Landscape of esophageal cancer in Northern Kenya: experience from Garissa Regional Cancer Center
Omar Abdihamid1†, Houda Abdourahman2†, Abdulsadiq Ibrahim1,Thinwa Kareu1, Abdullahi Hadi3, Abeid Omar4 and Miriam Mutebi5
1Garissa Regional Cancer Center, Garissa County Referral Hospital, PO Box 29-70100, Garissa, Kenya
2Department of Pathology, Hopital De Balbala Cheiko, Balbala, PO Box 669, Republic of Djibouti
3Department of Gastroenterology, University of Pretoria, PO Box Prinshof 349-Jr, Pretoria 0002, South Africa
4Kenyatta University Teaching Research and Referral Hospital, PO Box 7674 – 00100, GPO Nairobi, Kenya
5Department of Surgery, Aga Khan University, PO Box 30270-00100, Nairobi, Kenya
†Shared first authors
Abstract
Introduction: Esophageal cancer (EC) is the ninth most common cancer and the sixth leading cause of cancer deaths worldwide. More than 80% of cases and deaths from EC occur within developing countries. In Kenya, cancer is the second leading cause of non-communicable disease deaths, and the trend of cancer deaths is projected to increase as per the 2020 GLOBOCAN report showing 42,116 new cases annually with a mortality of 27,092 cases. EC is the leading cancer in men and the third most common in women in Kenya. The Garissa Regional Cancer Center (GRCC) is one of the three regional cancer centres in Kenya. Despite the rising EC incidence in the region, there is limited data about the clinicopathological features and treatment outcomes of EC, therefore, this is the first study to look at the landscape of EC in the northern Kenya region.
Methods: This was a retrospective study involving patients’ file review of confirmed EC cases diagnosed or treated at the GRCC from 2019 to 2023. Data collected from each patient’s chart included age, sex, risk factors, family history of EC, histological type, stage at diagnosis, treatment type and survival outcomes. For patients who were no longer in contact with the staff through clinic visits, the patients or their next of kin were contacted through phone calls for patients’ survival status. Data were collected and stored using the STATA software.
Results: Over the study period, 124 esophageal cases were identified, 64 (51.4%) were males and 60 (48.4%) were females with a mean age of 57.56 years. In terms of risk factors, hot beverage consumption was the highest (47 cases, 37.9%), followed by history of peptic ulcer disease (27 cases, 21.8%), smoking (8.9%) and gastresophageal reflux disease (2 cases, 1.6%). Stage of diagnosis at presentation was stage 1 (1 case, 0.8%), stage 2 (22 cases, 17.8%), stage 3 (25 cases, 20.2%), stage 4 (50 cases, 40.3%), not staged (26 cases, 21%). The majority had squamous cell carcinoma (SCC) (105 cases, 84.7%), followed by adenocarcinoma (5 cases, 4%), anaplastic (5 cases, 4%), SCC+ adenocarcinoma (1 case, 0.8%), unknown histology (8 cases, 3.2%). Nearly all patients had triple assessment (Endoscopy, histology and staging scans) accounting for 92 cases (74.2%), 24 cases (20%) had endoscopy+ histology only, and 8 cases (3.2%) had only imaging scans. In terms of family history of EC, 20 cases (16.1%) had a family history of EC.
Most of the patients were of ethnic Kenyan-Somali background (108 cases, Kenyan Somali, 87.1%) and majority were from Garissa County 96 cases (77.4%), 12 cases (9.7%) Wajir County, 12 cases (9.7%) from Tana River County and 4 cases (3.2%) from other counties. Many patients lacked health insurance (27 cases, 25.8%), while the majority paid out of pocket (92 cases,74.1%). Only 21% (26 cases) received chemotherapy alone, 5 cases (4%) got radiotherapy alone, 12.9% (16 cases) got chemoradiotherapy and a significant number of patients (77 cases, 62.1%) did not receive hospital-based cancer treatment.
Conclusion: This study is the first esophageal study at the GRCC and in northern Kenya in general. Our study confirmed the clinicopathological features of one of the most common cancers in Kenya and more so among Kenyan-Somalis.
The study also validates the predominance of histological subtypes of esophageal SCC with the late presentation, short survival and significant loss of follow-up. We recommend future EC studies employing a large prospective design with a large sample size to determine the impact of the new GRCC on the outcomes of EC patients and the local community.
Keywords: esophageal cancer, incidence, mortality, Garissa, Kenya
Correspondence to: Omar Abdihamid
Email: omaryhamidy@gmail.com
Published: 11/04/2024
Received: 10/10/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Esophageal cancer (EC) is the ninth most common cancer and the sixth leading cause of cancer deaths worldwide [1]. More than 80% of cases and deaths from EC occur within developing countries [2, 3]. The incidence of EC varies globally [4], with a higher incidence in areas such as Eastern Asia, South Central Asia, South Africa and Eastern Africa [3, 5]. Notably, EC continues to receive great attention due to its increasing incidence throughout the eastern Africa corridor, extending from Ethiopia to South Africa, heralding increasing number of collaborative and multicentre studies on the understanding the aetiologies of high EC incidences and the formation of working groups and consortiums such as African Esophageal Cancer Consortium [6] and the esophageal squamous cell carcinoma (ESCC) African PrEvention research [7]. This collaborative research has mainly employed case-control studies to investigate risk factors for ESCC such as hot beverages, oral hygiene and dental fluorosis, and alcohol [6–10].
