Primary thoracic synovial sarcomas: clinical profile and treatment outcomes of a rare entity managed at a tertiary care centre
Ghazal Tansir1, Sameer Rastogi1, Ekta Dhamija2, Shamim Ahmed Shamim3, Deepali Jain4, Adarsh Barwad4, Sunil Kumar5 and Rambha Pandey6
1Department of Medical Oncology, All India Institute of Medical Sciences, New Delhi 110029, India
2Department of Radiodiagnosis, All India Institute of Medical Sciences, New Delhi 110029, India
3Department of Nuclear Medicine, All India Institute of Medical Sciences, New Delhi 110029, India
4Department of Pathology, All India Institute of Medical Sciences, New Delhi 110029, India
5Department of Surgical Oncology, All India Institute of Medical Sciences, New Delhi 110029, India
6Department of Radiation Oncology, All India Institute of Medical Sciences, New Delhi 110029, India
Abstract
Introduction: Primary thoracic synovial sarcoma (PTSS) is a rare malignancy presenting with varying clinical manifestations. There is a paucity of data with few studies dedicated to this unique subset of neoplasms. We present our findings from one of the largest real-world studies among patients with PTSS.
Methods: This is a single-centre, real-world study in patients with PTSS included between 2017 and 2023. Survival estimates were obtained by the Kaplan-Meier method and Cox regression analysis.
Results: 24 patients with a median age of 34.5 years (range 16–54) presented with chest pain (n = 11, 45.8%) and dyspnea (n = 10, 41.6%). Predominant primary sites of disease were the lung (n = 12, 50%) and mediastinum (n = 6, 25%). The stage at presentation was unresectable locally advanced (n = 10, 41.6%), localised (n = 8, 33.3%) and metastatic (n = 6, 25%) with pulmonary metastases (n = 10, 62.5%) and pleural effusion (n = 4, 25%). 16 (66.6%) patients underwent surgical resection including 7 (43.8%) who received neoadjuvant chemotherapy (NACT). NACT was given in ten patients producing stable disease in 5 (50%) and partial response in 3 (30%) patients, respectively, with surgery performed in 7 (70%). 11 (62.5%) operated patients had a microscopically complete resection and 10 (41.6%) received postoperative radiotherapy. Anthracyclines were given in 23 (95.8%) patients in the first line, while pazopanib was the most common therapy in the second and third lines, respectively. At a median follow-up of 32 months (range 16.7–47.2), the median overall survival (OS) was 41 months (95% CI: 23.7–58.2) and 8 months (95% CI: 1–25.6) overall and in metastatic disease, respectively. Presentation with metastases (p = 0.01) and treatment with surgical resection (p = 0.005) were significantly associated with OS on univariate analysis.
Interpretation: The locally advanced nature of the disease at presentation signifies the need for early diagnosis and technically superior definitive therapies. The survival outcomes for metastatic disease remain poor and the need for novel therapies for advanced disease remains unmet so far.
Clinical trial registration: Not applicable
Keywords: synovial sarcoma, multidisciplinary treatment, primary thoracic sarcoma, thoracic oncology
Correspondence to: Sameer Rastogi
Email: samdoc_mamc@yahoo.com
Published: 06/09/2024
Received: 27/02/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Primary thoracic sarcomas constitute a rare subset of thoracic neoplasms. Pulmonary and chest wall sarcomas constitute 0.5% and 5% of all thoracic malignancies, respectively [1, 2]. While synovial sarcoma (SS) typically occurs at the extremities and trunk, primary thoracic synovial sarcoma (PTSS) has been described as less than 2% of all SS [3]. The primary subsites included among PTSS are the chest wall, mediastinum, lung and heart. Due to the involvement of vital mediastinal structures, clinical presentations may include respiratory and cardiac symptoms. Multidisciplinary treatment (MDT) includes surgery for resectable disease, with or without radiotherapy (RT) and adjuvant chemotherapy. Resection margin status and tumour size have been found to be important prognostic factors for localised disease [4]. Expertise in surgical resection and RT planning is paramount in cases of PTSS. Locally advanced or metastatic disease is managed with doxorubicin-based chemotherapeutic regimens, while subsequent therapies for advanced and metastatic disease are less well-defined. We conducted this retrospective study among patients with PTSS to examine the clinical characteristics, treatment patterns and outcomes of this rare disease.
Methods
This is a single-centre retrospective analysis of a prospectively maintained database evaluating patients with PTSS. The patients included in the study were registered in the sarcoma medical oncology clinic of All India Institute of Medical Sciences between May 2017 and January 2023. After clearance from the Institute Review Board, patient data were evaluated through hospital records including epidemiologic characteristics, stage of the disease, primary and metastatic sites, therapy administered, response rates and outcomes.
