Gastric cancer in Sub-Saharan Africa – a systematic review of primary data
Anishka Ramadhar1, Phoebe N Miller2, Mazvita Muchengeti1,3, Juliana Kagura1, Kathryn Chu4 and Cameron Gaskill5
1Division of Epidemiology and Biostatistics, School of Public Health, University of the Witwatersrand, Johannesburg, South Africa
2University of California San Francisco, San Francisco, CA, USA
3National Cancer Registry, National Health Laboratory Service, Johannesburg, South Africa
4Stellenbosch University, Faculty of Medicine and Health Sciences, Cape Town, South Africa
5University of California Davis, Davis, CA, USA
Abstract
Introduction: Gastric cancer (GC) is the third leading cause of global cancer-related mortality. Despite the shifting burden of GC to low-and middle-income countries, the data regarding incidence, treatment, and outcomes in these settings are sparse. The primary aim of this systematic review was to aggregate all available data on GC in sub-Saharan Africa (SSA) to describe the variability in incidence across the region.
Methods: Studies reporting population-based primary data on GC in SSA were considered. The inclusion was limited to primary studies published between January 1995 and March 2022 which comprised of adult patients in SSA with GC. Studies without accessible full text in either French or English language were excluded. Unadjusted GC incidence rates with their standard errors for each study were recalculated from the crude numerators and denominators provided in individual studies.
Results: A total of 5,626 articles were identified in the initial search, of which, 69 studies were retained. Reported incidence rates ranged from a high of 5.56 GC cases per 100,000 in Greater Meru Kenya to a low of 0.04 GC cases per 100,000 people in Benin City Nigeria. The overall crude pooled incidence was 1.20 GC cases per 100, 000 (95%CI 1.15–1.26) with a variability of 99.83% (I2 p < 0.001). From the 29 high-quality population-based registry studies the crude pooled incidence was 1.71 GC cases per 100,000 people (95%CI 1.56–21.88) with a variability of 99.60%.
Conclusion: This systemic review demonstrates that GC incidence is highly variable across SSA. The limited data on GC treatment, mortality, and survival presents a significant challenge to providing a complete epidemiologic description of the burden of GC in SSA. There is a need for further robust data collection, exploration, and research studies on cancer care in SSA, with continued assessment of primary data availability.
Keywords: gastric cancer, adenocarcinoma, Sub-Saharan Africa, systematic review, incidence, epidemiology
Correspondence to: Anishka Ramadhar
Email: anishkaramadhar@gmail.com
Published: 07/03/2024
Received: 19/09/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Strengths and limitations of this study
Limitations
• The employed search strategy is open to the biases of the search engines available to authors, potentially missing non-indexed reports published in regional journals.
• Calculations in the study were limited by the availability of regional and population- based estimates that were truly reflective of a given study’s sample population.
• Sparse GC data are available for the SSA countries.
• Histological confirmation was reported in less than 50% of the studies which may have positively skewed the GC incidence. Histological confirmation is needed for accurate data reporting and scarcity of histological information could affect the epidemiology findings for GC incidence.
Strengths
• This systematic review (SR) included studies which comprised of only primary data.
• This study is the only SR on GC in SSA.
• This study provides the most stringent review of high-quality data available to date to better inform efforts to bolster regional data collection.
Introduction
Globally gastric cancer (GC) is the fifth most common malignancy with over 1,250,000 new cases diagnosed annually. GC causes over 950,000 deaths each year and ranks as the third leading contributor to global cancer-related mortality [2]. While historically, the burden of GC has been attributed to higher-income countries, the shifting burden of non-communicable disease now attributes over 80% of GC-related deaths to low- and middle-income countries (LMICs) [2, 3]. The World Health Organisation 2020 report on cancer estimated 22,992 incident GC cases within Sub-Saharan Africa (SSA), amounting to over 20,000 deaths per year [4].
Current epidemiologic data on the burden of GC in LMICs is sparse [5]. GC is a multifactorial disease, impacted by genetic and environmental factors that result in wide epidemiologic variation. Up to 20-fold differences in incidence rates have been reported between different geographic regions [8]. Accurate epidemiologic data has historically been dependent on population-based registries. In the absence of high-quality data, cancer incidence, and mortality have been reported by relying on mathematical estimates, which may not appreciate the regional heterogenicity of GC rates or may under-represent GC incidence [9]. This is especially true for SSA, where only 10% of the population is included in population-based cancer registry data collection [10]. Further knowledge of GC incidence, treatment, and outcome is necessary for adequate treatment and public health planning.
The primary aim of this systematic review (SR) is to aggregate all available data on GC in SSA to describe the incidence rates and rate variability of GC in adults in SSA countries. Secondary aims include describing the treatment and mortality of GC in adults from SSA. The hypothesis is that GC is highly variable across the SSA region and this analysis has used primary studies to demonstrate this. The importance of GC variability indicates that the flow of risk factors to disease outcome differs by location. This is critical for public health planning which indicates that treatment and management approaches need to be customised to the variable GC situation at each location. This analysis will allow for a better understanding of the GC disease burden in SSA.
Methods
Eligibility criteria
Studies reporting population-based primary data on in SSA were considered. Country inclusion was in accordance with The World Bank definition of SSA. Primary data published between January 1995 and March 2022 which included adult patients in SSA with GC were included. Studies without accessible full text in either French or English language were excluded. All attempts to access texts and data were made, including contacting corresponding authors of publications. There was no involvement from any patients or the public in this SR.
Information sources, search strategy and study selection
The terms ‘GC’ OR ‘stomach cancer’ OR ‘gastric carcinoma’ OR ‘cancer of the stomach’ OR ‘stomach adenocarcinoma’ were queried when they appeared in the title, abstract or keyword of studies. The names of the SSA countries were applied without language restrictions. A full search strategy is included in Appendix 1. Published studies were identified through a comprehensive search of the following electronic databases; Web of Science, Embase, PubMed and Google Scholar. Duplicate studies were identified and removed. Abstracts were then screened by three authors (AR, PM, CG) to assess eligibility using the predetermined inclusion criteria. Full-text articles were then accessed and reviewed in detail to confirm appropriate inclusion and to extract relevant data. Additional citations were culled and included from the references of articles identified in the initial search. Citations were tracked using Zotero (6.0.8).
Publication bias and heterogeneity
Risk of bias was limited by maintaining a wide search criterion across multiple high quality electronic databases with a diversity of indexed articles.
Data extraction
Data collection included the following variables: surname of primary author, publication year, the country where the study was conducted, the study design, diagnostic method, the age group (age range including >18 years old), sample size, the number of GC reported, the overall cancer case number for the study population, the overall population size, regional or national population, differences by age or gender, treatment type, and the case fatality rate of GC in the study. Unadjusted GC incidence rates with their standard errors for each study were recalculated from the crude numerators and denominators provided in individual studies. Where denominators were not available regional and national population sizes were identified using a variety of sources such as macrotends.net, worldbank.org and countryeconomy.com [22–24]. The lowest population size value was used after verifying the values between various sources. Sub-group break downs (age, gender, race/ethnicity) were included. Age standardised rates were recorded without attempts to calculate backwards. Overall mortality and treatment strategies were recorded in crude numbers and percentages. To achieve a high level of reliability, three reviewers (AR, PM, CG) assessed the same articles and reconciled differences before adopting a final and complete data collection document.