In terms of diagnosis of EC, triple modalities such as endoscopic biopsy, histology confirmation and staging scans are the standard of care [11]. Emerging novel approaches to screening that may be cost-effective in EC endemic regions have been explored such as the use of Lugol’s iodine staining using endoscopy to detect precancerous lesions in the esophageal mucosa by Mwachiro et al [12] and the use of swallowable Cytosponge [13], a form of minimally invasive esophageal cytology sampling technique to improve early detection for this debilitating disease that presents at locally advanced stage in many EC hotspots in Africa [14–18].
Overall the standard diagnostic and treatment modality remains the use of multi-disciplinary evaluation comprising oncologists, surgeons, nutritionists and palliative care specialists among others [15]. The currently available treatment strategies for EC include surgery, chemotherapy, radiation therapy, molecular targeted therapy and immunotherapy therapy [16]. However, the prognosis remains poor, and the overall 5-year survival rate of less than 20% [17]. Ongoing prospective studies will shape guidelines-based management strategies for EC in the Eastern Africa region which could potentially be a benchmark for other low-and-middle-income countries plagued with the high incidence of ESCC [18]. Due to late-stage presentation, early initiation of nutrition intervention such as placement of a feeding tube and palliative care services including optimal pain control is vital in improving the overall survival and quality of life in EC patients [19–21].
In Kenya, cancer is the second leading cause of non-communicable disease deaths, and the trend of cancer deaths is projected to increase as per the 2020 GLOBOCAN report showing 42,116 new cases annually with a mortality of 27,092 cases [22]. The same GLOBOCAN data shows prostate and esophagus to be the leading cancers in men in Kenya, while breast, cervix and esophagus are the leading cancers in women, making EC the second most common cancer in males and the third most common in women [23].
There has been an upsurge of ESCC in Kenya over the years and is the most diagnosed cancer in both males and females and the leading cancer-related mortality in Kenya [6, 24–26]. Published studies regarding the epidemiological factors that may or may not drive the high incidence of ESCC in Kenya include poor diet and nutritional insufficiencies with high intake of red meat, use of alcohol and tobacco, environmental carcinogenic exposure, intake of hot beverages and genetic susceptibility citing a remarkable positive close family history of ECs [8, 27–30]. A study on geophagia, which is the intentional practice of consuming soil, especially during pregnancy is common along the African EC corridor, and its association with the development of EC has been investigated but did not appear to have a large effect on the overall risk of developing ESCC [31]. Similarly, a study from western Kenya by Menya et al [8] on the role of smoking and alcohol use especially traditional brews such as busaa and chang’aa showed a population attributable fraction for >2 drinks per day of 48%, contributing to half of the ESCC burden in Western Kenya. However, this concordance of alcohol intake with high incidences of ESCC is not seen among patients in northern Kenya where alcohol intake is almost non-contributory to ESCC incidence due to religious and cultural practices.
Challenges in cancer care in Kenya are multifold; patient education and poor health-seeking behaviour often contribute to late diagnosis, lack of awareness, poor uptake in cancer screening, diagnosis-related stigma, taboos, cultural barriers to seeking treatments, exorbitant cancer treatment costs, few oncology professionals and cancer care inequities remain main drivers of poor patient outcomes. The cost of cancer care creates a severe financial burden, catastrophic spending and assets liquidation, but the existing inequity in cancer care costs lives [32–34].
The Garissa Regional Cancer Center (GRCC) is affiliated with the Garissa County Referral Hospital in northern Kenya and was officially launched in February 2023, even though oncology services including chemotherapy and radiotherapy were offered from March 2022 [35]. Despite the availability of radiation and chemotherapy services at the GRCC, and rising ESCC incidence in Kenya, there is limited data about the clinicopathological features and treatment outcomes for this tumour type in this region. Therefore, this is the first study to look at the clinical pathological features of ESCC in the northern Kenya region.
Methods
This was a retrospective study involving patient files review of confirmed EC patients diagnosed or treated at the GRCC, a regional referral cancer centre from 2019 to 2023. Data collected on each patient’s chart included age, sex, risk factors, family history of EC, histological type, stage at diagnosis, treatment type and survival outcomes. For patients who were no longer in contact with the staff through clinic visits, the patients or their next of kin were contacted through phone calls for patients’ survival status. Data were collected and stored using the STATA software. As this was a descriptive retrospective chart review, ethics and review boards were exempted by the study institution.
Results
Over the study period, 124 esophageal cases were identified, 64 (51.4%) were males and 60 (48.4%) were females with a mean age of 57.56 years, citing nearly the same incidence of EC in terms of gender. In terms of risk factors, hot beverage consumption such as drinking hot tea was the highest (47 cases, 37.9%), followed by history of peptic ulcer disease (27 cases, 21.8%), smoking (8.9%), gastresophageal reflux disease (2 cases, 1.6%), unknown risk factors (7 cases, 5.6%) and 24.2% (30 cases) of the patients were lost to follow up with no identifiable risk factors. Alcohol intake as a risk factor for EC was captured in the patient files, in which all patients reported no history of alcohol intake, understandably due to religious and cultural practices.
