Spectrum of hepatitis B and hepatitis C-related cancers in India
Sivaranjini Kannusamy1,2,a, Amey Oak1,2,b, Sandhya Cheulkar1,2, Kamesh Maske1,2, Esha Dashmukhe1,2, Ashwini Patil1,2, Manisha Morajkar1,2, Manju Sengar1,3, Ganesh Balasubramaniam1 and Rajesh Dikshit1
1Homi Bhabha National Institute, Mumbai 400094, India
2Division of Cancer Care, Hospital Cancer Registries and Survival Studies, Centre for Cancer Epidemiology, Tata Memorial Centre, Mumbai 410210, India
3Department;; of Medical Oncology, Tata Memorial Centre, Mumbai 410210, India
ahttps://orcid.org/0000-0001-7414-8751
bhttps://orcid.org/0009-0004-1893-4191
Abstract
Introduction: Hepatitis-B virus infection contributes to 40%–50% of the Hepato-cellular carcinomas (HCC) in India, while hepatitis-C virus infection accounts for 12%–32% of cases. This study aimed at determining the patterns of cancers among patients with hepatitis B and C.
Materials and methods: This was a retrospective study of cancer patients with histologically proven diagnoses of cancer registered at Tata Memorial Hospital in Mumbai between 2017 and 2018. The proportional incidence ratio (PIR) was computed by dividing the observed number of site-specific cancer cases by the expected number.
Results: The study participants’ mean (SD) age was 48.69 (±16.91) years with a male-to-female ratio of 1.36. The prevalence of hepatitis B and C was 1.93% and 1.17%, respectively. Liver cancer showed the highest occurrence rate with notably increased PIR among individuals positive for hepatitis B (males: 14.41, females: 10.89) and hepatitis C (males: 7.15, females: 10.42). Furthermore, hepatitis B-positive patients showed elevated PIR for haemato-lymphoid malignancies such as multiple myeloma and non-Hodgkin’s lymphoma.
Limitation: The correlation between HBsAg and specific cancer types (PIRs) is limited by small case numbers, requiring careful interpretation of these findings.
Implications and conclusion: The PIR for liver cancer was heightened in both hepatitis B and C patients. Strengthened surveillance, including pre-screening for hepatitis B and C positive infection among cancer patients, as well as screening for HCCs among hepatitis seropositive individuals, is crucial to mitigate the incidence of HCC.
Keywords: cancer, hepatitis B, hepatitis C
Correspondence to: Sivaranjini Kannusamy
Email: sivaranjini.k27@gmail.com
Published: 06/09/2024
Received: 20/02/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cancer is responsible for one out of every six fatalities worldwide, or about 10 million deaths. Cancer-causing infections such as human papillomavirus and hepatitis cause one-third of all cancer cases in low and lower-middle-income nations [1].
Infection with the hepatitis B Virus (HBV) predominantly damages the liver, posing a significant risk of mortality from cirrhosis and hepatocellular cancer. Around 296 million individuals worldwide have chronic hepatitis B, and 820,000 died in 2019 as a result of cirrhosis and hepatocellular cancer [2]. In India, the prevalence of HBV infection ranges between 3.2% and 4.2% [3].
The most frequent histologic type is hepatocellular carcinoma (HCC), which accounts for 80% of all primary liver cancers [4]. In India, 40%–50% of HCC are caused by HBV infection, whereas 12%–32% are caused by hepatitis C Virus (HCV) infection [3]. HBV and HCV viruses have been found in a variety of extra-hepatic tissues, suggesting that they may speed up the oncogenesis of extra-hepatic cancers. Chronic HBV infection has been linked to a variety of extrahepatic malignancies, including gastric cancer, pancreatic cancer, intra- and extra-hepatic bile duct carcinoma, non-Hodgkin lymphoma (NHL), breast cancer, thyroid cancer, lung cancer and skin cancer [5]. HCV has been identified in extrahepatic organs and may, therefore, directly induce carcinogenesis by prolonged inflammation and oncogenesis of target tissues. Cholangiocarcinoma, pancreatic adenocarcinoma, papillary thyroid cancer, oral squamous cell cancer and renal cancer are the five most often reported non-HCC cancers associated with HCV [6, 7].