Quality criteria
The strengthening reporting of observational studies in epidemiology (STROBE) checklist was used to assess the quality of the included studies. The final studies included in this SR were assessed using the STROBE checklist and ranked on the following criteria: (1) Inclusion of study in Cancer in Five Continents according to the International Agency for Research on Cancer (IARC) or the data was from a population-based registry [25] (2) 100% histologic confirmation of cancer from a regional registry or national registry (3) >80% histologic confirmation of cancer from a hospital registry (4) histologic confirmation of cancer from any type of registry. The highest rank went to papers included in ‘Cancer in Five Continents’ and regional registries. The second highest rank included regionally or nationally representative studies that were >80% histologically confirmed. The third-ranking category included hospital or pathology registries that showed histological confirmation. The final ranking category included registries that were not histologically confirmed. The quality of studies was given a rank from one to four.
Data synthesis and analysis
Unadjusted incidence with their standard errors for each study was recalculated based on the information of crude numerators and either denominators provided by individual studies or the regional population sizes. Regional and national population sizes were identified within the paper and when not reported, they were identified using online sources such as macrotends.net, worldbank.org and countryeconomy.com. Incidence rate patterns of GC across the various nations in SSA after recalculation were summarised. Descriptive analysis was done for the GC treatment and mortality studies. All data was stored in excel (Microsoft Inc, Redmond, WA, USA) analyses were conducted using StataSE (version 15.1, College Station, Texas, USA).
Results
The database search returned a total of 5,626 articles including primary studies, case reports and reviews. Papers were collected from PubMed (694), Web of Science (4,887), and Google Scholar (45). After duplicates were removed there were 667 unique article titles left. Once the initial screening for titles including incidence of cancer in SSA was complete, 132 studies remained. From the 132 remaining studies, 14 papers were excluded by abstract alone, and the remaining 123 full-text articles were assessed for eligibility. References were searched but did not reveal additional relevant titles. After applying the selection criteria, 69 studies were finally retained in this review (Supplementary Figure S1).
From the 69 retained studies, we identified that the data collection was conducted across 50 unique study sites (regions or cities) and 23 countries. Most studies were conducted in East Africa [32] with Uganda appearing nine times across six unique locations, Zimbabwe appearing six times in both Harare and Bulawayo and Malawi appearing five times in Blantyre and Lilongwe (Supplementary Figure S2). West Africa had the second most studies [25]; Nigeria produced a total of nine studies across seven separate locations. East Africa and West Africa contributed 43% and 34% of the included studies respectively. North Africa provided studies from Sudan and South Africa provided four studies from Durban and the Eastern Cape (Table 1). Namibia, Botswana, South Sudan, Central African Republic, Chad, Niger and Mauritania are the SSA countries with missing primary GC data (Supplementary Figure S2).
Data was primarily extracted from regional registries, which accounted for 42% (N = 31) of the study sites, followed by retrospective reviews of hospital records (40%, N = 29). The remaining data were recorded from national registries (9%, N = 7) or retrospective reviews of pathology department data (8%, N = 6). Of the studies reviewed, 32% (N = 24) contained >90% histologically confirmed GC cases. A grading system was implemented to assess the quality of included studies: only 8% (N = 6) were from national registries and therefore ranked as the highest quality studies. Most studies (56%, N = 42) were from regional or national registries with <80% of the GC cases being histologically confirmed (Table 1).
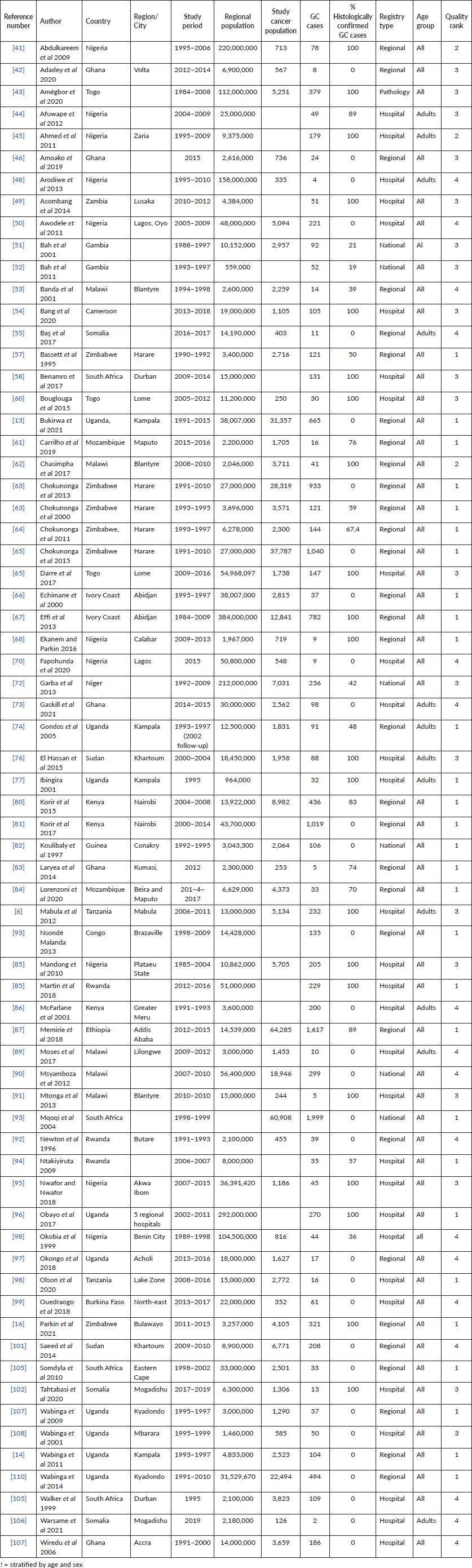
From the 69 registry-based primary data sets, the overall crude pooled incidence was 1.20 GC cases per 100,000 people (CI 1.15–1.26). The variability between incidence calculation was 99.83% (I2 p < 0.001) (Figure 1). From the 29 high-quality population-based registry studies the crude pooled incidence was 1.71 GC cases per 100,000 people (95%CI 1.56–21.88) and the variability between studies was 99.60% (Figure 2). The GC incidence variability for West Africa I2 = 99.62 (p = 0.00), Southern Africa I2 = 99.82 (p = 0.00) and East Africa I2 = 99.88 (p = 0.00) indicates significant inter- and intra-regional variability within SSA (Figure 1). The study site with the highest incidence was Greater Meru in Kenya with an incidence of 5.56 GC cases per 100,000 people. The study site with the lowest incidence was 0.04 GC cases per 100,000 people in Benin City of Nigeria (Figure 1).
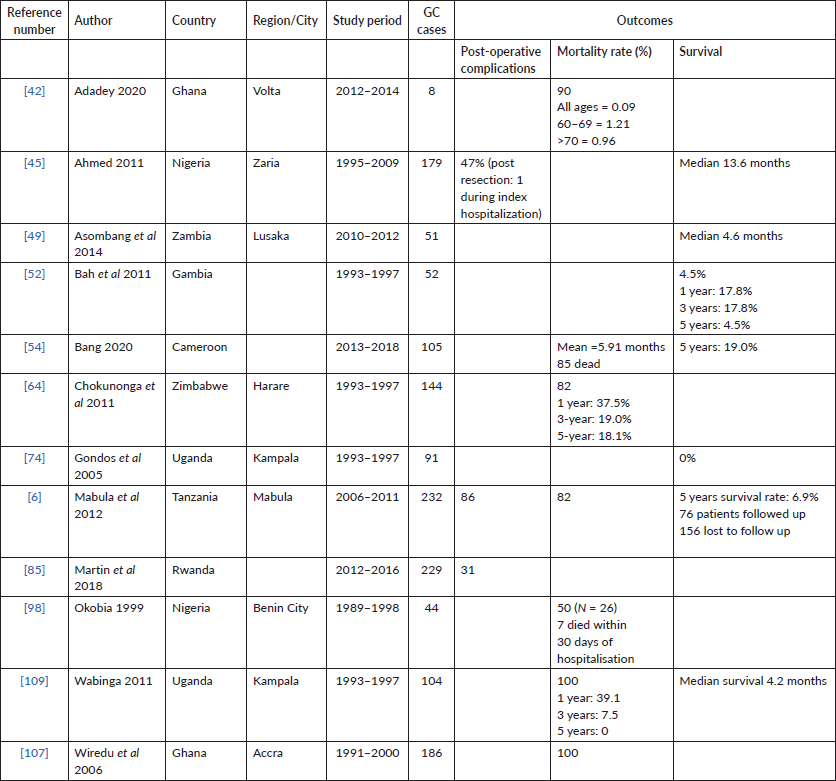
Twelve (16%) of the studies included treatment information and were primarily from Nigeria (N = 3) and Rwanda (N = 2). The majority describe either palliation or curative resection (N = 11) and a few described adjuncts like chemotherapy (N = 4), in Rwanda, South Africa, Tanzania and Cameroon. In Nigeria, between 47% and 86% of patients had surgery with at least half described as palliative.