Stage of diagnosis at presentation was stage 1 (1 case, 0.8%), stage 2 (22 cases, 17.8%), stage 3 (25 cases, 20.2%), stage 4 (50 cases, 40.3%), not staged (26 cases, 21%). The majority had squamous cell carcinoma (SCC) (105 cases, 84.7%), followed by adenocarcinoma (5 cases, 4%), anaplastic (5 cases, 4%), SCC+ adenocarcinoma (1 case, 0.8%), unknown histology (8 cases, 3.2%). In terms of diagnostic modality, nearly all patients especially following the launch of the new GRCC had triple assessment (Endoscopy, histology and staging scans) accounting for 92 cases (74.2%), 24 cases (20%) had endoscopy+ histology only, and 8 cases (3.2%) had only imaging scans. In terms of family history of EC, 20 cases (16.1%) had a family history of EC and 59 cases (47.6%) were lost to follow-up.
Given the homogenous ethnic demographic of northern Kenya, the majority of the patients were of ethnic Kenyan-Somali background (108 cases, Kenyan Somali, 87.1%), other tribes (7 cases, 5.6%) and lost to follow-up (9 cases, 7.3%). While most of the patients are from Garissa County, GRCC serves a huge catchment area of neighbouring counties, therefore, 96 cases (77.4%) were from Garissa County, 12 cases (9.7%) were from Wajir County, 12 cases (9.7%) from Tana River County and 4 cases (3.2%) from other counties.
Despite huge financial toxicity related to cancer costs in Kenya, many patients at the GRCC paid out of pocket for their treatment (92 cases,74.1%) while only (27 cases, 25.8%) paid through the National Hospital Insurance Fund (NHIF). Low uptake of the NHIF program is mainly due to a lack of education or awareness. There is a general lack of awareness of the existence and the potential benefits of the NHIF program among the public in northern Kenya. However, at the GRCC we are prospectively registering all new cancer patients for the NHIF program in order to improve awareness and uptake of all health insurance programs. Furthermore, fundraising from immediate family members, well-wishers and asset liquidation are common coping financial mechanisms (Table 1).
In terms of the treatment modalities and outcomes (Table 2), 21% (26 cases) received chemotherapy alone, 5 cases (4%) got radiotherapy alone, 12.9% (16 cases) got chemotherapy plus radiotherapy and a significant number of patients (77 cases, 62.1%) did not receive hospital-based cancer treatment. Of note, there were not any EC patients who underwent esophagectomy even after achieving pathological complete response following concurrent chemoradiation mainly due to a lack of or few qualified surgeons to perform such procedures at the GRCC or at other high-volume centres.
Owing to the advanced disease stage presentation, most patients present with cancer-related cachexia, and therefore, the rate of gastrostomy insertion for nutritional support among the EC patients was 23.4% (29 cases), while the stenting rate for relieving dysphagia was 2.4% (3 cases). Survival status was confirmed through phone calls to patients or their next of kin. 55 cases (44.4%) were lost to follow-up, 41.9% (52 cases) were reported to have died, and 17 patients (13.7%) were alive at the time of the study analysis. Overall, the 1–3 months survival rate was 16%, 3–6 months was 13%, 6–12 months survival was 18% and >12 months survival was 6%. Figures 1–6 show risk factors, family history of cancer, staging at diagnosis, histological sub-type, diagnostic modality and median survival in months, respectively.
Discussion
This is the first study that reviewed the clinicopathological patterns and presentations of EC patients and treatment outcomes at the GRCC, which is affiliated with the Garissa County Referral Hospital, Kenya. GRCC is the first cancer centre in the northern Kenya region in 60 years [36], heralding a huge milestone in the decentralisation of cancer in Kenya. The study finding also provides the first EC data specifically from the northern Kenya region that has a disproportionately high ESCC incidence [37], therefore, the findings are relevant and add to the much-needed data on the outcomes of EC in Kenya. Given, the lack of previous studies for comparison, and the homogenous Kenya Somali community in this region, this data is largely representative of both the ethnographic and epidemiological patterns of EC patients as well as their clinical outcomes.
Despite, the unique male predominance in EC in Africa [38, 39], our study showed a similar incidence of ESCC between males and females even in the absence of cigarette smoking and no alcohol use among both sexes. In our cohort, the majority of the ESCC patients were ethnic Kenyan Somalis (87%) from Garissa County (77%), followed by Wajir and Tana River counties at 9.8% and 10%, respectively, which correlates with the homogenous settlement of the ethnic Kenyan-Somali community in the northern Kenya region. This is similar to data from Somalia showing EC as the most common cancer with similar male and female EC incidence and late-stage disease presentation [40].