The lack of comprehensive data on the spectrum of malignancies among HBV and HCV patients in India underscores a critical gap in addressing these infections and developing effective prevention and management strategies. Tata Memorial Hospital (TMH), India’s largest tertiary referral cancer centre, plays a crucial role in addressing this issue. TMH, which treats patients from across the country, provides unique insights into cancer patterns among these groups. This study conducted at TMH focused on analyzing cancer patterns among HBV and HCV patients in Mumbai, India, from 2017 to 2018. Understanding the prevalent types of cancers associated with these infections can guide policies related to vaccination, screening, early detection and antiviral therapies. TMH’s findings not only benefit India but also offer valuable references globally for managing and preventing cancers linked to HBV and HCV.
Methodology
The current study was a retrospective study of cancer patients with histologically proven diagnosis of cancer registered at TMH in Mumbai from 2017 to 2018. After pre-test counseling, all cancer patients, both existing and new, presenting to TMH for diagnosis/treatment underwent screening for viral markers including hepatitis B surface antigen (HBsAg) and HCV. We specifically calculated the proportional incidence ratio (PIR), for cancer patients who tested positive for HBsAg using ELISA. Those individuals who tested positive for IgM anti-HBc antibodies and anti-HBs antibodies were categorized as HBsAg negative. This classification was grounded in the recognition that acute hepatitis B can manifest without transitioning into chronic infection in the majority of cases.
Cancer patients found positive for HBsAg or HCV testing at TMH are referred to the hospital’s gastrointestinal clinic. Each cancer patient has a distinct, comprehensive case file containing information about their sociodemographic characteristics, laboratory results and treatment regimens documented by the treating physician in the electronic medical record (EMR). Age, sex, residence, education, anatomic location, stage, histology of cancer and specifics of viral markers including HBsAg, HCV and HIV infection are abstracted from the EMR and uploaded into the hospital’s internal software. The International Classification of Diseases for Oncology [8] is emp;loyed by coding experts to classify cancer sites accurately.
The analysis of the data was performed in Microsoft Excel. We estimated the anticipated number of site-specific cancers among HBsAg and HCV-positive cancer cases utilizing the gender and age-specific proportions of each cancer site which were recorded in the Hospital Cancer Registry of TMH, Mumbai for the years 2017 and 2018. By dividing the overall number of cancer cases in each age group within the study data set by the corresponding age and site-specific proportions in the standard, the expected number of instances of a given cancer was calculated. To calculate the PIR, the observed number of site-specific cancer cases among HBsAg and HCV-positive cancer cases was divided by the expected number [9]. Since this study relied on hospital cancer data, only age-specific rates were calculated. Age-standardized rates necessitate population rates for calculation. A higher PIR indicates that, in HBsAg and HCV-positive cancer cases, the proportion of cancer at a given cancer site is higher than would be predicted based on the information from TMH’s hospital-based cancer registry. Under the presumption of a Poisson distribution, the estimated standard error of the PIR was used to calculate 95% confidence ranges for the observed instances. Each two-sided PIR test was deemed significant at a 5% level, and all tests were two-sided.
Results
A total of 71,001 cancer patients were registered during the period 2017 to 2018 in TMH, Mumbai. Table 1 describes the demographic characteristics of the patients and viral markers. The mean (SD) age of the study participants was 48.69 (±16.91) with nearly one-third being elderly. The male-to-female ratio was 1.36 in our study. Approximately (38.79%) of cancer patients hailed from the western region of India. The proportion of participants seropositive for HBsAg, HCV and both were 1,367 (1.93%), 831 (1.17%) and 34 (0.05%), respectively. The proportion of HIV-positive cancer patients was 418 (0.59%). A total o;f 523 patients who tested positive for HBsAg received antiviral treatment after consulting the hepatology clinic at TMH, while the treatment status of the remaining patients is unknown.
Table 1. Characteristics of cancer patients registered at TMH, Mumbai, India. Year: 2017–2018.
