Cancer service delivery and the impact of the COVID-19 pandemic in sub-Saharan Africa: a scoping review
Elochukwu F Ezenwankwo1,2,3, Chukwudi A Nnaji1,2,3 and Jennifer Moodley1,2,3
1School of Public Health and Family Medicine, University of Cape Town Faculty of Health Sciences, Cape Town 7925, Western Cape, South Africa
2SAMRC Gynaecology Cancer Research Centre, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa
3Cancer Research Initiative, University of Cape Town Faculty of Health Sciences, Cape Town, Western Cape, South Africa
Abstract
Background: The impact of the Coronavirus Disease 2019 (COVID-19) pandemic on health systems is widely reported worldwide. However, what remains unclear is the relative extent of the pandemic’s effects on cancer management in sub-Saharan Africa (SSA). This review provides an up-to-date synthesis of the literature to inform post-pandemic policy and practice efforts in the region.
Methods: Sources searched for published research include MEDLINE, PsycINFO, Cumulative Index to Nursing and Allied Health Literature, African Index Medicus, African Wide Information and Web of Science. Using predefined criteria, the retrieved citations were screened for primary research describing the direct and indirect impacts of the COVID-19 pandemic on the cancer care and service delivery landscape in SSA since March 2020. Evidence was summarised using narrative synthesis.
Results: Fourteen studies reporting findings from 19 SSA countries were included in this review. Studies were conducted mostly in the first wave of the pandemic (between March and July 2020) (10/14). The most commonly reported impact on cancer treatment (including surgery) were cancellations, delays and modifications (11/14). Half (7/14) of the studies reported on the impact of the pandemic on cancer care resource availability and service restructuring. Other notable impacts included temporary suspension, total cancellations or alterations in cancer screening (3/14) and diagnostic (3/14) services or programmes. Disruptions in cancer research and outreach activities were also reported (3/14). The availability and maintenance of cancer healthcare depended on multiple factors like availability of clinical supplies, existing oncology workforce, adequate supply of personal protective equipment and local pandemic mitigation measures. Notably, no studies reported on the impact of the pandemic on psychosocial support programmes, physiotherapy and other rehabilitation care for cancer patients.
Conclusion: Changes in cancer care and service delivery due to the COVID-19 pandemic varied considerably across countries in SSA. This review underscores the need for urgent actions to mitigate current setbacks while recommending evidence-based and contextualised approaches to revitalising cancer care in the post-pandemic era.
Keywords: cancer services, COVID-19 pandemic, sub-Saharan Africa
Correspondence to: Elochukwu F Ezenwankwo
Email: eznelo001@myuct.ac.za
Published: 08/12/2022
Received: 01/09/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Access to health services has remained suboptimal and below pre-pandemic levels in many countries following the declaration of the Coronavirus Disease 2019 (COVID-19) pandemic by the World Health Organization [1]. While the deleterious health systems impacts of the pandemic are global and widespread, evidence suggests that the disruptions are likely to be dire in low- and middle-income countries [2]. Cancer services remain one of the most widely impacted healthcare services, with changes seen throughout the entire continuum of care [3].
Cancer remains a major public health concern in sub-Saharan Africa (SSA), with more than 800,000 new cases and 520,000 associated deaths reported in 2020 [4]. By 2040, SSA will likely record over 1.5 million new cancer cases and 1 million deaths [4]. Currently, it accounts for a quarter of all deaths due to chronic, non-communicable diseases and one-seventh of all premature deaths in the region [4]. Many SSA countries have made significant progress along different strata of cancer prevention and control, even as major challenges still exist. In the last decade, countries like Rwanda, South Africa and Seychelles have achieved nearly 95% Population-level Human Papillomavirus (HPV) vaccination coverage for target school-age girls using national HPV immunisation programmes [5]. In Rwanda, for example, more than 98% of school-aged girls (i.e. ≥12 years) have completed a 3-dose schedule of HPV vaccination [6].
Although expanding at a relatively low rate, access to radiotherapy services reflects another area where the region has made considerable progress. Over the last decade, brachytherapy capacity for cervical cancer treatment has increased by almost 40% in SSA countries. Currently, nearly half of SSA countries have access to external beam radiotherapy, with a 21.5% net increase in the availability of mega units since 2012 [7]. Also, with increasing global cancer alliances and per capita government healthcare expenditure, some countries like Botswana, South Africa, Namibia, Mozambique, Rwanda, Malawi and Zambia have progressed to include early diagnosis and access to definitive cancer therapies as part of health coverage programmes [8]. The impact of the COVID-19 pandemic thus poses a threat to such recent cancer control gains in SSA.
Many countries around the globe have reported widely on the disruptive impacts of the COVID-19 pandemic on healthcare delivery [9–15]. At the beginning of the pandemic, many countries suspended or delayed cancer prevention and early diagnostic programmes in addition to many low/medium priority services, such as elective and non-emergency surgeries, outpatient clinics (i.e. palliative or adjuvant chemotherapy), radiotherapy procedures, in-person consultations and supportive care, consequently, leading to the globally reported large-scale reduction in care and service delivery [14, 15]. South Korea recorded a significant decline in screening rates for colorectal cancer (−23%), stomach cancer (−17%), breast cancer (−12%) and cervical cancer (−8%) in 2020 in comparison with the preceding year [9]. Across 41 cancer centres in India, one cohort study showed a 54% reduction in newly registered cancer patients, 46% reduction in patients who had follow-up visits, 37% reduction in outpatient chemotherapy, 49% reduction in major surgeries, 52% reduction in minor surgeries, 23% reduction in patients accessing radiotherapy, 38% reduction in pathological diagnostic tests, 43% reduction in radiological diagnostic tests and 29% reduction in palliative care referrals between March and May 2020 [10]. Less is known about the impact of the pandemic on cancer care and service delivery in the SSA region. Previous reviews were based on limited evidence and did not consider more recent literature [16]. Our research provides an updated, more extensive and contextualised literature summary to better support post-pandemic policy and practice efforts. While the pandemic might have eased, its impact will likely linger and continue to exacerbate the existing gaps in the cancer service delivery landscape in SSA. This review, therefore, seeks to inform and support efforts that are required to re‐evaluate regional priorities and re-organise local practices in order to restore and possibly strengthen cancer prevention and control services and programmes in the region.