The highest identifiable risk factor for ESCC in our study was a history of hot beverage intake which accounted for 58%, followed by a history of peptic ulcer disease at 33%. However, a case-control study in Kenya on the association between hot beverages and ESCC concluded a lack of true association [7]. Despite recording a history of peptic ulcer disease as a documented risk factor for EC in our study cohort at 33%, the association of peptic ulcer disease with ESCC is rare according to the literature [41], and cannot confer a positive association. Therefore, a case-control study is urgently needed to ascertain the aetiology of the high incidence of ESCC in the northern Kenya region. A family history of ECs as a risk factor for EC was identified in 16% of patients. However, in the present study, we solely depended on ascertaining the status of positive family history for EC based on qualitative means (phone call) with the registered next of kin. This strategy is clearly less reliable and can be affected by several factors such as recall bias, literacy level and certainty of EC diagnosis by the family members. In the future, emerging EC strategies such as opportunistic endoscopy screening for accompanying family members during the diagnosis of EC of patients [42] and the use of risk-stratified endoscopy screening for precancerous lesions can be explored to improve early detection in patients with positive family history.
Table 1. Social demographics and clinicopathological characteristics of EC patients at the GRCC.
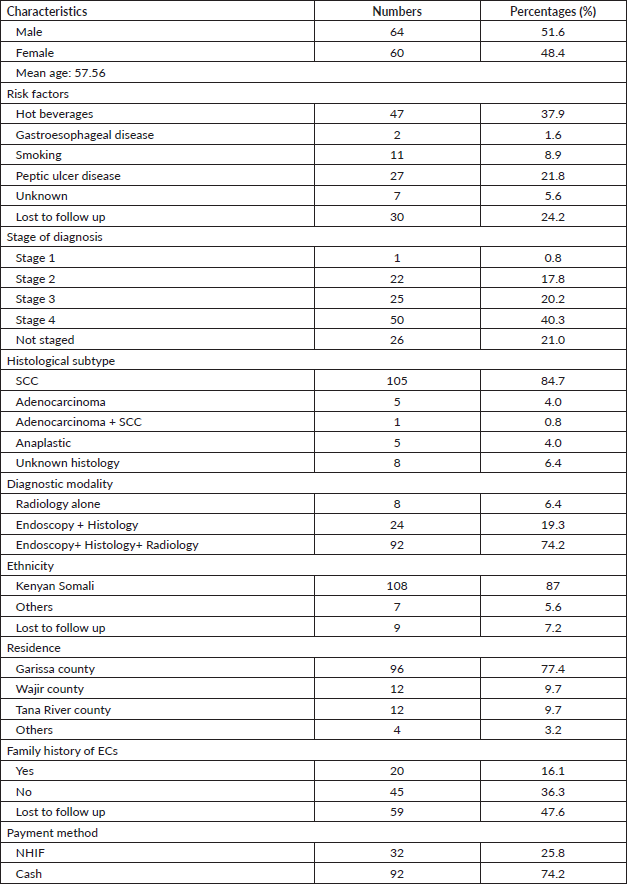
Table 2. Treatment types, supportive care and survival outcomes of EC patients.
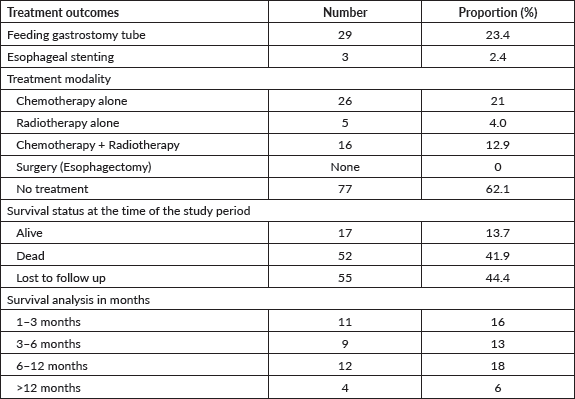
Figure 1. Risk factors of EC from patients’ files.
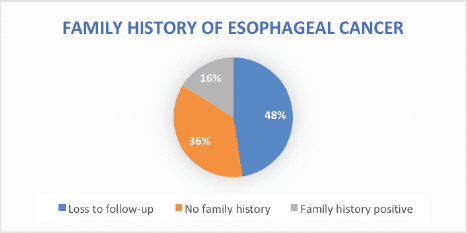
Figure 2. Family history of EC.
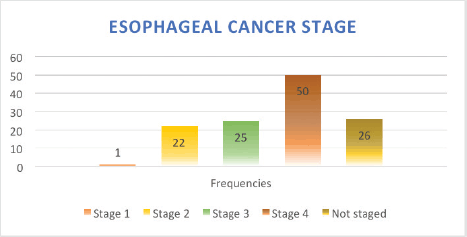
Figure 3. EC stage at presentation.
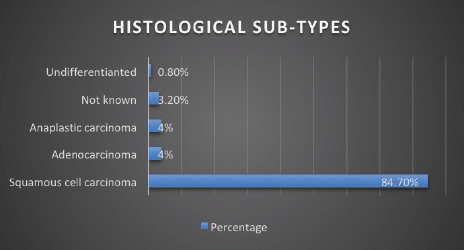
Figure 4. Histological subtype of EC.
