Prevalence and determinants of lymphedema in newly diagnosed Nigerian breast cancer patients using bioimpedance estimations
Funmilola Wuraola1,2,3, Olalekan Olasehinde1,2,3, Matteo Di Bernardo3, Patrick Akinyemi3, Israel Owoade3, Tajudeen Mohammed1,3, Adewale Aderounmu1,3, Samson Ogunleye3, Adeoluwa Adeleye3, Mary Ogunyemi3, Gregory Knapp3,4, Peter Kingham3,5 and Olusegun Alatise1,2,3
1Department of Surgery, Obafemi Awolowo University, Ile-Ife, Nigeria
2Department of Surgery, Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife, Nigeria
3African Research Group for Oncology, Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife, Nigeria
4Department of Surgery, Division of General Surgery, Dalhousie University, Halifax, Nova Scotia, NS B3H 4R2, Canada
5Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA
Abstract
Background: Breast cancer-related lymphedema (BCRL) is common and has significant impact on quality of life. Very little is known about BCRL in sub-Saharan Africa. Generally, BCRL has been mostly evaluated post treatment, with very limited data on the prevalence of pre-treatment BCRL at baseline. This study presents the prevalence and clinical associations of lymphedema among newly diagnosed, treatment-naive breast cancer patients in a Nigerian cohort using bioimpedance estimations.
Methods: Consecutively consenting, newly diagnosed, treatment-naive breast cancer patients were assessed for upper limb lymphedema using bioimpedance measurements of the extracellular fluid and the single-frequency bioelectrical impedance analysis value at 5 kHz. Patients were classified as having lymphedema if there was >10% difference in arm measurements or if the ratios of the arm measurements were >3 SD above a normative mean generated from representative controls. Regression analysis was performed to determine clinical variables associated with lymphedema.
Results: There were 154 breast cancer patients with a median age of 47 (40.0–56.8) years and a body mass index of 27 (23.5–30.9) kg/m2. The majority (70%) had stage III disease. All measurements were significantly higher in cases than controls. Using various definitions, the prevalence of lymphedema was between 11.7% and 14.3%. Various clinical variables relating to clinical stage were significantly associated with lymphedema.
Conclusion: The predominance of locally advanced disease in the Nigerian setting is associated with high pre-treatment lymphedema rates. This may set the stage for higher rates in the post-operative setting. Management of lymphedema should be incorporated into the treatment planning.
Keywords: breast, cancer, lymphedema, bioimpedance
Correspondence to: Dr Olalekan Olasehinde
Email: oolasehinde@oauife.edu.ng
Published: 09/02/2023
Received: 10/12/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Breast cancer-related lymphedema (BCRL) is a common complication of breast cancer treatment characterised by an abnormal collection of protein-rich fluid in the interstitial space. Lymphedema can occur following the disruption of the lymphatic drainage from the ipsilateral arm during axillary lymph node staging/resection [1–3]. There is significant heterogeneity in the reported incidence of lymphedema (2%–65%), which is largely attributable to highly variable diagnostic criteria.
Lymphatic disruption may result from extensive nodal metastasis, axillary surgery or irradiation [4–6]. Some patient-related factors such as obesity and recurrent cancer have also been implicated as predisposing factors [1, 2]. More recent data from the United States show some compelling evidence implicating race as a risk factor for developing lymphedema. Black women were observed to have higher odds of developing lymphedema compared to non-African American women [7].
Lymphedema, particularly in severe cases, can be challenging to manage and can have a significant impact on a number of quality of life indicators [5]. Most of the available treatment modalities aim to limit its progression and/or symptomatology as there is yet no proven curative treatment [8–10]. The prevention of lymphedema is considered the most pragmatic approach whenever feasible. To some extent, this has been made possible by the development of effective therapies and early detection of breast cancer, which has enabled the avoidance or de-escalation of axillary surgery.
In Nigeria, as in many parts of sub-Saharan Africa, the subject of lymphedema has been understudied with very limited data on its prevalence and management. The recent data implicating race as a risk factor, coupled with the high prevalence of locally advanced disease with nodal metastasis at the time of initial presentation are compelling reasons to evaluate this subject in the Nigerian setting. Even in early-stage disease, axillary management often entails axillary dissection in Nigeria, given the limited expertise and infrastructure for sentinel lymph node biopsy [11–13].
