Incurable advanced salivary gland tumours: a retrospective analysis and peek into the perplexing clinical and molecular intricacies from a tertiary care centre in India
Bipinesh Sansar1, Neha Singh2, Anuj Gupta1, Bal Krishna Mishra1, Abhishek Sharma1, Rahul Rai1, Pooja Gupta1 and Akhil Kapoor1
1Department of Medical Oncology, HBCH and MPMMCC, Varanasi 221005, India
2Department of Pathology, HBCH and MPMMCC, Varanasi 221005, India
Abstract
Background: Salivary gland tumours are rare cancers with variable course and prognosis. There is a paucity of data, especially for the advanced stages.
Materials and methods: This is a retrospective analysis carried out in our institute. All patients seeking treatment for incurable advanced salivary gland tumours from October 2018 to September 2022 were included. Relevant clinical data were collected and appropriate statistical analysis was applied.
Results: 30 patients were included in the analysis. The parotid gland was the most common site of origin (73%). Adenoid cystic carcinoma (ACC) and salivary duct carcinoma (SDC) were equally (37%) the most common pathological subtypes. The majority of patients were males (73%) and lungs (57%) were the most common site of metastases. On molecular analysis, SDC had high rates of androgen receptor (AR) (90%) and human epidermal growth factor receptor 2 (HER2) (55%) positivity. Mucoepidermoid carcinoma (MEC) had AR and HER2 positivity rates of 17% and 20%, respectively, while for ACC it was even lower. A variety of treatment regimens including hormonal therapy, anti-HER2 targeted therapy and chemotherapy were used in first-line treatment. With an overall response rate (ORR) of 10/21 (48%), only 9/21 (43%) went on to receive second-line treatment with an ORR of 4/9 (44%). The progression-free survival (PFS) with first-line treatment (PFS1) was a median of 5 months. The median PFS1 was worst for MEC. The median overall survival (OS) was 10 months. Median OS for ACC, SDC and MEC were 11, 10 and 7 months, respectively. At 24 months, ACC had much higher survival (50%) than others (10%) indicating a proportion of ACC with an indolent course.
Conclusion: Our analysis highlights the variable disease biology of advanced salivary gland tumours and throws light on the various possible treatment targets and strategies. Molecular profiling and advancement in targeted therapies are expected to increase survival in this group of rare cancers.
Keywords: salivary gland tumour, adenoid cystic, mucoepidermoid, mammary analogue secretory, salivary duct
Correspondence to: Akhil Kapoor
Email: kapoorakhil1987@gmail.com
Published: 21/09/2023
Received: 11/03/2023
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Salivary gland tumours are relatively rare cancers with incidence rates of 11.95 per 1,000,000 person-years [1, 2]. Although collectively termed together, their histology varies along with the presence of a gamut of molecular alterations which also differ based on the subtype [3, 4]. Their significant proportion are cured by surgery alone, while a few require definitive or adjuvant radiation therapy. Adjuvant systemic therapy is not recommended currently. This strategy leads to reasonably acceptable 5-year overall survival (OS) rates of 55%–85% [1]. However, sometimes these tumours may present upfront with locally advanced or metastatic disease or may recur with local and/or distant metastases which are not amenable to definitive treatment [5, 6].
These advanced salivary gland tumours consisting of stages 4A to 4C have a wide variation in their clinical behaviour ranging from indolent to highly aggressive disease courses. Also, the multitude of molecular alterations may affect the tempo of the disease and the responses to treatment [7].
Due to the rarity of these tumours, there is scarce data regarding real-life evidence of how the treatment pans out for these patients. Also, the limited number of randomised clinical trials for evaluating the efficacy of various therapeutic options leads to significant variations in clinical practice [8]. This has led to a lack of information on the number of patients who undergo molecular testing, those who receive treatment and the type of treatment received. Furthermore, there is a lack of clarity on how the various available treatments are sequenced and how it affects the outcome of these patients. For example, it is unknown whether initiating hormonal therapy as the first-line treatment is better than chemotherapy in androgen receptor (AR) positive cancers. Most of the time, the approach to chemotherapy is extrapolated from the guidelines for other head and neck cancers which have a different biology [6]. Thus, it is imperative that we know which treatment regimens are most effective in terms of responses elicited and how the prognosis in terms of survival varies with the current treatment patterns. The aim and purpose of our analysis is to summarise data from our country in terms of molecular tests done, various chemotherapeutic, hormonal and targeted treatment received and their effect on the outcome and comparison of the same with published data. We hypothesise a change in the treatment pattern in our country compared to other available data due to a paucity of widespread molecular testing for all patients.