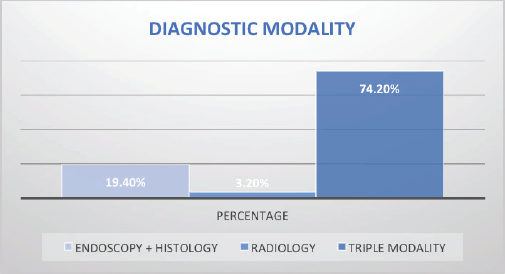
Figure 5. EC staging modality at diagnosis. Triple modality (Endoscopy, histology and CT scans).
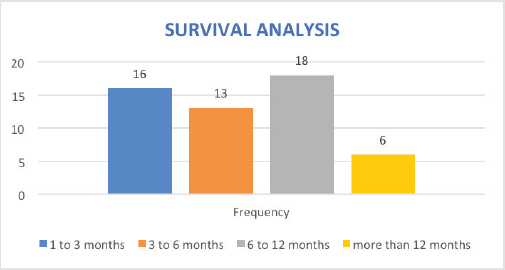
Figure 6. Overall survival analysis of EC patients in months.
Our data demonstrates ESCC as the most common histology representing over >80% of all cases, which is in keeping with other studies from East African countries including Kenya, Tanzania, Uganda [43–46] and South African countries [47] as well as incidences from China and Iran [48, 49]. More than 50% of the EC patients were in stage IV at presentation, data that is consistent with studies from regions with high prevalence of ESCC such as Ethiopia [50], Zambia, China [49] and India [51]. A significant 20% of the patients were not staged, owing to the limited diagnostic oncological services available before the launch of the new GRCC.
Accurate staging is critical in EC. Only 74% of our cohort had a conventional triple modality diagnosis (endoscopy, histology and staging scans), while others had staging scans alone or with histology alone. Furthermore, 21% of the patients in our study had no mode of cancer staging hence their stages were unknown, possibly due lack of cancer awareness and low literacy levels. Our findings are similar to the barriers to cancer diagnosis and treatment in other parts of Kenya. A study by Makau-Barasa et al [34] found the high cost of testing and treatment, low level of knowledge about cancer among the population and clinicians, poor health-seeking behaviours among the population, long distances to access diagnostic and treatment services, and few comprehensive cancer centres to be key barriers to improving early access to cancer diagnosis and treatment in Kenya [34]. However, progress has been made in recent years to improve access to cancer care, especially following the launch of three regional cancer centres and the launch of national action strategic plans for 2020–2030 [33].
The cost of cancer is a big determinant of cancer treatment compliance, and only 25.8% of our cohort had a form of health insurance, while 74% paid out of pocket for treatment, leading to catastrophic spending and financial toxicities. While cash payers were high in our cohort, family fundraisers, loans, patients’ assets liquidation and sponsorships are common financial coping mechanisms among cancer patients in Kenya. The low uptake of health insurance is mainly due to a lack of patient education, low health-seeking behaviour and a lack of incentives to enroll in such programs for patients in rural settings in Kenya. There is also a general lack of awareness of the existence, and the potential benefits of the NHIF program among the public in northern Kenya. However, the institution policy at the GRCC to prospectively register all new cancer patients for the NHIF program is currently implemented and is gradually improving awareness and uptake of the insurance programs.
Owing to the unavailability of cancer treatment services before the launch of GRCC, only 21% (26 cases) had palliative chemotherapy, 13% had chemoradiotherapy and 62% had no form of documented treatment and were lost to follow-up. Moreover, due to a lack of experienced surgical oncologists, esophagectomy is not routinely done even for patients who achieve a complete pathological response following neoadjuvant chemoradiotherapy. The low esophagectomy rate also highlights the broken linkages for referral for such patients to high-volume centres with expertise in doing esophagectomies. Due to late-stage presentation and resultant severe cachexia, 23.4% (29 cases) had a gastrostomy tube insertion for nutritional support before and during treatment and 2.4% (3 cases) had esophageal stenting done.
Survival analysis shows a significant number of our patients lost to follow-up (44%, 55 cases), indicating a poor follow-up pathway and dismal survivorship care. 42% (52 cases) were reported to have died at the time of study analysis, and only 14% (17 cases) were alive. While these data paint a dismal survival outcome, there is an overlap of patients’ data prior to the launch of the new GRCC which provides conventional cancer care and may have affected the final analysis. Also, since most patients were diagnosed at an advanced stage of cancer, the overall follow-up duration for median survival was too short, leading to the estimation of time-specific survival rates being less precise. Importantly, EC carries a high mortality in sub-Sahara Africa [19] with a study from Malawi showing a median time to death of 106 days (95% CI, 92 to 127) from diagnosis. Equally the 1, 2 and 3-year survival rates were 11% (95% CI, 8 to 15), 3% (95% CI, 1 to 6) and 0.9% (95% CI, 0.8 to 4), respectively [52]. We intend to carry out subsequent large sample-size studies that will reveal the survival outcomes of EC patients seen at the GRCC after receiving the currently available standard of care.