Anecdotally, lymphedema appears to be a common problem among newly diagnosed breast cancer patients in the Nigerian setting. To quantify this observation, the African Research Group for Oncology (ARGO) started collecting prospective data on lymphedema following modified radical mastectomy in Nigeria. This study presents the prevalence of lymphedema among newly diagnosed breast cancer patients prior to commencement of treatment. It is believed that this work will form an important building block for designing tailor-made interventions targeting BCRL in Nigeria.
Methods
Consecutive, newly diagnosed breast cancer patients presenting to Obafemi Awolowo University Teaching Hospital over a 3-year period (2019–2021) were recruited for enrolment in this study. The hospital has a prospectively maintained breast cancer database with over a 1,000 patients. The database captures clinical, pathological and treatment-related details with a standardised follow-up protocol that captures treatment outcomes.
Patients
The patient population in this study included newly diagnosed, treatment-naïve, histologically confirmed breast cancer patients. Relevant clinical details were obtained, including sociodemographic characteristics, anthropometric variables (e.g. weight and height), histopathology and stage at presentation. Patients with bilateral disease at presentation and those with previous breast or axillary operations were excluded.
Controls
Healthy individuals from the community who responded to an invitation for routine bioimpedance measurements served as controls. These controls were randomly matched in a 1:1 fashion to cases with age being less than or equal to 2 years apart, and body mass index (BMI) being less than or equal to 1 standard deviation (SD) of the BMI of the cases (5.5 kg/m2). Given that healthy controls did not present with a malignancy, the arm with the higher bioimpedance measurement was used as the affected side and compared to the measurement with the smaller value employed as the unaffected side.
Bioimpedance measurements
Patients and controls had bioimpedance assessments using the Inbody 770-2.0® (Biospace, Seoul, South Korea) bioimpedance machine. All measurements were performed by a trained staff using the same machine according to the manufacturer’s guide. Each participant stood barefooted on the two footplates with the heels on the rear sole electrodes and the front parts on the front electrodes. The two arms were kept away from the body with the four-fingers wrapped around the surface of the bottom hand electrode and the thumb on the oval electrode. Details of the bioimpedance measurements were subsequently presented on the screen and exported for analysis.
The bioimpedance estimations of the extracellular fluid (ECF) volume and single-frequency bioelectrical impedance analysis value at 5 kHz for both upper extremities were used in the determination of lymphedema estimates in this study.
The first set of lymphedema-related measurements was derived from the extracellular water (ECW) measurements for each arm. Comparing the affected to the unaffected side, the ratio of ECW of affected versus unaffected arm (continuous), and difference in ECW of >10% between the affected and unaffected arm (binary) were calculated for both cases and controls.
The second set of lymphedema-related measurements was derived from 5 kHz impedance measurements for each arm. Comparing the unaffected side to the affected side, we derived the ratio of 5 kHz impedance measurement of unaffected versus affected arm (continuous), and difference in 5 kHz measurements > 10% (binary) between unaffected versus affected arm.
Definition of lymphedema
Lymphedema was defined using various diagnostic criteria available in the literature [3, 14–18]:
(a) A difference of >10% in the ECW measurements of affected versus unaffected arm.
(b) A difference of >10% in the 5 kHz impedance measurements of unaffected versus affected arm.
(c) ECW ratio (affected versus unaffected arm) more than 3 SDs above the normative data (derived from controls).
(d) 5 kHz impedance ratio (unaffected versus affected arm) more than 3 SDs above the normative data (derived from controls).
Statistical analysis
All statistical analysis was done in R-studio 1.3.1073, using R v 4.0.2.
One-sided t-tests were used to compare measurements (continuous or binary) between cases and controls. Based on previously published data, a number of quantitative indicators were employed to understand the prevalence of lymphedema at presentation (>10% difference in measurements and bioimpedance ratios > 3 SD above controls), and if such measurements are associated with clinical data. We examined the relationship between a number of clinical variables and the presence of lymphedema, including affected side (right, left), sex (female, male), age (years), BMI (kg/m2), body surface area (BSA) (m2), waist circumference (cm), hip circumference (cm), mass size (cm), clinical T stage (1, 2, 3, 4), clinical N stage (1, 2, 3), clinical M stage (0, 1) and overall clinical stage (I, II, III, IV). All regression models were performed as univariate due to the close inter-relatedness of these clinical variables.