Materials and methods
This is a retrospective analysis carried out in the Medical Oncology Department of our institute. All patients seeking treatment for advanced salivary gland tumours from the period of October 2018 to September 2022 were included. The data were extracted from the electronic medical records by searching for the following keywords – ‘salivary gland tumours’, ‘adenoid cystic carcinoma’, ‘mucoepidermoid carcinoma’, ‘salivary duct carcinoma’ and ‘mammary analog secretory carcinoma’. Only advanced cases deemed to be unsuitable for curative treatment were selected for the analysis. Patients who were diagnosed at our centre but were not initiated on treatment or who did not complete at least 2 months of treatment were excluded. All the relevant clinical details like the duration and symptoms of presentation were noted. Past treatment details including the clinical stage and modalities of treatment administered were also noted. The relevant details post progression to advanced disease were studied to know the sites of progression and the treatment path adopted. For radiological response assessment, the Response Evaluation Criteria in Solid Tumours (RECIST) 1.1 criteria was used. For calculating overall response rates (ORRs), both partial response and stable disease as per RECIST criteria were included [9]. All the pathological details were noted including the molecular tests done and their interpretation. The molecular tests done included immunohistochemical (IHC) assays for AR and human epidermal growth factor receptor 2 (HER2) and next-generation sequencing (NGS) of tumour tissue wherever the results were available. NGS was done on SOPHiA Solid Tumour Plus Solution, which uses both DNA and RNA extracts. The outcome variables for our analysis were OS and progression-free survival (PFS). OS was calculated from the date of diagnosis till death. PFS was calculated from the date of the start of the therapy to the date of frank clinical or radiological progression. Patients lost to follow-up were contacted telephonically to know about their disease status. Those patients who could still not be traced in this manner were censored for the events of progression or death at an interval of 3 months post-last follow-up.
Statistical analysis
The statistical analysis was done using the Statistical Package for Social Sciences version 20. Mean, median and mode were calculated for quantitative data. The normality of data were assessed by using Q–Q plots. Univariate analysis was done to derive the p-value by chi-square calculation. OS and PFS curves were derived by Kaplan–Meier method. A p-value cut-off of less than 0.05 was considered significant.
Results
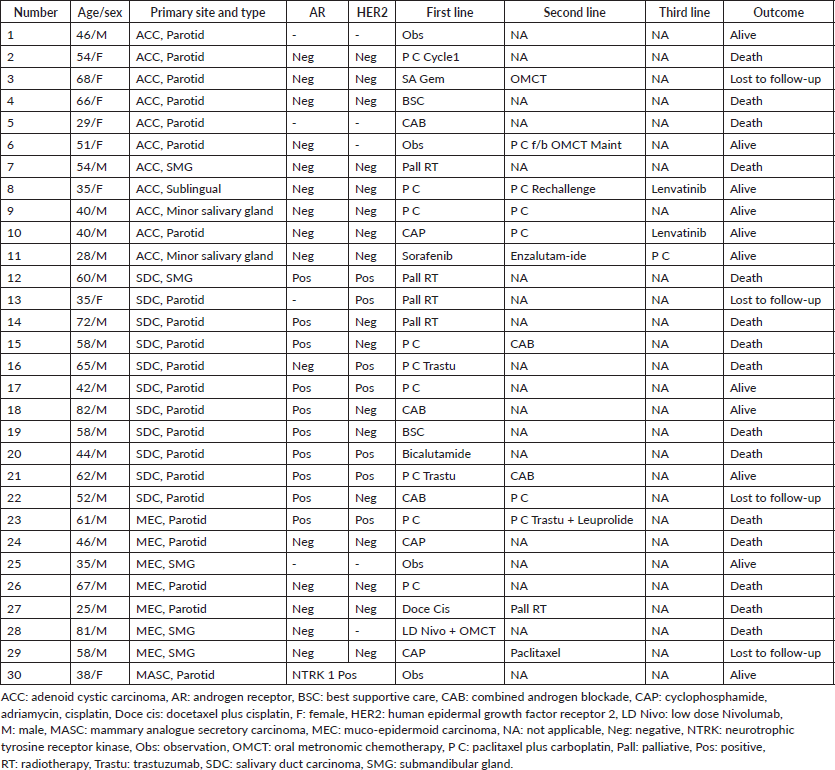
A total of 33 cases were identified. Out of these, one patient did not take any treatment at our centre, and for two patients follow-up details could not be traced after treatment initiation. So, these were excluded from the analysis. The basic clinical–pathological details are presented in Table 1.