Strengths and limitations
The current study is the first in the northern Kenya region to highlight the general burden of EC among patients seen at the GRCC. The study revealed barriers and opportunities to explore across the continuum of care of EC patients. The study data will influence policy formulations prospectively at both levels of government in designing programs that will improve early access to cancer treatment. The study limitation is mainly a single-centre experience and the retrospective nature that draws recall bias and underrepresentation of patients lost to follow-up or those who sought care elsewhere, thus underestimating their clinical and treatment outcomes. Similarly, the overlap of patients’ data who did not get standard of care and those treated at the new GRCC, may dilute the true patterns of EC outcomes.
Conclusion
This study is the first esophageal study at the GRCC and in northern Kenya in general. Our study confirmed the clinicopathological features of one of the most common cancers in Kenya and more so among Kenyan-Somalis. The study also validates the predominance of histological subtypes of ESCC with the late presentation, short survival and significant loss of follow-up. Our data demonstrates the need for the county government of Garissa in collaboration with the National Cancer Control Program to prioritise EC on the national health agenda, including promoting preventative strategies, cost-effective early screening, early detection and timely treatment, and designing interventions to improve treatment adherence such as using appointment reminders via mobile phones, setting up an up-to-date cancer registry, and enhancing referral pathways.
We recommend future EC studies employing a large prospective design with a large sample size to determine the impact of the new GRCC on the outcomes of EC patients and the local community. Also, given the absence of well-established EC risk factors such as alcohol consumption and cigarette habits among this community, the geographical location, and the ethnic background of homogenous Kenyan Somalis in the region, a culturally based and individualised case-control studies that incorporate local context and lifestyle practices as well as environmental studies is urgently needed to elucidate the high incidences of ESCC in this region.
Conflicts of interest
The authors declare that they have no conflict of interest.
Funding
None to be declared.
References
1. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
2. Cheng ML, Zhang L, and Borok M, et al (2015) The incidence of esophageal cancer in Eastern Africa: identification of a new geographic hot spot? Cancer Epidemiol 39(2) 143–149 https://doi.org/10.1016/j.canep.2015.01.001 PMID: 25662402 PMCID: 4470609
3. Wong MCS, Hamilton W, and Whiteman DC, et al (2018) Global incidence and mortality of esophageal cancer and their correlation with socioeconomic indicators temporal patterns and trends in 41 countries Sci Rep 8(1) 4522 https://doi.org/10.1038/s41598-018-19819-8 PMID: 29540708 PMCID: 5852053
4. Kamangar F, Nasrollahzadeh D, and Safiri S, et al (2020) The global, regional, and national burden of esophageal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017 Lancet Gastroenterol Hepatol 5(6) 582–597 https://doi.org/10.1016/S2468-1253(20)30007-8
5. Ferlay J, Soerjomataram I, and Dikshit R, et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012 Int J Cancer 136(5) E359–E386 https://doi.org/10.1002/ijc.29210
6. Van Loon K, Mwachiro MM, and Abnet CC, et al (2018) The African esophageal cancer consortium: a call to action J Glob Oncol 4 1–9
7. Middleton DR, Menya D, and Kigen N, et al (2019) Hot beverages and esophageal cancer risk in western Kenya: findings from the ESCCAPE case-control study Int J Cancer 144(11) 2669–2676 https://doi.org/10.1002/ijc.32032 PMCID: 6519248
8. Menya D, Kigen N, and Oduor M, et al (2019) Traditional and commercial alcohols and esophageal cancer risk in Kenya Int J Cancer 144(3) 459–469 https://doi.org/10.1002/ijc.31804
9. Mmbaga BT, Mwasamwaja A, and Mushi G, et al (2021) Missing and decayed teeth, oral hygiene and dental staining in relation to esophageal cancer risk: ESCCAPE case-control study in Kilimanjaro, Tanzania Int J Cancer 148(10) 2416–2428 https://doi.org/10.1002/ijc.33433 PMCID: 8048942
10. Menya D, Maina SK, and Kibosia C, et al (2019) Dental fluorosis and oral health in the African esophageal cancer corridor: findings from the Kenya ESCCAPE case-control study and a pan-African perspective Int J Cancer 145(1) 99–109 https://doi.org/10.1002/ijc.32086 PMCID: 6519293