Table 1. Characteristics of included studies describing GC incidence in SSA.
Twelve (16%) of studies included survival data, with the described time periods ranging from 1991 to 2018 across nine different countries. Median survivals were provided by approximately 33.3% of the studies (N = 3), and five studies described absolute survival in 5 years. The median survival periods reported ranged from 4.7 to 13.6 (Table 3).
Discussion
This SR is the first pooled analysis for GC incidence using primary data from SSA countries. While the pooled analysis shows an average incidence rate of 1.20 GC cases per 100,000 people (CI 1.15–1.26) in SSA, we demonstrate a significant incident variability between individual SSA countries and regions. Most data included in these studies were obtained from hospital registries or hospital data, with <10% included in national cancer registries and <50% containing histological confirmation. Analysis of pooled data limited to high-quality studies reported a similarly low incidence rate (2.12 cases/100,000 people) but retained significant variability. Less than 20% of studies reported treatment or mortality data.
The methodology used in this analysis is similar to other SRs and meta-analyses to estimate cancer incidence in SSA [10, 11], where reliance on population and hospital-based registries leads to similar incidence rate heterogeneity [10, 11]. We found significant heterogenicity (I2 >99%, p < 0.001) among the entire cohort and within geographic regional groups. This finding was maintained when the analysis was limited to only the highest quality data, as identified with the incidence of two East African nations, Ethiopia, and Rwanda with a GC incidence of 11.2 and 0.47 cases per 100,000 people respectively. The GC incidence rates in SSA are comparable to North America and Europe but far lower than Asia. The GLOBOCAN 2020 data shows the GC incidence rates were 5.7 in Northern America, 5.9 in Northern Europe, 4.6 in Western Europe, 8.5 in Western Europe and 22.4 in Eastern Asia where exposure to Helicobacter pylori is high and extensive genomic analysis have demonstrated genetic predisposition [31 ,39]. The regional variation of GC is well established, attributed to different risk factors, genetic predisposition, dietary practices, environmental exposures and access to healthcare [3, 4]. Helicobacter pylori is a key risk factor for GC and some SSA countries have a known high prevalence of this dependent on multiple factors including geographic elevation, water sources, and sanitation infrastructure [27]. Mozambique and Zimbabwe have a high consumption of smoked, salted, and pickled foods, which are known risk factors for GC [28]. Tobacco consumption is widespread in Ethiopia and Tanzania which may contribute to increased GC among involved populations [29]. Our findings of high incidence variability highlight the importance of local data to better understand the true incidence rate and specific risk factor for any given community.
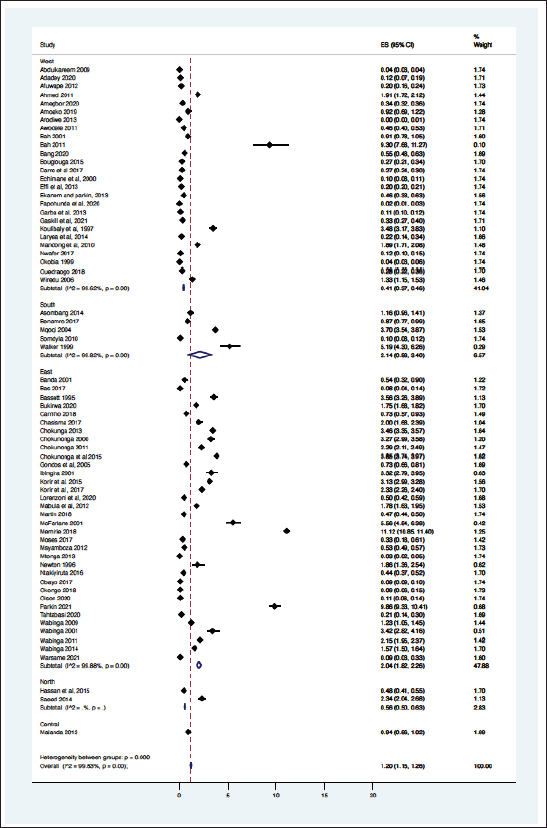
Figure 1. Crude incidence for all data sets describing GC in SSA (N = 69).
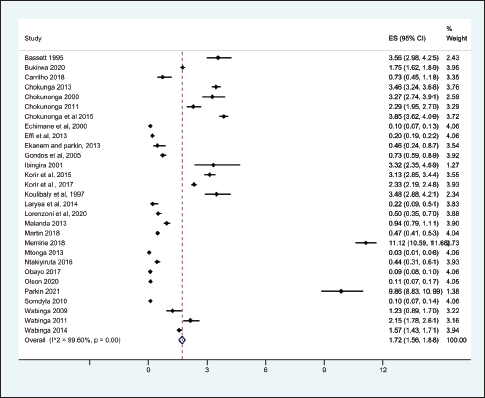
Figure 2. Crude incidence from the high-quality population-based registry studies describing GC in SSA.
Namibia, Botswana, South Sudan, Central African Republic, Chad, Niger and Mauritania has missing GC data, the North African and Southern African region provided limited GC data, whilst West and East Africa provided the bulk of the data used in this analysis. Nigeria, Malawi, Zimbabwe, and Uganda provided most of the data. This speaks to the longstanding, high quality successful cancer registries in SSA, including the Harare, Kampala and Eastern Cape population-based registries [13, 16, 17]. The large gap from missing data contributes to the true incidence of GC in SSA remaining unknown. A dire need for these registries to be replicated in other African countries remains, highlighted by the geographic variability in cancer epidemiology and existing gaps in GC diagnosis and care in SSA. The data quality is vastly different among SSA countries, closing the data gap and standardising the quality of data collection will minimise the incidence bias that is currently present for GC in SSA [38].
This SR incident results are similar to rates previously reported by modelling estimates [1]. The 2020 IARC (GLOBOCAN) estimated a SSA GC incidence rate of 2.1 cases per 100,000 people [7, 8]. Likewise, Institute for Health Metrics and Evaluation’s (IHME) Global Burden of Disease estimates a crude incidence rate of 3.21 cases per 100,000 [21]. The overall reported incidence rates are similar to these modelled, validating the accuracy of the average over the studies included. GLOBOCAN and IHME used different models to produce estimates of GC rates as a means to overcome the known limitations in primary data availability and quality on GC in SSA. However, these modelling methodologies are limited by the validity of assumptions regarding disease incidence and may not reflect the true disease burden in the population [26]. The GLOBOCAN methodology derived incidence rates from available registry information or from the average of rates from neighbouring countries, extrapolated over the known population of an entire country [8]. Only 12% of the countries in SSA had national data for these estimates, with 36% of countries incident rates relying on neighbouring country data [9]. The IHME rate was produced by estimating mortality rates for GC through registry information and then dividing by modelled mortality-to-incidence ratios. While this methodology maximizes data coverage, it is based on reported case fatality rates, which may overestimate incidence in areas with worse than expected mortality. The methodology utilised in this SR was stringent as only primary data were included in the analysis and regional and national population sizes were verified with global population databases [22–24]. While our results are comparable to the modelled estimates over SSA as a whole, they have the added benefit of improved GC incidence characterisation within subregions as evident by the high heterogenicity of reported rates [26]. These metrics may provide a more precise understanding of localised disease trends upon which disease prevention and management approaches may be planned.