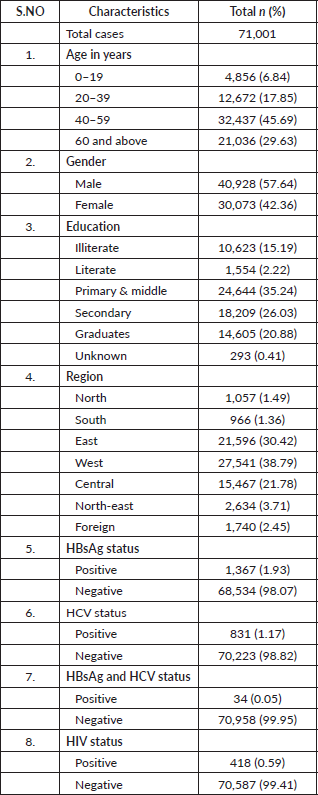
Table 2 outlines the cancer sites among HBsAg-positive cancer cases within the study group and provides estimates of PIR for the various cancer sites in males and females. Liver cancer emerged as the commonest site among HBsAg-positive cancer cases in both genders. The PIR for liver cancer was notably high in males at 14.41 (95% CI, 13.0–15.9), and in females at 10.89 (95% CI, 7.65–15.4), overall PIR of the liver was 16.31 (95% CI, 14.7–17.9). In males, there were elevated PIRs greater than 1 for nasopharynx, small intestine, mesothelioma, breast, renal pelvis, eye, secondary respiratory and digestive organs, as well as NHL and multiple myeloma. However, these increases were not statistically significant. For females, elevated PIRs greater than 1 were observed for the lip, nasopharynx, esophagus, colon, rectum, anus, other thoracic organs, bone, skin melanoma, soft tissue and peripheral nervous system, vulva, cervix, corpus uteri, ovary, other female genital organs, renal pelvis, other skin, as well as secondary respiratory and digestive organs. However, similar to males, these increases were not statistically significant.
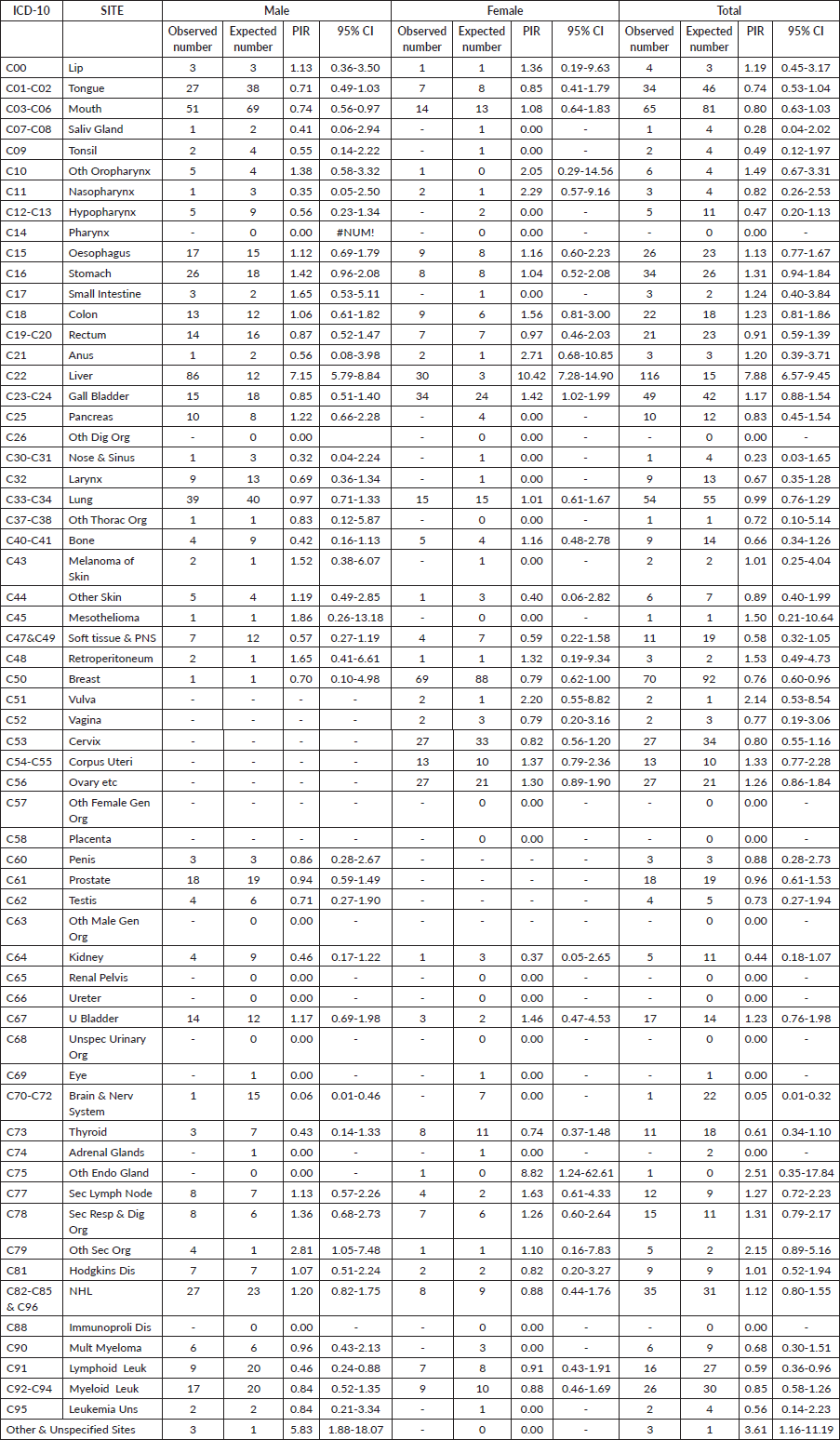
Table 3 illustrates the cancer sites among HCV-positive cancer cases in the study population and shows the estimates of PIR for the various cancer sites in males and females. The PIR was significantly higher for liver cancer in males 7.15 (95% CI, 5.79–8.84) and in females, 10.42 (95% CI, 7.28–14.90) and overall PIR was 7.88 (95% CI, 6.57–9.45). Elevated PIRs greater than 1 were observed in various cancer sites, including lip, other oropharynx, esophagus, stomach, small intestine, colon, rectum, anus, gall bladder, melanoma, mesothelioma, retroperitoneum, vulva, corpus uteri, ovary, urinary bladder, other endocrine glands, secondary lymph nodes, secondary respiratory and digestive organs, other secondary organs, Hodgkin’s disease, NHL and other unspecified sites. However, these increases were not found to be statistically significant.