The age range of patients was 25–82 years with a median of 52 years. The parotid gland was the most common site of origin (73%) while adenoid cystic carcinoma (ACC) and salivary duct carcinoma (SDC) were equally (37%) the most common pathological subtypes. The univariate analysis of various factors with the pathological subtypes is presented in Table 2. A male predominance (73%) was noted overall. Advanced mucoepidermoid carcinoma (MEC) was seen only in males while for advanced SDC, all except one patient was female. On the other hand, advanced ACC had a female predominance (55%). The majority of advanced tumours were recurrent (57%) rather than upfront advanced (43%). Among subtypes, ACC had the highest proportion (82%) of recurrent cases while other subtypes had a high proportion of upfront advanced cases. Lungs (57%) were the most common site of metastases, followed by bones. SDC (82%) and ACC (64%) had a high proportion of lung metastases but MEC had no incidence of the same.
The molecular profile of all tumours was analysed and the salient features are shown in Table 2. The AR and HER2 positivity rates are highest in SDCs while lowest in ACCs. The single case of Mammary analogue secretory carcinoma (MASC) was positive for pan-TRK IHC.
NGS was done in seven patients. One patient with SDC had mutations in the PIK3CA, HRAS and TP53 genes concurrently.
Table 1. Clinical–pathological details of patients included in the analysis.
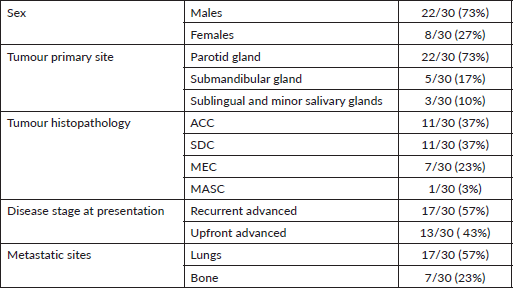
Table 2. Univariate analysis for association of various clinical factors with different subtypes of salivary gland tumours.
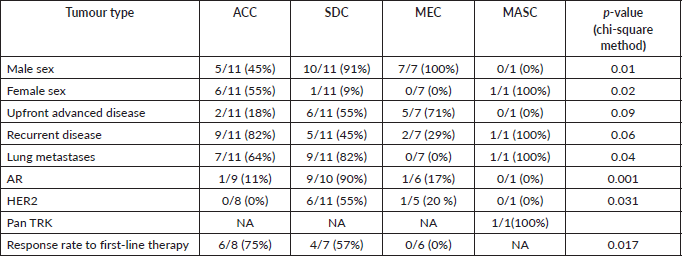
The treatment profile of all the patients is provided in the Supplementary Table 1. A variety of treatment regimens were used in first-line treatment of 21 patients who were started on systemic therapy. Four asymptomatic patients were kept on observation. The most common chemotherapy regimen was a combination of paclitaxel and carboplatin with or without trastuzumab based on the HER2 status, while the most common hormonal therapy was the combined androgen blockade (CAB) of bicalutamide and leuprolide. The ORR to first-line treatments was 10/21 ( 48%). Only 9/21 (43%) went on to receive second-line treatment in which the ORRs were 4/9 (44%). The best response to first-line therapy was seen in ACC (75%) while MEC had zero response rates.
