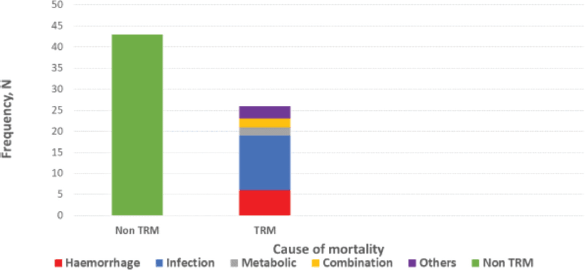
Figure 4. Causes of mortalities in study subjects.
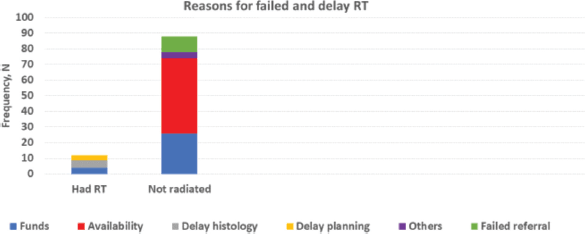
Figure 5. Radiotherapy services.
Predictors of treatment abandonment
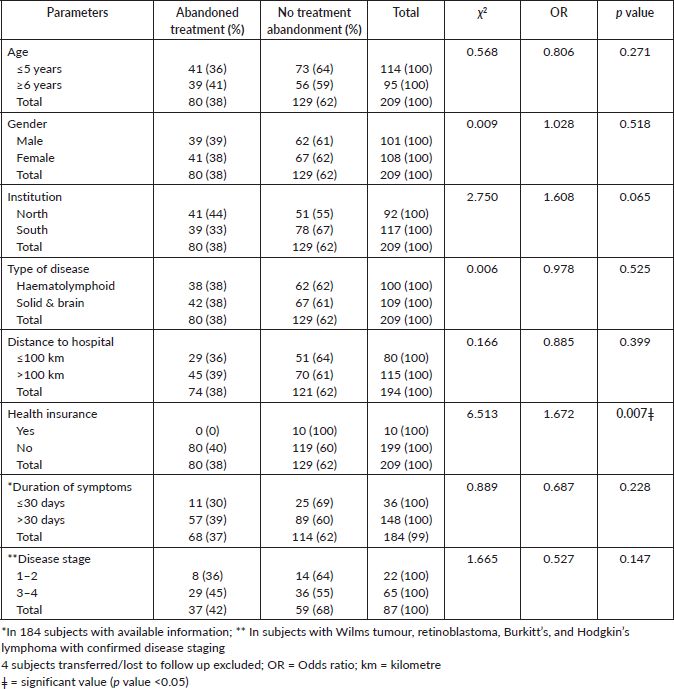
Treatment abandonment was more common in the north than the southern part of the country, 41/92 (45%) versus 39/117 (33%) respectively (p = 0.065). No subjects on health insurance abandoned treatment and treatment abandonment occurred solely in those with no health insurance (n = 10, χ2 = 0.012, p = 0.008). Age and gender were not risk factors for abandoning treatment (p = 0.271 and 0.518 respectively). There is a 1.6 odd of not abandoning treatment in subjects with health insurance, as shown in Table 3.
Table 3. Predictors of treatment abandonment.
