Incidence, risk factors and the prognostic role of thromboembolic events (TEEs) amongst patients with metastatic pancreatic adenocarcinoma (PAAD): a retrospective, single-center analysis
Cícero Gonzaga Santos1, Francisco de Assis Maia Jr1, Marcos Pedro Guedes Camandaroba1 and Victor Hugo Fonseca de Jesus2,3,4
1Department of Medical Oncology, A.C. Camargo Cancer Center, São Paulo, SP 01509-010, Brazil
2Department of Medical Oncology, Centro de Pesquisas Oncológicas (CEPON), Florianópolis, SC 88034-000, Brazil
3Department of Gastrointestinal Medical Oncology, Oncoclínicas, Florianópolis, 88015-020, Brazil
4Post-Graduate School, A.C. Camargo Cancer Center, São Paulo, SP 01509-010, Brazil
Abstract
Background: Thromboembolic events (TEEs) are frequent among patients with pancreatic adenocarcinoma (PAAD). We set out to estimate the incidence and establish predictive risk factors for TEE and estimate the impact of TEEs on the overall survival (OS) of patients with metastatic PAAD.
Methods: This is a retrospective, single-center study. We included patients with a pathologically confirmed diagnosis of PAAD with distant metastases treated at AC Camargo Cancer Center from 2016 to 2021. We used the competitive risk survival models to estimate the cumulative incidence of TEE. Risk factors for the development of TEEs were evaluated using the competitive risk and logistic regression models. The impact of TEEs on OS was assessed using both landmark and time-dependent covariate Cox survival analyses.
Results: The study population consists of 199 patients. The cumulative incidence of TEEs in 1, 6 and 24 months were 10.1%, 19.3% and 30.2%, respectively. Log10(CA 19-9) was the only factor independently associated with increased risk of TEEs in the logistic regression (Odds ratio = 1.03; 95% confidence interval (95%CI), 1.00–1.06; p = 0.030) and competitive risk survival (Subdistribution hazard ratio = 1.14; 95%CI, 1.02–1.27; p = 0.019) models. In the landmark analysis, early TEEs (within 1 month of diagnosis) were not associated with inferior OS. In the time-dependent covariate Cox proportional hazard model, TEEs were not found to be statistically associated with inferior OS, although there was a trend towards it (Hazard ratio = 1.59; 95%CI, 0.99–2.54; p = 0.051).
Conclusion: TEEs occur in a large fraction of patients with metastatic PAAD. Statistical models with higher predictive performance are currently needed. For the time being, consideration for prophylactic anticoagulation should be done on an individual basis.
Keywords: thromboembolic, thrombosis, embolism, metastatic, pancreatic, cancer
Correspondence to: Victor Hugo Fonseca de Jesus
Email: victor.jesus@cepon.org.br
Published: 14/08/2024
Received: 06/04/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Pancreatic adenocarcinoma (PAAD) is the 6th most common cause of cancer-related death worldwide [1]. Its epidemiological burden is growing in both developed and developing countries [2, 3], and PAAD is expected to become the 2nd most common cause of cancer-related death in the USA by 2026 [4]. Indeed, among women, PAAD has already surpassed colorectal cancer as the 3rd most common cause of cancer-related death in the USA [5]. Therefore, there is a need for a deep understanding of the mechanism underpinning PAAD morbidity and mortality.
Thromboembolic events (TEEs) are one of the most common clinical complications among patients with PAAD. In contemporary series, TEEs have been shown to occur in 14%–35% of patients with PAAD [6–14], the highest incidence rate amongst all human malignancies [15]. These rates are even higher when we consider patients with metastatic disease [6, 10, 16]. The high frequency of TEEs amongst patients with metastatic PAAD has spurred the use of prophylactic anticoagulation amongst these patients. Indeed, several randomised clinical trials (RCTs) have shown a significant decrease in the incidence of TEEs for patients with solid tumours (including PAAD) undergoing prophylactic anticoagulation [17–21].
However, despite the significant burden of TEEs in patients with PAAD, autopsy series show that TEEs rarely are the underlying mechanism of death of patients with PA [22, 23], and this holds especially in recent years when better imaging techniques have become available to diagnose TEEs [23]. Perhaps, this minor role of TEEs in PAAD-associated mortality explains the disappointing overall survival (OS) outcomes of RCTs assessing the role of prophylactic anticoagulation for patients with metastatic PAAD [17, 18].
Therefore, the net benefit of prophylactic anticoagulation for patients with metastatic PAAD remains disputable. To better understand its potential usefulness, we set out to investigate the frequency and prognostic role of TEEs amongst patients with PAAD treated at the A.C. Camargo Cancer Center and also looked for potential risk factors for the development of TEEs in this population.
Methods
Study design
This is a retrospective, unicentric, descriptive and analytic study. We gathered routinely collected data from the electronic medical records of patients with metastatic PAAD. Due to its retrospective nature, the need for an informed consent term was waived. This study was approved by the A.C. Camargo Cancer Center Internal Ethics Committee (CAAE: 65909422.5.0000.5432).
Population
We included patients aged 18 years old and above with pathologically confirmed diagnosis of PAAD with radiological or pathological confirmation of metastatic disease from 1st January 2016 to 31st December 2021. We excluded patients who received chemotherapy or the best supportive care in other institutions and those with concurrent metastases from other solid tumours.
Variables
We collected demographic, clinical and pathological data from patients’ electronic medical records. We identified patients who experienced TEEs by searching clinical notes and imaging reports within patients’ electronic medical records. Thereafter, we collected details regarding TEEs (such as type of TEE (arterial versus venous), TEE site and symptoms at TEE diagnosis).
Procedures
At the diagnosis of metastatic disease, patients were staged using computed tomography (CT) and/or magnetic resonance imaging (MRI). Patients undergoing chemotherapy underwent routine assessment of chemotherapy activity with CT and/or MRI every 8–12 weeks. They were not screened for TEEs using other imaging modalities (such as color Doppler ultrasound) unless they were symptomatic.
