Real-world analysis of the use of lenvatinib in differentiated thyroid cancers
Zoya Peelay, Deevyashali Parekh, Vijay M Patil, Vanita Noronha, Nandini Menon and Kumar Prabhash
Department of Medical Oncology, Tata Memorial Hospital, HBNI, Parel, Mumbai 400012, India
Abstract
Introduction: Lenvatinib is one of the approved treatments for radioiodine-refractory differentiated thyroid cancers. However, there is very limited data from India on real-world efficacy and adverse events of Lenvatinib and hence this analysis was performed.
Methods: This was a retrospective analysis in which patients of radioiodine-refractory differentiated thyroid cancer as per the SELECT study criteria, who received lenvatinib, were selected for the study over the last 4 years. The baseline demographic characteristics, adverse events of lenvatinib, the date of progression and the date of overall survival (OS) were extracted from the electronic medical records of Tata Memorial Hospital. SPSS version 20 was used for analysis.
Results: The median starting dose of lenvatinib was 20 mg. Fifteen events for progression had occurred and the median progression-free survival (PFS) was 12.2 months [95% CI: 4.4–not available (NA)]. The events for OS analysis were 12. The median OS was 35.3 months (95% CI: 11.4–NA). There was no impact on starting dose on PFS or OS.
Conclusion: The real-world data of Lenvatinib suggest a lot of variability in the starting dose. In spite of this variability, the response rates and OS are similar to that noted in pivotal study. This suggests a case for need for more studies evaluating lower doses of Lenvatinib.
Keywords: lenvatinib, thyroid cancer, papillary, follicular, radioiodine refractory, dedifferentiated
Correspondence to: Kumar Prabhash
Email: kumarprabhashtmh@gmail.com
Published: 30/01/2023
Received: 13/10/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Thyroid cancers are a relatively uncommon malignancy in India; however, amongst them, differentiated thyroid cancers are one of the commonest malignancies. Differentiated thyroid cancers are treated with surgery followed by radioiodine therapy as per the risk stratification. In spite of this treatment, nearly two third of patients will become radioiodine refractory over their lifetime. When the patients become radioiodine refractory, treatment options are very limited. Lenvatinib was not available in India until recently and hence these patients were treated with sorafenib. Lenvatinib became available in India in 2018 and has been used frequently since then.
Lenvatinib is a multi-kinase inhibitor. Different doses of lenvatinib are used in different cancer settings and there remain concerns with respect to adverse events of lenvatinib. Grade 3 and above adverse events are seen in more than 50% of patients. Thus, frequent dose modifications are required. There is very limited data from India about the efficacy, compliance and side effects of lenvatinib, hence this study was contemplated. Patients who have radioiodine refractory thyroid cancers and are treated with lenvatinib over the past 4 years were included in the study. The primary objective was to study the efficacy of lenvatinib and the secondary objective was to study the adverse events and compliance in dedifferentiated thyroid cancers.
Methods
Selection of patients
The Head and Neck Medical oncology unit of Tata Memorial Center maintains a prospective database of all the patients undergoing chemotherapy under their care. Patients were selected from this database for the current analysis. The criteria to select these patients were as follows:
1. Adult patients of age ≥ 18 years
2. Diagnosed with thyroid cancer
3. Treated with lenvatinib
4. Time period from January 2018 to December 2021
5. The patients who satisfied all the above-mentioned four criteria were selected.
Data collection
The data regarding baseline characteristics, previous treatment details, lenvatinib administration, compliance to it, adverse events on it, response, date of progression, site of progression, date of death and status at last follow-up were curated from the prospective database and electronic medical records.
Endpoint definitions
Response assessment was done as per RECIST 1.1 criteria and the adverse events were recorded at every visit based on Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Progression-free survival (PFS) was defined as time from start of lenvatinib to progression or death whichever occurred earlier. Overall survival (OS) was defined as the time from the start of lenvatinib to death.
Statistical analysis
Descriptive statistics were performed. The Kaplan–Meier method was used for the estimation of PFS and OS. The impact of starting dose of lenvatinib on PFS and OS was estimated using Cox regression analysis. A p-value of 0.05 was considered as significant.
