Clinicopathological features, response patterns, outcomes and BRAF status in patients with advanced acral melanoma: a preliminary Peruvian study
Denisse Castro1,2, Brady Beltrán1,2, Oscar Carnero3,4, Mauricio Póstigo5, Wilhelm Valdivia6, Cinthia Figueroa7, Manuel Leiva8, Marco López9 and Virgilio E Failoc-Rojas10,11
1Departamento de Oncología y Radioterapia, Hospital Nacional Edgardo Rebagliati Martins, EsSalud, Jesús María 15072, Lima, Peru.
2Centro de Investigación de Medicina de Precisión, Universidad de San Martin de Porres, La Molina 15024, Lima, Peru.
3Universidad Católica de Santa María, Arequipa 04013, Peru.
4Clinica Valle Sur, Arequipa 04001, Perú.
5Departamento de Patología, Hospital Carlos Alberto Seguín Escobedo, EsSalud, Arequipa 04400, Peru.
6Departamento de Oncología, Hospital Nacional Adolfo Guevara Velasco, EsSalud, Cusco 80108, Peru.
7Departamento de Oncología, Hospital Almanzor Aquinaga Asenjo, EsSalud, Chiclayo 14001, Peru.
8Departamento de Oncología, Hospital Nacional Alberto Sabogal Sologuren, EsSalud, Callao 07011, Lima, Peru.
9Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
10Universidad San Ignacio de Loyola, Lima 15024, Peru
11Análisis estadísticos, MedStat-Educación e Investigación, Lima 15072, Peru
Abstract
Background: Globally, acral melanoma (AM) is underrepresented in most clinical trials, being predominant in Caucasian populations. Latin America is a niche that needs to be explored. Therefore, this study aimed to determine the clinical features, response patterns, outcomes and v-raf murine sarcoma viral oncogene homolog B1 (BRAF) status in Peruvian patients with advanced AM.
Methods: We retrospectively reviewed the medical records of 19 patients with advanced AM who received immunotherapy (IO) in first- or subsequent-line therapy. The samples were analysed, and their mutational state was performed by deoxyribonucleic acid sequencing, focusing primarily on the most frequently mutated gene, BRAF. Descriptive statistics were used to assess the baseline characteristics. Overall survival was estimated using the Kaplan-Meier method.
Results: The median age was 64 years and 63.2% were men. Plantar was the site most frequently affected (84.2%). The most frequent stage was stage III (68.4%), with 26.4% receiving adjuvant therapy. The majority of cases exhibited a Breslow thickness of >4 mm (52%), a Clark level of IV/V (89.4%), and all patients presented ulceration and a high range of mitosis. During follow-up, all patients experienced recurrent advanced disease, with 52.6% developing visceral metastasis. Patients who received IO as first or subsequent line had an overall response rate (ORR) of 33.3%, and those who received it as first-line therapy had an ORR of 40%. Twenty-one percent of the patients harbored BRAF V6000E mutation and, showing an ORR of 50% compared to wild-type individuals (44.4%) after the first line of treatment.
Conclusion: Our preliminary study reported that AM has poor clinico-pathological features and response rates to IO in Peruvian patients. However, those who received IO as a first-line treatment or harbored the BRAF mutation appeared to have a slightly better response than wild-type patients.
Keywords: melanoma, cutaneous malignant, treatment outcome, proto-oncogene proteins B-raf
Correspondence to: Virgilio Failoc
Email: virgiliofr@gmail.com
Published: 27/08/2024
Received: 22/04/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (
Introduction
Acral melanoma (AM) is a different subtype of cutaneous melanoma (CM) that originates on the acral surfaces: palms, soles or subungual bed [1, 2]. Its incidence widely varies between different populations. In Caucasians, AM is a rare neoplasia, accounting for 1%–7% of all CM [3]. On the contrary, in countries in Asia and Latin America, AM is the most common subtype of CM. In Asia, it accounts for 47%–86%, while in Mexico it represents 44%, in Colombia 43.7%, and in Peru 35%–61.2% [4–9].