Chest wall, mediastinal, pulmonary and cardiac sites were included in the study and staged as localised, locally advanced unresectable and metastatic disease. Staging as locally advanced unresectable disease was deemed as per the expert thoracic surgeons in the multidisciplinary tumour board meetings. The histopathological diagnosis of SS had been reviewed by the expert sarcoma pathologists (AB and AM) at our institution. Statistical analysis was done through SPSS 26 (SPSS, Chicago, IL). Nominal data were entered as numbers (%) and continuous data as median and mean values as applicable. Progression-free survival (PFS) was calculated from the date of initiation of treatment to the first date of documented progressive disease or death from any cause. Overall survival (OS) was calculated by the Kaplan-Meier method from the date of diagnosis to death from any cause; patients alive or lost to follow up were censored. Prognostic variables were analysed for association with OS and PFS by the chi square test and multivariate analysis was carried out by Cox regression analysis.
Results
24 patients with PTSS were included with a median age of 34.5 years (range 16–54) and marginal male predominance (n = 14, 58.3%). The presenting ECOG performance status (PS) was 1 in 13 (54.1%), 2/3 in 10 (41.6%) and 4 in 1 (4.1%) patients, respectively. The most common baseline symptoms were chest pain (n = 11, 45.8%), dyspnea (n = 10, 41.6%) and cough (n = 7, 29.1%). The distribution of stages at baseline diagnosis included 8 (33.3%) localised, 10 (41.6%) unresectable locally advanced and 6 (25%) metastatic diseases. At the time of data analysis for this study, this distribution comprised 3 (12.5%) localised, 5 (20.8%) locally advanced and 16 (66.6%) metastatic disease, respectively. 3 (12.5%) patients were misdiagnosed as lung cancers at peripheral centres during the initial evaluation, but were later found to have PTSS upon presenting to us.
The baseline median tumour size was 10 cm (range 2–20). The most common primary locations included the lung (n = 12, 50%), mediastinum (n = 6, 25%), chest wall (n = 4, 16.6%) and other sites such as cardiac SS in 1 (4.1%) patient. Among the 16 patients with metastatic disease, the common sites of metastases were lung (n = 10, 62.5%), pleural effusion (n = 4, 25%) and pleura (n = 3, 18.7%). 19 (79.1%) patients were positive for SS18 rearrangement by break-apart FISH while histopathologic tissue was not sufficient for molecular testing in the remainder. The clinico-pathological details of the study cohort are summarised in Table 1.
Table 1. Clinical details of patients with PTSS.
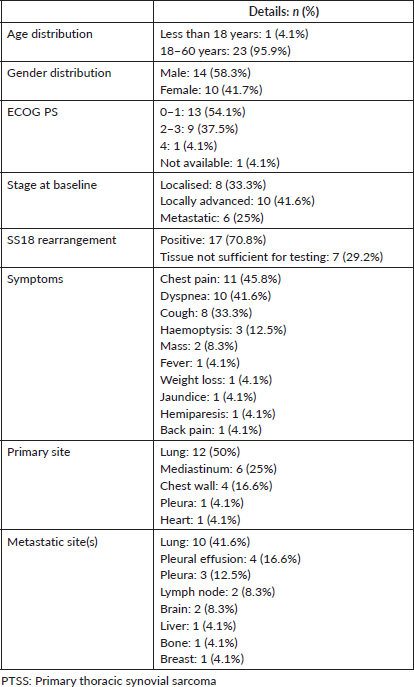
Curative management was planned based on MDT meetings between departments of medical oncology, surgical oncology and radiation oncology. Neoadjuvant chemotherapy (NACT) was administered in ten patients including 9 (90%) patients with locally advanced and 1 (10%) patient who had metastatic disease with a single site of metastases. Primary sites of disease managed with NACT were the lung (n = 5, 50%), mediastinum (n = 3, 30%) and chest wall (n = 2, 20%), with a median size of 12 cm (range 5–20). Indications for NACT decided by the MDT included cardiac and/or great vessel involvement (n = 4, 40%), extensive disease not amenable to complete surgery (n = 3, 30%), impending respiratory compromise (n = 2, 20%) and risk of nerve damage due to brachial plexus involvement (n = 1, 10%). NACT produced stable disease in 5 (50%), partial response in 3 (30%), complete response in 1 (10%) and local disease progression in 1 (10%) patients each. 16 (66.6%) patients underwent surgical resection, among whom 9 (56.2%) had upfront surgery and 7 (43.8%) were operated on after NACT. 11 (62.5%) had a complete resection (R0), 2 (18.7%) microscopically incomplete (R1) resection and 2 (12.5%) had the macroscopic residual disease (R2) (Table 2). 3 out of the 10 (30%) patients could not undergo surgery due to persistent extensive disease (n = 1, 33.3%), cardiac involvement (n = 1, 33.3%), negative consent by the patient due to the risk of brachial plexus damage (n = 1, 33.3%). RT was a part of the treatment regimen in 12 (50%) patients and 10 (41.6%) received postoperative RT while the remainder 2 (8.3%) received definitive RT for surgically unresectable disease.