Outcomes
The study’s primary outcome was the incidence of TEEs. TEEs were defined as any radiologically proven thrombus affecting the arterial, venous, or intracardiac compartments, with or without symptoms (incidental). We included TEEs that occurred one month before the diagnosis of metastatic PAAD and thereafter. OS was defined as the time from the diagnosis of metastatic disease to death or last follow-up visit. We considered that the TEE was the likely cause of death when this was the primary diagnostic hypothesis leading to the patient’s demise according to the treating physician’s impressions or review of the patient’s medical record [24]. Significant bleeding during anticoagulation was defined as a drop of 2 or more hemoglobin points secondary to bleeding [25]. A recurrent TEE was defined as a thrombosis and/or embolism affecting a previously uninvolved site or one that had (clinical or radiological) interval documentation of resolution [26].
Statistical analysis
We used absolute values and ratios to describe the distributions of categorical variables and median values and interquartile ranges (IQRs) to outline the distribution of numerical variables. We employed the competitive risk survival model (risk of TEE versus risk of death) to describe the cumulative incidence rates of TEEs [27]. Survival curves were generated using the Kaplan-Meier method and compared using the log-rank test. Median follow-up was calculated with the reverse Kaplan-Meier method. Patients without an event were censored at the last visit. Patients whose TEE was diagnosed up to a month before the detection of metastatic disease had the date of TEE set to the date of diagnosis of metastases. We generated univariate and multivariate logistic regression (with odds ratios (ORs)) and competing risk survival (with subdistribution hazard ratios (SHRs)) models to look for predictors of TEEs. We evaluated the performance of baseline cancer antigen 19-9 (CA 19-9) levels in predicting TEE using the receiver operating characteristic (ROC) curve (calculated through the closest topleft model with 95% confidence intervals generated with 2,000 bootstrap replicates). We assessed the prognostic role of early TEE (≤ 1 month from diagnosis of metastatic disease) in OS in a landmark analysis. We excluded patients who died in the first month after the diagnosis of metastatic disease and assessed the prognostic role of TEEs within the first month of diagnosis. Also, as TEE status can vary during patients’ disease course (from no TEE in metastatic disease to TEE during metastatic disease), we employed time-dependent covariate Cox proportional hazard models to look for the prognostic role of TEE. We assumed that Eastern Cooperative Oncology Group (ECOG) performance status, presence of liver metastasis, CA 19-9 levels, number of metastatic sites and TEE were time-dependent covariates. Here, we excluded patients with missing values of CA 19-9 (a constraint of the survival package for the time-dependent covariate Cox proportional hazard model). Variables with p-values <0.25 in the univariate analyses were used to build multivariate models [28]. A two-tailed p-value <0.05 was considered statistically significant. Statistical analyses were performed using the software project R version 4.3.2 (packages tidycmprsk and survival).
Results
Study population
We identified 297 patients with metastatic PAAD in our database search. We excluded 98 patients for the following reasons: diagnosis of metastatic disease before 1st January 2016 or after 31st December 2021 (34 patients), concomitant primary tumours (26 patients), nom-metastatic disease (18 patients), diagnosis of non-pancreatic peri-ampullary tumour (7 patients), treatment outside A.C. Camargo Cancer Center (5 patients) and other reasons (8 patients). Therefore, the study population consists of 199 patients – Supplementary Figure 1.
Table 1 describes the characteristics of the study population. Median age was 67 years (IQR: 58–73) and males and females were equally represented. Most patients had an ECOG performance status of 0 or 1 (N = 168; 84.5%) at the diagnosis of metastatic disease. Most patients presented with de novo metastases (N = 131; 65.8%) and liver metastases were present in 64.8% (N = 129) of the patients. The median CA 19-9 level was 540.0 UI/mL (IQR: 74.8–4995.8). Thirty-seven patients (18.6%) had a personal history of TEE and 55 patients (27.7%) were on anti-platelet drugs and/or anticoagulants at baseline. Most patients (N = 121; 60.8%) were classified as intermediate risk according to the Khorana risk score.
Table 1. Overall characteristics of the population.
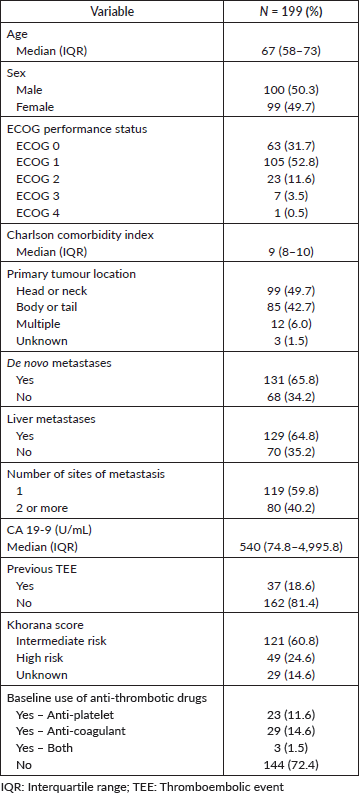
Table 2. Cumulative incidence of TEEs.
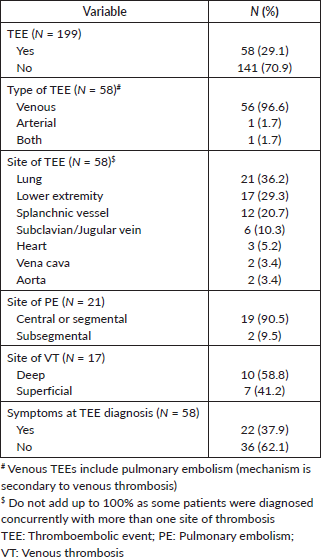
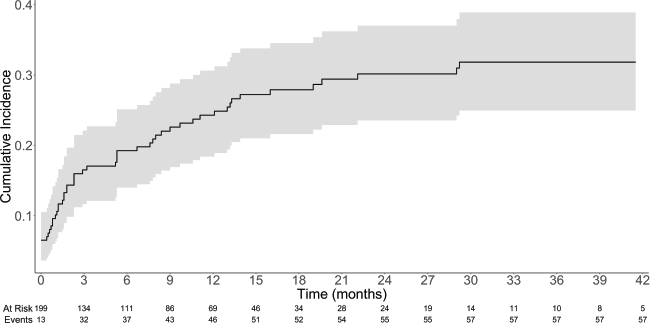
Figure 1. Cumulative incidence of TEEs with 95% confidence interval.