Results
Baseline characteristics
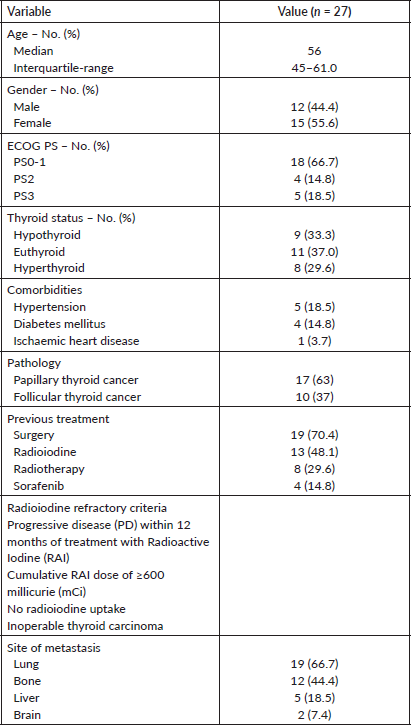
We identified 27 patients who had received Lenvatinib. The baseline characteristics are provided in Table 1. All patients had metastatic disease and were radioiodine refractory.
Table 1. Baseline characteristics of patients treated with lenvatinib. ECOG, Eastern Cooperative Oncology Group; PS, Performance status.
Compliance
Table 2 shows the adverse event profile. The starting dose was 24 mg in 10 (37%), 20 mg in 4 (14.8%), 18 mg in 8 (29.6%), 14 mg in 3 (11.1%) and 10 mg in 2 (7.4%) patients. The median starting dose of lenvatinib was 20 mg. The dose escalation and de-escalation was performed in three patients, two of them had a starting dose of 10 mg which was escalated to 14 mg and in one patient the dose was increased from 20 mg to 24 mg. The dose interruption had to be done in 17 patients, the dose reduction had to be done in 15 patients and the number of interruptions was single and multiple in 5 and 12 patients, respectively. The dose settled was 24 mg in 4 (14.8%), 20 mg in 5 (18.5%), 18 mg in 4 (14.8%), 14 mg in 7 (25.9%) and 10 mg in 7 (25.9%) patients (Figure 1). Lenvatinib had to be stopped due to toxicity in 4 patients (14.8%), recurrent proteinuria in 2 (7.4%) patients and hand foot syndrome (HFS) in 2 (7.4%) patients.
Outcome
The response rates are shown in Table 3. The best response among the four patients previously treated with lenvatinib was partial response in one patient and stable disease (SD) in three patients. The median follow-up was 19.7 months. Fifteen events for progression had occurred and the median PFS was 12.2 months [95% CI: 4.4–not available (NA)]. The 1- and 2-year PFS was 52.2% (95% CI: 30.2–70.4) and 34.3% (95%CI: 14.5–55.2), respectively (Figure 2). There was no impact of starting dose on PFS. (Hazard ratio: 1.020; 95% CI: 0.890–1.170, p = 0.764). The events for OS analysis were 12. The median OS was 35.3 months (95% CI: 11.4–NA). The 1- and 3-year OS was 70.0% (95% CI: 48.7–83.7) and 29.9% (95% CI: 19.6–69.1), respectively (Figure 3). There was no impact of starting dose on OS (Hazard ratio: 1.090; 95% CI: 0.930–1.280, p = 0.303). With regard to subjects receiving treatment without a surgical thyroidectomy due to inoperable cancer, the outcomes in neoadjuvant chemotherapy (NACT) lenvatinib for these patients were 50% response rate and Median OS was 25.56 months (95% CI: 7.9–43.23).
Discussion
To the best of authors’ knowledge, this is one of the first such sets of data from India looking at real-world applicability of lenvatinib in thyroid cancer. Lenvatinib was not available in India for a long period of time. The use of lenvatinib has been associated with a high amount of adverse events [1–7]. For the use of Lenvatinib in multiple other sites, the doses used are variable ranging from 10 mg to 24 mg [1–7]. However, because of the adverse event profile, there was a concern if trial data could be applicable in a real-world setting [8]. This is the novelty of this study.
Table 2. Adverse events due to lenvatinib and their grading. HFS, Hand foot syndrome.
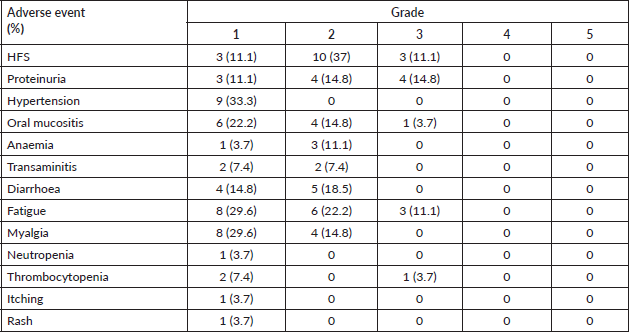
Table 3. Response assessment from baseline till treatment completion.