The median follow-up of all patients was 18 months with the range being 6–69 months. The PFS with first-line treatment (PFS1) was a median of 5 months ( 95% confidence interval (CI); 0.5–9.4 months) as depicted in Figure 1. The median PFS1 was worst for MEC followed by ACC and SDC respectively as shown in Figure 2. After 17 months of follow-up, ACC had a better PFS1 than SDC indicating a subgroup with a better prognosis. The PFS with second-line treatment (PFS2) was a median of 4 months (95% CI; 2.5–5.4 months). The median OS was 10 months ( 95% CI; 4.9–15 months) depicted in Figure 3. Median OS for ACC, SDC and MEC were 11, 10 and 7 months, respectively, as depicted in Figure 4. ACC has the best survival while MEC has the worst. At 24 months, ACC had much higher survival (50%) than others (10%).
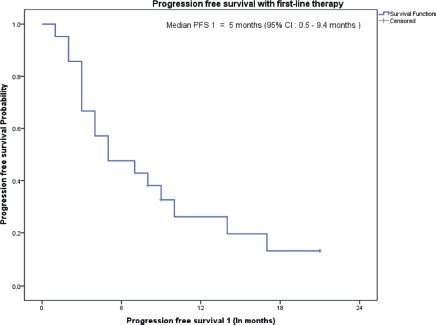
Figure 1. PFS on first-line treatment.
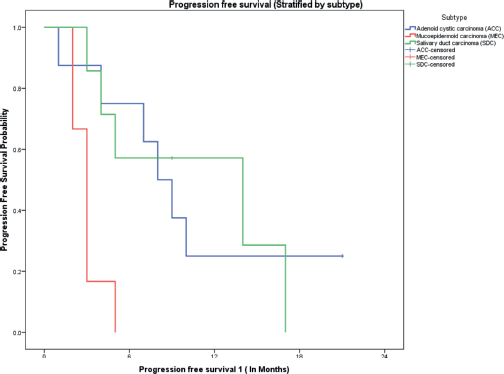
Figure 2. PFS on first-line therapy stratified by pathological subtypes.
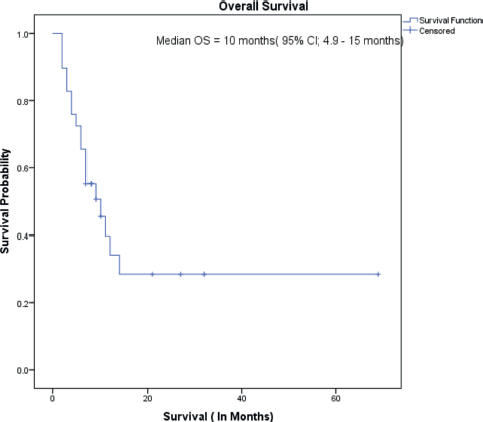
Figure 3. Overall survival.
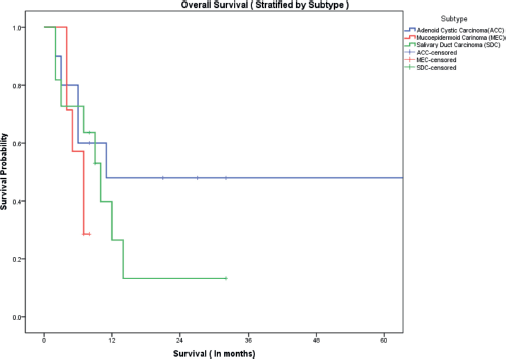
Figure 4. OS stratified by subtype.
Discussion
We would like to restate our aim of bringing out the clinical, pathological, molecular and treatment heterogeneity of advanced salivary gland tumours and the patterns observed at a tertiary care centre in our country. There is a paucity of data focussing on this group of patients in advanced stages [10]. Our study adds value by providing important inputs relevant to the treatment in the current era of molecular profiling.