Median 2 lines (range 1–6) of medical therapy (chemotherapy and/or targeted therapy) were given, including median 4 (range 3–6) cycles of neo(adjuvant) chemotherapy. 23 (95.8%) patients received anthracycline-based chemotherapy regimens in the first-line setting, either as (neo) adjuvant or palliative treatment. The most common medical regimens used in the 2nd line (n = 12, 50%) for advanced/metastatic disease included pazopanib (n = 5, 41.6%), gemcitabine with/without docetaxel (n = 4, 33.3%) and high dose ifosfamide (n = 2, 16.6%). Table 3 summarises the chemotherapy regimens used for the patients across all lines of treatment. Dexrazoxane was used prophylactically for cardio-protection in 2 (8.3%) patients. 1 patient who received dexrazoxane has been given postoperative mediastinal RT.
14 (58.3%) patients of the cohort are currently alive, including seven living with metastatic disease. At a median follow-up of 32 months (range 16.7–47.2), the median OS was 41 months (95% CI: 23.7–58.2) and the 5-year OS was 37% (95% CI: 9.5%–64.4%) (Figure 1). The median OS as per disease stage was not reached for localised disease, 44 months (95% CI: 4.1–83.8) for locally advanced and 8 months (95% CI: 1–25.6) for metastatic disease (Figure 2). The median PFS with first-line of therapy was 12 months (95% CI: 10.5–13.4) (Figure 3), while it was 3 months (95% CI: 1.4–4.5) and 7 months (3.6–10.4) with second and third-lines, respectively (Table 3).
Metastatic disease at presentation (p = 0.01) and treatment with surgical resection (p = 0.005) (p = 0.005) were significantly associated with OS per univariate analysis but not by multivariate analysis (Table 4). Age, gender, ECOG PS (0 to 1 versus 2 and above), primary site (pulmonary versus non-pulmonary) and lymph node involvement were not found to be significantly associated with OS. Among the patients who underwent surgery, resection status, administration of adjuvant chemotherapy or adjuvant RT were not found to be associated with OS.
Discussion
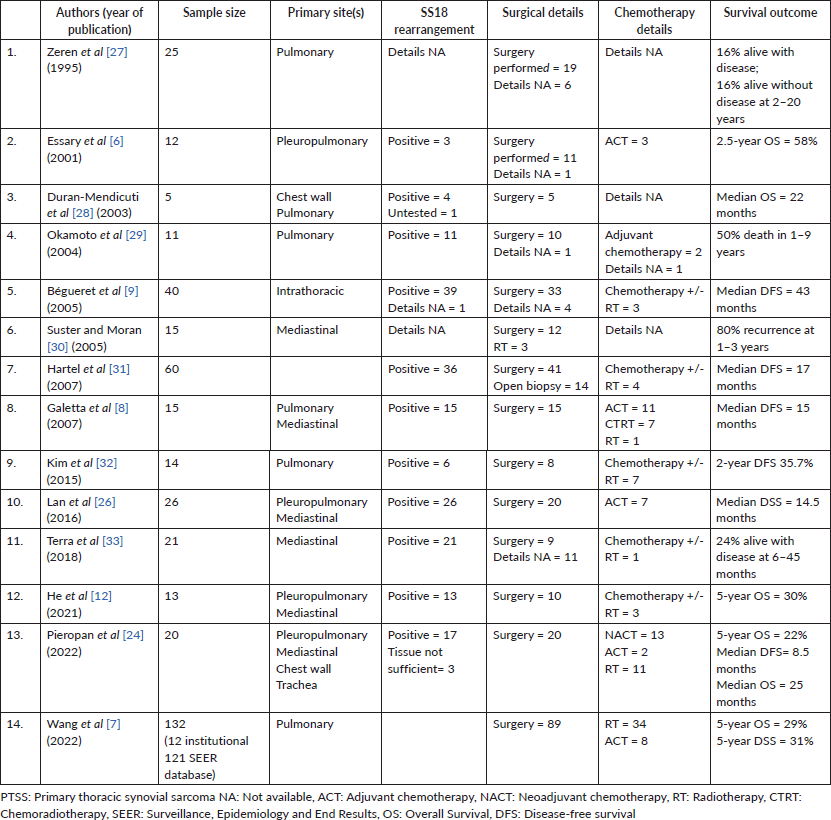
This is one of the largest contemporary studies covering all sub-sites of PTSS and its symptomatology, clinico-epidemiological profile, management and survival outcomes. Previous studies on primary thoracic sarcomas of all histologies have included patients with SS such as the analysis by Spraker et al [5] among 365 patients that included 43 patients with pulmonary SS. Studies that have included PTSS exclusively are few and with a limited number of patients due to the rarity of the condition (Table 5).