Methods
This review aimed to provide a comprehensive synthesis of the evidence from peer-reviewed studies that considered how the COVID-19 pandemic had affected cancer care and service delivery in SSA since the pandemic began. To achieve this, a scoping review of the literature was conducted using the modified framework of Levac et al [17]. Findings are reported according to the Preferred Reporting Items for
Systematic reviews and Meta-Analyses extension for Scoping Reviews guidelines [18]. Consent to participate or institutional review board approval was not sought for this review as, rather than the collection of primary data, publicly available peer-reviewed literature was utilised as evidence source. Details of the protocol are available as part of a review protocol registered on the International Prospective Register of Systematic Reviews (CRD42022343362).
Our review included studies that considered changes in service provision for cancer patients or at-risk individuals (i.e. cancer screening services). Eligibility was not restricted by study design, publication date or publication language as long as data/findings were from countries in SSA. To be eligible, studies needed to account for the impact of the COVID-19 pandemic on any or different aspects of cancer care or service provision, namely, screening, diagnosis, treatment (including surgery), survivorship, resource availability, service restructuring, research and outreach, based on self-report, health service data or patient/provider experience. For international networks and collaborations or studies focusing on the wider health system impacts, studies were included if they provided country and/or cancer-specific findings. Expert panel discussions highlighting major constraints to continuing service delivery in (countries within) the region were also considered if they were peer-reviewed. This review excluded other non-primary articles, including reviews, commentaries and viewpoint articles.
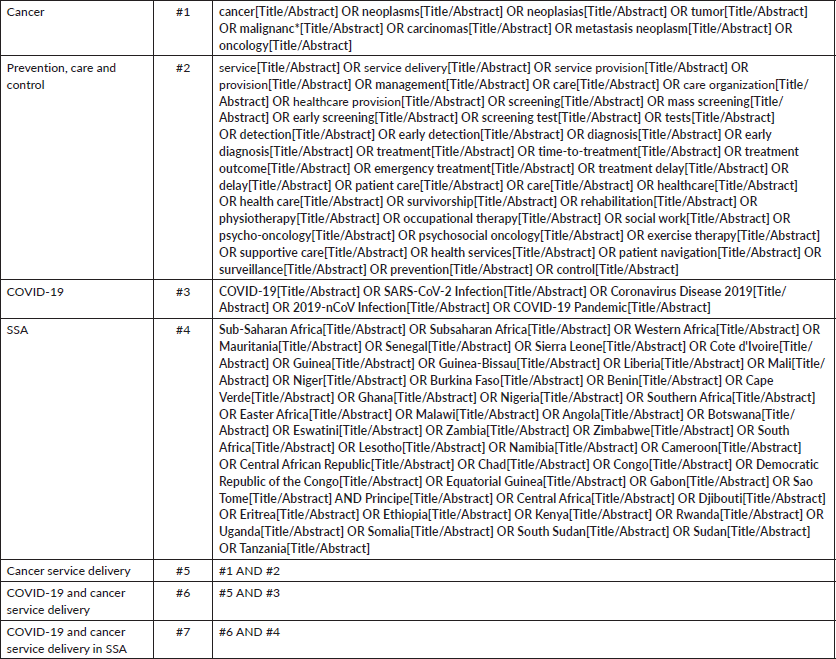
MEDLINE (via PubMed), APA PsycINFO, Cumulative Index to Nursing and Allied Health Literature, African Index Medicus, African Wide Information and Web of Science (ESCI & SCI-EXPANDED) were searched for primary research published between March 2020 and June 2022. EE and CN developed the search strategy using a well-defined systematic approach [19]. In developing our search strategy, Medical Subject Headings (MeSH), keywords and other search items were sought and combined using appropriate Boolean operators. Specifically, search strings were designed to be sensitive to the broad array of alternative terminologies and keywords related to the COVID-19 pandemic and cancer service delivery (See Supplementary Table 1). To capture studies with data and findings from SSA, this review implemented a location filter containing all countries currently classified as part of SSA using the World Bank classification. Additionally, recent systematic reviews of cancer and COVID-19 literature were scanned for relevant citations.
Identified records were moved to RefWorks software for de-duplication and then Microsoft Excel Spreadsheet for screening. Article selection was implemented at two levels: the first level involved the screening of the titles and abstracts of the retrieved papers to identify potentially eligible studies (performed by EE and verified by CN). The second level involved the assessment of full texts of potentially eligible studies identified in the previous step based on the review’s eligibility criteria (performed independently by EE and CN). Differences in opinions at different points in the study selection process were resolved by discussion in consultation with JM.
Data extraction was performed by EE and verified by CN based on a pre-specified form developed and piloted by the review team. Data were abstracted for a broad range of variables, including authors’ details (author, year and country), study aim, study design, participants’ characteristics, results and authors’ main conclusion. Aggregation of results was performed using a thematic narrative synthesis approach. Findings were summarised and reported based on key cancer management domains, namely, screening, diagnosis, treatment, survivorship, resource availability, service restructuring, research and outreach to map changes across the entire landscape of care.