The ACC group had the maximum percentage of recurrent cases (82%). This might be due to their indolent nature and long natural history leading to more patients being detected at early resectable stages. This will lead to more chances of them undergoing definitive surgeries and later presenting with recurrence. On the other hand, the SDC and MEC groups present more commonly with upfront unresectable or metastatic disease due to their aggressive tempo [7].
There is a lack of consensus on the first-line treatment option to be used as evidenced by the use of as many as seven different regimens. Also, some patients may be mildly symptomatic or asymptomatic at presentation for whom observation may be a plausible option [7]. The PFS of just 5 months on first-line treatments along with a less than 50% ORR indicates that the disease is not very sensitive to chemotherapy and targeted treatments and may progress rapidly indicating the complexity of underlying driver pathways [11]. However, 43% of patients were able to receive second-line therapies, thus suggesting that disease biology in this subset of patients is favourable leading to an indolent course. Again, a wide variety of second-line regimens were used. Some of these treatment regimens were extrapolated from the head and neck treatment data.
Comparing our data on ACC with other data available from meta-analyses and other studies, some similarities are appreciated [12]. The ORR with platinum-based combinations was in the range of 50%–80% compared to our data of 75% [13–16]. Tyrosine kinase inhibitors like lenvatinib, sunitinib, axitinib and sorafenib were associated with high rates of stable disease 60%–90% [17–21]. Our patients treated on lenvatinib and sorafenib also demonstrated high disease control rates even in the third-line setting. This suggests that these agents can be used from the second line onward and even may be a suitable maintenance strategy post-first-line taxane-platinum combination therapy. Also, watchful observation seems to be an excellent option in asymptomatic patients [22]. However. the median OS was only 11 months despite a 2-year survival of 50% indicating the need to identify this subset of aggressive ACCs [23]. Although the numbers are small, female patients in our dataset had the most aggressive disease (progression within 6 months of treatment initiation) and may warrant more potent therapy [24].
SDCs having near universal AR and very high HER2 positivity rates respectively are seen even in our patients [25–28]. The response rates in a systematic review of these patients were 60%–70%,18%–53% and 10%–50% with HER2 targeted therapy, androgen blockade therapy, and chemotherapy respectively [29–34]. Our response rates are similar with a 57% response rate. Although CAB was not very effective in our patients, a reasonable conclusion cannot be reached due to the low sample size. The available data regarding PFS 1 and OS is scarce but our patients had a median PFS1 and OS comparable with those with ACC. But, the 2-year survival of less than 20% indicates aggressiveness. As discussed by Dalin et al [26], the molecular signature similarities with apocrine breast cancer may be extrapolated in SDC with reference to future research in treatment options [26]. Thus, HER2-directed therapy combined with chemotherapy followed by CAB and anti-HER2 therapy as maintenance seems to be a sound strategy.
MECs had the poorest response to treatment and equally poor survival. This, along with the lack of adequate data, calls for more research on molecular niches and effective treatment strategies [35–37]. We found a case of MASC which was positive for IHC for pan-TRK. This IHC was utilised in line with the available literature suggesting the benefits of pan-TRK IHC as a time and tissue-efficient screen for NTRK fusions [38]. Recently, the European Society of Medical Oncology has released guidelines for the management of salivary gland tumours emphasising molecular testing [39]. A report by Kapoor et al [10] also highlighted the importance of identifying molecular targets and proposed a treatment algorithm for this rare disease. So, it is expected that a NGS-based testing strategy will soon become the standard of care.
Conclusion
Our analysis highlights the variable disease biology of advanced salivary gland tumours, especially the indolent nature of ACC compared to others. It also throws light on the various possible treatment targets and strategies like CAB and its combination with anti-HER2 therapy. Molecular profiling and advancement in targeted therapies are expected to increase the survival in this group of rare cancers by enabling a more personalised treatment approach.
Conflicts of interest
The authors report no conflicts of interest.
Data sharing
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Funding
None of the co-authors have received any financial aid in carrying out this analysis and neither do they have any conflicting financial or ethical interests with reference to this manuscript.