Characteristics of the TEEs
Overall, 58 patients (29.1%) developed TEEs. The median time from diagnosis of metastatic disease to TTE was 2.3 months (IQR: 0.53–9.53). The cumulative incidence rates of TEE in 1, 3, 6 and 12 months were 10.1%, 16.5%, 19.3% and 24.3%, respectively – Table 2 and Figure 1. Table 3 describes the characteristics of the TEEs. Pulmonary embolism (PE; N = 21; 36.2%) and lower extremity venous thrombosis (LEVT; N = 17; 29.3%) were the most frequent types of events. Most PE events (N = 19; 90.5%) were central or segmental and most LEVT events (N
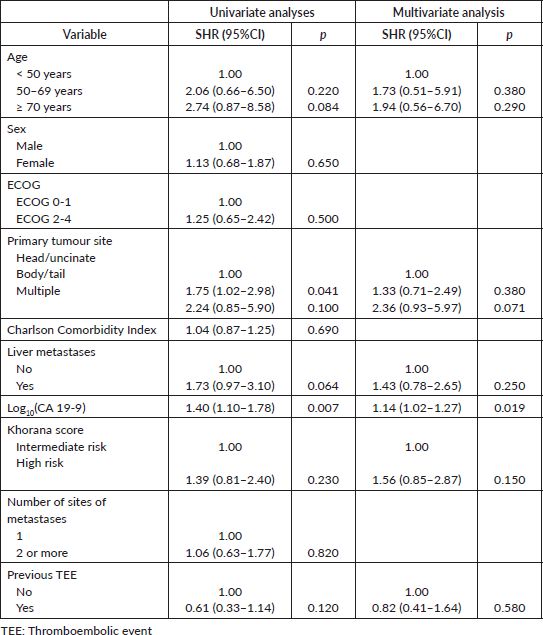
= 10; 58.8%) affected deep veins of the lower extremities. Most patients were asymptomatic at the diagnosis of TEEs (N = 36; 62.1%). Most TEE episodes occurred before (N = 17; 29.3%) or during (N = 20; 34.5%) first-line therapy – Supplementary Table 1. When TEEs occurred during chemotherapy, they most often occurred when patients were receiving FOLFIRINOX (N = 11; 19.0%) or gemcitabine plus nab-paclitaxel (N = 10; 17.2%) – Supplementary Table 2. In both the logistic regression (OR = 1.03; 95% confidence interval (95%CI), 1.00–1.06; p = 0.030) and competitive risk survival (SHR = 1.14; 95%CI, 1.02–1.27; p = 0.019) models, elevated log10(CA 19-9) levels were the only predictor of development of TEEs in the multivariate analyses – Supplementary Tables 3 and 4. Also, in the competitive risk survival analysis, multiple primary sites showed a trend toward higher risk of TEEs (SHR = 2.36; 95%CI, 0.93–5.97; p = 0.071). In logistic regression, CA 19-9 levels ≥757 UI/mL were associated with increased risk of TEE (AUC = 0.614; sensitivity = 0.593; specificity = 0.606) – Supplementary Figure 2.
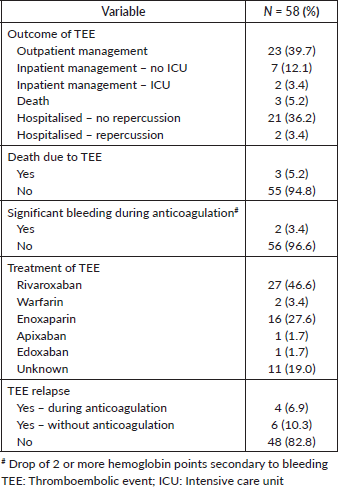
Outcomes of TEEs
Table 4 describes the clinical outcomes of the 58 patients diagnosed with TEEs. Most patients were managed in the outpatient setting (N = 23; 39.7%) or were admitted to the medical ward with no significant clinical repercussions from the TEE (N = 21; 36.2%). Three patients (5.2% of patients with TEEs and 1.5% of all patients) had TEEs as the likely cause of death. The most commonly used drugs for the management of the TEE were rivaroxaban (N = 27; 46.6%) and enoxaparin (N = 16; N = 27.6%). Two patients (3.4%) experienced significant bleeding after anticoagulation. New TEEs occurred in ten patients (17.2%), mostly after withdrawal of anticoagulation (N = 6; 10.3%).
Table 3. Characteristics of the TEEs.
Survival analysis
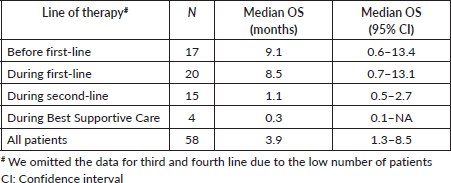
Median follow-up was 29.5 months (95%CI, 25.9–40.5). Forty-two patients (21.1%) were lost to follow-up. Median OS was 11.2 months (95%CI, 9.7–13.6)–Supplementary Figure 3. Median OS after the diagnosis of TEE was 3.9 months (95%CI, 1.3–8.5), ranging from 0.3 months in BSC to 9.1 months before first-line–Supplementary Table 5. There was no statistically significant difference between the OS times of patients with asymptomatic (5.6 months; 95%CI, 2.5–10.6) and symptomatic TEEs (1.9 months; 95%CI, 0.5–8.5), although there was a trend toward increased OS in the former group (p = 0.07). In a landmark survival analysis, liver metastases (HR = 1.67; 95%CI, 1.11–2.51; p = 0.014) and increased log10(CA 19-9) levels (HR = 1.09; 95%CI, 1.02–1.17; p = 0.013) were associated with worse OS, while TEE in the first month was not – Supplementary Table 6. Of note, among patients who died in the first month after the diagnosis of metastatic disease, five (31.2%) experienced TEEs before death.