The demographic profile of our patients is concordant with that described in previous studies on SS wherein most patients are aged 40 years or less with an even gender distribution or a slight male predominance [3]. The clinical presentations in our patients depended on the primary site of involvement with a majority presenting with chest pain and respiratory complaints such as dyspnea and cough, as has been observed previously [6]. Thoracic sarcomas usually present in advanced stages due to their non-specific clinical presentations [7]. More than 40% of our patients had locally advanced unresectable disease at the time of baseline diagnosis and a median tumour size of 10 cm. This was also observed by Galetta et al [8] in their series on PTSS in which the tumours were similarly large with a median size of 8 cm.
The most reported primary sub-site in the previous studies on PTSS is pulmonary, which was also observed among our patients [6]. Molecular testing for translocation (X;18) assumes importance in the cases of PTSS with equivocal radio-pathological diagnoses [9]. The site of disease sometimes leads to constraints in obtaining tissue specimens for translocation testing. Despite this, we were able to perform molecular testing and demonstrate SS18 rearrangement in 79% of our patients. Extremely rare and aggressive sub-sites such as cardiac SS are included in our cohort, which has only been described in a few case reports and presents with a fulminant clinical course as in our patient [10].
Table 2. Details of curative therapy received by patients with PTSS.
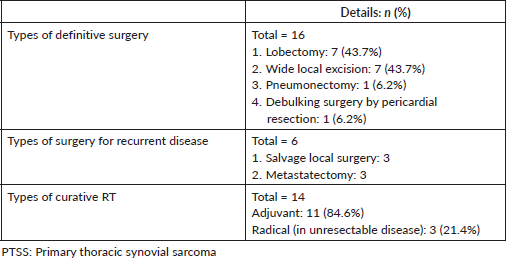
Table 3. Details of medical treatment received by patients with PTSS.
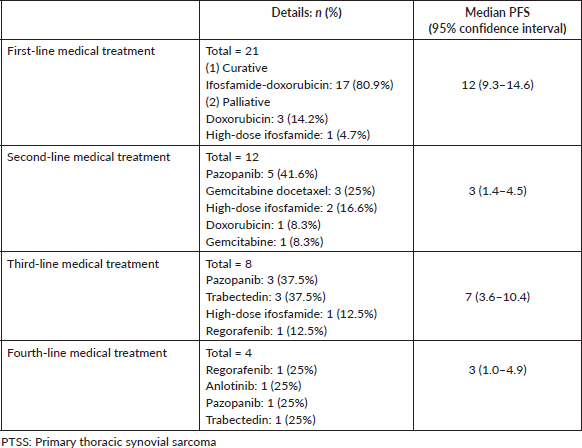
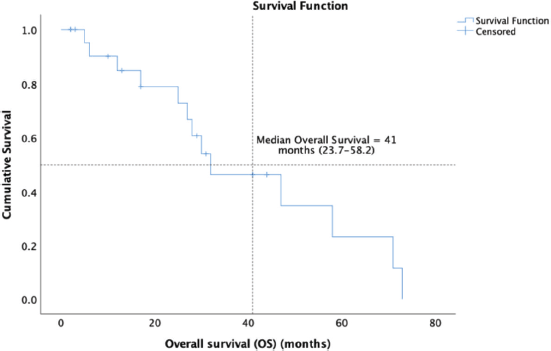
Figure 1. Kaplan-Meier analysis showing OS of patients with PTSS.