Results
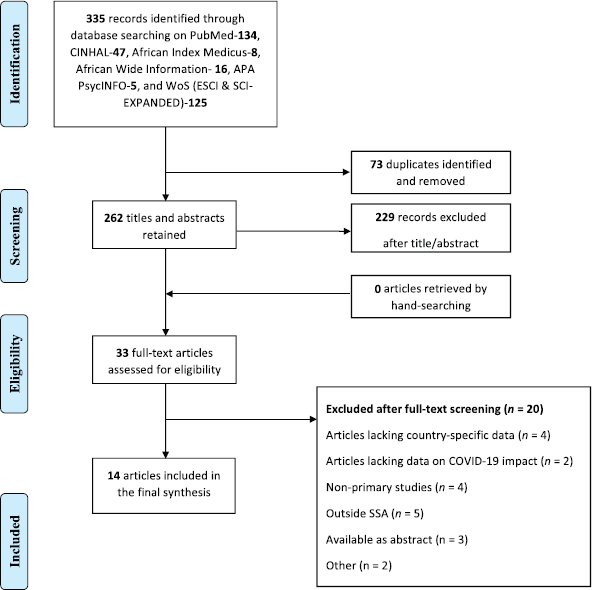
Fourteen studies reporting findings from 19 SSA countries were included in this narrative synthesis (Table 1) [2, 20–32]. Details of article screening and selection are provided in Figure 1. Studies were conducted largely in the first wave of the pandemic (i.e. between March and July 2020) [2, 20, 22, 24, 25, 27–31]. Geographically, the majority of the included studies were from South Africa (n = 7) [2, 22–24, 28, 29, 31], Kenya (n = 5) [20–22, 25, 31] and Nigeria (n = 5) [22, 26, 30–32]. Other countries were Namibia (n = 2) [26, 31], Uganda (n = 1) [26], Zambia (n = 3) [26, 27, 31], Ethiopia (n = 2) [26, 31], Cameroon (n = 2) [27, 31], Rwanda (n = 2) [27, 31], Côte d’Ivoire (n = 1) [27], Botswana (n = 1) [31], Zimbabwe (n = 1) [31], Mozambique (n = 1) [31], Burkina Faso (n = 1) [31], Tanzania (n = 2) [22, 31], Cabo Verde (n = 1) [31], Republic of Congo (n = 1) [31], Ghana (n = 1) [22] and Sudan (n = 1) [31]. See Table 1 for an extensive description of included studies.
Table 1. Characteristics of included studies.
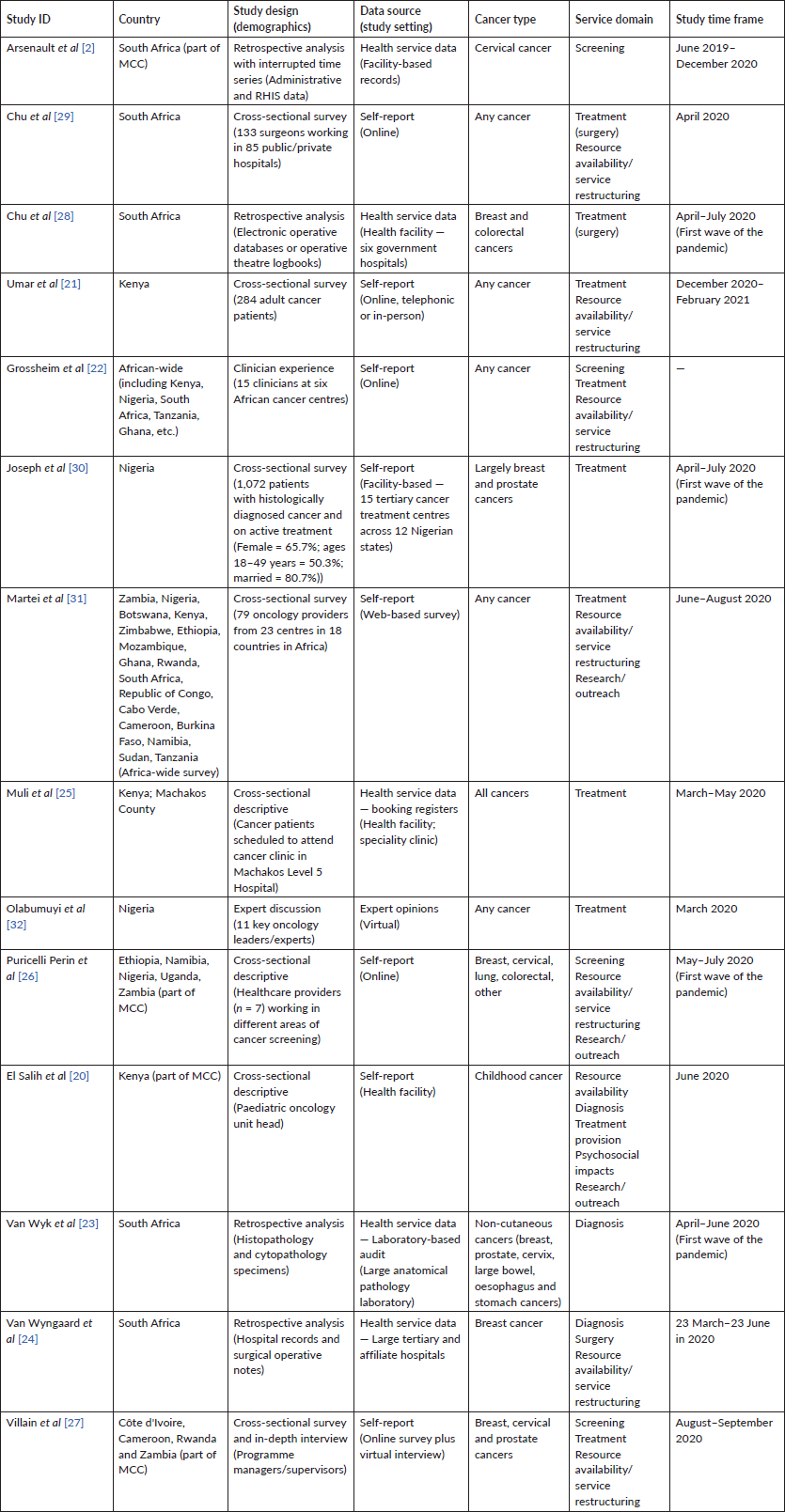
Source: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. doi:10.1371/journal.pmed1000097
For more information, visit www.prisma-statement.org.