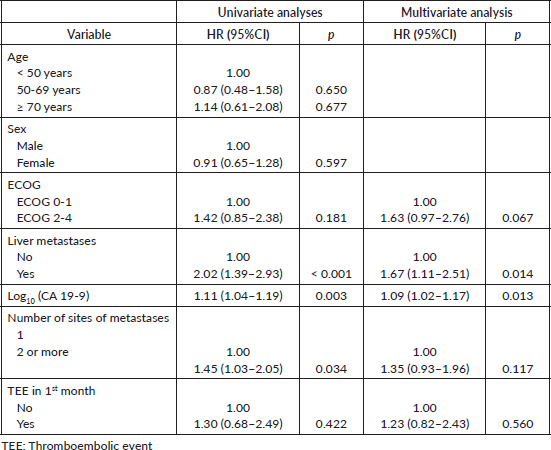
In time-dependent covariate Cox proportional hazard models, 35 patients were excluded due to missing values for the CA 19-9 either at diagnosis of metastatic disease or TEE. In the multivariate model, ECOG 2-4 (HR = 1.92; 95%CI, 1.19–3.10; p = 0.007), presence of liver metastasis (HR = 1.85; 95%CI, 1.21–2.83; p = 0.004) and increased log10(CA 19-9) levels (HR = 1.10; 95%CI, 1.02–1.18; p = 0.009) were associated with worse OS. TEE was not associated with OS in this analysis despite the p-value being close to the statistical significance threshold – Table 5.
Table 4. Outcomes and treatment characteristics of the TEEs.
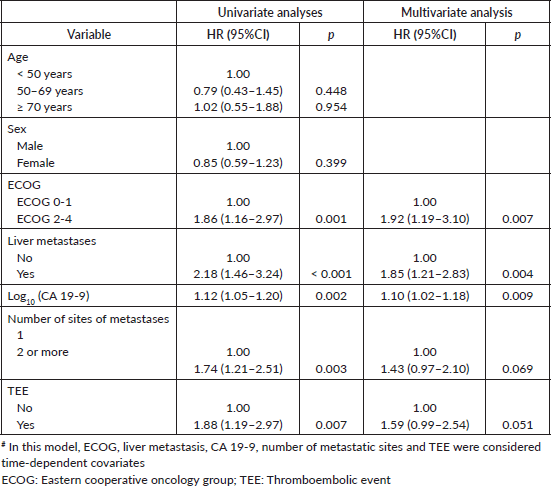
Table 5. Time-dependent covariate Cox proportional hazard models for OS# (N = 164).
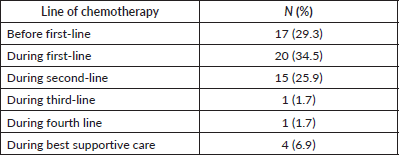
Discussion
In this study, we show that nearly one in three patients with metastatic PAAD will develop TEEs either at diagnosis or thereafter. Most TEEs will be asymptomatic and despite the high incidence of TEEs, they were rarely considered to be the cause of death in our cohort. In our time-dependent covariate Cox proportional hazard model, TEEs were associated with a trend toward inferior OS (not statistically significant). Importantly, the Khorana score was not able to identify patients with metastatic PAAD at the highest risk of developing TEE and only CA 19-9 levels were associated with increased risk of TEEs.
PAAD is associated with the highest rates of TEEs among all malignancies [15]. TEEs have been shown to occur in up to 57%–67% of patients with PAAD in early autopsy series [29, 30] and 14%–35% in contemporary clinical series [6–14]. These variable rates are secondary to differences in patient population (Asian patients experience TEE less often than Western ones) [31], definition of TEE (inclusion or not of arterial thrombotic events), treatment setting (chemotherapy is associated with increased risk of TEE) [9], duration of follow-up and composition of the population. Patients with metastatic PAAD have a 2.5–3.7 times higher risk of developing TEE [8, 16]. Therefore, we believe the relatively high incidence of TEEs found in this study can be explained by the inclusion of patients exclusively with metastatic disease, the use of a broad definition of TEE and the longer follow-up of patients from a Western population.
Given the high frequency of TEEs in patients with advanced PAAD, multiple studies have been undertaken to assess the role of prophylactic anticoagulation in this population. In the FRAGEM and the CONKO-004 studies, in addition to chemotherapy, patients with advanced PAAD
were given prophylactic dalteparin or enoxaparin, respectively [17, 18]. Both studies meet the primary endpoint of reduction in the incidence of TEEs. However, both failed to show improvements in OS for patients treated with prophylactic anticoagulation. While these studies were likely underpowered to evaluate differences in survival outcomes, other factors might explain the lack of OS benefit in favour of prophylactic anticoagulation. Patients with metastatic PAAD undergoing chemotherapy have their disease status checked every 8–12 weeks using CT or MRI. Recent studies suggest 50%–77% of all TEEs occurring in patients with PAAD are incidental [7, 9, 10, 14, 16, 32, 33], and CT and MRI are often able to identify such TEEs. Therefore, patients can be promptly started on therapeutic anticoagulants before they become symptomatic.
In our study, 62% of patients were asymptomatic at the onset of the TEE and these patients had a trend toward longer survival after TEE when compared to those with symptomatic TEE. While there is controversy as to whether incidental TEEs are associated with improved OS when compared to symptomatic episodes, treatment of TEE in the asymptomatic phase theoretically leads to a reduction in the number of deaths directly related to TEE. This might explain data from retrospective studies suggesting the risk of death as a direct consequence of a TEE in patients with PAAD is very low (0%–1.3%) [7, 9, 14, 33]. Accordingly, in the pre-specified analysis of patients with PAAD with high-risk Khorana scores from the CASSINI study, the risk of VTE-related death was only 0.7% in the up-to-date 180 observation period [21]. Our study shows similar findings, with 1.5% of patients dying as a direct consequence of TEE. Therefore, the risk of TEE-related death seems to be rather low in the contemporary management of patients with metastatic PAAD.