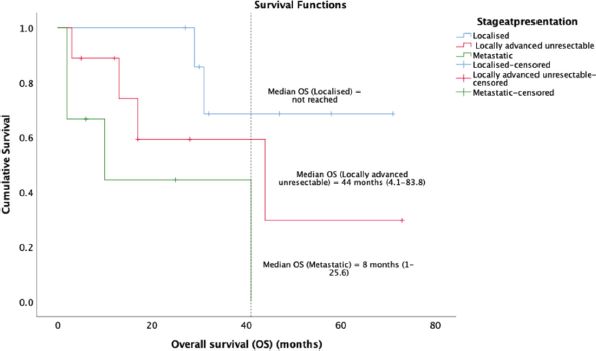
Figure 2. Kaplan-Meier analysis showing OS of patients with PTSS classified as per stage of disease at presentation.
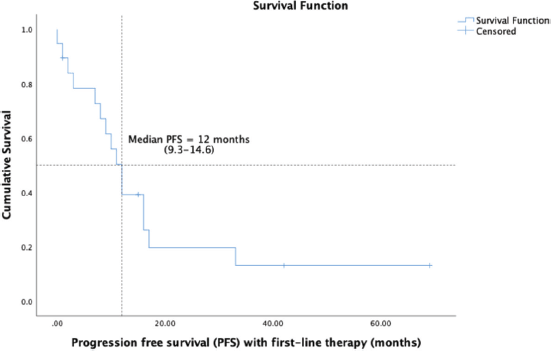
Figure 3. Kaplan-Meier analysis showing PFS of patients with PTSS.
Table 4. Cox-regression analysis of factors associated with OS in patients with PTSS.
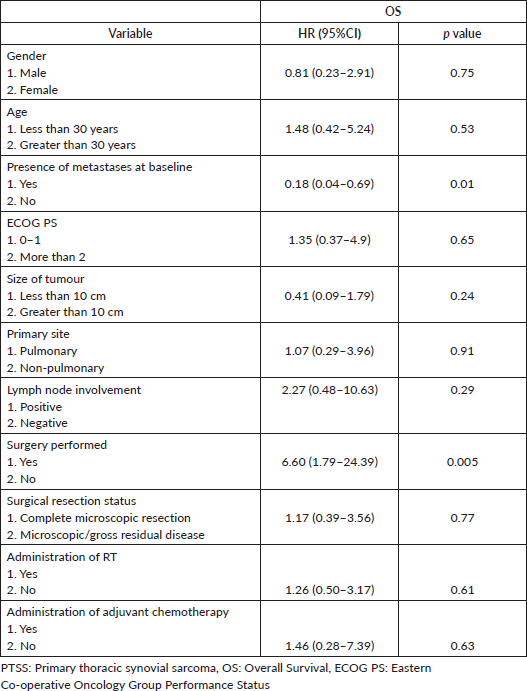
Table 5. Summary of previous published case series on PTSS.
Lung (41.6%), pleural effusion (16.6%) and pleura (12.5%) were the commonest sites of metastases in our series which have also been reported among patients with PTSS previously [11]. We observed some atypical metastatic sites, including lymph nodes (8.3%), brain (8.3%), breast (4.1%) and bone (4.1%). These rare sites were also reported among PTSS by Wang and Li [10] with nodal (9.9%), bone (2.5%) and brain (1.7%) metastases. A series on pulmonary sarcomas which had 12% patients with localised SS found a significant proportion of nodal metastases (16%) in pulmonary sarcomas [5]. This finding led the authors to opine that mediastinal nodal evaluation could be a routine part of the baseline evaluation of localised PTSS.
MDT is a key part of the management of sarcomas and is especially useful for PTSS which can be locally advanced and complex to treat. He et al [12] demonstrated a survival benefit among patients with PTSS who underwent MDT evaluation prior to initiation of treatment. Complete surgical resection in combination with RT and/or chemotherapy are the preferred treatment modalities. Among our patients, 66.6% underwent surgery as part of their treatment with 68.7% R0 resections. This is comparable to the proportion of R0 resections (63%) reported among primary pulmonary sarcomas in a previous series comprising of 22 patients [4].
The role of preoperative chemotherapy in PTSS remains poorly defined due to the lack of prospective data and the risk of selection bias in interpreting retrospective studies [13]. Among our patients (45.8%) who received anthracycline-based NACT, the objective response was a comparable 40% and delayed surgery was performed in 70% of patients with unresectable disease at baseline. Since PTSS tumours can be technically challenging to resect, administration of NACT can increase the number of complete surgeries.