In most SSA nations, access to healthcare is limited with decreased treatment capacity leading to increased mortality from GC [30]. Less than 20% of all the studies provide information for treatment or survival. From the available data, most surgeries reported were palliative. This is possibly a result of poor surveillance, late-stage presentation, and lack of treatment options upon presentation [5]. Late-stage GC diagnosis, healthcare costs, inadequate diagnostic tools and limited healthcare workers all contribute to restricted GC treatment in SSA [5, 6]. The African enigma which hypothesizes the disassociation between GC and H. pylori infection is important to consider in further research [108]. The scarcity of robust data for GC in SSA, and the association between H. pylori and GC in SSA not being widely researched may indicate the African enigma is not as convincing as prior belief [108, 109]. Despite these limitations, treatment data are essential to establishing context-relevant treatment guidelines [33]. Future data collection could focus on the current standard of GC care in SSA and available palliative services.
Table 2. Treatment data reported in available studies on GC from SSA.
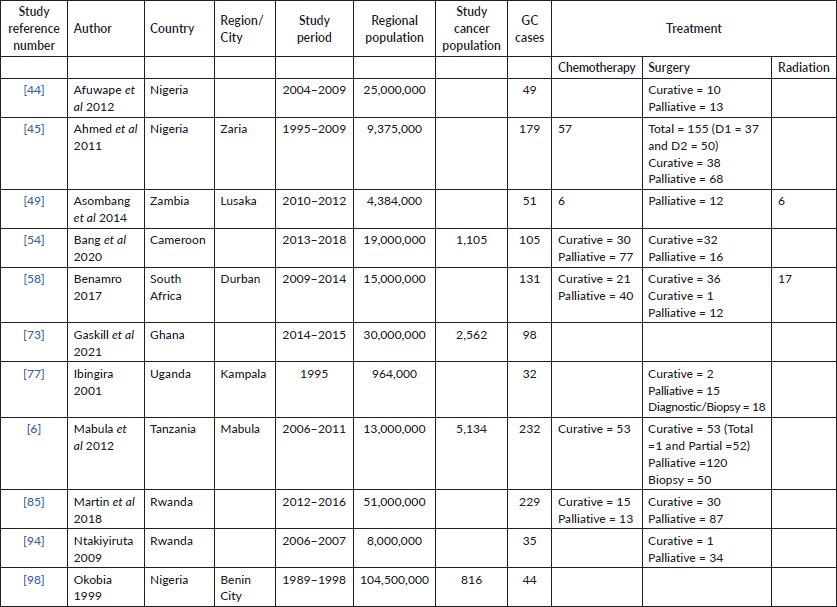
Table 3. Mortality data reported in available studies on GC from SSA.
Population-based GC disease registries such as the successful Harare, Eastern Cape and Kampala registries identifies demographic and geographic discrepancies in incidence and mortality rates which is valuable for improving cancer care and developing population specific control measures [34, 37]. Granular data collected from hospitals, clinics and laboratories will give better clarity of the epidemiology and risk factors that shape the local epidemiology of GC. This information on GC in SSA is vital to achieve a better understanding of the local and regional disease landscape and demonstrate the impact of local public health improvements accounting for the high regional variability of GC in SSA [35]. Establishing and maintaining population-based registries at local levels pose a challenge to most SSA regions due to limited healthcare infrastructure, shortage of trained data collectors, high costs, time involvement, and accuracy needed for proper data collection [36]. Disease registry success may be possible by focussing on local-level data collection that is aligned with and monitored by national data collection guidelines to ensure adherence to high-quality standards. Regional and national health systems will benefit from investing in GC surveillance programmes, national control programmes and training of health workers for accurate data collection [34, 40].
This review was limited by several factors. The employed search strategy is open to the biases of the search engines available to authors, potentially missing non-indexed reports published in regional journals. Additionally, our calculations were limited by the availability of regional and population-based estimates that were truly reflective of a given study’s sample population. Despite these limitations, we provide the most stringent review of high-quality data available to date to better inform efforts to bolster regional data collection. Moreover, we likely underestimate the true burden of GC disease in SSA as the studies that do exist are limited by selection bias for those who receive care for GC.
Conclusion
This study demonstrates the high variability of GC incidence across SSA, independent of data quality. We calculated an overall rate of 1.20 cases per 100,000 people, similar to modelled estimated but highlighting the large regions devoid of primary data. The variable GC incidence rates from the contributing studies of this SR highlight the need for further development of population-based cancer registries [19, 20].
Conflicts of interest
Our authors have no competing interests and the contents of this manuscript have not been published elsewhere. There are no conflicts of interest to disclose.
Funding
PROSPERO number CRD42022341498.
No external funding or funding by any author has been received for this study
References
1. International agency for Research on Cancer, The Global Cancer Observatory (2021) Sub-Saharan African Hub Fact Sheet, Globocan 2020 (Lyon: International agency for Research on Cancer, The Global Cancer Observatory)
2. Asombang AW, Rahman R, and Ibdah JA (2014) Gastric cancer in Africa: current management and outcomes World J Gastroenterol 20(14) 3875–3879 https://doi.org/10.3748/wjg.v20.i14.3875 PMID: 24833842 PMCID: 3983443
3. Morhason-Bello IO, Odedina F, and Rebbeck TR, et al (2013) Challenges and opportunities in cancer control in Africa: a perspective from the African Organisation for Research and Training in Cancer Lancet Oncol 14 e142–e151 https://doi.org/10.1016/S1470-2045(12)70482-5 PMID: 23561745
4. Hamdi Y, Abdeljaoued-Tej I, and Zatchi AA, et al (2021) Cancer in Africa: the untold story Front Oncol 11 650117 https://doi.org/10.3389/fonc.2021.650117 PMID: 33937056 PMCID: 8082106
5. Kingham TP, Alatise OI, and Vanderpuye V, et al (2014) Treatment of cancer in sub-Saharan Africa Lancet Oncol 14 e158–e167 https://doi.org/10.1016/S1470-2045(12)70472-2
6. Mabula JB, Mchembe MD, and Koy M, et al (2012) Gastric cancer at a university teaching hospital in northwestern Tanzania: a retrospective review of 232 cases World J Surg Oncol 10 257 https://doi.org/10.1186/1477-7819-10-257 PMID: 23181624 PMCID: 3527214
7. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
8. Globocan 2020 WHO report
9. Ferlay J, Ervik M, and Lam F, et al . Global Cancer Observatory: Cancer Today (Lyon: International Agency for Research on Cancer) [https://GCo.iarc.fr/today] Date accessed: 09/10/18
10. Cumberbatch MG, Jubber I, and Black PC, et al (2018) Epidemiology of bladder cancer: a systematic review and contemporary update of risk factors in 2018 Eur Urol 74 784–795 https://doi.org/10.1016/j.eururo.2018.09.001 PMID: 30268659
11. Adeloye D, Sowunmi OY, and Jacobs W, et al (2018) Estimating the incidence of breast cancer in Africa: a systematic review and meta-analysis J Glob Health 8(1) 010419 https://doi.org/10.7189/jogh.08.010419 PMID: 29740502 PMCID: 5903682
12. Ajani JA, D’Amico TA, and Bentrem DJ, et al (2022) Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology J Nat Compr Cancer Netw 20(2) 167–192 https://doi.org/10.6004/jnccn.2022.0008
13. Bukirwa P, Wabinga H, and Nambooze S, et al (2021) Trends in the incidence of cancer in Kampala, Uganda, 1991 to 2015 Int J Cancer 148 2129–2138 https://doi.org/10.1002/ijc.33373