Unlike sun-exposed CM subtypes such as superficial spreading and lentiginous melanoma, AM is rarely related to a precursor nevi or ultraviolet involvement [10]. Furthermore, the Cancer Genome Atlas reported that AM has a much lower mutational burden and frequency of BRAF mutation frequency than other CM subtypes [11–13], which is associated with lower effectiveness of checkpoint inhibitors, such as nivolumab and/or pembrolizumab, compared with sun-exposed CM [14].
Globally, AM is underrepresented in most clinical trials due to the predominant inclusion of study populations of European descent (Caucasian). Furthermore, gene-environment interactions vary between populations, potentially influencing disease development and treatment response [15]. Studies focusing on AM in Latin American populations remain largely unexplored, as research has been conducted primarily in Asian countries. Furthermore, there is a scarcity of published data on AM. Therefore, this study aimed to evaluate the clinical features, response patterns and survival outcomes in a Peruvian cohort of advanced AM. Also, we examined the v-raf murine sarcoma viral oncogene homolog B1 (BRAF) status in our population.
Methods
Study population
This retrospective cohort study included all patients with AM diagnoses in five Peruvian national cancer centers from January 2013 to April 2019. The inclusion criteria were as follows: histopathological diagnosis of advanced AM, patients aged ≥18 years, Eastern Cooperative Oncology Group performance status (ECOG PS) 0-2, eligible to receive systemic immunotherapy (IO) for a first or subsequent line of treatment, and clinical history with complete clinical information and follow-up. Patients with a second neoplasm, prior treatment in other healthcare centers, untreated or uncontrolled brain or leptomeningeal metastasis, and insufficient material in skin tissue for next-generation sequencing (NGS) were excluded from the study.
In our cohort, patients could receive IO with nivolumab as the first or subsequent line of treatment (second or third line) according to the protocols established at our institutions in Peru. This means that both treatment-naïve patients and those who had undergone previous chemotherapy were eligible to receive IO with nivolumab.
Approval was obtained from the Institutional Review Board and Ethics Committee of five hospitals in Peru. Data were stored securely and anonymously, and the entire population was included using a census-type sampling approach.
Variables
The variables included were age, sex, location of the melanoma (palmar or plantar), ECOG PS, clinical stage according to the 8th edition of the American Joint Committee on Cancer, sentinel lymph node biopsy (SLNB), complete lymph node dissection (CLND), involvement of nodular lymphoma, adjuvant therapy, Breslow thickness, Clark level, ulceration, mitosis count, surgical margin, BRAF status, lactate dehydrogenase (LDH) levels, visceral metastases, treatment regimen, overall response rate (ORR), response to IO [complete response (CR); partial response (PR); stable disease (SD); progression of the disease (PD)], progression-free survival (PFS) and overall survival (OS).
Mutation analysis
Tumour biopsy samples were used for deoxyribonucleic acid (DNA) isolation. A mutational screen was performed, covering a set of 50 frequently mutated genes in solid tumours, using the NEBNext DirectTM cancer hotspot panel obtained from New England Biolabs (Ipswich, MA). After target enrichment, libraries were constructed and indexed according to the manufacturer’s instructions. Subsequently, the libraries were pooled and sequenced at the paired end on Illumina’s miSeq platform (Illumina, San Diego, CA).
Our analysis focuses primarily on the most frequently mutated gene, BRAF. All samples were confirmed for BRAF mutations using Sanger sequencing to ensure optimal sensitivity and specificity in our analysis. The BRAF amplicons were amplified by polymerase chain reaction, purified and subjected to bidirectional sequencing on an ABI3730xl DNA analyzer (Applied Biosystems, Foster City, CA). Chromatogram analysis was performed using Mutation Surveyor Software (Softgenetics, State College, PA).
Statistical methods
Statistical analyzes were performed with Stata/SE version 16.1 (StataCorp. 2019. Stata statistical software: Release 16. College Station, TX) for Windows 10 Pro x64 bits.