For cases only, linear regression models were used for continuous measurements to understand how various clinical variables may be statistically relevant in predicting lymphedema. This was done for all continuous lymphedema measurements that were statistically significant (in comparing cases and controls) in the methodologies above. Logistic regression models were used for binary measurements in a similar fashion. This was done for all binary lymphedema measurements that were statistically significant (in comparing cases and controls) in the tests above. The lymphedema measurement that showed the lowest incidence (difference in ECW > 10%) was selected for the univariate analysis. This was quite representative of the clinical associations that were observed with the other lymphedema measurements. For all statistical analysis, p value was set at 0.05.
Results
Baseline characteristics
A total of 154 Nigerian breast cancer patients were included in this study (Table 1). From a total of 783 healthy volunteers, 148 healthy controls were matched to the cases. The median age and BMI of the breast cancer patients were 47 (40.0–56.8) years and 27 (23.45–30.97) kg/m2, respectively. In the control group, median age was 46 (40–53) years while BMI was 27.75 (23.62–30.55) kg/m2. The median tumour size was 8.5 (6–14) cm with the majority (70%) having stage III disease.
Table 1. Clinical characteristics of cases and controls.
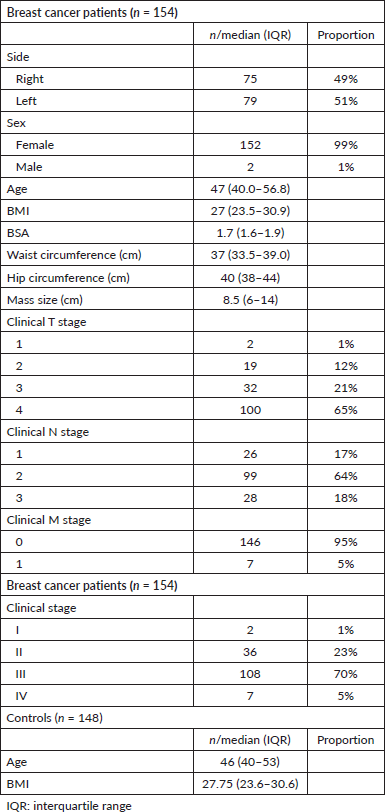
Table 2. Prevalence of lymphedema.

Table 3. Comparison of cases and controls.
Bioimpedance estimated prevalence of lymphedema
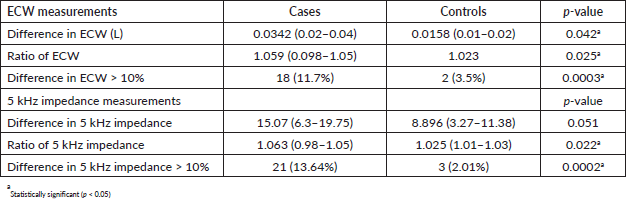
The proportion of patients with lymphedema based on the difference of >10% in the ECW arm measurements was 11.7% while 12.3% of the patients were adjudged to have lymphedema using ECW ratio of more than 3 SDs above the normative data (>1.091).
Using a single-frequency bioimpedance measurement at 5 kHz, the proportion of patients with lymphedema was 13.6% based on a difference of >10% between the arms, while it was 14.3% using the 5 kHz impedance ratio greater than 3 SDs above the normative data (>1.095) (Table 2).
Comparison of cases and controls
The prevalence of lymphedema among breast cancer patients was significantly higher than in controls (11.7%–14.3% versus 0%–2.0%). A comparison of all but one bioimpedance measurements (difference in 5 kHz impedance) between cases and controls showed statistically significant differences in all measured quantities (Table 3).
Table 4. Clinical determinants of lymphedema.