Figure 1. PRISMA flow diagram detailing study screening and selection.
Disruptions due to the COVID-19 pandemic were reported mostly for non-cutaneous cancers and core aspects of cancer services such as screening [2, 26, 27], diagnosis [20, 23, 24], treatment (including surgery) [20–22, 24, 25, 27–32], resource availability/service restructuring [20–22, 26, 27, 29, 31] and research/outreach [20, 26, 31]. While five studies reported findings from retrospective and/or interrupted time-series analyses using health service data [2, 23–25, 28], a majority of the included studies involved self-reported surveys of adult cancer patients [21, 30] and oncology providers [20, 22, 23, 26, 27, 29, 31, 32] (Table 1). Three studies [21, 29, 30] reported findings from nationwide surveys within SSA countries, whereas four studies reported findings from international networks of collaborations beyond the sub-Saharan region [2, 20, 26, 27]. Table 2 highlights the impact of the COVID-19 pandemic across various aspects of cancer care and service delivery.
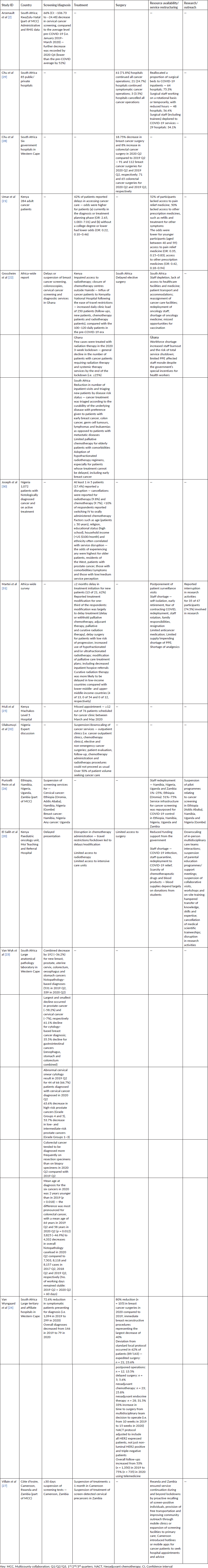
Table 2. Summary of COVID-19 impacts on cancer care.
Cancer screening
Four studies reported findings for COVID-19 impacts on cancer screening (mainly cervical cancer) in the SSA region [2, 22, 26, 27]. In KwaZulu Natal (South Africa), one study revealed a 66% (confidence interval (CI): −106.73 to −24.48) decrease in cervical cancer screening in March 2020, compared to the average level pre-COVID-19 (i.e. January 2019 to March 2020), and over 50% reduction by December 2020 following an interrupted time series analysis of Administrative and Routine Health Information System (RHIS) data [2]. Grossheim et al [22], in their qualitative study with oncology providers, reported delays or temporary suspension of breast cancer screening, colonoscopies, cervical cancer screening and diagnostic services in Ghana. In one multi-country survey, cervical and/or breast cancer screening was suspended in (some parts of) Ethiopia, Namibia and Nigeria in the first wave of the pandemic, according to oncology providers [26]. In another multi-country survey, clinicians reported cancelling at least 30 days of screening tests relating to breast, cervical and prostate cancers in Cameroon and Zambia [27].
Cancer diagnosis
In South Africa, one study reported a combined decrease of 36% for new breast, prostate, uterine, cervical, colorectal, oesophageal and stomach cancer (histopathology-based) diagnoses in the second quarter of 2020 (531 in the second quarter of 2019 and 339 in the second quarter of 2020) following a laboratory-based audit of one large anatomical pathology laboratory in Western Cape Province [23]. While the largest and smallest declines were recorded in prostate cancer (58.2%) and cervical cancer (7%), respectively, the study found a 61.1% decline for cytology-based breast cancer diagnosis and a 35.5% decline for gastrointestinal cancers (oesophagus, stomach and colorectum combined) [23]. The study further reported a 63.6% decrease in high-risk prostate cancers (grades 4 and 5) and a 53.7% decrease in low- and intermediate-risk prostate cancers (grades 1 to 3) [23]. The mean age at diagnosis for the six cancers in 2020 was 2 years younger than in 2019 — the difference was most pronounced for colorectal cancer, with a mean age of 64 years in the second quarter of 2019 and 58 years in the second quarter of 2020 [23]. In a different retrospective analysis involving hospital records in Western Cape, Van Wyngaard et al [24] found a 72.6% reduction in symptomatic patients presenting for diagnosis (i.e. 1,094 in 2019 to 299 in 2020) and a 45.9% reduction in overall diagnoses from 146 in 2019 to 79 in 2020. There is also evidence of the impact of the pandemic on paediatric cancer diagnosis. El Salih et al [20] reported delayed presentation among children with childhood cancers in one of Kenya’s largest teaching/referral hospital in a multi-country cross‐sectional study involving heads of paediatric oncology units.
Cancer treatment
The most commonly reported impact of the pandemic was related to cancer treatment (including surgery), with 11 out of 14 studies reporting on this. Martei et al [31] reported a ≤2 months delay in treatment initiation for new patients (13 of 21, 62%) in a web-based survey of 79 oncology providers from 23 centres across 18 countries in Africa. One-third of the respondents reported changes in their treatment plans, including treatment delay (i.e. delay or withholding of palliative chemotherapy, adjuvant therapy, palliative and curative radiation therapy, etc.); increased use of hypofractionated and/or ultrafractionated radiotherapy; modification of palliative care treatment plans and decreased inpatient hospice referrals [31]. The study also found that curative radiation therapy was more likely to be delayed in low-income countries than in lower-middle- and upper-middle-income countries [31].