Discussion
This study details the prevalence of different cancer sites among individuals who are positive for HBsAg and HCV. We evaluated PIR for various cancer sites for HBsAg and HCV-positive cases seeking care at TMH using age and gender-specific proportions of each cancer site from the Hospital Cancer Registry of TMH as a reference. The prevalence of HBsAg and HCV positivity in our study was 1.93% and 1.17%, respectively.
Infection with HBV is widely known as a risk factor for HCC [10]. Our findings revealed that the PIR was considerably higher for HCC in persons who were seropositive for HBsAg and HCV when compared to the data from TMH’s hospital cancer registry. In both genders, HBsAg seropositive subjects also had higher PIR for certain non-liver cancers such as multiple myeloma, NHL, leukemia and Hodgkin lymphoma. Our findings on the association between HBsAg with HCC, leukemia and lymphoma align with a large case-control study conducted among the Chinese population. A case-control study observed significant associations between HBsAg and HCC, gastrointestinal cancers, NHL and leukemia [10]. A population-based cohort study in China and a few other prospective studies also established an association of HBsAg with liver cancer and non-liver cancer especially digestive system cancers, oral cancers and lymphoma [11–13]. Studies across the globe have shown strong evidence of an association between HCV and HCC [14]. Our study findings were also consistent with elevated PIR for HCC among HCV seropositive patients.