Descriptive statistics were used to assess baseline characteristics, presenting categorical variables in frequencies and percentages, and numerical variables in median and ranges. Kaplan-Meier analyzes were used to determine the OS of patients, and this method was selected to assess the survival function over time. Results are presented in months; all patients were followed for a minimum of 1 year.
Results
A total of 19 patients aged 18 and over from 5 hospitals in Peru were included: Hospital Nacional Edgardo Rebagliati Martins (Lima), Hospital Nacional Alberto Sabogal Sologuren (Lima), Hospital Carlos Alberto Segun Escobedo (Arequipa), Hospital Nacional Adolfo Guevara Velasco (Cusco) and Hospital Almanzor Aquinaga Asenjo (Chiclayo).
According to the baseline clinical characteristics, 94.7% of the patients exhibited ECOG PS 0-1, the plantar surface being the site most frequently affected (84.2%) followed by the palms (15.8%). All patients underwent primary tumour resection as the definitive treatment; however, approximately 50% underwent an SLNB. Furthermore, the most frequent stage was stage III with 68.4%, and 26.4% of those received adjuvant therapy (Table 1).
According to pathological and molecular characteristics at baseline, most cases exhibited a Breslow thickness of >4 mm and a Clark level of IV/V in 52% and 89.4% of cases, respectively. All patients presented ulceration and a high range of mitosis (≥ 1 mitosis/mm2). NGS analysis revealed the presence of the BRAF gene mutation in four cases (21.1%), all of which contained the V600E gene mutation (Table 2).
During follow-up, all patients experienced recurrent advanced disease, with 52.6% developing visceral metastasis. Patients with recurrent advanced AM received first-line treatment as follows: nivolumab 78% (n = 10), chemotherapy 21.1% (n = 6) and palliative radiotherapy 28.6% (n = 3). Overall, patients who received IO as the first or subsequent line had an ORR of 33.3% and those who received it as first-line therapy had an ORR of 40%. Among those who harbored the V600E mutation (n = 4), with two patients receiving IO, the ORR was 50%, compared to 44.4% in wild-type patients Table 3.
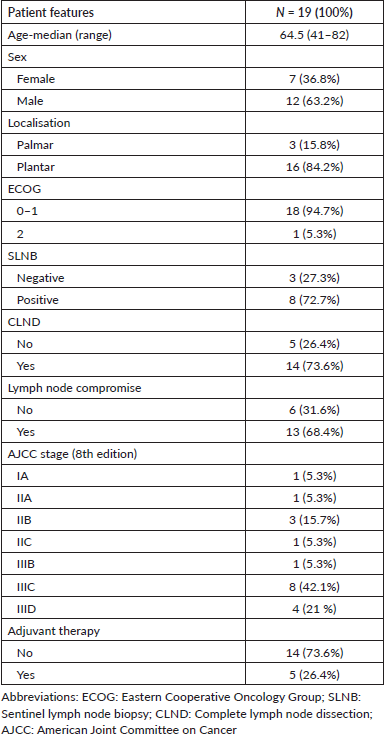
It was observed that as nivolumab use was introduced later, the progression rate increased (the progression rate in the first, second and third lines was 44.4%, 75% and 100%, respectively). Furthermore, the mortality rate was 31.5% for those who received nivolumab as a first-line treatment compared to those who received it as a subsequent-line treatment (second line: 50% and third line: 100%) Figure 1.
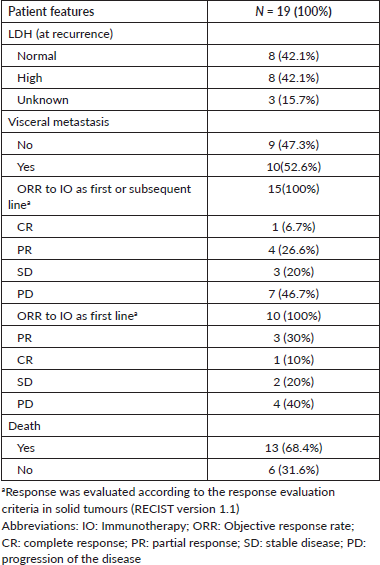
In general, the median OS of all patients was 55.5 months. The OS for patients with BRAF V600E mutation was 94.5 months, compared to 29.5 months for wild-type patients (Figure 2). No differences in survival were observed according to sex. The median OS in clinical stage II was longer than in stage III at diagnosis (56 months versus 46 months).