In Nigeria, 1 in 5 adult cancer patients reported at least an alteration in their treatment course during lockdown, according to one national survey [31]. In another study, oncology providers alluded to an over 50% reduction in patient volume in Nigeria [32]. According to the clinicians, several outpatient clinics, chemotherapy clinics, radiotherapy procedures, patient evaluation and follow-up visits were either downscaled or suspended [32]. In the national survey by Joseph et al [30], nearly 10% of the participating 1,072 patients reported switching from intravenous to orally administered chemotherapy, with over 18% reporting total cancellation of radiotherapy and chemotherapy treatments. Factors such as age (patients ≥ 50 years), religion, educational status (high school), household income (< US $100/month) and ethnicity often correlated with cancer treatment service disruption, with the odds of experiencing any disruption being highest for older patients, patients residing in the western region, patients with prostate cancer, patients with comorbidities/symptoms and patients with relatively lower perception of their treatment [30].
In Kenya, Grossheim et al [22] reported impaired access to radiotherapy and closure of chemotherapy centres outside Nairobi, and consequently, an influx of cancer patients following the ease of travel restrictions — daily clinic load, i.e., in Kenyatta National Hospital increased to 250 patients (i.e. follow-ups, new patients, chemotherapy patients and radiotherapy patients), in comparison with the 100–120 daily patients in the pre-COVID-19 era. In another survey, 42% of adult Kenyan patients reported delays in accessing cancer care — odds were higher for patients (a) currently in the diagnosis or treatment planning phase (OR: 2.65, 1.003–7.01) and (b) without a college degree or lower (OR: 0.22, 0.10–0.46) [21]. Similarly, disrupted access to chemotherapy and radiotherapy (including intensive care unit) was reported in Kenya for children with cancer, according to the multi-country cross‐sectional study by El Salih et al [20].
Following the outbreak of COVID-19 in Cameroon, cancer treatment was suspended nationally for at least 1 month [27]. Similarly, there was a nationwide suspension of treatment of screen‐detected cervical precancers in Zambia [27]. In Ghana, only a few cases were treated with radiation therapy in the 2020 3-week lockdown, with many patients receiving hypofractionated therapy [22]. By the end of the lockdown, the decline in cancer patients seeking radiation and systemic therapies had reached 25%, according to Grossheim et al [22].
Many clinicians in South Africa reported a reduction in inpatient visits in their hospitals/centres and an increase in patient triage based on disease risk status — i.e., patients with early breast cancer, colon cancer, germ cell tumours, lymphomas and leukaemias were prioritised over those with metastatic diseases [22]. Limited access to palliative chemotherapy for elderly patients with comorbidities was also reported [22]. Many centres adopted hypofractionated radiotherapy regimens for patients with early breast cancer and others whose treatment could not be delayed [22].
Seven studies, including two multi-country surveys, provided findings for the limited access, including delays, in cancer surgeries across multiple centres in the region, which mostly affected elective and non-emergency (low risk) breast cancer surgeries [22, 24, 28, 29, 31, 32] or childhood cancers [20]. In the South African study conducted between March and June 2020, by Van Wyngaard et al [24], of the 62% (89/143) of patients with altered treatment courses, 23% received expedited surgery (n = 21), 19% had their surgeries either delayed (n = 5) or postponed (n = 12), while 57% received neoadjuvant chemotherapy (n = 23) or neoadjuvant endocrine therapy (n = 28). Management course was altered as part of the triage process for reasons including a high risk of severe disease from COVID-19 in the perioperative period and limited access to the operating facilities [24]. The study also reported a 33% increase in time to surgery from the multidisciplinary team’s decision to operate (i.e. from 10 weeks in 2019 to 15 weeks in 2020); an appreciable increase in follow-ups from 53% (n = 1,350) in 2019 to 75% (n = 735) in 2020 using telemedicine; and the adjustment of their neoadjuvant chemotherapy protocol to include all Human Epidermal Growth Factor Receptor 2 (HER2) expressed patients, not just non-luminal HER2 positive and triple-negative patients [24]. Specifically, the study found an 80% reduction (n = 105) in breast cancer surgeries in 2020 compared to 2019, with the reduction in immediate breast reconstructive procedures performed in the hospital representing the largest decrease ever (i.e. 40%).
In a different survey conducted in April 2020, 61 (71.8%) South African hospitals maintained all cancer surgeries; however, 21 (24.7%) maintained surgeries for symptomatic cancers, while 3 (3.5%) hospitals cancelled all operations relating to cancer [29]. In another South African study (a retrospective analysis), although not statistically significant, Chu et al [28] reported an 18.75% decrease in breast cancer surgery and an 8% increase in colorectal cancer surgery in the second quarter of 2020 in comparison to the corresponding period in 2019. In Ghana, surgical delays increased the demand for neoadjuvant therapy, with some patients avoiding upfront surgery and palliative chemotherapy [22].
Resource availability
Workforce shortages were reported in Ghana, South Africa, Namibia, Uganda, Zambia, Ethiopia, Nigeria and Kenya [20, 22, 29, 31]. Reasons for these shortages included (fear of contracting) COVID-19 infection, staff quarantine, staff rotation, staff resignation, family responsibilities and redeployment to COVID-19 control [20, 22, 29, 31]. For instance, countries such as Namibia, Nigeria, Uganda, Zambia and Ethiopia (Oromia) reported as high as 25%–75% redeployment of staff involved in cancer screening services [26]. In South Africa, redeployment of surgical staff (including trainees) was reported in at least 29 hospitals, while 48 hospitals permitted surgical staff on a rotational basis or temporary appointment, with reduced hours [29]. In Ghana, workforce shortage increased staff burnout and the risk of total service shutdown [22].