Our study findings suggest a significant association between HCC and hepatitis B and C infection. However, the elevated PIR for hematological malignancies among HBsAg-positive individuals may stem from an additional mandatory investigation of HBsAg core and envelope antigens in patients with hematological cancer. Clinicians managing hepatitis B and C infection-positive patients should remain vigilant for malignancies. The National Viral Hepatitis Control Program recommends routine HCC surveillance with abdominal ultrasound and alpha-fetoprotein testing every 6 months for individuals with cirrhosis, a family history of HCC, an age of more than 40 years and an elevated HBV DNA level of more than 2,000 IU/mL [15]. Further reactivation of the HCV in patients receiving chemotherapy has resulted in a deleterious clinical course. So, universal pre-chemotherapy HCV testing for patients with hematological malignancies is recommended by current guidelines [16]. Strengthening surveillance by pre-screening for hepatitis B and C positive infection among cancer patients as well as HCCs among hepatitis seropositivity is important to reduce the incidence of HCC. The paper also highlights the crucial role of addressing HBV and HCV treatments in managing cancer risks, particularly due to their well-established links with specific cancers such as HCC and liver cancer. It stresses the importance of managing viral infections to decrease associated cancer risks, emphasizing the significance of screening, early detection and prompt treatment to prevent the development of these cancers.
Table 2. Details of cancer sites and PIR for the cancer sites in the 1,367 patients with HBsAg and cancer registered at TMH, Mumbai, India. Year: 2017–2018.
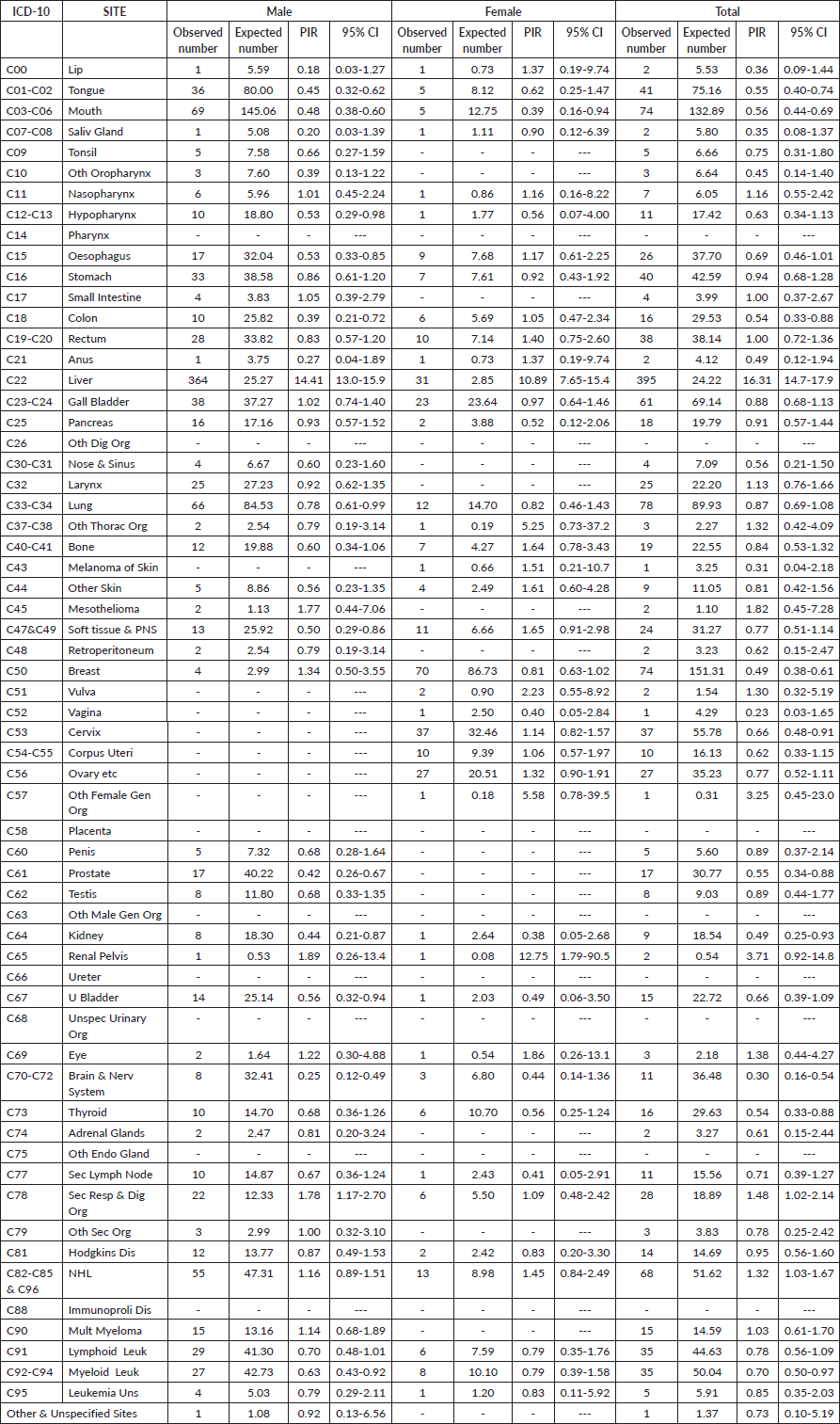
Table 3. Details of cancer sites and PIR for the cancer sites in the 831 patients with HCV and cancer registered at TMH, Mumbai, India. Year: 2017–2018.