14. Wabinga H, Parkin DM, and Nambooze S, et al (2011) Cancer survival in Kampala, Uganda, 1993−1997 IARC Sci Publ 162 243–247
15. Chokunonga E, Windridge P, and Sasieni P, et al (2016) Black–white differences in cancer risk in Harare, Zimbabwe, during 1991–2010 Int J Cancer 138 1416–1421 https://doi.org/10.1002/ijc.29883
16. Parkin DM, Chingonzoh T, and Vuma S, et al (2021) Changes in the incidence of cancer in Bulawayo, Zimbabwe over a 50-year period Cancer Epidemiol Biomarkers Prev 30(5) 867–873 https://doi.org/10.1158/1055-9965.EPI-20-0669 PMID: 33619023
17. Somdyala NI, Bradshaw D, and Gelderblom WC, et al (2010) Cancer incidence in a rural population of South Africa, 1998–2002 Int J Cancer 127 2420–2429 https://doi.org/10.1002/ijc.25246 PMID: 20162610
18. Somdyala NI, Parkin DM, and Sithole N, et al (2015) Trends in cancer incidence in rural Eastern Cape Province; South Africa, 1998–2012 Int J Cancer 136 E470–E474 https://doi.org/10.1002/ijc.29224
19. Moodley J, Constant D, and Mwaka AD, et al (2021) Anticipated help seeking behaviour and barriers to seeking care for possible breast and cervical cancer symptoms in Uganda and South Africa ecancermedicalscience 15 1171 https://doi.org/10.3332/ecancer.2021.1171 PMID: 33680085 PMCID: 7929770
20. Lubuzo B, Ginindza T, and Hlongwana K (2019) Exploring barriers to lung cancer patient access, diagnosis, referral and treatment in Kwazulu-Natal, South Africa: the health providers’ perspectives Transl Lung Cancer Res 8(4) 380–391 https://doi.org/10.21037/tlcr.2019.08.17 PMID: 31555513 PMCID: 6749129
21. Institute for Health Metrics and Evaluation (IHME) report
22. https://www.macrotrends.net/countries
23. https://data.worldbank.org/
24. https://countryeconomy.com/demography/population
26. Ioannidis JPA (2020) Coronavirus disease 2019: the harms of exaggerated information and non-evidence-based measures Eur J Clin Invest 50(4) e13222 https://doi.org/10.1111/eci.13222 PMID: 32191341 PMCID: 7163529
27. Fock KM and Ang TL (2010) Epidemiology of Helicobacter pylori infection and gastric cancer in Asia J Gastroenterol Hepatol 25(4) 479–486 https://doi.org/10.1111/j.1440-1746.2009.06188.x PMID: 20370726
28. Marques-Vidal P, Ravasco P, and Ermelinda Camilo M (2004) Gastric cancer: epidemiology, prevention, and therapy Nutr Cancer 48(2) 149–156
29. Akinyemiju T, Abera S, and Ahmed M, et al (2017) The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the Global, Regional, and National level: results from the global burden of disease study 2015 JAMA Oncol 3(10) 1683–1691 https://doi.org/10.1001/jamaoncol.2017.3055 PMID: 28983565 PMCID: 5824275
30. Ferlay J, Soerjomataram I, and Ervik M, et al (2013) GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 (Lyon: International Agency for Research on Cancer) [http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx]
31. Ferlay J, Ervik M, and Lam F, et al (2023) Global Cancer Observatory: Cancer Today (Lyon: International Agency for Research on Cancer) [https://gco.iarc.fr/today/home] Date accessed: 26/04/23
32. Rawla P and Barsouk A (2019) Epidemiology of gastric cancer: global trends, risk factors and prevention Prz Gastroenterol 14(1) 26–38 PMID: 30944675 PMCID: 6444111
33. Sexton RE, Al Hallak MN, and Diab M, et al (2020) Gastric cancer: a comprehensive review of current and future treatment strategies Cancer Metastasis Rev 39(4) 1179–1203 https://doi.org/10.1007/s10555-020-09925-3 PMID: 32894370 PMCID: 7680370
34. Gliklich RE, Dreyer NA, and Leavy MB (2014) Registries for Evaluating Patient Outcomes: A User’s Guide 3rd edn (Rockville: Agency for Healthcare Research and Quality) [https://www.ncbi.nlm.nih.gov/books/NBK208643/]
35. Allemani C, Matsuda T, and Di Carlo V, et al (2018) Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries Lancet 391(10125) 1023–1075 https://doi.org/10.1016/S0140-6736(17)33326-3 PMID: 29395269 PMCID: 5879496
36. Selvin E, Parrinello CM, and Sacks DB, et al (2014) Trends in prevalence and control of diabetes in the United States, 1988-1994 and 1999-2010 Ann Intern Med 160(8) 517–525 https://doi.org/10.7326/M13-2411 PMID: 24733192 PMCID: 4442608
37. Cruz-Flores S, Rabinstein A, and Biller J, et al (2011) Racial-ethnic disparities in stroke care: the American experience: a statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke 42(7) 2091–2116 https://doi.org/10.1161/STR.0b013e3182213e24 PMID: 21617147
38. Gini A, Jansen EEL, and Zielonke N, et al (2020) Impact of colorectal cancer screening on cancer-specific mortality in Europe: a systematic review Eur J Cancer 127 224–235 https://doi.org/10.1016/j.ejca.2019.12.014 PMID: 31932176
39. Katoh H and Ishikawa S (2021) Lifestyles, genetics, and future perspectives on gastric cancer in east Asian populations J Hum Genet 66 887–899 https://doi.org/10.1038/s10038-021-00960-8 PMID: 34267306 PMCID: 8384627
40. Maione L and Chanson P (2019) National acromegaly registries Best Pract Res Clin Endocrinol Metabol 33(2) 101264 https://doi.org/10.1016/j.beem.2019.02.001
41. Abdulkareem FB, Faduyile FA, and Daramola AO, et al (2009) Malignant gastrointestinal tumours in south western Nigeria: a histopathologic analysis of 713 cases West Afr J Med 28(3) 173–176
42. Adadey SM, Languon S, and Ayee R, et al (2020) Incidence and mortality of cancer in the Volta Region of Ghana Exp Biol Med 245(12) 1058–1065 https://doi.org/10.1177/1535370220931514
43. Amégbor K, Darre T, and Ayéna KD, et al (2011) Cancers in Togo from 1984 to 2008: epidemiological and pathological aspects of 5251 cases J Cancer Epidemiol 2011 319872 https://doi.org/10.1155/2011/319872 PMID: 22007216 PMCID: 3189592
44. Afuwape OO, Irabor DO, and Ladipo JK, et al (2012) A review of the current profile of gastric cancer presentation in the university college hospital Ibadan, a tertiary health care institution in the tropics J Gastrointest Cancer 43(2) 177–180 https://doi.org/10.1007/s12029-011-9259-z
45. Ahmed A, Ukwenya AY, and Makama JG, et al (2011) Management and outcome of gastric carcinoma in Zaria, Nigeria Afr Health Sci 11(3) 353–361
46. Amoako YA, Awuah B, and Larsen-Reindorf R, et al (2019) Malignant tumours in urban Ghana: evidence from the city of Kumasi BMC Cancer 19(1) 267 [ https://doi.org/10.1186/s12885-019-5480-0 PMID: 30909876 PMCID: 6434839
47. Joutei HA, Mahfoud W, and Sadaoui I, et al (2020) Étude des caractéristiques épidémiologiques cliniques et anatomopathologiques de l’adénocarcinome gastrique chez une population Marocaine Ann Pathol 40(6) 442–446 https://doi.org/10.1016/j.annpat.2020.04.014
48. Arodiwe EB, Ike SO, and Nwokediuko SC, et al (2013) Pattern of cancer deaths in the medical wards of a teaching hospital in South East Nigeria Niger J Clin Pract 16(4) 505–510 https://doi.org/10.4103/1119-3077.116901 PMID: 23974748