Anthracyclines and high-dose ifosfamide have shown efficacy in advanced SS with response rates varying from 25% to 60% in the first-line setting [14–17]. Our patients with advanced disease not previously exposed to anthracyclines were managed with single-agent doxorubicin, except for 1 patient who received ifosfamide. Agents that have shown anti-tumour activity in later lines in SS include trabectedin [18], pazopanib [19], regorafenib [20] and anlotinib [21] while immunotherapy and EZH2 inhibitors have produced dismal results [13, 22]. Among our patients, both cytotoxic chemotherapy and targeted agents were used in equal numbers including the first reported use of anlotinib (Figure 4) in PTSS so far. The use of dexrazoxane with anthracyclines among patients with sarcomas is currently inconsistent despite the demonstrated reduction in risk of clinical and subclinical chemotherapy-related cardiac adverse effects [23]. Dexrazoxane could be used with anthracyclines in PTSS, especially left-sided tumours, as was done in our patient who was planned for adjuvant mediastinal RT.
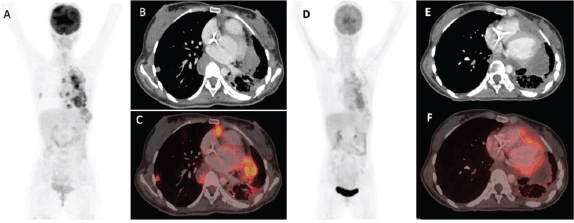
Figure 4. Fluorodeoxyglucose-positron emission tomography (PET) images of a patient with mediastinal SS treated with Anlotinib. (a): Pre-anlotinib maximum intensity projection (MIP), trans-axial computer tomography (CT) (b): And fused PET/CT (c): Images showing multiple variable sized lung subpleural and parenchymal nodules with some showing necrotic changes in bilateral lungs. (d–f): Post-anlotinib images showing decrease in size, uptake and number of most of the parenchymal and subpleural lesions in bilateral lungs with increase in associated necrotic component.
The survival outcomes of PTSS have been demonstrated to be poorer than SS (including all sub-sites) at a 5-year OS of 22% [24] in the former compared to 67% in the latter [25]. This was also observed by Lan et al [26] among pleuropulmonary and mediastinal SS where the median OS was 14.5 months in comparison to that reported in extremity SS (52 months). The median OS of 41 months in our study compares favourably to prior reports, especially driven by localised and locally advanced stages. However, survival outcomes were dismal among our patients with metastatic PTSS, emphasising the poor responses to palliative treatments. The stage of disease and surgical resection were the prognostic variables found significantly associated with OS on univariate analysis. The assessment for other variables could have been limited due to the small sample size of our cohort. In previous analyses, the prognostic variables associated with OS include ECOG PS ≥2, tumour size greater than 5 cm, incomplete surgical resection, non-administration of adjuvant chemotherapy and poor response to chemotherapy [8, 24, 26].
The limitations of our study include the constraints associated with its retrospective design. Also, a larger number of subjects would allow for a robust assessment of prognostic variables having an impact on the survival of patients with PTSS. However, our single-centre insight on this ultra-rare disease contributes to the paucity of currently reported literature.
Conclusion
PTSS is a rare subset of thoracic malignancies that affects young individuals and presents with respiratory symptoms, large tumour size and advanced stage. The thoracic location poses challenges in terms of diagnosis and definitive management. Patients managed at non-expert centres may be misdiagnosed, leading to delayed or inadequate treatment. Multidisciplinary care and better palliative treatment options are required for the management of PTSS.
List of abbreviations
CI, Confidence interval; DFS, Disease-free survival; ECOG, Eastern Cooperative Oncology Group; MDT, Multidisciplinary treatment; OS, Overall survival; PTSS, Primary thoracic synovial sarcoma; SS, Synovial sarcoma.
Author acknowledgments
GT takes responsibility for the authenticity of the manuscript, including the data and analysis.
GT performed data entry, analysis, data interpretation and manuscript writing. SR performed the conceptualisation of the study, interpretation of results and manuscript proofreading. ED and SAS provided information regarding baseline radiological details and response assessments of the study patients. DJ and AB provided details of histopathologic findings and molecular testing of the study patients. SK provided details on the surgical management of the study patients.
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
The authors have not received any financial support for the conduct of this study.
Ethical approval
Ethical approval for the study has been obtained from the Institution Review Board of the All India Institute of Medical Sciences (IEC-678/01.10.2021).