Table 1. Baseline clinical features of AM.
Discussion
In our study, we found that our population had poor clinico-pathological features, poor response to IO, and a prevalence of 21% of BRAF mutations. However, those who received IO as a first-line treatment or harbored the BRAF mutation appeared to have a slightly better response than wild-type patients.
Overall, our results confirmed that the plantar site was the most frequent location, similar to findings from previous studies conducted in Latin American and Asian populations [5, 16–18]. Additionally, our patients exhibited more advanced disease, and poorer pathological features. This aligns with other studies that found that Asian and Hispanic patients exhibited more advanced disease staging [5, 17, 18], thicker Breslow depth [7, 17, 18] and more ulcerative lesions [5, 7, 17, 18] compared to Caucasians [19].
In addition, we found that patients with advanced AM who received nivolumab in the first or subsequent line had a modest response, with an ORR of 33.3%. However, patients who received it as a first-line treatment achieved a higher ORR of 40%. This is similar to data published in the United States, which reported an ORR of 32% in first-line treatment with monotherapy using nivolumab or pembrolizumab, with a median OS of 31.7 months [20]. However, our results were higher than those of Asian studies from Japan and China, which reported ORRs ranging from 16.5% to 18.8% [21–23]. A larger study from Japan with 193 AM patients found that monotherapy with nivolumab or pembrolizumab achieved an ORR of 16.5% and a median OS of 18.1 months [22]. Nevertheless, a Japanese study showed a slightly improved result of 42.9% with a combination of anti-PD1 and anti-CTLA-4 in patients with AM [24]. This suggests that the efficacy of IO in AM might be influenced by ethnicity.
Moreover, our study revealed that patients treated with nivolumab as a front-line therapy exhibited superior outcomes compared to those treated in subsequent lines. Additionally, delayed initiation of correlated with increased rates of disease progression and mortality. These findings are consistent with existing literature, which shows the 2-year survival probability was higher with first-line therapy (0.5) compared to second-line (0.26), and third-line (0.14) therapy in advanced CM [25]. Thus, initiating IO in the first-line setting is recommended over subsequent lines, for both AM and sun-exposure CM.
Our main findings can be well explained by previous reports that found income, education, and social welfare might contribute to delayed diagnosis in these populations, leading to poorer clinical features and prognosis compared to Caucasian patients [26]. These differences between populations reflect the complex etiology of the disease, which includes the existence of different ethnic groups within these populations and the disparity in healthcare access [27].
Table 2. Baseline pathological and molecular features of AM.
Table 3. Clinical features and treatment outcomes at recurrent advanced AM.
Also, we found that the BRAF V600E mutation (21%) was present in the overall population. The frequency of BRAF mutations in AM is low and has been underreported compared to CM exposed to the sun, exhibiting differences between Caucasian, Asian and Latin American countries [28–34]. In Caucasian populations, the frequency of BRAF mutation in AM has been reported to range from 8% to 15% [20, 28, 29]. However, a recent and larger study from the United States determined a higher frequency of BRAF mutations (21.3%), which was similar to our findings. In Asia, the prevalence of the BRAF mutation varied between countries, with a frequency of 13.8%–15% reported in China [31, 35], Korea, 19.4% [32], and India, 31% [33]. On the other hand, Brazil reported a frequency of 31% BRAF V600E/V600K mutations among all AM cases [34]. The contrast in BRAF frequency among populations denotes clear ethnic and racial differences between Caucasian, Asian and Hispanic populations.