49. Asombang AW, Kayamba V, and Turner-Moss E, et al (2014) Gastric malignancy survival in Zambia, Southern Africa: a two year follow up study Med J Zamb 41 13–18
50. Awodele O, Adeyomoye AA, and Awodele DF, et al (2011) Cancer distribution pattern in south-western Nigeria Tanzan J Health Res 13(2) 125–131 https://doi.org/10.4314/thrb.v13i2.55226 PMID: 25566610
51. Bah E, Parkin DM, and Hall AJ, et al (2001) Cancer in the Gambia: 1988-97 Br J Cancer 84(9) 1207–1214 https://doi.org/10.1054/bjoc.2001.1730 PMID: 11336472 PMCID: 2363873
52. Bah E, Sam O, and Whittle H, et al (2011) Cancer survival in the Gambia, 1993-1997 IARC Sci Publ 162 97–100
53. Banda LT, Parkin DM, and Dzamalala CP, et al (2001) Cancer incidence in Blantyre, Malawi 1994-1998 Trop Med Int Health 6(4) 296–304 https://doi.org/10.1046/j.1365-3156.2001.00707.x PMID: 11348520
54. Bang GA, Savom EP, and Oumarou BN, et al (2020) Clinical epidemiology and mortality risk factors of gastric cancer in a sub-Saharan African setting: a retrospective analysis of 120 cases in Yaoundé (Cameroon) Pan Afr Med J 37 104
55. Baş Y, Hassan HA, and Adıgüzel C, et al (2017) The distribution of cancer cases in Somalia Semin Oncol 44(3) 178–186 https://doi.org/10.1053/j.seminoncol.2017.10.007
56. Bassène ML, Sy D, and Dia D, et al (2015) Le cancer gastrique : étude descriptive de 101 cas dans le centre d’endoscopie digestive du CHU Aristide Le Dantec [Stomach cancer: a descriptive study of 101 cases at the gastrointestinal endoscopy center at Aristide Le Dantec University Hospital] Med Sante Trop 25(4) 377–380 PMID: 26680270
57. Bassett MT, Chokunonga E, and Mauchaza B, et al (1995) Cancer in the African population of Harare, Zimbabwe, 1990-1992 Int J Cancer 63(1) 29–36 https://doi.org/10.1002/ijc.2910630107 PMID: 7558448
58. Benamro F, Sartorius B, and Clarke DL, et al (2017) The spectrum of gastric cancer as seen in a large quaternary hospital in KwaZulu-Natal, South Africa South Afr Med J 107(2) 130–133 https://doi.org/10.7196/SAMJ.2017.v107i2.11383
59. Bodalal Z, Azzuz R, and Bendardaf R (2014) Cancers in Eastern Libya: first results from Benghazi Medical Center World J Gastroenterol 20(20) 6293–6301 https://doi.org/10.3748/wjg.v20.i20.6293 PMID: 24876750 PMCID: 4033467
60. Bouglouga O, Lawson-Ananissoh LM, and Bagny A, et al (2015) Cancer de l’estomac: aspects épidémiologiques, cliniques et histologiques au CHU Campus de Lomé (Togo) [Stomach cancer: epidemiological, clinical and histological aspects at the Lome Campus teaching hospital (Togo)] Med Sante Trop 25(1) 65–68 PMID: 25786857
61. Carrilho C, Fontes F, and Tulsidás S, et al (2019) Cancer incidence in Mozambique in 2015-2016: data from the Maputo Central Hospital Cancer Registry Eur J Cancer Prev 28(4) 373–376 https://doi.org/10.1097/CEJ.0000000000000457
62. Chasimpha SJD, Parkin DM, and Masamba L, et al (2017) Three-year cancer incidence in Blantyre, Malawi (2008-2010) Int J Cancer 141(4) 694–700 https://doi.org/10.1002/ijc.30777 PMID: 28493322 PMCID: 5999322
63. Chokunonga E, Borok MZ, and Chirenje ZM, et al (2013) Trends in the incidence of cancer in the black population of Harare, Zimbabwe 1991-2010 Int J Cancer 133(3) 721–729 https://doi.org/10.1002/ijc.28063 PMID: 23364833
64. Chokunonga E, Borok MZ, and Chirenje ZM, et al (2011) Cancer survival in Harare, Zimbabwe, 1993-1997 IARC Sci Publ 162 249–255
65. Darre T, Kpatcha TM, and Bagny A, et al (2017) Descriptive epidemiology of cancers in Togo from 2009 to 2016 Asian Pac J Cancer Prev APJCP 18(12) 3407–3411 PMID: 29286611 PMCID: 5980902
66. Echimane AK, Ahnoux AA, and Adoubi I, et al (2000) Cancer incidence in Abidjan, Ivory Coast: first results from the cancer registry, 1995-1997 Cancer 89(3) 653–663 https://doi.org/10.1002/1097-0142(20000801)89:3<653::AID-CNCR22>3.0.CO;2-Z PMID: 10931466
67. Effi AB, Koffi KE, and Doukouré B, et al (2013) Épidémiologie descriptive des cancers en Côte d’Ivoire Bull Cancer 100(2) 119–125 https://doi.org/10.1684/bdc.2013.1695 PMID: 23406565
68. Ekanem IO and Parkin DM (2016) Five year cancer incidence in Calabar, Nigeria (2009-2013) Cancer Epidemiol 42 167–172 https://doi.org/10.1016/j.canep.2016.04.014 PMID: 27164305
69. Elghali MA, Gouader A, and Bouriga R, et al (2018) Gastric cancers in Central Tunisia: evolution specificities through two decades and relation with Helicobacter pylori Oncology 95(2) 121–128 https://doi.org/10.1159/000488488 PMID: 29694966
70. Fapohunda A, Fakolade A, and Omiye J, et al (2020) Cancer presentation patterns in Lagos, Nigeria: experience from a private cancer center J Public Health Afr 11(2) 1138 https://doi.org/10.4081/jphia.2020.1138
71. Feuchtner J, Mathewos A, and Solomon A, et al (2019) Addis Ababa population-based pattern of cancer therapy, Ethiopia PLoS One 14(9) e0219519 https://doi.org/10.1371/journal.pone.0219519 PMID: 31536505 PMCID: 6752935
72. Garba SM, Zaki HM, and Arfaoui A, et al (2013) Épidémiologie des cancers au Niger, 1992 à 2009 Bull Cancer 100(2) 127–133 https://doi.org/10.1684/bdc.2013.1699 PMID: 23420007
73. Gaskill CE, Gyedu A, and Stewart B, et al (2021) Improving global surgical oncology benchmarks: defining the unmet need for cancer surgery in Ghana World J Surg 45(9) 2661–2669 https://doi.org/10.1007/s00268-021-06197-y PMID: 34152449
74. Gondos A, Brenner H, and Wabinga H, et al (2005) Cancer survival in Kampala, Uganda Br J Cancer 92(9) 1808–1812 https://doi.org/10.1038/sj.bjc.6602540 PMID: 15827554 PMCID: 2362045
75. Gyorki DE, Muyco A, and Kushner AL, et al (2012) Cancer surgery in low-income countries: an unmet need Arch Surg 147(12) 1135–1140 https://doi.org/10.1001/archsurg.2012.1265 PMID: 23248017
76. El Hassan A, El Hassan L, and Mudawi H, et al (2008) Malignant gastric tumors in Sudan: a report from a single pathology center Hematol/Oncol Stem Cell Ther 1(2) 130–132 https://doi.org/10.1016/S1658-3876(08)50044-6 PMID: 20063541
77. Ibingira CB (2001) Management of cancer of the stomach in Mulago Hospital Kampala, Uganda East Afr Med J 78(5) 233–237 https://doi.org/10.4314/eamj.v78i5.9044
78. Jedy-Agba E, Curado MP, and Ogunbiyi O, et al (2012) Cancer incidence in Nigeria: a report from population-based cancer registries Cancer Epidemiol 36(5) e271–e278 https://doi.org/10.1016/j.canep.2012.04.007 PMID: 22621842 PMCID: 3438369
79. Johnson O, Ersumo T, and Ali A (2000) Gastric carcinoma at Tikur Anbessa Hospital, Addis Ababa East Afr Med J 77(1) 27–30 PMID: 10944835
80. Korir A, Okerosi N, and Ronoh V, et al (2015) Incidence of cancer in Nairobi, Kenya (2004-2008) Int J Cancer 137(9) 2053–2059 https://doi.org/10.1002/ijc.29674 PMID: 26139540