References
1. Nakahashi N, Emori M, and Tsuchie H, et al (2019) Treatment outcome of chest wall soft tissue sarcomas: analysis of prognostic factors J Surg Oncol 120 1235–1240 https://doi.org/10.1002/jso.25708 PMID: 31536137
2. Mjid M, Blibech H, and Toujani S, et al (2015) Rare primary pulmonary tumors Eur Respir J 46(suppl 59)
3. Ferrari A, Gronchi A, and Casanova M, et al (2004) Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution Cancer 101(3) 627–634 https://doi.org/10.1002/cncr.20386 PMID: 15274077
4. Gołota J, Osowiecka K, and Orłowski T (2018) Primary pulmonary sarcoma – long-term treatment outcomes and prognostic factors Kardiochir Torakochirurgia Pol 15(3) 162–169
5. Spraker MB, Bair E, and Bair R, et al (2013) An analysis of patient characteristics and clinical outcomes in primary pulmonary sarcoma J Thorac Oncol 8(2) 147–151 https://doi.org/10.1097/JTO.0b013e318277401f
6. Essary LR, Vargas SO, and Fletcher CDM (2002) Primary pleuropulmonary synovial sarcoma Cancer 94(2) 459–469 https://doi.org/10.1002/cncr.10188 PMID: 11905413
7. Wang W, Guo J, and Bao M, et al (2022) Primary pulmonary synovial sarcoma JTCVS Open 10 404–414 https://doi.org/10.1016/j.xjon.2022.02.014 PMID: 36004273 PMCID: 9390693
8. Galetta D, Pelosi G, and Leo F, et al (2007) Primary thoracic synovial sarcoma: factors affecting long-term survival J Thorac Cardiovasc Surg 134(3) 808–809.e1 https://doi.org/10.1016/j.jtcvs.2007.05.036 PMID: 17723844
9. Bégueret H, Galateau-Salle F, and Guillou L, et al (2005) Primary intrathoracic synovial sarcoma: a clinicopathologic study of 40 t(X;18)-positive cases from the French Sarcoma Group and the Mesopath Group Am J Surg Pathol 29(3) 339–346 https://doi.org/10.1097/01.pas.0000147401.95391.9a PMID: 15725802
10. Wang JG and Li NN (2013) Primary cardiac synovial sarcoma Ann Thorac Surg 95(6) 2202–2209 https://doi.org/10.1016/j.athoracsur.2013.01.030 PMID: 23647858
11. Telugu RB, Kodiatte TA, and Sakthi D, et al (2022) Primary pulmonary synovial sarcoma: a clinicopathological study of 22 cases Malays J Pathol 44(2) 215–224 PMID: 36043584
12. He H, Yang L, and Peng Y, et al (2021) The value of multidisciplinary team (MDT) management in the diagnosis and treatment of primary intrathoracic synovial sarcomas: a single-center experience J Thorac Dis 13(2) 600–612 https://doi.org/10.21037/jtd-20-2887 PMID: 33717533 PMCID: 7947479
13. Stacchiotti S and Van Tine BA (2018) Synovial sarcoma: current concepts and future perspectives JCO 36(2) 180–187 https://doi.org/10.1200/JCO.2017.75.1941
14. Sleijfer S, Ouali M, and van Glabbeke M, et al (2010) Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: an exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG) Eur J Cancer 46(1) 72–83 https://doi.org/10.1016/j.ejca.2009.09.022
15. Eilber FC, Brennan MF, and Eilber FR, et al (2007) Chemotherapy is associated with improved survival in adult patients with primary extremity synovial sarcoma Ann Surg 246 105–113 https://doi.org/10.1097/01.sla.0000262787.88639.2b PMID: 17592298 PMCID: 1899195
16. Spurrell EL, Fisher C, and Thomas JM, et al (2005) Prognostic factors in advanced synovial sarcoma: an analysis of 104 patients treated at the Royal Marsden Hospital Ann Oncol 16(3) 437–444 https://doi.org/10.1093/annonc/mdi082 PMID: 15653701
17. Ferrari A, De Salvo GL, and Brennan B, et al (2015) Synovial sarcoma in children and adolescents: the European Pediatric Soft Tissue Sarcoma Study Group prospective trial (EpSSG NRSTS 2005) Ann Oncol 26(3) 567–572 https://doi.org/10.1093/annonc/mdu562
18. Kawai A, Araki N, and Sugiura H, et al (2015) Trabectedin monotherapy after standard chemotherapy versus best supportive care in patients with advanced, translocation-related sarcoma: a randomised, open-label, phase 2 study Lancet Oncol 16(4) 406–416 https://doi.org/10.1016/S1470-2045(15)70098-7 PMID: 25795406