Among all cancer sites, the prevalence of HIV infection was 0.5%. NHL was the most common cancer among HIV-positive patients accounting for 108 (26.09%). In female HIV-positive cancer patients, cervical cancer was the leading type, representing 70 (42.17%). Among males, mouth cancer was the most prevalent, comprising 42 (16.94%). These findings underscore the importance of targeted cancer screening and prevention strategies for HIV-positive individuals.
Our study demonstrates several significant strengths. First, we analyzed a substantial dataset of 710,001 cancer cases, enabling a comprehensive exploration of the relationship between HBsAg and HCC. This extensive dataset also facilitated an examination of how HBsAg and HCV relate to various cancer types, offering a broad perspective on viral influences in cancer development. Additionally, our adherence to standardized guidelines for evaluating seromarker prevalence ensures the reliability and consistency of our findings. A key contribution of our research is confirming HBsAg and HCV infections as notable risk factors for liver cancer (HCC). By establishing this connection, we emphasize the importance of viral screening and effective management strategies to reduce the burden of liver cancer, particularly in regions where these infections are prevalent.
However, despite these strengths, our study faces several limitations that merit consideration. The retrospective nature of data collection, relying on hospital records to ascertain HBsAg and HCV status, introduces potential biases and challenges in establishing temporal relationships between viral infections and carcinogenesis. It is difficult to determine definitively whether HBsAg or HCV infection preceded the development of cancer in these cases. Retrieving h;epatitis treatment information from the EMR is challenging for patients receiving care from facilities outside of TMH. Moreover, because our study was conducted within a hospital setting, we faced limitations in calculating standardized rates, which are more robust for comparisons across different populations. Furthermore, some of our statistical analyses were constrained by small case numbers in certain cancer sites (PIRs), necessitating cautious interpretation of these findings.
Conclusion
In our study, the prevalence of hepatitis B and C was 1.9% and 1.10%, in all cancer sites, respectively. The PIR was higher for liver cancer across both genders in cancer patients who tested positive for HBsAg and HCV. The necessity for primary and secondary preventive efforts to decrease the incidence of HCC is highlighted by the elevated PIR for liver cancer among HBsAg and HCV patients.
Acknowledgment
We would like to acknowledge the staff in the Division of Cancer Care, Hospital Cancer Registries and Survival Studies for their immense contribution and continuous technical support for completing the study.
Conflicts of interest
There are no conflict of interest to declare.
Funding
This study received no funding.
Informed consent
The database of the hospital-based cancer registry is securely maintained by authorized study personnel. Measures have been ensured to safeguard the user ID and password for accessing the data ensuring the patient rights are upheld and protected.
Author contributions
- Dr Sivaranjini Kannusamy: Conceptualization, design, statistical analysis, data interpretation and manuscript writing.
- Dr Amey Oak: Provided knowledgeable direction and oversight to ensure the study’s methodological rigor and contributed to manuscript writing.
- Mrs Sandhya Cheulkar: Contributed to data validation and interpretation.
- Dr Kamesh Maske: Contributed to the conceptualization and manuscript writing.