81. Korir A, Yu Wang E, and Sasieni P, et al (2017) Cancer risks in Nairobi (2000-2014) by ethnic group Int J Cancer 140(4) 788–797 https://doi.org/10.1002/ijc.30502
82. Koulibaly M, Kabba IS, and Cissé A, et al (1997) Cancer incidence in Conakry, Guinea: first results from the cancer registry 1992-1995 Int J Cancer 70(1) 39–45 https://doi.org/10.1002/(SICI)1097-0215(19970106)70:1<39::AID-IJC6>3.0.CO;2-7 PMID: 8985088
83. Laryea DO, Awuah B, and Amoako YA, et al (2014) Cancer incidence in Ghana, 2012: evidence from a population-based cancer registry BMC Cancer 14 362 https://doi.org/10.1186/1471-2407-14-362 PMID: 24884730 PMCID: 4046022
84. Lorenzoni CF, Ferro J, and Carrilho C, et al (2020) Cancer in Mozambique: results from two population-based cancer registries Int J Cancer 147(6) 1629–1637 https://doi.org/10.1002/ijc.32953 PMID: 32142162
85. Martin AN, Silverstein A, and Ssebuufu R, et al (2018) Impact of delayed care on surgical management of patients with gastric cancer in a low-resource setting J Surg Oncol 118(8) 1237–1242 https://doi.org/10.1002/jso.25286 PMID: 30380140 PMCID: 6250579
86. McFarlane G, Forman D, and Sitas F (2001) A minimum estimate for the incidence of gastric cancer in Eastern Kenya Br J Cancer 85(9) 1322–1325 https://doi.org/10.1054/bjoc.2001.1994 PMID: 11720468 PMCID: 2375245
87. Memirie ST, Habtemariam MK, and Asefa M, et al (2018) Estimates of cancer incidence in Ethiopia in 2015 using population-based registry data J Glob Oncol 4 1–11 PMID: 30241262 PMCID: 6223441
88. Missaoui N, Trabelsi A, and Parkin DM, et al (2010) Trends in the incidence of cancer in the Sousse region, Tunisia, 1993-2006 Int J Cancer 127(11) 2669–2677 https://doi.org/10.1002/ijc.25490 PMID: 20521249
89. Moses A, Mwafongo A, and Chikasema M, et al (2017) Risk factors for common cancers among patients at Kamuzu Central Hospital in Lilongwe, Malawi: a retrospective cohort study Malawi Med J 29(2) 136–141 https://doi.org/10.4314/mmj.v29i2.11 PMID: 28955421 PMCID: 5610284
90. Msyamboza KP, Dzamalala C, and Mdokwe C, et al (2012) Burden of cancer in Malawi; common types, incidence and trends: national population-based cancer registry BMC Res Notes 5 149 https://doi.org/10.1186/1756-0500-5-149 PMID: 22424105 PMCID: 3327635
91. Mtonga P, Masamba L, and Milner D, et al (2013) Biopsy case mix and diagnostic yield at a Malawian central hospital Malawi Med J 25(3) 62–64 PMID: 24358421 PMCID: 3859990
92. Newton R, Ngilimana PJ, and Grulich A, et al (1996) Cancer in Rwanda Int J Cancer 66(1) 75–81 https://doi.org/10.1002/(SICI)1097-0215(19960328)66:1<75::AID-IJC14>3.0.CO;2-A PMID: 8608971
93. Nsondé Malanda J, Nkoua Mbon JB, and Bambara AT, et al (2013) Douze années de fonctionnement du registre des cancers de Brazzaville [Twelve years of working of Brazzaville cancer registry] Bull Cancer 100(2) 135–139 https://doi.org/10.1684/bdc.2013.1701 PMID: 23406573
94. Ntakiyiruta G (2009) Gastric cancers at Kibogora Hospital East Cent Afr J Surg (Online) 14(1) 130–134
95. Nwafor CC and Nwafor NN (2018) The pattern and distribution of cancers in Akwa Ibom State, Nigeria Niger J Clin Pract 21(5) 603–608 https://doi.org/10.4103/njcp.njcp_316_17 PMID: 29735861
96. Obayo S, Lukwago L, and Orem J, et al (2017) Gastrointestinal malignancies at five regional referral hospitals in Uganda Afr Health Sci 17(4) 1051–1058 https://doi.org/10.4314/ahs.v17i4.13
97. Okongo F, Ogwang DM, and Liu B, et al (2019) Cancer incidence in Northern Uganda (2013–2016) Int J Cancer 144 2985–2991 https://doi.org/10.1002/ijc.32053
98. Olson AC, Afyusisye F, and Egger J, et al (2020) Cancer incidence and treatment utilization patterns at a regional cancer center in Tanzania from 2008-2016: initial report of 2,772 cases Cancer Epidemiol 67 101772 https://doi.org/10.1016/j.canep.2020.101772
99. Ouedraogo S, Ouedraogo S, and Kambire JL, et al (2018) Profil épidémiologique, clinique, histologique et thérapeutique des cancers digestifs primitifs dans les régions nord et est du Burkina Faso Bull Cancer 105(12) 1119–1125 https://doi.org/10.1016/j.bulcan.2018.09.001 PMID: 30392708
100. Parkin DM, Nambooze S, and Wabwire-Mangen F, et al (2010) Changing cancer incidence in Kampala, Uganda, 1991-2006 Int J Cancer 126(5) 1187–1195 https://doi.org/10.1002/ijc.24838
101. Saeed IE, Weng HY, and Mohamed KH, et al (2014) Cancer incidence in Khartoum, Sudan: first results from the cancer registry, 2009-2010 Cancer Med 3(4) 1075–1084 https://doi.org/10.1002/cam4.254 PMID: 24821265 PMCID: 4303176
102. Tahtabasi M, Mohamud Abdullahi I, and Kalayci M, et al (2020) Cancer incidence and distribution at a tertiary care hospital in Somalia from 2017 to 2020: an initial report of 1306 cases Cancer Manag Res 12 8599–8611 https://doi.org/10.2147/CMAR.S277202 PMID: 33061565 PMCID: 7534047
103. Wabinga HR, Parkin DM, and Wabwire-Mangen F, et al (2000) Trends in cancer incidence in Kyadondo County, Uganda, 1960-1997 Br J Cancer 82(9) 1585–1592 PMID: 10789729 PMCID: 2363394
104. Wabinga HR (2002) Pattern of cancer in Mbarara, Uganda East Afr Med J 79(4) 193–197 https://doi.org/10.4314/eamj.v79i4.8877
105. Walker AR and Halse J (1999) Pattern of cancer in Indian patients hospitalized in Durban, South Africa Eur J Cancer Prev 8(3) 247–254 https://doi.org/10.1097/00008469-199906000-00013 PMID: 10443954
106. Warsame AA, Sönmez RE, and Muse MM, et al (2021) Prevalence of cancer related to sociodemographic characteristics and prevention strategies in Mogadishu, Somalia Bangladesh J Med Sci 20(4) 756–761 https://doi.org/10.3329/bjms.v20i4.54130
107. Wiredu EK and Armah HB (2006) Cancer mortality patterns in Ghana: a 10-year review of autopsies and hospital mortality BMC Public Health 6 159 [10.1186/1471-2458-6-159] https://doi.org/10.1186/1471-2458-6-159 PMID: 16787544 PMCID: 1539002
108. Dong SXM (2023) “Peptic ulcers are an infectious disease caused by Helicobacter pylori” is an illusion in medical research Preprints 2023020297
109. Cavadas B, Leite M, and Pedro N, et al (2021) Shedding light on the African enigma: in vitro testing of Homo sapiens-Helicobacter pylori coevolution Microorganisms 9 240 https://doi.org/10.3390/microorganisms9020240
Appendix: GC SR
Methods – search strategy
Articles applicable to this GC SR were identified by a PubMed, Google Scholar and Web of Science search between 1st January 1995 and 1st June 2022. GC related search terms comprising ‘GC’, ‘stomach neoplasm’, ‘gastric neoplasm’, ‘cancer of the stomach’, ‘GC’ and ‘stomach cancer’ were applied to the search. The SSA country name and the SSA regional was applied with no language restriction.