11. Waters JK and Reznik SI (2022) Update on management of squamous cell esophageal cancer Curr Oncol Rep 24(3) 375–385 https://doi.org/10.1007/s11912-021-01153-4 PMID: 35142974
12. Mwachiro MM and Dawsey SM (2021) Larger esophageal Lugol’s unstained lesions need more follow-up: size as an important predictor for risk of premalignant lesions Gastrointest Endosc 93(5) 1074–1076 https://doi.org/10.1016/j.gie.2020.11.010 PMID: 33875143 PMCID: 9831127
13. Middleton DRS, Mmbaga BT, and O’Donovan M, et al (2021) Minimally invasive esophageal sponge cytology sampling is feasible in a Tanzanian community setting Int J Cancer 148(5) 1208–1218 https://doi.org/10.1002/ijc.33366 PMCID: 7839767
14. Kayamba V (2019) Esophageal cancer hotspots in Africa Lancet Gastroenterol Hepatol 4(11) 818–820 https://doi.org/10.1016/S2468-1253(19)30253-5 PMID: 31609235
15. He S, Xu J, and Liu X, et al (2021) Advances and challenges in the treatment of esophageal cancer Acta Pharm Sin B 11(11) 3379–3392 https://doi.org/10.1016/j.apsb.2021.03.008 PMID: 34900524 PMCID: 8642427
16. Sheikh M, Roshandel G, and McCormack V, et al (2023) Current status and future prospects for esophageal cancer Cancers (Basel) 15(3) 765 https://doi.org/10.3390/cancers15030765 PMID: 36765722 PMCID: 9913274
17. Yang J, Liu X, and Cao S, et al (2020) Understanding esophageal cancer: the challenges and opportunities for the next decade Front Oncol 10 1727 https://doi.org/10.3389/fonc.2020.01727 PMID: 33014854 PMCID: 7511760
18. Buckle GC, Mrema A, and Mwachiro M, et al (2022) Treatment outcomes of esophageal cancer in Eastern Africa: protocol of a multi-center, prospective, observational, open cohort study BMC Cancer 22(1) 82 https://doi.org/10.1186/s12885-021-09124-5 PMID: 35045815 PMCID: 8772224
19. Asombang AW, Chishinga N, and Nkhoma A, et al (2019) Systematic review and meta-analysis of esophageal cancer in Africa: epidemiology, risk factors, management and outcomes World J Gastroenterol 25(31) 4512–4533 https://doi.org/10.3748/wjg.v25.i31.4512 PMID: 31496629 PMCID: 6710188
20. Mwachiro M, Parker R, and Lando J, et al (2022) Predictors of adverse events and early mortality after esophageal stent placement in a low resource setting: a series of 3823 patients in Kenya Endosc Int Open 10(4) E479–e487 https://doi.org/10.1055/a-1783-9829 PMID: 35433219 PMCID: 9010091
21. Birla R, Achim F, and Marica C, et al (2020) Palliation of dysphagia in patients with unresectable esophageal tumours – current methods and indications – a review Dig Med Res 3 https://doi.org/10.21037/dmr-20-67
22. IARC (2020) Kenya Source: Globocan 2020 (Lyon: IARC)
23. Ministry of Health, Kenya (2019) Kenya Cancer Policy-2019-2030 [
24. Menya D, Oduor M, and Kigen N, et al (2018) Cancer epidemiology fieldwork in a resource-limited setting: experience from the western Kenya ESCCAPE esophageal cancer case-control pilot study Cancer Epidemiol 57 45–52 https://doi.org/10.1016/j.canep.2018.09.006 PMID: 30300838 PMCID: 6289835
25. Odera JO, Odera E, and Githang’a J, et al (2017) Esophageal cancer in Kenya Am J Dig Dis (Madison) 4(3) 23–33 PMID: 29082268 PMCID: 5659304
26. Yang CS and Chen XL (2021) Research on esophageal cancer: with personal perspectives from studies in China and Kenya Int J Cancer 149(2) 264–276 https://doi.org/10.1002/ijc.33421 PMCID: 8141013
27. Maiyoh GK and Tuei VC (2019) Rising cancer incidence and role of the evolving diet in Kenya Nutr Cancer 71(4) 531–546 https://doi.org/10.1080/01635581.2018.1542010 PMID: 30638068
28. Mwachiro MM, Pritchett N, and Calafat AM, et al (2021) Indoor wood combustion, carcinogenic exposure and esophageal cancer in southwest Kenya Environ Int 152 106485 https://doi.org/10.1016/j.envint.2021.106485 PMID: 33689906 PMCID: 8832867
29. Mwachiro MM, Parker RK, and Pritchett NR, et al (2019) Investigating tea temperature and content as risk factors for esophageal cancer in an endemic region of Western Kenya: validation of a questionnaire and analysis of polycyclic aromatic hydrocarbon content Cancer Epidemiol 60 60–66 https://doi.org/10.1016/j.canep.2019.03.010 PMID: 30925281 PMCID: 6559237
30. Patel K, Mining S, and Wakhisi J, et al (2011) TP53 mutations, human papilloma virus DNA and inflammation markers in esophageal squamous cell carcinoma from the Rift Valley, a high-incidence area in Kenya BMC Res Notes 4 469 https://doi.org/10.1186/1756-0500-4-469 PMID: 22040862 PMCID: 3216406