El Salih et al [20] reported reduced government funding and scarcity of chemotherapeutic drugs and blood products for children with cancer in Moi Teaching and Referral Hospital. In one survey of 284 adult cancer patients across Kenya, 52% of participants lacked access to pain relief medicine, while 50% lacked access to other prescription medicines, such as refills and treatment for other symptoms — the odds were lower for younger participants (aged between 40 and 59): access to pain relief medicine (Odds Ratio (OR): 0.35, 0.15–0.83); and access to other prescription medicines (OR: 0.42, 0.18–0.94) [21]. In the multinational survey by Martei et al [31], oncology providers reported shortages of anticancer medication, analgesics and personal protective equipment and postponement of patient surveillance visits [31]. Some clinicians reported the lack of access to healthcare facilities and cancer drugs for cancer patients, in addition to limited access to transport and accommodations for patients [22]. There was also a report of missed opportunities for Human papillomavirus (HPV) vaccination [22]. In Ghana, limited personal protective equipment (PPE) affected staff morale despite the government’s special incentives for health workers [22].
Cancer service restructuring
Many cancer facilities were repurposed for COVID-19 services in Ethiopia, Namibia, Nigeria, Uganda, Zambia and South Africa [22, 26, 29]. In South Africa, at least 64 hospitals reported reallocating some surgical beds to COVID-19 inpatients [29].
Rwanda and Zambia ensured service continuation during and beyond lockdowns by proactively recalling screen‐positive individuals, providing free transportation, improving community outreach through mobile clinics and by extending and expanding screening facilities to primary care [27]. Cameroon introduced hotlines or mobile apps for cancer patients to seek hospital appointments and advice [27].
Cancer research, outreach and support services
One multi-country survey [26] reported the suspension of pilot programmes relating to cancer screening in Ethiopia (Addis Ababa), Namibia, Uganda and Nigeria (Gombe). Another study [20] reported downscaling of in-person multidisciplinary care teams interactions; suspension of parental education programmes/support meetings; suspension of collaborative visits, workshops and on-site training; hampered transfer of knowledge, skills and expertise; cancellation of medical scientific traineeships and disruption in research activities in one Kenyan paediatric oncology unit. According to Martei et al [31], 35 of 47 participants (74.5%) involved in cancer research reported interruption in their research participation. Worthy of note is that no study provided findings on the direct or indirect effects of the pandemic on psychosocial support programmes or physiotherapy and other rehabilitation care for cancer patients.
Discussion
This review points to a substantial impact of the COVID-19 pandemic on cancer service delivery and oncology landscape in SSA, with definitive or implied disruptions in cancer screening and early diagnosis, access to treatment (including surgery), service delivery infrastructure (i.e. health facilities, oncology workforce, access to cancer medicine and other clinical supplies, etc.), resource allocation and research/outreach programmes [2, 20, 29-32, 21–28]. It provides an up-to-date evidence base for informing and supporting COVID-19 responsive policies and practices in the region. Although studies were from 19 countries (representing just about 41% of the countries in the region), much of the findings were from South Africa, Kenya and Nigeria. This is likely a combined reflection of the geographical differences in COVID-19 burden and the varied research capacity and health system vulnerabilities across countries in the region [2, 20, 30–32, 21–26, 28, 29].
The temporary suspension or cancellation of cancer screening services and programmes as reported in (some parts of) Ghana, Nigeria, Uganda, Ethiopia, Namibia, South Africa, Cameroon and Zambia reflects attempts by countries to mitigate the spread of the COVID-19 virus. Many countries recorded substantial reductions in screening procedures for breast, prostate and colorectal cancers; however, the current evidence reveals the highest pandemic-related effects on cervical cancer screening, with some centres reporting as high as 66% reductions in comparison to pre-pandemic periods [2, 22, 26, 27]. These delays are likely to further exacerbate current challenges with scaling up access to cancer screening in SSA countries, where most cancer patients are diagnosed at advanced or metastatic stages [4, 23, 24]. Currently, breast and cervical cancer dominate the SSA cancer burden, with deaths from cervical and breast cancers accounting for nearly 26.4% of cancer deaths reported in the region in 2020 [4]. The marked decline in symptomatic patients presenting at medical facilities for diagnosis, for example, in South Africa, and the reduction in early breast, cervical, colorectal and prostate cancer diagnoses, together with the suspension of screening programmes, raise major concerns over missed opportunities for earlier stage diagnosis [4]. Further, this underscores the need to integrate support for cancer screening and timely diagnosis programmes in national post-pandemic plans [4].
A variety of changes, delays and modifications in cancer treatment (including chemotherapy, radiation therapy and surgery) schedules were reported. For most centres, the cancellation of outpatient clinics led to several modifications in curative and palliative care treatment plans resulting in limited or delayed access to cancer treatment, mainly for childhood cancer patients [20] or older adults with advanced or metastatic cancers [21, 22, 30–32]. The long-term impact of treatment delays and cancellations is not known and requires ongoing monitoring. Mitigating the impact of delayed or cancelled treatment will require the optimisation of cancer referral and patient navigation systems to ease barriers to early treatment while addressing supply-side challenges with the availability of anticancer medicines and treatment commodities in the face of a global supply chain crisis. Besides, cancer treatment facilities and health systems need to brace for the resource challenges that may accompany the influx of cancer patients returning to care following the ease of travel restrictions.