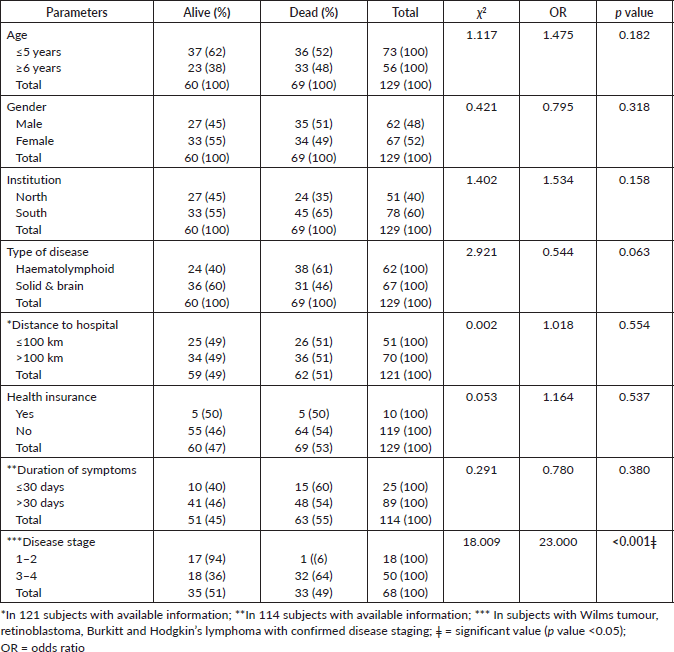
Neither the distance from the facility of care to the home nor the access to health insurance were predictors of deaths. Subjects with advanced disease (WT, RB, HL and BL) had 23 odds of death compared to those with stages 1 and 2 disease at diagnosis, as shown in Table 4. Subjects with haematolymphoid diseases had a higher death rate compared to other malignancies. In all, 71% subjects with ALL died (n = 34/48) compared to 14/28 (50%) in retinoblastoma, 16/38 (42%) WT, 2/5 (40%) BL, 2/9 (22%) HL and one mortality in LGG (χ2 = 12.692, p = 0.020). Subjects with advanced diseases had a higher mortality rate than those with disease stages 1 or 2, as shown in Table 4.
Table 4. Predictors of death in study participants.
Discussion
To attain the goal of WHO GICC by the year 2030, there is a need for countries to evaluate where they are and strategically work towards the goal. The current study was done to access the current situation of children diagnosed with the six indexed malignancies and also evaluate factors that would hinder the required survival rate by the year 2030. The number of study participants in the current study is rather low despite being a multicentre study in major centres. The lack of national data on childhood cancer in Nigeria makes it difficult to extrapolate if this is as a result of preference for traditional care as against orthodox medicine or poor hospital data registry.
Acute lymphoblastic leukaemia is the commonest malignancy in our cohort with cancer. However, ALL tends to be commoner in the South while Burkitt lymphoma and Hodgkin’s lymphoma are commoner in the Northern part of Nigeria. The shift from HIV-related malignancies as the predominant malignancies has been previously documented and the pattern of disease now mirrors what obtains in HICs [12, 13]. The persistent high proportion of HIV related malignancies in the North has also been reported by Suleiman et al [14] The national shift to predominance of haematologic malignancies perhaps is as a result of clinical findings of a wide coverage of highly active antiretroviral therapy and winning in prevention of maternal-to-child transfusion of HIV. Likewise, the prevalence of low-grade glioma is low. This finding was also reported by Suleiman et al [14] The reduced immunity and increase malaria infection in individuals with low socioeconomic status and chronic malnutrition have been documented as a risk factors for BL; however, the evaluation of this parameters as contributor to regional disparities of BL is not within the scope of our study [15, 16]. The reason for the few brain tumours in our cohort is unclear. In a study done over a decade ago in an institution in the southwestern part of Nigeria, an approximately 0.76 cases per year low-grade astrocytoma was reported [17]. There is paucity of data on paediatric brain tumours in Nigeria, and this study further buttresses the need for research in this aspect of childhood cancer. In addition, multidisciplinary paediatric brain tumour clinics will enhance adequate capturing of cases.
In our cohort, the proportion of male subjects with BL and HL were remarkably higher than females. This is in keeping with the literature. The explanation for the increase risk of lymphoma in males is unknown with postulation of likely environmental or genetic factor [18, 19]. In addition, the proportion of female subjects with Wilms tumour in the present study was widely higher than males, this accounted for the overall gender distribution in our cohort. Girls have a higher risk of Wilms tumour than boys but the explanation for this risk is unclear [20].
The majority of our cohort presented late to the hospital and advanced disease stages were commonly found. This trend of delayed presentation has been reported in other LMICs; however, the time to presentation in our cohort is higher than 35 days reported by Ribeiro et al in Brazil and 68 days in Ethiopia [21, 22]. Children with retinoblastoma tend to present late. This was also reported by Gardie et al [22] in Egypt. The delayed presentation inadvertently resulted in more advanced diseases in study participants. As a country, there is a need to put measures in place to combat delayed diagnosis as this is crucial to GICC's goal achievement.