31. Narh CT, Dzamalala CP, and Mmbaga BT, et al (2021) Geophagia and risk of squamous cell esophageal cancer in the African esophageal cancer corridor: findings from the ESCCAPE multicountry case-control studies Int J Cancer 149(6) 1274–1283 https://doi.org/10.1002/ijc.33688 PMID: 34004024 PMCID: 8411422
32. Abdihamid O (2022) Closing the gap in cancer care in Kenya in 2022 Lancet Oncol 23(6) 715–716 https://doi.org/10.1016/S1470-2045(22)00087-0 PMID: 35550268
33. Makau-Barasa LK, Greene S, and Othieno-Abinya NA, et al (2020) A review of Kenya’s cancer policies to improve access to cancer testing and treatment in the country Health Res Policy Syst 18(1) 2 https://doi.org/10.1186/s12961-019-0506-2 PMID: 31910868 PMCID: 6947983
34. Makau-Barasa LK, Greene SB, and Othieno-Abinya NA, et al (2018) Improving access to cancer testing and treatment in Kenya J Glob Oncol 4 1–8 PMID: 30241200 PMCID: 6180746
35. Abdihamid O (2022) Garissa Cancer Center: Inaugural Oncology Facility in Northern Kenya region in 60 Years (Berlin: The Cancer Community, Springer Nature)
36. Abdihamid O (2022) Garissa Cancer Center: Inaugural Oncology Facility in Northern Kenya region in 60 Years (Berlin: Springer Nature, Research Communications)
37. Abdihamid O (2022) The Upsurge of Esophageal Cancer in Ethnic Somali patients in Kenya: A Call to Action (Berlin: Springer Nature, Research Communications)
38. Kachala R (2010) Systematic review: epidemiology of esophageal cancer in Sub-Saharan Africa Malawi Med J 22(3) 65–70 https://doi.org/10.4314/mmj.v22i3.62190
39. Middleton DRS, Bouaoun L, and Hanisch R, et al (2018) Esophageal cancer male to female incidence ratios in Africa: a systematic review and meta-analysis of geographic, time and age trends Cancer Epidemiol 53 119–128 https://doi.org/10.1016/j.canep.2018.01.020 PMID: 29414631 PMCID: 5871654
40. Tahtabasi M, Mohamud Abdullahi I, and Kalayci M, et al (2020) Cancer incidence and distribution at a Tertiary Care Hospital in Somalia from 2017 to 2020: an initial report of 1306 cases Cancer Manag Res 12 8599–8611 https://doi.org/10.2147/CMAR.S277202 PMID: 33061565 PMCID: 7534047
41. Moses FM, Wong RK, and Peura DA (1983) Squamous cell carcinoma of the esophagus associated with peptic duodenal ulcer Am J Gastroenterol 78(11) 712–714 PMID: 6637959
42. Lando JO, Mwachiro MM, and Parker RK, et al (2022) Prevalence of esophageal squamous dysplasia in relatives of patients with esophageal cancer in Southwestern Kenya Cancer Epidemiol 78 102141 https://doi.org/10.1016/j.canep.2022.102141 PMID: 35299153
43. Patel K, Wakhisi J, and Mining S, et al (2013) Esophageal cancer, the topmost cancer at MTRH in the Rift Valley, Kenya, and its potential risk factors ISRN Oncol 2013 503249
44. Mmbaga EJ, Deardorff KV, and Mushi B, et al (2018) Characteristics of esophageal cancer cases in Tanzania J Glob Oncol 4 1–10
45. Obayo S, Mulumba Y, and Thompson CL, et al (2023) Clinicopathological characteristics and treatment outcomes of esophageal cancer patients in Uganda Ecancermedicalscience 17 1576 https://doi.org/10.3332/ecancer.2023.1576 PMID: 37533943 PMCID: 10393309
46. McHembe MD, Rambau PF, and Chalya PL, et al (2013) Endoscopic and clinicopathological patterns of esophageal cancer in Tanzania: experiences from two tertiary health institutions World J Surg Oncol 11(1) 257 https://doi.org/10.1186/1477-7819-11-257 PMID: 24094270 PMCID: 3850722
47. Asombang AW, Kasongo N, and Muyutu J, et al (2021) Descriptive analysis of esophageal cancer in Zambia using the cancer disease hospital database: young age, late stage at presentation Pan Afr Med J 39 12 PMID: 34394803 PMCID: 8348283
48. Aledavood A, Anvari K, and Sabouri G (2011) Esophageal cancer in Northeast of Iran Iran J Cancer Prev 4(3) 125–129 PMID: 26328051 PMCID: 4551295
49. Jiang YX, Zhang DW, and Chen Y, et al (2015) The characteristics of esophageal squamous cell carcinoma: an analysis of 1317 cases in southeastern China Contemp Oncol (Pozn) 19(2) 137–141 PMID: 26034392 PMCID: 4444447
50. Hassen HY, Teka MA, and Addisse A (2020) Survival status of esophageal cancer patients and its determinants in Ethiopia: a facility based retrospective cohort study Front Oncol 10 594342 https://doi.org/10.3389/fonc.2020.594342
51. Cherian JV, Sivaraman R, and Muthusamy AK, et al (2007) Carcinoma of the esophagus in Tamil Nadu (South India): 16-year trends from a tertiary center J Gastrointestin Liver Dis 16(3) 245–249 PMID: 17925916
52. Kaimila B, Chen Y, and Mulima G, et al (2023) Survival after diagnosis of esophageal squamous cell carcinoma in Malawi JCO Glob Oncol 9 e2300173 https://doi.org/10.1200/GO.23.00173 PMID: 37944090 PMCID: 10645405