19. van der Graaf WTA, Blay JY, and Chawla SP, et al (2012) Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial Lancet 379(9829) 1879–1886 https://doi.org/10.1016/S0140-6736(12)60651-5 PMID: 22595799
20. Mir O, Brodowicz T, and Italiano A, et al (2016) Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial Lancet Oncol 17(12) 1732–1742 https://doi.org/10.1016/S1470-2045(16)30507-1 PMID: 27751846
21. Van Tine BA, Chawla SP, and Trent JC, et al (2021) A phase III study (APROMISS) of AL3818 (Catequentinib, Anlotinib) hydrochloride monotherapy in subjects with metastatic or advanced synovial sarcoma JCO 39(15_suppl) 11505 https://doi.org/10.1200/JCO.2021.39.15_suppl.11505
22. Schoffski P, Agulnik M, and Stacchiotti S, et al (2017) Phase 2 multicenter study of the EZH2 inhibitor tazemetostat in adults with synovial sarcoma (NCT02601950) JCO 35(15_suppl) 11057 https://doi.org/10.1200/JCO.2017.35.15_suppl.11057
23. Benjamin RS and Minotti G (2021) Doxorubicin-dexrazoxane from day 1 for soft-tissue sarcomas: the road to cardioprotection Clin Cancer Res 27(14) 3809–3811 https://doi.org/10.1158/1078-0432.CCR-21-1376 PMID: 33990361
24. Pieropan S, Mercier O, and Mitilian D, et al (2022) Feasibility and long-term outcomes of surgery for primary thoracic synovial sarcoma Interact Cardiovasc Thorac Surg 35(4) ivac238 https://doi.org/10.1093/icvts/ivac238 PMID: 36066443 PMCID: 9492245
25. Xu G, Aiba H, and Yamamoto N, et al (2021) Efficacy of perioperative chemotherapy for synovial sarcoma: a retrospective analysis of a Nationwide database in Japan BMC Cancer 21(1) 773 https://doi.org/10.1186/s12885-021-08485-1 PMID: 34217231 PMCID: 8255009
26. Lan T, Chen H, and Xiong B, et al (2016) Primary pleuropulmonary and mediastinal synovial sarcoma: a clinicopathologic and molecular study of 26 genetically confirmed cases in the largest institution of southwest China Diagn Pathol 11(1) 62 https://doi.org/10.1186/s13000-016-0513-3 PMID: 27401493 PMCID: 4939734
27. Zeren H, Moran CA, and Suster S, et al (1995) Primary pulmonary sarcomas with features of monophasic synovial sarcoma: a clinicopathological, immunohistochemical, and ultrastructural study of 25 cases Hum Pathol 26(5) 474–480 https://doi.org/10.1016/0046-8177(95)90242-2 PMID: 7750931
28. Duran-Mendicuti A, Costello P, and Vargas SO (2003) Primary synovial sarcoma of the chest: radiographic and clinicopathologic correlation J Thorac Imaging 18(2) 87–93 https://doi.org/10.1097/00005382-200304000-00006 PMID: 12700482
29. Okamoto S, Hisaoka M, and Daa T, et al (2004) Primary pulmonary synovial sarcoma: a clinicopathologic, immunohistochemical, and molecular study of 11 cases Hum Pathol 35(7) 850–856 https://doi.org/10.1016/j.humpath.2004.02.011 PMID: 15257548
30. Suster S and Moran CA (2005) Primary synovial sarcomas of the mediastinum: a clinicopathologic, immunohistochemical, and ultrastructural study of 15 cases Am J Surg Pathol 29(5) 569–578 https://doi.org/10.1097/01.pas.0000157934.50936.3e PMID: 15832079
31. Hartel PH, Fanburg-Smith JC, and Frazier AA, et al (2007) Primary pulmonary and mediastinal synovial sarcoma: a clinicopathologic study of 60 cases and comparison with five prior series Mod Pathol 20(7) 760–769 https://doi.org/10.1038/modpathol.3800795 PMID: 17464314
32. Kim GH, Kim MY, and Koo HJ, et al (2015) Primary pulmonary synovial sarcoma in a Tertiary Referral Center: clinical characteristics, CT, and 18F-FDG PET findings, with pathologic correlations Medicine (Baltimore) 94(34) e1392 https://doi.org/10.1097/MD.0000000000001392 PMID: 26313782 PMCID: 4602937
33. Terra SBSP, Aesif SW, and Maleszewski JJ, et al (2018) Mediastinal synovial sarcoma: clinicopathologic analysis of 21 cases with molecular confirmation Am J Surg Pathol 42(6) 761–766 https://doi.org/10.1097/PAS.0000000000001050 PMID: 29543673