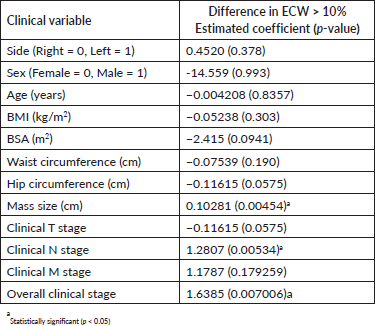
Clinical determinants of lymphedema
Of the clinical variables tested, mass size, nodal staging and overall clinical staging were the only factors that were significantly associated with the development of lymphedema. Age, BMI and other clinical variables were not significantly associated with the development of lymphedema (Table 4).
Discussion
BCRL has been mostly evaluated following treatment. This study focused on disease-related lymphedema using bioimpedance measurements among newly diagnosed, treatment-naive breast cancer patients. Evaluating the prevalence of lymphedema at baseline provides some insight into preoperative factors that might be associated with its occurrence and also allows for comparison of pre-and post-treatment values to determine whether treatments worsen or resolve it. For the first time in sub-Saharan Africa, we report a baseline incidence of lymphedema of 11.7%–14.3% in breast cancer patients at presentation, particularly in patients presenting with locally advanced disease, which comprised the majority of the study population. The high prevalence of lymphedema justifies routine surveillance at the time of initial presentation as early diagnosis may prompt the inclusion of preventive and therapeutic measures in the treatment plan. Currently, there are no clear-cut guidelines or recommendations on the management of lymphedema in the pre-operative setting and how it might affect decision-making regarding surgery and irradiation. Presumably in these patients, the surgical plan will be individualised to minimise further lymphatic disruption and worsening of lymphedema post-operatively.
Traditionally, arm circumference measurement has been the most commonly used method of diagnosing lymphedema. The most common criterion for lymphedema diagnosis using arm circumference is greater than or equal to 2 cm inter-limb difference at any single location, or greater than or equal to 200 mL volume difference. Given that arm circumference measurements assess the volume of the entire limb, it might be affected by changes in muscle and fat mass [15, 19]. This study adopted the use of bioimpedance measurements given its proven validity and improved accuracy over routine arm measurement. Bioimpedance analysis measures body response to applied electrical current and calculates the body fluid volume [14, 20]. The technology can differentiate ECF from total limb volume. Bioimpedance is known to diagnose lymphedema at least 10 months before it becomes clinically apparent [21]. It also compares favourably with optoelectric perometry which uses infrared light emitted from a frame that passes over the limb [17, 22, 23]. Although considered the gold standard, the cost of perometry limits its applicability in resource-constrained settings. Bioimpedance, which is less expensive, is therefore a more attractive option especially in resource-limited settings. In sub-Saharan Africa, the use of bioimpedance for diagnosing lymphedema is quite novel and to date there is no data for comparison. Various diagnostic criteria exist in the literature for defining lymphedema. With the use of bioimpedance, the commonly used criteria are the ECW and single-frequency bioimpedance measurements used in this study. These parameters have been evaluated in various studies with good diagnostic accuracy [3, 16]. The lymphedema rates obtained from the two parameters either by comparing the affected to the unaffected arm or by comparing ratios of patients to controls yielded comparable results.
A baseline prevalence of lymphedema > 10% prior intervention is quite concerning. The patient population in this study may partially explain this high rate. The majority had locally advanced lesions with axillary involvement at presentation (70% had stage III disease). Although the data on pre-operative lymphedema is limited, it appears to be much less common in contemporary series from North America. In a large prospective cohort of women undergoing pre- and post-operative lymphedema screening at Massachusetts General Hospital, only 2.5% of women had an ipsilateral arm volume measurement that was >10% compared to the contralateral side [24]. Early diagnosis of breast cancer reduces the risk of axillary involvement and consequently the risk of lymphedema at the time of presentation. This study clearly identifies three variables: mass size, N stage and overall stage as positive predictors of lymphedema. All of these indicate that advanced disease stage is correlated to and may increase the risks of lymphedema at presentation. Different from several other studies on BCRL, this analysis found no association between lymphedema and BMI [25]. Such associations have been demonstrated among patients evaluated post-treatment rather than newly diagnosed patients as presented in this series.