- Mrs Esha Dashmukhe, Mrs Ashwini Patil and Ms Manisha Morajkar: Contributed to data abstraction and validation.
- Dr Manju Sengar: Made substantial contributions in drafting the final report.
- Dr Ganesh Balasubramaniam: Substantial contributions to the study administration and conceptualization.
- Dr Rajesh Dikshit: Contributed to Conceptualization, design, management of the study and assisted in drafting the final report.
References
1. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
2. World Health Organization (2022) Hepatitis B: Fact Sheet [EB/OL] (Geneva: World Health Organization) [http://www.who.int/mediacentre/factsheets/fs204/zh/] Date accessed: 24/06/24
3. Viral; Hepatitis – The Silent Disease Facts and Treatment Guidelines (2015) (Dhaka: Directorate General of Health Service, Ministry of Health and Family Welfare, Government of India)
4. Harris PS, Hansen RM, and Gray ME, et al (2019) Hepatocellular carcinoma surveillance: an evidence-based approach World J Gastroenterol 25(13) 1550–1559 https://doi.org/10.3748/wjg.v25.i13.1550 PMID: 30983815 PMCID: 6452232
5. Sayiner M, Golabi P, and Younossi ZM (2019) Disease burden of hepatocellular carcinoma: a global perspective Dig Dis Sci 64(4) 910–917 https://doi.org/10.1007/s10620-019-05537-2 PMID: 30835028
6. Thrift AP, El-Serag HB, and Kanwal F (2017) Global epidemiology and burden of HCV infection and HCV-related disease Nat Rev Gastroenterol Hepatol 14(2) 122–132 https://doi.org/10.1038/nrgastro.2016.176
7. Bhadoria AS, Khwairakpam G, and Grover GS, et al (2022) Viral hepatitis as a public health concern: a narrative review about the current scenario and the way forward Cureus 14(2) e21907 PMID: 35265429 PMCID: 8898569
8. World Health Organization (2013) International Classification of Diseases for Oncology (ICD-O) [Internet] 3rd edn (Geneva: World Health Organization) [https://apps.who.int/iris/handle/10665/96612] Date accessed: 15/03/22
9. Boyle P and Parkin DM (1991) Statistical methods for registries Cancer Registration Principles and Methods eds OM Jensen, DM Parkin, and R Maclennan, et al (Lyon: IARC scientific publications) pp 126–158
10. Tian T, Song C, and Jiang L, et al (2020) Hepatitis B virus infection and the risk of cancer among the Chinese population Int J Cancer 147(11) 3075–3084 https://doi.org/10.1002/ijc.33130 PMID: 32478856
11. Kamiza AB, Su FH, and Wang WC, et al (2016) Chronic hepatitis infection is associated with extrahepatic cancer development: a nationwide population-based study in Taiwan BMC Cancer 16(1) 861 https://doi.org/10.1186/s12885-016-2918-5 PMID: 27821099 PMCID: 5100218
12. Ulcickas Yood M, Quesenberry CP, and Guo D, et al (2007) Incidence of non-Hodgkin’s lymphoma among individuals with chronic hepatitis B virus infection Hepatology 46(1) 107–112 https://doi.org/10.1002/hep.21642 PMID: 17526021
13. Sundquist K, Sundquist J, and Ji J (2014) Risk of hepatocellular carcinoma and cancers at other sites among patients diagnosed with chronic hepatitis B virus infection in Sweden: HBV and cancer J Med Virol 86(1) 18–22 https://doi.org/10.1002/jmv.23754
14. Perz JF, Armstrong GL, and Farrington LA, et al (2006) The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide J Hepatol 45 529–538 https://doi.org/10.1016/j.jhep.2006.05.013 PMID: 16879891
15. National Guidelines for Diagnosis & Management of Viral Hepatitis (2018) (New Delhi: Ministry of Health and Family Welfare, Government of India)
16. Li YR, Hu TH, and Chen WC, et al (2021) Screening and prevention of hepatitis C virus reactivation during chemotherapy World J Gastroenterol 27(31) 5181–5188 https://doi.org/10.3748/wjg.v27.i31.5181 PMID: 34497443 PMCID: 8384748