The final search terms used was ‘(Cancer OR mass OR malignancy OR adenocarcinoma OR carcinoma OR neoplasm OR tumour) AND (Gastric OR stomach) AND (Botswana OR Burkina Faso OR Burundi OR Cabo Verde OR Cameroon OR central African republic OR Chad OR Comoros OR Democratic Republic of the Congo OR Congo OR Cote D’Ivoire OR Djibouti OR Equatorial Guinea OR Eritrea OR Swaziland OR Ethiopia OR Gabon OR Gambia OR Ghana OR Guinea OR Guinea Bissau OR Kenya OR Lesotho OR Liberia OR Madagascar OR Malawi OR Mali OR Mauritania OR Mauritius OR Mozambique OR Namibia OR Niger OR Nigeria OR Rwanda OR Sao Tome OR Senegal OR Seychelles OR Sierra Leone OR Somalia OR South Africa OR Sudan OR Tanzania OR Togo OR Uganda OR Zambia OR Zimbabwe OR SSA OR Africa)’
The participants included will be from primary GC studies from 1st January1995 to 1st June 2022 comprising of adult patients above the age of 18 years in SSA who have or had GC. Randomised control trials, cohort studies, case-control studies, and cross-sectional primary data studies of adult GC patients in SSA were included in this analysis. The primary exposure is GC. The primary outcomes of interest are incidence, treatment, and mortality. Studies that describe treatment interventions for GC including both surgery and chemoradiation were included. GC studies conducted outside of SSA or inclusive of non-African countries, paediatric cancer studies, benign gastric tumours, and gastric lymphoma were excluded. Letters to the editor, case reports, case series, narrative reviews, commentaries, perspectives, literature reviews, meta-analyses, medical reports, and non-peer reviewed publications were excluded from the analysis.
All references identified after deployment of the search strategy were exported using Zotero software. All records obtained from the various databases were combined in a single Zotero folder and all duplicates removed. The final search results underwent a title review to assess if the abstracts may contain relevant information for the study. After elimination of non-relevant titles, an abstract review was done prior to a full text review of pertinent studies. A data extraction form was employed on Microsoft Excel to record information on; the surname of primary author, year of publication, country of study, diagnostic method, registry type, study design, age group (age rages including >18 years old), sample size, GC case numbers, overall cancer case number for study population, overall population size, age or gender stratification, treatment type and numbers, and the case fatality rate of GC in the study. References of all relevant articles for additional data sources missed during our search was scanned and included where full texts were retrieved. References of pertinent reviews were also scanned.
5,558 records were identified with the online database search. An initial record screening and removal of duplicated records resulted in 667 studies remaining. Five hundred and thirty-five records were excluded after title screening and 132 study abstracts were screened. After the abstract screening 14 studies were excluded and 123 studies were assessed for eligibility by full text reading and reference reviewal. A total of 85 studies were included in this SR after 38 full text articles were excluded due to outcomes of interest not being reported.
Two reviewers (CG and PM) independently evaluated the titles of studies obtained from the searches. All three reviewers (CG, PM and AR) reviewed abstracts of papers obtained, after which the full texts of potentially eligible papers were retrieved. The three reviewers independently reviewed the full text of each potentially eligible study, compared their results, and resolved any discrepancies by discussion. For duplicate studies published in more than one report, the study reporting the largest sample size was considered. Studies with inaccessible full text either online or from the corresponding author was excluded. Methodological quality and risk of bias of included studies were assessed using the STROBE checklist. https://www.strobe-statement.org/
A SR with descriptive analysis was performed after the final data collection. To determine the incidence of GC, the crude GC case numbers (numerators), the regional population and the population of people with diagnosis of cancer (denominators) were considered. Overall mortality and treatment strategies are reported in crude numbers and percentages. Incidence patterns of GC across the various nations in SSA are summarised after the recalculation of incidence was done.
A narrative summary of findings regarding overall mortality and treatment strategies are reported due to the heterogeneity and paucity of data. All data analyses were conducted using StataSE (version 15.1, College Station, Texas, USA).
GC in SSA – an SR of primary data
Supplementary figures
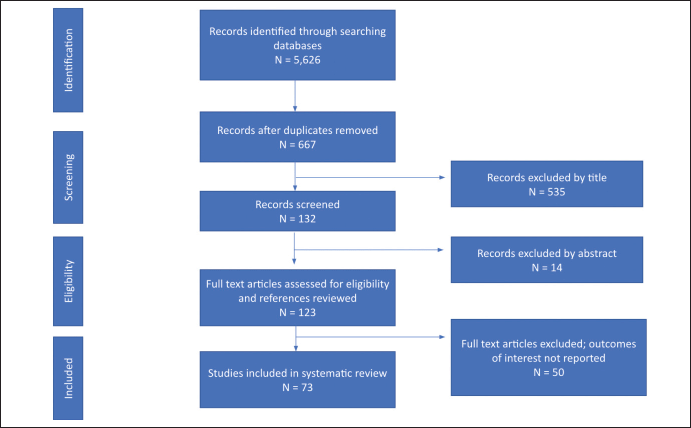
Figure S1. PRISMA flowchart diagram of the study selection.
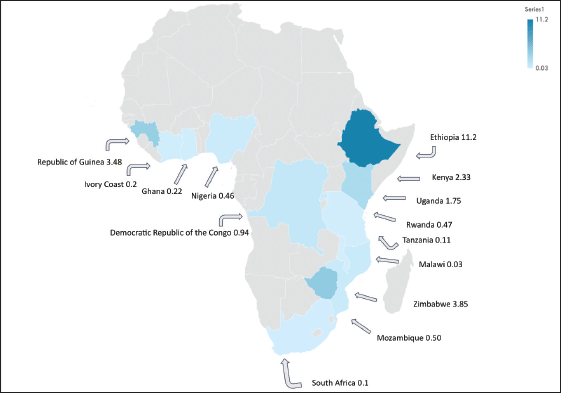
Figure S2. Gastric incidence map with case incidence per 100,000 people1.
1 Estimated size incidence cases per 100,000 described here. Data selected from the most recently published high-quality population-based registry reports