References
1. Carvalho AL, Nishimoto IN, and Califano JA, et al (2005) Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database Int J Cancer 114 806–816 https://doi.org/10.1002/ijc.20740
2. Boukheris H, Curtis RE, and Land CE, et al (2009) Incidence of carcinoma of the major salivary glands according to the WHO classification, 1992 to 2006: a population-based study in the United States Cancer Epidemiol Biomarkers Prev 18(11) 2899–2906 Epub 2009 Oct 27 https://doi.org/10.1158/1055-9965.EPI-09-0638 PMID: 19861510 PMCID: 2779732
3. Iyer J, Hariharan A, and Cao UMN (2021) An overview on the histogenesis and morphogenesis of salivary gland neoplasms and evolving diagnostic approaches Cancers 13(15) 3910 https://doi.org/10.3390/cancers13153910 PMID: 34359811 PMCID: 8345412
4. Bobati SS, Patil BV, and Dombale VD (2017) Histopathological study of salivary gland tumors J Oral Maxillofac Pathol 21(1) 46–50 https://doi.org/10.4103/0973-029X.203762 PMID: 28479686 PMCID: 5406818
5. Wang X, Luo Y, and Li M, et al (2017) Management of salivary gland carcinomas – a review Oncotarget 8(3) 3946–3956 https://doi.org/10.18632/oncotarget.13952 PMCID: 5354805
6. Geiger JL, Ismaila N, and Beadle B, et al (2021) Management of salivary gland malignancy: ASCO guideline J Clin Oncol 39(17) 1909–1941 https://doi.org/10.1200/JCO.21.00449 PMID: 33900808
7. Son E, Panwar A, and Mosher CH, et al (2018) Cancers of the major salivary gland J Oncol Pract 14(2) 99–108 https://doi.org/10.1200/JOP.2017.026856 PMID: 29436307
8. Mizrachi A, Bachar G, and Unger Y, et al (2017) Submandibular salivary gland tumors: clinical course and outcome of a 20-year multicenter study Ear Nose Throat J 96(3) E17–E20 https://doi.org/10.1177/014556131709600320 PMID: 28346650
9. Ruchalski K, Braschi-Amirfarzan M, and Douek M, et al (2021) A primer on RECIST 1.1 for oncologic imaging in clinical drug trials Radiol Imaging Cancer 3(3) e210008 https://doi.org/10.1148/rycan.2021210008 PMID: 33988475 PMCID: 8183261
10. Kapoor A, Noronha V, and Chougule A, et al (2020) Molecular tumor board: case 4 salivary gland cancer Cancer Res Stat Treat 3(3) 554–563 https://doi.org/10.4103/CRST.CRST_258_20
11. Yousaf A, Sulong S, and Abdullah B, et al (2022) Heterogeneity of genetic landscapes in salivary gland tumors and their critical roles in current management Medeni Med J 37(2) 194–202 https://doi.org/10.4274/MMJ.galenos.2022.63139 PMID: 35735183 PMCID: 9234367
12. Ko JJ, Siever JE, and Hao D, et al (2016) Adenoid cystic carcinoma of head and neck: clinical predictors of outcome from a Canadian centre Curr Oncol 23(1) 26–33 Epub 2016 Feb 18 https://doi.org/10.3747/co.23.2898 PMID: 26966401 PMCID: 4754057
13. Airoldi M, Fornari G, and Pedani F, et al (2000) Paclitaxel and carboplatin for recurrent salivary gland malignancies Anticancer Res 20(5C) 3781–3783 PMID: 11268454
14. Licitra L, Cavina R, and Grandi C, et al (1996) Cisplatin, doxorubicin and cyclophosphamide in advanced salivary gland carcinoma. A phase II trial of 22 patients Ann Oncol 7(6) 640–642 https://doi.org/10.1093/oxfordjournals.annonc.a010684 PMID: 8879381
15. Nakano K, Sato Y, and Sasaki T, et al (2016) Combination chemotherapy of carboplatin and paclitaxel for advanced/metastatic salivary gland carcinoma patients: differences in responses by different pathological diagnoses Acta Otolaryngol 136(9) 948–951 Epub 2016 Apr 20 https://doi.org/10.3109/00016489.2016.1170876 PMID: 27094013