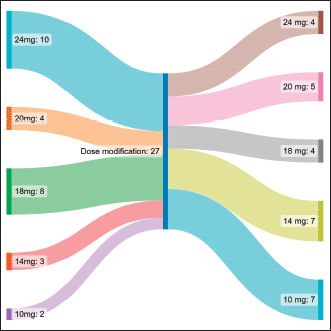
Figure 1. Sankey diagram representing the change in dose in patients taking Lenvatinib.
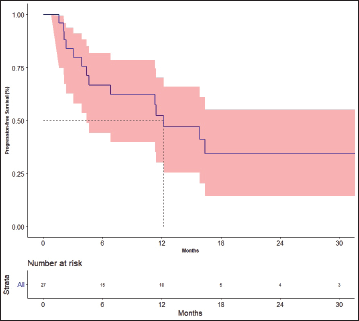
Figure 2. Progression-free survival.
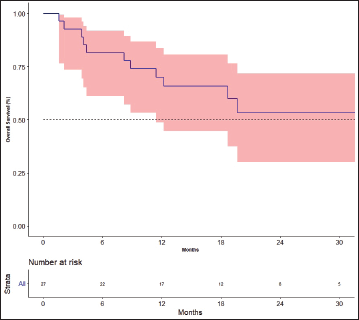
Figure 3. Overall survival.
Compared to the SELECT trial [9], which also studied the efficacy of Lenvatinib, our study reported a slightly lower median PFS (12.2 versus 18.3 months). The overall response rates were similar, 62.9% in our study versus 64.7% in SELECT. This difference may be due to certain key differences between the two studies. 66.7% of the patients in our study were Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0-1 while 95% of patients in SELECT were ECOG PS 0-1. This might explain the lower PFS from our study.
The adverse event profile was similar in both studies. However, this was in spite of a lower dose of Lenvatinib started in 63% (17 out of 27 patients). The dose interruptions and dose reductions with Lenvatinib in literature are in the tune of 50%–97% [10–15]. Physician discretion was used in our study for the starting dose and depending on tolerances the dose was either escalated or de-escalated as was done in other similar studies [16, 17]. The use of 24 mg starting dose is associated with a survival benefit in literature [9, 18, 19], as is the lack of dose interruptions [20]. Hence, it is important to select a starting dose of 24 mg for patients. In our study, ten patients were started with a 24 mg dose and out of them, four could continue on the dose. Our study suggests that if an effective dose escalation and de-escalation is used, the starting dose of Lenvatinib is unlikely to impact outcomes.
The study has a few inherent limitations. This was a retrospective study in a single centre. Furthermore, our sample size is relatively small to draw robust, reliable conclusions.
Conclusion
The real-world data of Lenvatinib suggest a lot of variability in the starting dose. In spite of this variability, the response rates and OS are similar to that noted in pivotal study. This suggests a case for need for more studies evaluating lower doses of Lenvatinib.
Acknowledgments
None.
Conflicts of interest
None.
Financial declaration
No funding was obtained for this study.
References
1. Capdevila J, Fazio N, and Lopez C, et al (2021) Lenvatinib in patients with advanced grade 1/2 pancreatic and gastrointestinal neuroendocrine tumors: results of the phase II TALENT trial (GETNE1509) J Clin Oncol 39 2304–2312 https://doi.org/10.1200/JCO.20.03368 PMID: 33945297
2. Finn RS, Ikeda M, and Zhu AX, et al (2020) Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma J Clin Oncol 38 2960–2970 https://doi.org/10.1200/JCO.20.00808 PMID: 32716739 PMCID: 7479760
3. Kudo M, Finn RS, and Qin S, et al (2018) Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial Lancet 391 1163–1173 https://doi.org/10.1016/S0140-6736(18)30207-1 PMID: 29433850
4. Arance A, de la Cruz-Merino L, and Petrella TM, et al (2022) Phase II LEAP-004 study of lenvatinib plus pembrolizumab for melanoma with confirmed progression on a programmed cell death protein-1 or programmed death ligand 1 inhibitor given as monotherapy or in combination J Clin Oncol 2022 JCO2200221
5. Taylor MH, Lee C-H, and Makker V, et al (2020) Phase IB/II trial of lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma, endometrial cancer, and other selected advanced solid tumors J Clin Oncol 38 1154–1163 https://doi.org/10.1200/JCO.19.01598 PMID: 31961766 PMCID: 7145588