In our study, the risk of significant bleeding during therapeutic anticoagulation for patients with TEEs was relatively low at 3.4%. Nonetheless, other retrospective series suggest the risk of bleeding in patients with PAAD undergoing anticoagulation has been underappreciated and that rates of bleeding in clinical trials might underestimate the true incidence of this complication in this population [9, 10]. Indeed, in a large prospective series from Japan, PAAD patients had the highest risk of bleeding among six different tumour types, suggesting these patients might be more prone to bleeding complications [31].
Given that most patients with metastatic PAAD will not develop TEEs, that many of these episodes are asymptomatic, and that these patients might be at greater risk of bleeding, the use of prophylactic anticoagulation could be guided by risk algorithms. The Khorana score has been devised and validated in large cohorts of patients with cancer [34]. In brief, information on primary tumour site, body mass index (BMI), pre-chemotherapy platelet and leukocyte counts and hemoglobin level (or use of red blood cell growth factors) are used to assign patients to a three-tier risk classifier. By definition, all patients with PAAD are classified as having an intermediate or high risk of TEE. However, despite the wide acceptance of the Khorana score, multiple studies have failed to show that the Khorana score adequately predicts the risk of TEEs amongst patients with PAAD [13, 14, 16, 32]. Likewise, we failed to identify the Khorana score as a predictor of the risk of TEE amongst patients with metastatic PAAD. It has been suggested that in the presence of PAAD, many items of the Khorana score have poor predictive value [16]. Moreover, PAAD patients comprised <2% of patients in the original cohort used to develop the score and in the validation cohorts. Finally, one limitation of the Khorana score is that it has been devised to predict the risk of symptomatic TEEs. Indeed, limited data suggest the Khorana score might be accurate only in predicting symptomatic TEEs [13, 35]. Therefore, one cannot recommend the use of the Khorana score to predict the risk of TEEs in patients with metastatic PAAD.
Besides the presence of metastatic disease, multiple risk factors for the development of TEEs amongst patients with PAAD have been suggested. Treatment with chemotherapy, liver metastases [33, 36], previous TEE [31], previous anti-thrombotic treatment [37], obesity (BMI ≥30 kg/m2) [31, 37], non-O ABO blood type [37] and primary tumour site in body/tail [7, 16, 37] have all been associated with increased risk of TEE. In our study, the only variable independently associated with increased risk of TEE in both logistic regression and the competing risk survival models was higher baseline CA 19-9 level. Jeong et al [7] found among patients with advanced PAAD, those with CA 19-9 levels >1,000 UI/mL at baseline had a 2.7 times higher risk of TEE when compared with those with lower values. Given this cut-off is rather arbitrary, we set out to establish an optimal threshold value based on our logistic regression model. Disappointingly, in our study the performance of baseline CA 19-9 level as an isolated predictor of TEE was modest, suggesting other risk factors and/or the temporal evolution of this tumour marker should be taken into account. In the latter sense, Peippo et al [38] suggested that CA 19-9 doubling time could be used to identify those at greater risk of developing TEEs, as patients with TEE show shorter CA 19-9 doubling time than those who do not experience TEE (median: 2.7 versus 16.1 months; p = 0.026). One should highlight that while some studies have failed to identify elevated baseline CA 19-9 levels as risk factors for TEEs (perhaps due to issues related to study design) [16, 37], CA 19-9 is associated with thrombin generation in treatment-naïve patients [39] and is an important prognostic factor for patients with metastatic PAAD [40, 41]. Therefore, it seems reasonable to consider CA 19-9 levels in the decision-making process of whether or not to offer prophylactic anticoagulation to patients with metastatic PAAD.
Whether or not TEEs are associated with inferior OS amongst patients with advanced PAAD has been a matter of debate. In a recent meta-analysis, TEEs have been associated with inferior OS (HR = 1.38; p < 0.001) and disease-free survival (HR = 2.42; p < 0.001) [42]. Interestingly, this worse prognosis seems to be driven by the stronger prognostic impact of early (before therapy initiation) TEEs. In our study, we analysed the prognostic role of TEE in OS using a time-dependent covariate model to account for the fact that TEEs can occur at any time during the clinical evolution of patients with metastatic PAAD. We show that patients with TEEs had a trend toward worse OS (HR = 1.59; p = 0.051). Interestingly, in our landmark analysis excluding patients with early death (within 1 month of the diagnosis of metastatic PAAD), early TEEs were not associated with inferior OS, perhaps due to insufficient statistical power or because many of these patients were able to receive active chemotherapy regimens that changed the natural history of the disease. Alternatively, by excluding patients who died within 30 days of the diagnosis of metastatic disease, we might have selected patients with TEE with favourable characteristics, blurring the difference between the two treatment groups. In any case, our study ratifies the negative prognostic impact of TEEs in the disease course of patients with metastatic PAAD.
Our study has limitations. It is susceptible to biases related to its retrospective design and, as a unicentric investigation, we acknowledge our findings might not be immediately applicable to other populations. Also, the relatively modest sample size of this study possibly rendered it underpowered to establish the relationship between some predictive factors and the risk of TEE. Also, we were not able to find a clinically optimal CA 19-9 threshold that identifies patients with the highest risk of developing TEEs. Finally, as patients were not systematically submitted to an autopsy, we might have underestimated the true frequency of deaths secondary to TEE. However, our study has strengths. In our analysis, we included only patients with metastatic PAAD. Moreover, we described in detail the risk and natural history of patients experiencing TEEs. Finally, using statistically sound methods, we confirmed the prognostic role of TEEs in the clinical course of patients with metastatic PAAD.