While it is acknowledged that high rates of both treatment abandonment and deaths are generally common in low-resource regions like Africa, the wide disparity in their prevalence amongst African countries cannot be ignored. A similar treatment abandonment rate was reported in an institutional-based study in the South-eastern part of Nigeria (51.2%), while 7% treatment abandonment was seen in a five-country study involving Ghana, Malawi, Cameroon, Zimbabwe and Kenya, and 42% in Ethiopia [23–25]. Studies have reported factors such as funding and preference for alternative medicine as a reason for treatment abandonment [24, 26]. As long as out-of-pocket financing of childhood cancer remains the norm in Nigeria, no significant impact can be made to reduce treatment abandonment. There is a need for the government to collaborate with international bodies on making treatment for childhood cancer free. There is a need for huge governmental and non-governmental investments to increase access to all cancer treatments and support, for example, through expansion of insurance coverage to the informal sector and for childhood cancer treatment. In addition, childhood cancer awareness programmes and education of the populace will enhance early presentation and improve survival outcome.
Our study reveals the potential positive impact of health insurance in that none of the few participants (< 5%) on health insurance abandoned treatment despite the fact that the scheme enrolled cancer patients, benefiting indirectly with respect to supportive investigations and non-oncologic medications. Childhood cancer treatment is yet to be included in the National Health Insurance Scheme in Nigeria. To attain the GICC goal, there is a need to expand the health insurance coverage beyond the current level of less than 5% enrolee and include childhood cancer treatment in the health insurance package [27].
The case fatality rate from our cohort was quite high compared to the other LMICs. In South Africa and Tanzania, Davidson et al [29] had a 77% survival rate and 18.9% deaths occurred in Tanzania, as reported by Majaliwa et al [28]. Subjects with ALL, Wilms tumour and retinoblastoma had the highest death ratio, 49%, 23% and 20%, respectively.
The high incidence of treatment-related mortality (death in the absence of progressive disease) in our cohort (38%), mostly due to febrile neutropenia and bleeding, is quite concerning, possibly reflecting weak resources for early detection and responses to such anticipated adverse events. This may also be due to the advanced disease at presentation, concomitant with a limited supportive care system. Along with enhanced supportive care system encompassing improved access to high-tech laboratory, molecular and blood product support, there is the additional need for evidence-informed adaptation of national treatment protocol with less treatment toxicity, contextualised to the peculiarity of late presentation in advanced disease stage when there is compromised reserve to withstand drug toxicities.
Although radiotherapy is a cornerstone treatment modality for WT, HL, RB and progressive LGG, the majority of the children who required radiotherapy could not access it (93%) due to unavailability of functional radiotherapy services, lack of funds, delayed histological results reporting and delayed clinical decision for radiotherapy. For Wilms tumour, radiotherapy within 4 weeks post-surgery has been documented to have a better prognosis, but none of our study participants commenced radiotherapy within a month after surgery. At a current estimated cost of 600 dollars per treatment, radiotherapy services may remain out of reach for a large proportion of children in a country where the average citizens earn 46 dollars. This further threatens the attainment of the GICC goal in Nigeria and further calls for concerted inter-sectorial interventions to subsidise radiotherapy services for childhood cancers. Additionally, timely cancer therapy hinges hugely on prompt histological diagnosis. Unfortunately, we observed significant delayed histological diagnosis, in addition to late presentation. Enormous manpower brain-drain in Nigeria’s health sector continues to worsen. There is thus a need for novel proactive measures to facilitate both histological investigations and reporting, for example, through AI.
Expectedly, children with advanced disease stage and haematolymphoid malignancies had increased odds of death, as similarly reported from both HIC and low- and middle-income countries. This partly explains our finding of low survival of 28%, a far cry from GICC goal of 60%! Worse still, these deaths occur mostly within 2 years post-diagnosis, similar to a study by Israels et al [30] who reported a mortality rate of 15% within the first 3 months among five sub-Saharan African countries (Nigeria not included). High rate of early deaths further strengthens the urgent need for strategies to enhance early detection and diagnosis and robust supportive care services.
Strengths and limitation
Compared to several previously published single-centre cross-sectional or case-control studies, the multicentre ambi-directional cohort design of our study makes our study more representative of the current clinic-epidemiologic and resource challenge profile of paediatric oncologic services, specifically for GICC 6, in Nigeria. The combination of retro- and prospective aspects provided more data for better information. Conducting solely a retrospective study over a long period may be faced with the problem of data missingness, while a solely prospective study over a longer period may be limited by research resources. However, although all six Nigerian geopolitical zones were represented, we acknowledge that there are variations in the sociocultural contexts across each zone which may impact presentation, diagnosis and care.