6. Motzer R, Alekseev B, and Rha S-Y, et al (2021) Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma N Engl J Med 384 1289–1300 https://doi.org/10.1056/NEJMoa2035716 PMID: 33616314
7. Wirth LJ, Brose MS, and Sherman EJ, et al (2021) Open-label, single-arm, multicenter, phase II trial of lenvatinib for the treatment of patients with anaplastic thyroid cancer J Clin Oncol 39 2359–2366 https://doi.org/10.1200/JCO.20.03093 PMID: 33961488 PMCID: 8280094
8. Aydemirli MD, Kapiteijn E, and Ferrier KRM, et al (2020) Effectiveness and toxicity of lenvatinib in refractory thyroid cancer: Dutch real-life data Eur J Endocrinol 182 131–138 https://doi.org/10.1530/EJE-19-0763
9. Schlumberger M, Tahara M, and Wirth LJ, et al (2015) Lenvatinib versus placebo in radioiodine-refractory thyroid cancer N Engl J Med 372 621–630 https://doi.org/10.1056/NEJMoa1406470 PMID: 25671254
10. Koehler VF, Berg E, and Adam P, et al (2021) Real-world efficacy and safety of multi-tyrosine kinase inhibitors in radioiodine refractory thyroid cancer Thyroid 31 1531–1541 PMID: 34405734
11. Kish JK, Chatterjee D, and Wan Y, et al (2020) Lenvatinib and subsequent therapy for radioactive iodine-refractory differentiated thyroid cancer: a real-world study of clinical effectiveness in the United States Adv Ther 37 2841–2852 https://doi.org/10.1007/s12325-020-01362-6 PMID: 32382946 PMCID: 7467445
12. Locati LD, Piovesan A, and Durante C, et al (2019) Real-world efficacy and safety of lenvatinib: data from a compassionate use in the treatment of radioactive iodine-refractory differentiated thyroid cancer patients in Italy Eur J Cancer 118 35–40 https://doi.org/10.1016/j.ejca.2019.05.031 PMID: 31299580
13. Rendl G, Sipos B, and Becherer A, et al (2020) Real-world data for lenvatinib in radioiodine-refractory differentiated thyroid cancer (RELEVANT): a retrospective multicentric analysis of clinical practice in Austria Int J Endocrinol 2020 8834148 https://doi.org/10.1155/2020/8834148 PMID: 33312196 PMCID: 7719524
14. Song E, Kim M, and Kim EY, et al (2020) Lenvatinib for radioactive iodine-refractory differentiated thyroid carcinoma and candidate biomarkers associated with survival: a multicenter study in Korea Thyroid 30 732–738 https://doi.org/10.1089/thy.2019.0476 PMID: 31910091
15. Jiang H-J, Chang Y-H, and Chen Y-H, et al (2021) Low dose of lenvatinib treatment for patients of radioiodine-refractory differentiated thyroid carcinoma – a real-world experience Cancer Manag Res 13 7139–7148 https://doi.org/10.2147/CMAR.S326255 PMCID: 8449554
16. Cabanillas ME, Ryder M, and Jimenez C (2019) Targeted therapy for advanced thyroid cancer: kinase inhibitors and beyond Endocr Rev 40 1573–1604 https://doi.org/10.1210/er.2019-00007 PMID: 31322645 PMCID: 7341904
17. Cabanillas ME and Habra MA (2016) Lenvatinib: role in thyroid cancer and other solid tumors Cancer Treat Rev 42 47–55 https://doi.org/10.1016/j.ctrv.2015.11.003
18. Schlumberger M, Jarzab B, and Cabanillas ME, et al (2016) A phase II trial of the multitargeted tyrosine kinase inhibitor lenvatinib (E7080) in advanced medullary thyroid cancer Clin Cancer Res 22 44–53 https://doi.org/10.1158/1078-0432.CCR-15-1127
19. Brose MS, Panaseykin Y, and Konda B, et al (2022) A randomized study of lenvatinib 18 mg vs 24 mg in patients with radioiodine-refractory differentiated thyroid cancer J Clin Endocrinol Metab 107 776–787 https://doi.org/10.1210/clinem/dgab731 PMCID: 8852210
20. Tahara M, Brose MS, and Wirth LJ, et al (2019) Impact of dose interruption on the efficacy of lenvatinib in a phase 3 study in patients with radioiodine-refractory differentiated thyroid cancer Eur J Cancer 106 61–68 https://doi.org/10.1016/j.ejca.2018.10.002