Interestingly, although the assessed population was small, patients with AM who harbored BRAF mutations showed slightly better results (ORR: 50%) compared to those with wild-type (ORR:44%), suggesting a potentially greater benefit from IO comparable to sun-exposed CM. In contrast to our results, a Japanese study reported a higher ORR in BRAF wild-type AM patients compared to those with BRAF mutations after anti PD-1 monotherapy (20.7% versus 8.1%; p = 0.04) [36]. Similarly, Shoushtari et al [20] in the United States found that patients with BRAF mutations did not respond to anti PD-1 monotherapy. However, a more recent study with a larger patient cohort, including AM (11.5%), found that the combination of anti-LAG3 and nivolumab had a better median PFS compared to nivolumab monotherapy (10.6 versus 4.6 months), regardless of BRAF mutation status [37].
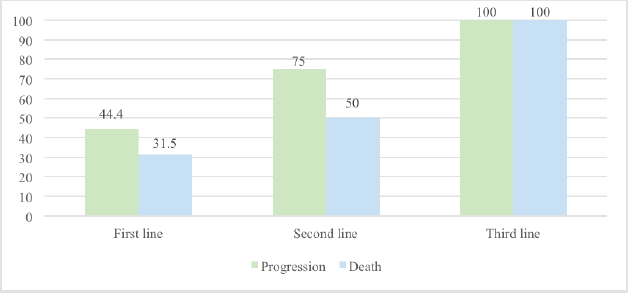
Figure 1. Progression and mortality rate in advanced AM based on the timing of IO introduction.
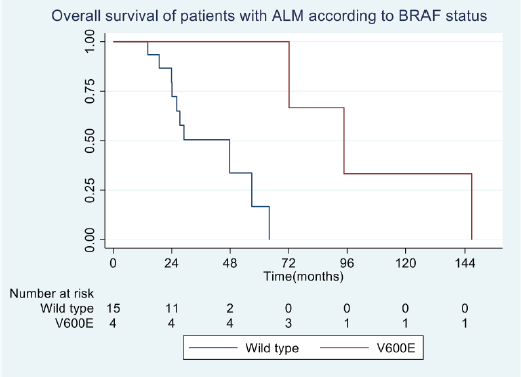
Figure 2. OS in patients with advanced AM according to BRAF status.
Our study had limitations. Specifically, it was a retrospective study with a small sample size of patients with AM. However, our aim is to improve the reliability and generalisability of our findings by incorporating more patients in future prospective cohort studies. The challenge of conducting analyses with small sample sizes is that they increase the risk of Type II errors, reducing statistical power to identify significant differences between groups or to perform subgroup analyses. Additionally, anti-BRAF therapy was not available in our institution for those with BRAF mutations, preventing us from testing its efficacy in our study population.
Additionally, our study provides valuable information and raises awareness about the influence of race/ethnicity, mutational status, timing of treatment delivery and efficacy in AM. More research should be conducted with a larger sample size in a multicenter study in Latin American countries.
In conclusion, our study showed that Peruvian patients with AM have poor clinico-pathological features, and limited response to IO. We found a BRAF mutation prevalence of 21% among our cohort. Also, patients treated IO as first-line therapy or those with BRAF mutations showed slightly better responses compared to wild-type patients. Nevertheless, there remains a critical need for a deeper understanding of this distinct disease. Collaborative efforts involving larger, comprehensive prospective studies across Latin American countries are crucial to fully elucidate the genetic, molecular, and ethnic factors influencing treatment patterns and survival outcomes in AM.
Acknowledgment
The authors thank the medical staff of each hospital for providing excellent care for our patients.
Conflicts of interest
None of the authors had personal, financial, commercial, or academic competing interests.
Funding
There was no financial support for this research.
Availability of data and materials
All data generated or analysed during this study are included in this article.
Informed consent
This study was carried out in accordance with the ethical standards of the responsible institution for human subjects, as well as with the Declaration of Helsinki.
Ethics approval and consent to participate
This study was carried out in accordance with the Declaration of Helsinki. The approval was obtained from the Institutional Review Board and Ethics Committee of each institution for the use of skin samples and data. Written informed consent was obtained from all participants. This data was securely and anonymously stored.