In conclusion, ALL was the commonest of GICC-6. We report high incidence of treatment-abandonment, overall-mortality and TRM among Nigerian children with cancers; these factors threaten the attainment of GICC goal by Nigeria. There is thus an urgent need to enhance access/coverage of health-insurance for oncologic services, including radiotherapy, laboratory diagnostics and efficient referral pathways and linkages. Moreover, continuing multi-disciplinary and collaborative education of oncology care-providers in evidence-based locally-contextualised treatment protocols is required for optimal outcomes. Further prospective study to monitor progress and outcomes of advocacy should go side-by-side with these.
List of abbreviations
ALL, Acute lymphoblastic leukaemia; BL, Burkitt lymphoma; GICC, Global Initiative for Childhood Cancer; HIC, High-income country; HL, Hodgkin’s lymphoma; LGG, Low-grade glioma; LMIC, Low-middle income country; RB, Retinoblastoma; RT, Radiotherapy; TRM, Treatment-related mortality; WHO, World Health Organisation; WT, Wilms tumour.
Conflicts of interest
The authors hereby declare no conflicts of interest.
Funding
There are no financial conflicts of interest to disclose.
References
1. WHO Childhood Cancer (Childhood Cancer (who.int)) Date accessed: 02/04/24
2. WHO Africa Will Account for 50 Percent Childhood Cancer Burden by 2050 Date accessed: 02/04/24
3. World Health Organization WHO Global Initiative for Childhood Cancer: An Overview Date accessed: 10/03/25
4. van Heerden J, Elhuni E, and van Staaden H, et al (2023) Global initiative for childhood cancer: increased implementation of core projects in Africa 45(1) 16–20
5. World Population Dashboard -Nigeria | United Nations Population Fund (unfpa.org) Date accessed: 02/04/25
6. Akinsete AM, Odugbemi BA, and Ogundowole GE, et al (2019) Pediatric oncology in Nigeria: a panoramic view J Glob Oncol 5(5) 1–7 PMID: 31268812 PMCID: 6690627
7. Alexander S, Pole JD, and Gibson P, et al (2015) Classification of treatment-related mortality in children with cancer: a systematic assessment Lancet Oncol 16(16) e604e10 https://doi.org/10.1016/S1470-2045(15)00197-7
8. Mostert S, Arora RS, and Arreola M, et al (2011) Abandonment of treatment for childhood cancer: position statement of a SIOP PODC Working Group Lancet Oncol 12 719–720 https://doi.org/10.1016/S1470-2045(11)70128-0 PMID: 21719348
9. National Cancer Institute NCI Dictionary of Cancer Terms Date accessed: 08/06/24
10. Sweet-Cordero A, Antillon F, and Baez F, et al (1999) Factors that influence abandonment of care among children with cancer in Guatemala Pediatr Blood Cancer 33(1) 151
11. Medeiros BC (2018) Interpretation of clinical endpoints in trials of acute myeloid leukaemia Leuk Res 68 32–39 https://doi.org/10.1016/j.leukres.2018.02.002 PMID: 29524739
12. Babatunde TO, Akang EE, and Ogun GO, et al (2015) Pattern of childhood cancer in University College Hospital, Ibadan during 1991-2010 and comparison with the previous three decades Paediatr Int Child Health 35(2) 144–150 https://doi.org/10.1179/2046905514Y.0000000132 PMID: 25975278
13. Johnston WT, Erdmann F, and Newton R, et al (2021) Childhood cancer: estimating regional and global incidence Cancer Epidemiol 71(Part B) 101662 https://doi.org/10.1016/j.canep.2019.101662
14. Sulieman DE, Adamu AS, and Ezenkwa US, et al (2024) Suleiman DIncidence of childhood cancers in the North East geopolitical zone of Nigeria Front Oncol 14 1379968 https://doi.org/10.3389/fonc.2024.1379968