Consistent with previous reviews, we found that, in general, palliative care treatments were affected more frequently than curative intent treatments [21, 22, 30–32]. While scaling down palliative care during the pandemic may be consistent with many international recommendations for managing individuals with highly compromised immune systems [33–36], efforts must be made to re-escalate care for this population to prevent the worsening of symptoms and rapid disease progression, including cancer metastasis. Even as the risk of severe COVID-19 disease and hospitalisation persists for this population, countries can adopt a phased return of palliative care based on the local pandemic situation and capacity for response and outbreak containment.
Pandemic-related interruptions in cancer surgeries were found largely among low-risk cancer patients (i.e. patients seeking elective and non-emergency surgeries) with fewer instances of total cancellation of surgical services [28, 31, 32]. Reductions in cancer surgeries were attributed to the shortage of surgical oncologists and other oncology providers, limited access to operating theatres and the heightened concern over the increased risk of COVID-19 infection in the perioperative periods [29]. Evidence from studies assessing the effect of cancer surgery delays on cancer outcomes suggests that delays in surgical treatment are associated with adverse oncological outcomes [37]. As countries take post-pandemic measures to restore cancer surgery capacity, further research is needed to ascertain the effect of surgery disruptions on cancer progression and survival in the affected population of cancer patients for future pandemics. Modifying surgical care plans may warrant routine integration of neo-adjuvant therapies to downstage cancer disease and minimise any risk of metastases due to surgical delays [38].
Our review identified multiple factors associated with the availability and maintenance of cancer healthcare in SSA during the pandemic, including travel logistics and limited funding, reduced oncology workforce (i.e. redeployment to COVID-19 relief, in some cases up to 75%), limited clinical supplies (i.e. anticancer drugs, blood products, pain medications, etc.) and medical equipment, access to health facilities (including operating rooms), limited supply of personal protective equipment, as well as state and local COVID-19 prevention and control measures [20, 22, 29, 31]. In addition, disruption in research and training activities evidenced by reports of cancellation or downscaling of in-person multidisciplinary care teams interactions; parental education programmes/support meetings; medical scientific traineeships; collaborative visits, workshops and on-site training impacted the ability to transfer knowledge, skills and expertise among stakeholders [20, 31]. These warrant efforts to ensure the availability of resources for cancer research, such as through better funding, strengthening collaboration and leveraging technological tools for virtual collaborative research engagement and research capacity building.
While underscoring the need for urgent actions to mitigate current setbacks in the region, this review also highlights the need to strengthen routine facility- and population-based cancer data and reporting systems. This remains critical for building reliable cancer data and research infrastructure for informing cancer control priorities and interventions. The fact that the combined evidence draws largely from self-reported data holds implications for building disaster (including pandemic) resilient cancer healthcare systems in SSA. Of the 14 included studies, only five reported findings based on (retrospective or time-series) analyses of health service data by comparing pandemic and pre-pandemic situations [2, 23–25, 28]. The dearth of such primary studies partly shows a lack of investment in data infostructure before the pandemic and the inability of the current cancer care infrastructure to strengthen and support health service data. It also complicates any effort to establish the full impact of the pandemic and the ability of many countries to re-escalate cancer services. Among other demands, transforming health systems in the aftermath of the pandemic warrants optimising health service data infrastructure in the region. In addition to adequate funding, such effort requires strengthening routine facility and community reporting systems and building capacity to analyse and use health facility data.
Also worthy of note is that no study provided findings on the direct or indirect effects of the pandemic on psychosocial support programmes or physiotherapy and other rehabilitation care for cancer patients. Postponements and delays in cancer treatment, in addition to movement restrictions and financial constraints, place an enormous emotional and psychological burden on cancer patients and their relatives [39]. Treatment delays and cancellations of follow-up visits might have further led to increased anxiety over cancer progression or recurrence. Many patients also experience complications like cancer-related fatigue, chronic pain, lymphedema, aerobic weakness, bowel and urinary incontinence, sexual dysfunction, osteoporosis, increased frailty and risk of falling and require assistance to return to work and other day-to-day activities [40]. Before the pandemic, evidence had shown the beneficial outcomes of physiotherapy, occupational therapy, exercise-based rehabilitation, social work and other non-pharmacological interventions for cancer patients [40–45]. Cancer patients also generally engage in key positive health behaviours such as sufficient exercise, healthy eating, limiting alcohol and not smoking to effectively navigate cancer treatment and maximise survival outcomes [40–42, 46]. The ability to maintain these positive health behaviours also may have been compromised by the pandemic [47]. The dearth of evidence on how the pandemic has impacted these services, which are critical for building resilient cancer management systems, represents an important gap in the literature and negatively impacts efforts to support and strengthen these services.
This review has made important findings from a substantial array of literature sources; however, it has some limitations. As this was a scoping review, quality appraisal of the included studies, the majority of which were descriptive and analytical cross-sectional surveys, was not undertaken. While the current findings improve our understanding of the state of cancer healthcare in SSA since the pandemic began, the limited number, the largely descriptive nature and the limited representativeness of the included studies limit the interpretation and generalisability of our findings and recommendations. That most findings were from lower- and upper-middle countries in SSA with disproportionately stronger health systems than the low-income countries in the region points to another major limitation. Given the underrepresentation of studies from low-income countries, the evidence of the pandemic’s impact may be underestimated. Furthermore, the variability in the magnitude of the reported effects of the pandemic on the different aspects of cancer care across different contexts limits the aggregation, interpretation and applicability of the findings and recommendations in diverse social and health system contexts in SSA.