Conclusion
Currently, international guidelines suggest prophylactic anticoagulation should be considered for patients at intermediate or high risk of TEEs [43, 44]. However, the slow acceptance of this recommendation translates into the reluctance from many clinicians to administer potentially harmful drugs to patients when RCTs have failed to show any OS benefit. Looking ahead, we should be able to design more accurate prediction models to determine the risk of TEE amongst patients with metastatic PAAD, perhaps using dynamic and molecular biomarkers. This way, we can optimize benefits while minimizing risks, discomfort and costs of prophylactic anticoagulation in this patient population. Until then, we believe a patient-centered discussion using the currently available risk factors for the development of TEEs and bleeding can be used to decide whether the benefits of prophylactic anticoagulation outweigh its potential harms for a given patient.
List of abbreviations
AUC, Area under the curve; BMI, Body mass index; CA 19-9, Cancer antigen 19-9; CT, Computed tomography; ECOG, Eastern Cooperative Oncology Group; HR, Hazard ratio; ICU, Intensive care unit; IQR, Interquartile range; LEVT, Lower extremity venous thrombosis; MRI, Magnetic resonance imaging; OR, Odds ratio; OS, Overall survival; PAAD, Pancreatic adenocarcinoma; PE, Pulmonary embolism; RCT, Randomised controlled trials; ROC, Receiver operating characteristic; SHR, Subdistribution hazard ratio; TEE, Thromboembolic event; VT, Venous thrombosis.
Conflicts of interest
The authors have no conflict of interest to declare.
Funding
This study had no funding source.
Author contributions
Cícero Gonzaga Santos: conceptualisation, data collection, writing and visualisation; Francisco de Assis Maia Jr: data collection, writing and visualisation; Marcos Pedro Guedes Camandaroba: conceptualisation, writing and visualisation; Victor Hugo Fonseca de Jesus: conceptualisation, statistical analysis, writing and visualisation.
References
1. Globocan Cancer today [https://gco.iarc.fr/today/] Date accessed: 10/02/24
2. Quante AS, Ming C, and Rottmann M, et al (2016) Projections of cancer incidence and cancer-related deaths in Germany by 2020 and 2030 Cancer Med 5(9) 2649–2656 https://doi.org/10.1002/cam4.767 PMID: 27356493 PMCID: 5055190
3. Barbosa IR, Santos CA, and Souza DLB (2018) Pancreatic cancer in Brazil: mortality trends and projections until 2029 Arq Gastroenterol 55 230–236 [https://www.scielo.br/j/ag/a/QhLS6XbnJqdXdmWN6XGNMtC/abstract/?lang=en] https://doi.org/10.1590/s0004-2803.201800000-59
4. Rahib L, Wehner MR, and Matrisian LM, et al (2021) Estimated projection of US cancer incidence and death to 2040 JAMA Netw Open 4(4) e214708 https://doi.org/10.1001/jamanetworkopen.2021.4708 PMID: 33825840 PMCID: 8027914
5. Siegel RL, Giaquinto AN, and Jemal A (2024) Cancer statistics, 2024 CA Cancer J Clin 74(1) 12–49 https://doi.org/10.3322/caac.21820 PMID: 38230766
6. Chan LL, Lam KY, and Lam DCM, et al (2023) Risks and impacts of thromboembolism in patients with pancreatic cancer Hong Kong Med J 29(5) 396–403 PMID: 37789507
7. Jeong HT, Bae JH, and Kim HG, et al (2023) Venous thromboembolism in patients with advanced pancreatic cancer receiving palliative chemotherapy: incidence and effect on prognosis Korean J Gastroenterol 81(3) 109–120 https://doi.org/10.4166/kjg.2022.137 PMID: 36960693
8. Laderman L, Sreekrishnanilayam K, and Pandey RK, et al (2023) Venous thromboembolism in metastatic pancreatic cancer Eur J Haematol 110(6) 706–714 https://doi.org/10.1111/ejh.13955 PMID: 36941225
9. Suzuki T, Hori R, and Takeuchi K, et al (2021) Venous thromboembolism in Japanese patients with pancreatic cancer Clin Appl Thromb Hemost 27 https://doi.org/10.1177/10760296211051766 PMID: 34730013 PMCID: 8573688
10. Hanna-Sawires RG, Groen JV, and Hamming A, et al (2021) Incidence, timing and risk factors of venous thromboembolic events in patients with pancreatic cancer Thromb Res 207 134–139 https://doi.org/10.1016/j.thromres.2021.08.002 PMID: 34628229
11. Riedl JM, Schwarzenbacher E, and Moik F, et al (2022) Patterns of thromboembolism in patients with advanced pancreatic cancer undergoing first-line chemotherapy with FOLFIRINOX or gemcitabine/nab-paclitaxel Thromb Haemost 122(4) 633–645 https://doi.org/10.1055/a-1548-4847