Consent for publication
The manuscript has not been submitted for publication or consideration elsewhere.
Author contributions
Conceptualisation: DC. Methodology: DC and VEFR. Initial manuscript draft: DC. Data analysis: VEFR. Interpretation: All authors. Writing – Review and editing: DC and VEFR. All authors read and approved the final version of the manuscript.
References
1. Arrington JH, Reed RJ, and Ichinose H, et al (1977) Plantar lentiginous melanoma: a distinctive variant of human cutaneous malignant melanoma Am J Surg Pathol 1(2) 131–143 https://doi.org/10.1097/00000478-197706000-00004 PMID: 602975
2. Nagore E, Pereda C, and Botella-Estrada R, et al (2009) Acral lentiginous melanoma presents distinct clinical profile with high cancer susceptibility Cancer Causes Control 20(1) 115–119 https://doi.org/10.1007/s10552-008-9221-y
3. Bradford PT, Goldstein AM, and McMaster ML, et al (2009) Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005 Arch Dermatol 145(4) 427–434 https://doi.org/10.1001/archdermatol.2008.609 PMID: 19380664 PMCID: 2735055
4. Kato T, Suetake T, and Tabata N, et al (1999) Epidemiology and prognosis of plantar melanoma in 62 Japanese patients over a 28-year period Int J Dermatol [Internet] 38(7) 515–519 Date accessed: 20/03/24 https://doi.org/10.1046/j.1365-4362.1999.00736.x PMID: 10440280
5. Chi Z, Li S, and Sheng X, et al (2011) Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: a study of 522 consecutive cases BMC Cancer 11 85 https://doi.org/10.1186/1471-2407-11-85 PMID: 21349197 PMCID: 3056833
6. Lino-Silva LS, Domínguez-Rodríguez JA, and Aguilar-Romero JM, et al (2016) Melanoma in Mexico: clinicopathologic features in a population with predominance of acral lentiginous subtype Ann Surg Oncol 23(13) 4189–4194 https://doi.org/10.1245/s10434-016-5394-x PMID: 27401447
7. Pozzobon F, Fierro E, and Acosta Á, et al (2013) Características del melanoma cutáneo primario en el Instituto Nacional de Cancerología 2006-2010 Rev Colomb Cancerol 17(3) 111–118 https://doi.org/10.1016/S0123-9015(13)70013-1
8. Coras N, Morales D, and Yabar A, et al (2013) Prognosis of melanoma in Peru: an analysis of 410 cases ASCO Meet Abstr [Internet] 31(15_suppl) e20023 [http://meeting.ascopubs.org/cgi/content/abstract/31/15_suppl/e20023]
9. Lozano-Espinoza N, Ramos W, and Galarza C, et al (2009) Melanoma cutáneo y mucoso: epidemiología, características clínicas y metástasis a distancia en un hospital de Lima-Perú. Periodo 1996-2007 Dermatología Peru [Internet] 19(4) 314–321 [http://sisbib.unmsm.edu.pe/BVRevistas/dermatologia/v19_n4/pdf/a04v19n4.pdf]
10. Goydos JS and Shoen SL (2016) Acral lentiginous melanoma Cancer Treat Res 167 321–329 https://doi.org/10.1007/978-3-319-22539-5_14
11. Hayward NK, Wilmott JS, and Waddell N, et al (2017) Whole-genome landscapes of major melanoma subtypes Nature 545(7653) 175–180 https://doi.org/10.1038/nature22071 PMID: 28467829
12. Schadendorf D, van Akkooi ACJ, and Berking C, et al (2018) Melanoma Lancet 392 971–984 https://doi.org/10.1016/S0140-6736(18)31559-9 PMID: 30238891
13. Furney SJ, Turajlic S, and Stamp G, et al (2014) The mutational burden of acral melanoma revealed by whole-genome sequencing and comparative analysis Pigment Cell Melanoma Res 27(5) 835–838 https://doi.org/10.1111/pcmr.12279 PMID: 24913711