15. Muzame AB, Ngeiywa M, and Makwali J, et al (2020) Effect of demographic and socio-economic factors on occurrence and co-occurrence of Plasmodium falciparum malaria and endemic Burkitt’s lymphoma among children in Western Kenya Strateg J Bus Change Manag 7(4) 975–984
16. Williams CKO, Foroni L, and Luzzatto L, et al (2014) Childhood leukaemia and lymphoma: African experience supports a role for environmental factors in leukaemogenesis Ecancermedicalscience 8 https://doi.org/10.3332/ecancer.2014.478 PMID: 25435906 PMCID: 4239129
17. Uche EO, Shokunbi MT, and Malomo AO, et al (2013) Paediatric brain tumours in Nigeria: clinical profile, management strategies, and outcome Child’s Nerv System 29(7) 1131–1135 https://doi.org/10.1007/s00381-013-2105-9
18. Xing Z, Cao P, and Li Z, et al (2024) Sex differences in the incidence, mortality, and survival outcomes of lymphoma subtypes [Internet] [https://www.researchsquare.com/article/rs-4358947/v1]
19. Boerma EG, Van Imhoff GW, and Appel IM, et al (2004) Gender and age-related differences in Burkitt lymphoma - epidemiological and clinical data from the Netherlands Eur J Cancer 40(18) 2781–2787 https://doi.org/10.1016/j.ejca.2004.09.004 PMID: 15571961
20. Caldwell BT, Wilcox DT, and Cost NG (2017) Current Management for Pediatric Urologic Oncology vol 64, Advances in Pediatrics (Academic Press Inc) pp 191–223
21. Ribeiro ILA, de Melo ACR, and Santiago BM, et al (2023) Time to diagnosis of paediatric cancer and factors that require attention J Public Health 31 397–404 https://doi.org/10.1007/s10389-021-01517-x
22. Gardie Y, Wassie M, and Wodajo S, et al (2023) Delay in diagnosis and associated factors among children with cancer admitted at pediatric oncology ward, University of Gondar comprehensive specialized hospital, Ethiopia: a retrospective cross-sectional study BMC Cancer 23(1) 1–7 https://doi.org/10.1186/s12885-023-10873-8
23. Nri-Ezedi CA, Ulasi TO, and Menkiti FE, et al (2024) Epidemiological trends and treatment abandonment of paediatric solid tumours in a Nigerian tertiary hospital: a seven-year review (2016–2022) BMC Cancer 943
24. Chagaluka G, Afungchwi GM, and Landman L, et al (2021) Treatment abandonment: a report from the collaborative African network for childhood cancer care and research—CANCaRe Africa Pediatr Blood Cancer 68(12) https://doi.org/10.1002/pbc.29367 PMID: 34549506
25. Hordofa DF, Ahmed M, and Birhanu Z, et al (2024) Childhood cancer presentation and initial outcomes in Ethiopia: findings from a recently opened pediatric oncology unit PLOS Global Public Health 4(7 July) https://doi.org/10.1371/journal.pgph.0003379 PMID: 38985815 PMCID: 11236196
26. Friedrich P, Lam CG, and Kaur G, et al (2016) Determinants of treatment abandonment in childhood cancer: results from a global survey PLoS One 11(10) e0163090 https://doi.org/10.1371/journal.pone.0163090 PMID: 27736871 PMCID: 5063311
27. Eze OI, Iseolorunkanmi A, and Adeloye D (2024) The National Health Insurance Scheme (NHIS) in Nigeria: current issues and implementation challenges J Glob Health Econ Policy 4 e2024002
28. Majaliwa E, Smith ER, and Cotache-Condor C, et al (2023) Childhood and adolescent cancer care at a Tertiary Hospital in Northern Tanzania: a retrospective study JCO Glob Oncol 9(9) e2200263 https://doi.org/10.1200/GO.22.00263 PMID: 37384861 PMCID: 10497254
29. Davidson A, Moodley J, and Pillay K, et al (2022) The University of Cape Town’s paediatric cancer database: results from the first years (2019–2021) SAJO 6 a234 https://doi.org/10.4102/sajo.v6i0.234
30. Israels T, Afungchwi GM, and Chagaluka G, et al (2021) Early death and treatment-related mortality: a report from SUCCOUR - supportive care for children with cancer in Africa Pediatr Blood Cancer 68(9) 1–6