12. Berger AK, Singh HM, and Werft W, et al (2017) High prevalence of incidental and symptomatic venous thromboembolic events in patients with advanced pancreatic cancer under palliative chemotherapy: a retrospective cohort study Pancreatology 17(4) 629–634 https://doi.org/10.1016/j.pan.2017.04.012 PMID: 28462862
13. Muñoz Martín AJ, García Alfonso P, and Rupérez Blanco AB, et al (2014) Incidence of venous thromboembolism (VTE) in ambulatory pancreatic cancer patients receiving chemotherapy and analysis of Khorana’s predictive model Clin Transl Oncol 16(10) 927–930 https://doi.org/10.1007/s12094-014-1165-y PMID: 24643701
14. García Adrián S, González AR, and de Castro EM, et al (2022) Incidence, risk factors, and evolution of venous thromboembolic events in patients diagnosed with pancreatic carcinoma and treated with chemotherapy on an outpatient basis Eur J Intern Med 105 30–37 https://doi.org/10.1016/j.ejim.2022.07.020 PMID: 35931614
15. Horsted F, West J, and Grainge MJ (2012) Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis PLoS Med 9(7) e1001275 https://doi.org/10.1371/journal.pmed.1001275 PMID: 22859911 PMCID: 3409130
16. Frere C, Bournet B, and Gourgou S, et al (2020) Incidence of venous thromboembolism in patients with newly diagnosed pancreatic cancer and factors associated with outcomes Gastroenterology 158(5) 1346–1358.e4 https://doi.org/10.1053/j.gastro.2019.12.009
17. Pelzer U, Opitz B, and Deutschinoff G, et al (2015) Efficacy of prophylactic low-molecular weight heparin for ambulatory patients with advanced pancreatic cancer: outcomes from the CONKO-004 trial J Clin Oncol 33(18) 2028–2034 https://doi.org/10.1200/JCO.2014.55.1481 PMID: 25987694
18. Maraveyas A, Waters J, and Roy R, et al (2012) Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer Eur J Cancer 48(9) 1283–1292 https://doi.org/10.1016/j.ejca.2011.10.017
19. Agnelli G, Gussoni G, and Bianchini C, et al (2009) Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study Lancet Oncol 10(10) 943–949 https://doi.org/10.1016/S1470-2045(09)70232-3 PMID: 19726226
20. Agnelli G, George DJ, and Kakkar AK, et al (2012) Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer N Engl J Med 366(7) 601–609 https://doi.org/10.1056/NEJMoa1108898 PMID: 22335737
21. Vadhan-Raj S, McNamara MG, and Venerito M, et al (2020) Rivaroxaban thromboprophylaxis in ambulatory patients with pancreatic cancer: results from a pre-specified subgroup analysis of the randomized CASSINI study Cancer Med 9(17) 6196–6204 https://doi.org/10.1002/cam4.3269 PMID: 32663379 PMCID: 7476843
22. Douglass HO and Penetrante RB (1990) Pancreatic cancer. Why patients die Int J Pancreatol 7(1-3) 135–140 https://doi.org/10.1007/BF02924230 PMID: 2081919
23. Matsuda Y, Hagio M, and Naito Z, et al (2012) Clinicopathological features of 30 autopsy cases of pancreatic carcinoma J Nippon Med Sch 79(6) 459–467 https://doi.org/10.1272/jnms.79.459
24. Tritschler T, Kraaijpoel N, and Girard P, et al (2020) Definition of pulmonary embolism-related death and classification of the cause of death in venous thromboembolism studies: communication from the SSC of the ISTH J Thromb Haemost 18(6) 1495–1500 https://doi.org/10.1111/jth.14769 PMID: 32496023
25. Kaatz S, Ahmad D, and Spyropoulos AC, et al (2015) Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH J Thromb Haemost 13(11) 2119–2126 https://doi.org/10.1111/jth.13140
26. Heit JA, Lahr BD, and Petterson TM, et al (2011) Heparin and warfarin anticoagulation intensity as predictors of recurrence after deep vein thrombosis or pulmonary embolism: a population-based cohort study Blood 118(18) 4992–4999 https://doi.org/10.1182/blood-2011-05-357343 PMID: 21890644 PMCID: 3208304
27. Zhang Z (2017) Survival analysis in the presence of competing risks Ann Transl Med 5(3) 47 https://doi.org/10.21037/atm.2016.08.62 PMID: 28251126 PMCID: 5326634
28. Hosmer D, Lemeshow S, and May S (2008) Model development Applied Survival Analysis: Regression Modeling of Time-to-Event Data 2nd edn (Hoboken: Wiley Inter-Science) pp 132–168 https://doi.org/10.1002/9780470258019.ch5
29. Mikal S and Campbell AJ (1950) Carcinoma of the pancreas; diagnostic and operative criteria based on 100 consecutive autopsies Surgery 28(6) 963–969 PMID: 14787831