16. Schramm VL Jr, Srodes C, and Myers EN (1981) Cisplatin therapy for adenoid cystic carcinoma Arch Otolaryngol 107(12) 739–741 https://doi.org/10.1001/archotol.1981.00790480015004 PMID: 6274284
17. Ho AL, Dunn L, and Sherman EJ, et al (2016) A phase II study of axitinib (AG-013736) in patients with incurable adenoid cystic carcinoma Ann Oncol 27(10) 1902–1908 Epub 2016 Aug 26 https://doi.org/10.1093/annonc/mdw287 PMID: 27566443 PMCID: 5035791
18. Hotte SJ, Winquist EW, and Lamont E, et al (2005) Imatinib mesylate in patients with adenoid cystic cancers of the salivary glands expressing c-kit: a Princess Margaret Hospital phase II consortium study J Clin Oncol 23(3) 585–590 https://doi.org/10.1200/JCO.2005.06.125 PMID: 15659505
19. Chau NG, Hotte SJ, and Chen EX, et al (2012) A phase II study of sunitinib in recurrent and/or metastatic adenoid cystic carcinoma (ACC) of the salivary glands: current progress and challenges in evaluating molecularly targeted agents in ACC Ann Oncol 23(6) 1562–1570 Epub 2011 Nov 11 https://doi.org/10.1093/annonc/mdr522
20. Tchekmedyian V, Sherman EJ, and Dunn L, et al (2019) Phase II study of lenvatinib in patients with progressive, recurrent or metastatic adenoid cystic carcinoma J Clin Oncol 37(18) 1529–1537 Epub 2019 Apr 2 https://doi.org/10.1200/JCO.18.01859 PMID: 30939095 PMCID: 6599407
21. Thomson DJ, Silva P, and Denton K, et al (2015) Phase II trial of sorafenib in advanced salivary adenoid cystic carcinoma of the head and neck Head Neck 37(2) 182–187 Epub 2014 Mar 13 https://doi.org/10.1002/hed.23577
22. Lorini L, Ardighieri L, and Bozzola A, et al (2021) Prognosis and management of recurrent and/or metastatic head and neck adenoid cystic carcinoma Oral Oncol 115 105213 Epub 2021 Feb 9 https://doi.org/10.1016/j.oraloncology.2021.105213 PMID: 33578204
23. Cantù G (2021) Adenoid cystic carcinoma. An indolent but aggressive tumour. Part B: treatment and prognosis Acta Otorhinolaryngol Ital 41(4) 296–307 https://doi.org/10.14639/0392-100X-N1729 PMID: 34533533 PMCID: 8448184
24. Marcinow A, Ozer E, and Teknos T, et al (2014) Clinicopathologic predictors of recurrence and overall survival in adenoid cystic carcinoma of the head and neck: a single institutional experience at a tertiary care center Head Neck 36(12) 1705–1711 Epub 2014 Feb 1 https://doi.org/10.1002/hed.23523
25. Boon E, Bel M, and van Boxtel W, et al (2018) A clinicopathological study and prognostic factor analysis of 177 salivary duct carcinoma patients from The Netherlands Int J Cancer 143(4) 758–766 Epub 2018 Mar 23 https://doi.org/10.1002/ijc.31353 PMID: 29492965 PMCID: 6055864
26. Dalin MG, Desrichard A, and Katabi N, et al (2016) Comprehensive molecular characterization of salivary duct carcinoma reveals actionable targets and similarity to apocrine breast cancer Clin Cancer Res 22(18) 4623–4633 Epub 2016 Apr 21 https://doi.org/10.1158/1078-0432.CCR-16-0637 PMID: 27103403 PMCID: 5026550
27. Takase S, Kano S, and Tada Y, et al (2017) Biomarker immunoprofile in salivary duct carcinomas: clinicopathological and prognostic implications with evaluation of the revised classification Oncotarget 8(35) 59023–59035 https://doi.org/10.18632/oncotarget.19812 PMID: 28938615 PMCID: 5601711