14. Kaunitz GJ, Cottrell TR, and Lilo M, et al (2017) Melanoma subtypes demonstrate distinct PD-L1 expression profiles Lab Investig 97(9) 1063–1071 https://doi.org/10.1038/labinvest.2017.64 PMID: 28737763 PMCID: 5685163
15. Dimitriou F, Krattinger R, and Ramelyte E, et al (2018) The world of melanoma: epidemiologic, genetic, and anatomic differences of melanoma across the globe Curr Oncol Rep [Internet] 20(11) 87 Date accessed: 19/03/24 https://doi.org/10.1007/s11912-018-0732-8 PMID: 30250984
16. Gui J, Guo Z, and Wu D (2022) Clinical features, molecular pathology, and immune microenvironmental characteristics of acral melanoma J Transl Med [Internet] 20(1) 367 Date accessed: 12/07/24 https://doi.org/10.1186/s12967-022-03532-2 PMID: 35974375 PMCID: 9382740
17. Castaneda CA, Torres-Cabala C, and Castillo M, et al (2017) Tumor infiltrating lymphocytes in acral lentiginous melanoma: a study of a large cohort of cases from Latin America Clin Transl Oncol [Internet] 19(12) 1478–1488 Date accessed: 12/07/24 https://doi.org/10.1007/s12094-017-1685-3 PMID: 28577153
18. Huang K, Xu Y, and Gabriel EM, et al (2020) Comparative analysis of acral melanoma in Chinese and Caucasian patients J Skin Cancer [Internet] 2020 5169051 Date accessed: 12/07/24 https://doi.org/10.1155/2020/5169051 PMID: 33083061 PMCID: 7557897
19. Behbahani S, Malerba S, and Samie FH (2020) Acral lentiginous melanoma: clinicopathological characteristics and survival outcomes in the US National Cancer Database 2004-2016 Br J Dermatol [Internet] 183(5) 952–954 Date accessed: 12/07/24 https://doi.org/10.1111/bjd.19211 PMID: 32407556
20. Shoushtari AN, Munhoz RR, and Kuk D, et al (2016) The efficacy of anti-PD-1 agents in acral and mucosal melanoma Cancer 122(21) 3354–3362 https://doi.org/10.1002/cncr.30259 PMID: 27533633 PMCID: 5134420
21. Zhao L, Yang Y, and Ma B, et al (2019) Factors influencing the efficacy of anti-PD-1 therapy in Chinese patients with advanced melanoma J Oncol 2019 6454989 https://doi.org/10.1155/2019/6454989 PMID: 31662753 PMCID: 6791241
22. Nakamura Y, Namikawa K, and Yoshino K, et al (2019) Real-world efficacy of anti-PD-1 antibodies in advanced acral melanoma patients: a retrospective, multicenter study (JAMP study) J Clin Oncol 37(15_suppl) 9529 https://doi.org/10.1200/JCO.2019.37.15_suppl.9529
23. Maeda T, Yoshino K, and Nagai K, et al (2019) Efficacy of nivolumab monotherapy against acral lentiginous melanoma and mucosal melanoma in Asian patients Br J Dermatol 180 1230–1231 https://doi.org/10.1111/bjd.17434
24. Namikawa K, Kiyohara Y, and Takenouchi T, et al (2018) Efficacy and safety of nivolumab in combination with ipilimumab in Japanese patients with advanced melanoma: an open-label, single-arm, multicentre phase II study Eur J Cancer [Internet] 105 114–126 Date accessed: 19/03/24 https://doi.org/10.1016/j.ejca.2018.09.025 PMID: 30447539
25. O’Sullivan DE, Boyne DJ, and Gogna P, et al (2023) Understanding real-world treatment patterns and clinical outcomes among metastatic melanoma patients in Alberta, Canada Curr Oncol [Internet] 30(4) 4166–4176 Date accessed: 22/03/24 https://doi.org/10.3390/curroncol30040317 PMID: 37185430 PMCID: 10136717