30. Ambrus JL, Ambrus CM, and Mink IB, et al (1975) Causes of death in cancer patients J Med 6(1) 61–64 PMID: 1056415
31. Okusaka T, Saiura A, and Shimada K, et al (2023) Incidence and risk factors for venous thromboembolism in the cancer-VTE registry pancreatic cancer subcohort J Gastroenterol 58(12) 1261–1271 https://doi.org/10.1007/s00535-023-02033-3 PMID: 37676492 PMCID: 10657787
32. van Es N, Franke VF, and Middeldorp S, et al (2017) The Khorana score for the prediction of venous thromboembolism in patients with pancreatic cancer Thromb Res 150 30–32 https://doi.org/10.1016/j.thromres.2016.12.013
33. Ishigaki K, Nakai Y, and Isayama H, et al (2017) Thromboembolisms in advanced pancreatic cancer: a retrospective analysis of 475 patients Pancreas 46(8) 1069–1075 https://doi.org/10.1097/MPA.0000000000000889 PMID: 28787333
34. Khorana AA, Kuderer NM, and Culakova E, et al (2008) Development and validation of a predictive model for chemotherapy-associated thrombosis Blood 111(10) 4902–4907 https://doi.org/10.1182/blood-2007-10-116327 PMID: 18216292 PMCID: 2384124
35. Price L, Nguyen M, and Picozzi V, et al (2010) Portal vein thrombosis in pancreatic cancer: natural history, risk factors, and implications for patient management Proceedings of the 7th Gastrointestinal Cancer Symposium (Orlando, 22–24 January 2010) – (Abstract 143)
36. Afsar CU, Gunaldi M, and Kum P, et al (2014) Pancreatic carcinoma, thrombosis and mean platelet volume: single center experience from the southeast region of Turkey Asian Pac J Cancer Prev 15(21) 9143–9146 https://doi.org/10.7314/APJCP.2014.15.21.9143 PMID: 25422192
37. Li D, Pise MN, and Overman MJ, et al (2015) ABO non-O type as a risk factor for thrombosis in patients with pancreatic cancer Cancer Med 4(11) 1651–1658 https://doi.org/10.1002/cam4.513 PMID: 26275671 PMCID: 4673991
38. Peippo MH, Kurki S, and Seppänen H, et al (2020) CA 19-9 doubling time in pancreatic cancer as a predictor of venous thromboembolism: a hospital database study Acta Oncol 59(2) 237–241 https://doi.org/10.1080/0284186X.2019.1679881
39. Mattila N, Hisada Y, and Przybyla B, et al (2021) Levels of the cancer biomarker CA 19-9 are associated with thrombin generation in plasma from treatment-naïve pancreatic cancer patients Thromb Res 199 21–31 https://doi.org/10.1016/j.thromres.2020.12.018 PMID: 33385797
40. Saad ED, Machado MC, and Wajsbrot D, et al (2002) Pretreatment CA 19-9 level as a prognostic factor in patients with advanced pancreatic cancer treated with gemcitabine Int J Gastrointest Cancer 32(1) 35–41 https://doi.org/10.1385/IJGC:32:1:35
41. Wasan HS, Springett GM, and Chodkiewicz C, et al (2009) CA 19-9 as a biomarker in advanced pancreatic cancer patients randomised to gemcitabine plus axitinib or gemcitabine alone Br J Cancer 101(7) 1162–1167 https://doi.org/10.1038/sj.bjc.6605243 PMID: 19724276 PMCID: 2768104
42. Su K, Duan R, and Wu Y (2024) Prognostic value of venous thromboembolism in patients with advanced pancreatic cancer: a systematic review and meta-analysis Front Oncol 14 1331706 https://doi.org/10.3389/fonc.2024.1331706 PMID: 38390258 PMCID: 10882063
43. National Comprehensive Cancer Network (NCCN) (2023) Cancer-Associated Venous Thromboembolic Disease [https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf] Date accessed: 01/04/24
44. Falanga A, Ay C, and Di Nisio M, et al (2023) Venous thromboembolism in cancer patients: ESMO clinical practice guideline Ann Oncol 34(5) 452–467 https://doi.org/10.1016/j.annonc.2022.12.014 PMID: 36638869
Supplementary
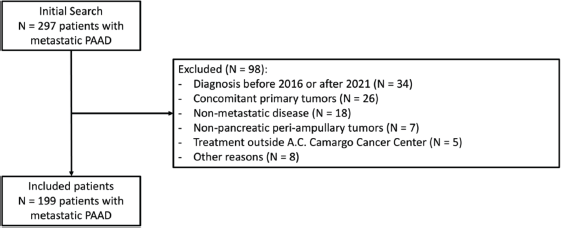
Supplementary Figure 1. Flow diagram of study population.
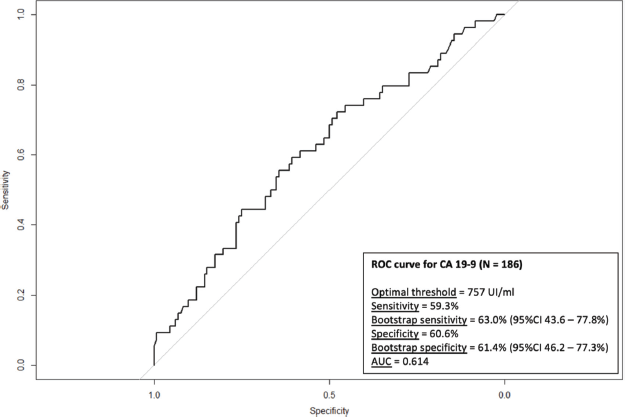
Supplementary Figure 2. ROC curve for the risk of TEE according to CA 19-9 levels.
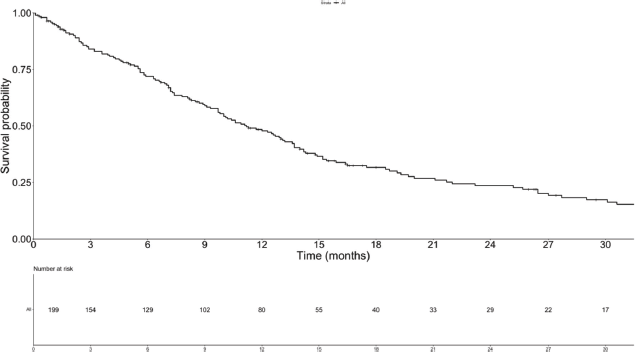
Supplementary Figure 3. OS of the study population.
Supplementary Table 1. Line of chemotherapy at the episode of TEE.
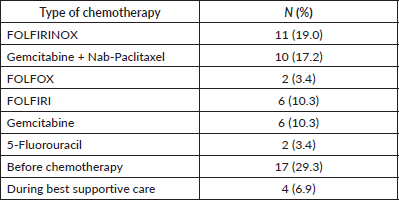
Supplementary Table 2. Type of chemotherapy at the episode of TEE.
Supplementary Table 3. OR for TEEs with previous TEE.
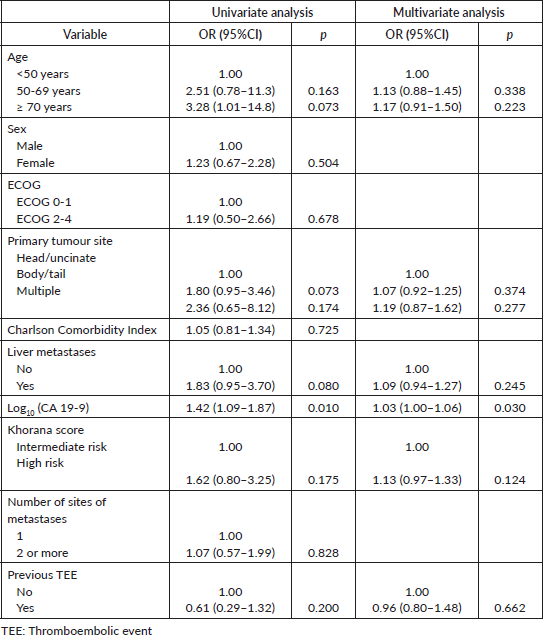
Supplementary Table 4. Sub-distribution hazard ratios (SHR) for TEEs.
Supplementary Table 5. OS after the diagnosis of TEE according to line of therapy.
Supplementary Table 6. Cox proportional hazard models for OS in landmark survival analysis.