28. Schmitt NC, Kang H, and Sharma A (2017) Salivary duct carcinoma: an aggressive salivary gland malignancy with opportunities for targeted therapy Oral Oncol 74 40–48 Epub 2017 Sep 21 https://doi.org/10.1016/j.oraloncology.2017.09.008 PMID: 29103750 PMCID: 5685667
29. Corrêa TS, Matos GDR, and Segura M, et al (2018) Second-line treatment of HER2-positive salivary gland tumor: ado-trastuzumab emtansine (T-DM1) after progression on trastuzumab Case Rep Oncol 11(2) 252–257 https://doi.org/10.1159/000488669 PMID: 29867432 PMCID: 5981674
30. Viscuse PV, Price KA, and Garcia JJ, et al (2019) First line androgen deprivation therapy vs. chemotherapy for patients with androgen receptor positive recurrent or metastatic salivary gland carcinoma – a retrospective study Front Oncol 9 701 https://doi.org/10.3389/fonc.2019.00701
31. Uijen MJM, Lassche G, and van Engen-van Grunsven, et al (2020) Systemic therapy in the management of recurrent or metastatic salivary duct carcinoma: a systematic review Cancer Treat Rev 89 102069 Epub 2020 Jul 15 https://doi.org/10.1016/j.ctrv.2020.102069 PMID: 32717621
32. Takahashi H, Tada Y, and Saotome T, et al (2019) Phase II trial of trastuzumab and docetaxel in patients with human epidermal growth factor receptor 2-positive salivary duct carcinoma J Clin Oncol 37(2) 125–134 Epub 2018 Nov 19 https://doi.org/10.1200/JCO.18.00545
33. Locati LD, Perrone F, and Cortelazzi B, et al (2016) Clinical activity of androgen deprivation therapy in patients with metastatic/relapsed androgen receptor-positive salivary gland cancers Head Neck 38(5) 724–731 Epub 2015 Jun 25 https://doi.org/10.1002/hed.23940
34. Fushimi C, Tada Y, and Takahashi H, et al (2018) A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma Ann Oncol 29(4) 979–984 https://doi.org/10.1093/annonc/mdx771 PMCID: 5913639
35. Nakano T, Yamamoto H, and Hashimoto K, et al (2013) HER 2 and EGFR gene copy number alterations are predominant in high‐grade salivary mucoepidermoid carcinoma irrespective of MAML 2 fusion status Histopathology 63(3) 378–392 https://doi.org/10.1111/his.12183 PMID: 23855785
36. Ullah A, Khan J, and Waheed A, et al (2023) Mucoepidermoid carcinoma of the salivary gland: demographics and comparative analysis in U.S. children and adults with future perspective of management Cancers 15(1) 250 https://doi.org/10.3390/cancers15010250 PMID: 36612247 PMCID: 9818327
37. Wang K, McDermott JD, and Schrock AB, et al (2017) Comprehensive genomic profiling of salivary mucoepidermoid carcinomas reveals frequentBAP1, PIK3CA, and other actionable genomic alterations Ann Oncol 28(4) 748–753 https://doi.org/10.1093/annonc/mdw689 PMID: 28327999
38. Hechtman JF, Benayed R, and Hyman DM, et al (2017) Pan-Trk immunohistochemistry is an efficient and reliable screen for the detection of NTRK fusions Am J Surg Pathol 41(11) 1547–1551 https://doi.org/10.1097/PAS.0000000000000911 PMID: 28719467 PMCID: 5636652
39. van Herpen C, Vander Poorten V, and Skalova A, et al (2022) Salivary gland cancer: ESMO-European Reference Network on Rare Adult Solid Cancers (EURACAN) clinical practice guideline for diagnosis, treatment and follow-up ESMO Open 7(6) 100602 Epub 2022 Nov 2 https://doi.org/10.1016/j.esmoop.2022.100602 PMID: 36567082 PMCID: 9808465
Supplementary Table 1. Treatment details of all patients.