The need to explore the subject of lymphedema among blacks is substantiated by recent data from the United States. In a cohort of 276 patients who had undergone axillary lymph node dissection, a lymphedema rate of 24.7% was reported, with black women having a 3.8-fold increased risk of developing lymphedema compared to white women after controlling for known-confounders [7]. Our study represents the first step in exploring the subject in details among a homogenously black population. The post-operative lymph oedema rate in our cohort is being evaluated in an ongoing study, and this will be compared with earlier studies. A study comparing lymphedema rates in stage and treatment comparable patients across different races and regions of the world using the same surveillance technique might be an interesting undertaking.
Findings from this study were based solely on bioimpedance measurements. Hence, we did not determine the proportion of patients with clinically significant lymphedema presenting with arm symptoms such as pain, heaviness or swelling. This has been taken into consideration in an ongoing longitudinal study evaluating the prevalence and determinants of BCRL in the same institution. It will also be interesting to know how pre-treatment lymphedema relates to the development of post-treatment lymphedema.
Conclusion
This study has provided evidence that suggests a high prevalence of pre-treatment BCRL in a Nigerian cohort. The findings of this study provide a strong basis to explore this subject in greater detail.
Acknowledgments
The authors appreciate the efforts of Mr Gbenga Ogunleye and other ARGO research staff for their help with patient recruitment.
Funding
This study did not receive any funding for the execution of this project.
Conflicts of interest
The authors have no relevant financial or non-financial conflicts of interest.
Author contributions
All authors contributed to the study conception, design and execution.
Data availability
The datasets used during the current study are not publicly available. It can however be made available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study received necessary approval from the ethical review board. All procedures were carried out in accordance with relevant guidelines and regulations. Consent was obtained from participants before participation.
References
1. Ayre K and Parker C (2019) Lymphedema after treatment of breast cancer: a comprehensive review J Unexplored Med Data 4 5
2. He L, Qu H, and Wu Q, et al (2020) Lymphedema in survivors of breast cancer Oncol Lett 19(3) 2085–2096 PMID: 32194706 PMCID: 7039097
3. Kim L, Jeon JY, and Sung IY, et al (2011) Prediction of treatment outcome with bioimpedance measurements in breast cancer related lymphedema patients Ann Rehabil Med 35(5) 687–693 https://doi.org/10.5535/arm.2011.35.5.687
4. Tandra P, Kallam A, and Krishnamurthy J, et al (2019) Identification and management of lymphedema in patients with breast cancer J Oncol Pract 15(5) 255–262 https://doi.org/10.1200/JOP.18.00141 PMID: 31009281
5. Fish ML, Grover R, and Schwarz GS (2020) Quality-of-life outcomes in surgical vs nonsurgical treatment of breast cancer–related lymphedema: a systematic review JAMA Surg 155(6) 513 https://doi.org/10.1001/jamasurg.2020.0230 PMID: 32347903
6. Ozaslan C and Kuru B (2004) Lymphedema after treatment of breast cancer Am J Surg 187(1) 69–72 https://doi.org/10.1016/j.amjsurg.2002.12.003 PMID: 14706589
7. Barrio AV, Montagna G, and Sevilimedu V, et al (2022) Impact of race and ethnicity on incidence and severity of breast cancer related lymphedema after axillary lymph node dissection: results of a prospective screening study Cancer Res 15 82
8. Li L, Yuan L, and Chen X, et al (2016) Current treatments for breast cancer-related lymphoedema: a systematic review Asian Pac J Cancer Prev APJCP 17(11) 4875–4883 PMID: 28030915 PMCID: 5454690