26. Behbahani S, Malerba S, and Samie FH (2021) Racial and ethnic differences in the clinical presentation and outcomes of acral lentiginous melanoma Br J Dermatol [Internet] 184(1) 158–160 Date accessed: 12/07/24 https://doi.org/10.1111/bjd.19406 PMID: 32683697
27. Raval NS, Hodges WT, and Ugwu-Dike PO, et al (2022) Racial and socioeconomic differences in acral lentiginous melanoma outcomes: a surveillance, epidemiology, and end results analysis J Am Acad Dermatol [Internet] 87(4) 866 [/pmc/articles/PMC/] Date accessed: 12/07/24 https://doi.org/10.1016/j.jaad.2021.11.023 PMCID: 9925091
28. Saldanha G, Potter L, and DaForno P, et al (2006) Cutaneous melanoma subtypes show different BRAF and NRAS mutation frequencies Clin Cancer Res [Internet] 12(15) 4499–4505 Date accessed: 19/03/24 https://doi.org/10.1158/1078-0432.CCR-05-2447 PMID: 16899595
29. Lang J and MacKie RM (2005) Prevalence of exon 15 BRAF mutations in primary melanoma of the superficial spreading, nodular, acral, and lentigo maligna subtypes J Invest Dermatol 125(3) 575–579 https://doi.org/10.1111/j.0022-202X.2005.23833.x PMID: 16117801
30. Yeh I, Jorgenson E, and Shen L, et al (2019) Targeted genomic profiling of acral melanoma J Natl Cancer Inst 111(10) 1068–1077 https://doi.org/10.1093/jnci/djz005 PMID: 30657954 PMCID: 6792090
31. Si L, Kong Y, and Xu X, et al (2012) Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort Eur J Cancer 48(1) 94–100 https://doi.org/10.1016/j.ejca.2011.06.056
32. Hong JW, Lee S, and Kim DC, et al (2014) Prognostic and clinicopathologic associations of BRAF mutation in primary acral lentiginous melanoma in Korean patients: a preliminary study Ann Dermatol 26(2) 195–202 https://doi.org/10.5021/ad.2014.26.2.195 PMID: 24882974 PMCID: 4037672
33. Ahmad F, Avabhrath N, and Natarajan S, et al (2019) Molecular evaluation of BRAF V600 mutation and its association with clinicopathological characteristics: first findings from Indian malignant melanoma patients Cancer Genet [Internet] 231–232 46–53 [https://pubmed.ncbi.nlm.nih.gov/30803557/] Date accessed: 19/03/24 https://doi.org/10.1016/j.cancergen.2019.01.003
34. Fernandes M, Barcelos D, and Comodo AN, et al (2019) Acral lentiginous melanomas harbour intratumor heterogeneity in BRAF exon 15, with mutations distinct from V600E/V600K Am J Dermatopathol 41(10) 733–740 https://doi.org/10.1097/DAD.0000000000001418 PMID: 31021835
35. Niu HT, Zhou QM, and Wang F, et al (2013) Identification of anaplastic lymphoma kinase break points and oncogenic mutation profiles in acral/mucosal melanomas Pigment Cell Melanoma Res 26(5) 646–653 https://doi.org/10.1111/pcmr.12129 PMID: 23751074
36. Nakamura Y, Namikawa K, and Yoshino K, et al (2020) Anti-PD1 checkpoint inhibitor therapy in acral melanoma: a multicenter study of 193 Japanese patients Ann Oncol Off J Eur Soc Med Oncol [Internet] 31(9) 1198–1206 [https://pubmed.ncbi.nlm.nih.gov//] Date accessed: 19/03/24 https://doi.org/10.1016/j.annonc.2020.05.031 PMID: 32522691
37. Tawbi HA, Schadendorf D, and Lipson EJ, et al (2022) Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma N Engl J Med [Internet] 386(1) 24–34 [https://www.nejm.org/doi/full/10.1056/nejmoa2109970] Date accessed: 19/03/24 https://doi.org/10.1056/NEJMoa2109970 PMID: 34986285 PMCID: 9844513