9. Fu MR (2014) Breast cancer-related lymphedema: symptoms, diagnosis, risk reduction, and management World J Clin Oncol 5(3) 241–247 https://doi.org/10.5306/wjco.v5.i3.241 PMID: 25114841 PMCID: 4127597
10. Marchica P, D’arpa S, and Magno S, et al (2021) Integrated treatment of breast cancer-related lymphedema: a descriptive review of the state of the art Anticancer Res 41(7) 3233–3246 https://doi.org/10.21873/anticanres.15109 PMID: 34230117
11. Olasehinde O, Alatise O, and Omisore A, et al (2021) Contemporary management of breast cancer in Nigeria: insights from an institutional database Int J Cancer 148(12) 2906–2914 https://doi.org/10.1002/ijc.33484 PMID: 33506499 PMCID: 8394611
12. Awofeso O, Roberts AA, and Salako O, et al (2018) Prevalence and pattern of late-stage presentation in women with breast and cervical cancers in Lagos university teaching hospital, Nigeria Niger Med J 59(6) 74–79 https://doi.org/10.4103/nmj.NMJ_112_17
13. Ayandipo OO, Ogun GO, and Adepoju OJ, et al (2020) Impact of axillary node-positivity and surgical resection margins on survival of women treated for breast cancer in Ibadan, Nigeria Ecancermedicalscience 14 1084 https://doi.org/10.3332/ecancer.2020.1084 PMID: 32863878 PMCID: 7434507
14. Warren AG, Janz BA, and Slavin SA, et al (2007) The use of bioimpedance analysis to evaluate lymphedema Ann Plast Surg 58(5) 541–543 https://doi.org/10.1097/01.sap.0000244977.84130.cf PMID: 17452840
15. Hayes S, Cornish B, and Newman B (2022) Comparison of methods to diagnose lymphoedema among breast cancer survivors: 6-month follow-up Breast Cancer Res Treat 89 221–226 https://doi.org/10.1007/s10549-004-2045-x
16. Lim SM, Han Y, and Kim SI, et al (2019) Utilization of bioelectrical impedance analysis for detection of lymphedema in breast cancer survivors: a prospective cross sectional study BMC Cancer 19(1) 669 https://doi.org/10.1186/s12885-019-5840-9 PMID: 31286884 PMCID: 6613266
17. Bundred NJ, Stockton C, and Keeley V, et al (2015) Comparison of multi-frequency bioimpedance with perometry for the early detection and intervention of lymphoedema after axillary node clearance for breast cancer Breast Cancer Res Treat 151(1) 121–129 https://doi.org/10.1007/s10549-015-3357-8 PMID: 25850535
18. Liu S, Zhao Q, and Ren X, et al (2021) Determination of bioelectrical impedance thresholds for early detection of breast cancer-related lymphedema Int J Med Sci 18(13) 2990–2996 https://doi.org/10.7150/ijms.53812 PMID: 34220327 PMCID: 8241780
19. Bland KL, Perczyk R, and Du W, et al (2003) Can a practicing surgeon detect early lymphedema reliably? Am J Surg 186(5) 509–513 https://doi.org/10.1016/j.amjsurg.2003.07.003 PMID: 14599616
20. Böhm A and Heitmann BL (2013) The use of bioelectrical impedance analysis for body composition in epidemiological studies Eur J Clin Nutr 67(S1) S79–S85 https://doi.org/10.1038/ejcn.2012.168 PMID: 23299875
21. Cornish BH, Chapman M, and Hirst C, et al (2001) Early diagnosis of lymphedema using multiple frequency bioimpedance Lymphology 34(1) 2–11 PMID: 11307661
22. Ward LC, Czerniec S, and Kilbreath SL, et al (2009) Operational equivalence of bioimpedance indices and perometry for the assessment of unilateral arm lymphedema Lymphat Res Biol 7(2) 81–85 https://doi.org/10.1089/lrb.2008.1027 PMID: 19522677
23. Czerniec SA, Ward LC, and Lee MJ, et al (2011) Segmental measurement of breast cancer-related arm lymphoedema using perometry and bioimpedance spectroscopy Support Care Cancer 19(5) 703–710 https://doi.org/10.1007/s00520-010-0896-8
24. Sun F, Skolny MN, and Swaroop MN, et al (2016) The need for preoperative baseline arm measurement to accurately quantify breast cancer-related lymphedema Breast Cancer Res Treat 157(2) 229–240 [doi: 10.1007/s10549-016-3821-0] https://doi.org/10.1007/s10549-016-3821-0 PMID: 27154787
25. Tandra P, Kallam A, and Krishnamurthy J, et al (2019) Identification and management of lymphedema in patients with breast cancer J Oncol Pract 15(5) 255–262 [doi: 10.1200/JOP.18.00141] https://doi.org/10.1200/JOP.18.00141 PMID: 31009281