Conclusion
Available evidence demonstrates substantial disruption and wide variation in the availability and maintenance of cancer care in SSA since the beginning of the pandemic. Even as the pandemic continues to ease, its impact will likely linger and continue to exacerbate the prevailing gaps in cancer healthcare. Overall, the review’s findings underscore the need for cancer programmes, decision-makers and health services managers to critically take stock of the pandemic’s effect, re-evaluate local practices and implement post-pandemic actions that reflect current cancer service delivery priorities. Specifically, this review underscores the need for urgent actions to mitigate current setbacks while recommending evidence-based and contextualised approaches to revitalising cancer care in the post-pandemic era. Findings further underscore the need to strengthen routine facility – and population-based cancer data and reporting systems in SSA, which are critical for building reliable cancer data and research infrastructure for informing cancer control priorities and interventions.
List of abbreviations
PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analysis; CINAHL, Cumulative Index to Nursing and Allied Health Literature;WoS, Web of Science; SSA, Sub-Saharan Africa; COVID-19, Coronavirus disease of 2019.
Declarations
Ethics approval and consent to participate
This is a scoping review of publicly available peer-reviewed literature, with no primary data collection. Hence, consent to participate or institutional review board approval is not warranted.
Consent for publication
Not applicable.
Availability of data and materials
All data generated and analysed during this study are included in this manuscript and its supplementary information files.
Conflicts of interest
The authors declare that they have no competing interests.
Funding
Not applicable.
Authors’ contributions
EE, CN and JM conceptualised the study. EE and CN developed the review protocol; led data collection, analysis and interpretation; and drafted the first version manuscript. JM provided critical insights and reviewed the final draft; all authors read and approved the final manuscript.
Acknowledgments
Not applicable.
References
1. Pujolar G, Oliver-Anglès A, and Vargas I, et al (2022) Changes in access to health services during the COVID-19 pandemic: a scoping review Int J Environ Res Public Health [Internet] 19(3) 1749 Date accessed: 19/07/22 https://doi.org/10.3390/ijerph19031749 PMID: 35162772 PMCID: 8834942
2. Arsenault C, Gage A, and Kim MK, et al (2022) COVID-19 and resilience of healthcare systems in ten countries Nat Med [Internet] 28(6) 1314–1324 [https://www.nature.com/articles/s41591-022-01750-1] Date accessed: 27/06/22 https://doi.org/10.1038/s41591-022-01750-1 PMID: 35288697 PMCID: 9205770
3. Pulse Survey on Continuity of Essential Health Services During the COVID-19 Pandemic: Interim Report, 27 August 2020 [Internet] [https://www.who.int/publications/i/item/WHO-2019-nCoV-EHS_continuity-survey-2020.1] Date accessed: 19/07/22
4. Bray F, Parkin DM, and Gnangnon F, et al (2022) Cancer in sub-Saharan Africa in 2020: a review of current estimates of the national burden, data gaps, and future needs Lancet Oncol [Internet] 23(6) 719–728 [http://www.thelancet.com/article/S1470204522002704/fulltext] Date accessed: 08/06/22 https://doi.org/10.1016/S1470-2045(22)00270-4 PMID: 35550275
5. Black E and Richmond R (2018) Prevention of cervical cancer in sub-Saharan Africa: the advantages and challenges of HPV vaccination Vaccines [Internet] 6(3) [/pmc/articles/PMC<a href=http://www.ncbi.nlm.nih.gov/pmc/articles/PMC6161067>6161067/] Date accessed: 22/08/22 https://doi.org/10.3390/vaccines6030061 PMID: 30205561 PMCID: 6161067
6. Sayinzoga F, Umulisa MC, and Sibomana H, et al (2020) Human papillomavirus vaccine coverage in Rwanda: a population-level analysis by birth cohort Vaccine [Internet] 38(24) 4001 [/pmc/articles/PMC<a href=http://www.ncbi.nlm.nih.gov/pmc/articles/PMC7221340>7221340/] Date accessed: 22/08/22 https://doi.org/10.1016/j.vaccine.2020.04.021 PMID: 32336599 PMCID: 7221340
7. Elmore SNC, Polo A, and Bourque JM, et al (2021) Radiotherapy resources in Africa: an international atomic energy agency update and analysis of projected needs Lancet Oncol [Internet] 22(9) e391 [/pmc/articles/PMC<a href=http://www.ncbi.nlm.nih.gov/pmc/articles/PMC8675892>8675892/] Date accessed: 23/08/22 https://doi.org/10.1016/S1470-2045(21)00351-X PMID: 34478675 PMCID: 8675892
8. Current Health Expenditure (% of GDP) – Sub-Saharan Africa Data [Internet] [https://data.worldbank.org/indicator/SH.XPD.CHEX.GD.ZS?locations=ZG] Date accessed: 22/08/22
9. Lee K, Lee YY, and Suh M, et al (2022) Impact of COVID-19 on cancer screening in South Korea Sci Rep [Internet] 12(1) 11380 Date accessed: 19/07/22 https://doi.org/10.1038/s41598-022-15778-3 PMID: 35790880 PMCID: 9255521
10. Ranganathan P, Sengar M, and Chinnaswamy G, et al (2021) Impact of COVID-19 on cancer care in India: a cohort study Lancet Oncol [Internet] 22(7) 970–976 [http://www.thelancet.com/article/S1470204521002400/fulltext] Date accessed: 01/07/21 https://doi.org/10.1016/S1470-2045(21)00240-0 PMID: 34051879 PMCID: 8159191
11. Chen RC, Haynes K, and Du S, et al (2021) Association of cancer screening deficit in the United States with the COVID-19 pandemic JAMA Oncol 7(6) 878–884 https://doi.org/10.1001/jamaoncol.2021.0884 PMID: 33914015 PMCID: 8085759
12. Patt D, Gordan L, and Diaz M, et al (2020) Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors JCO Clin Cancer Inform [Internet] 4(4) 1059–1071 Date accessed: 19/07/22 https://doi.org/10.1200/CCI.20.00134 PMID: 33253013 PMCID: 7713534
13. Watt T, Sullivan R, and Aggarwal A (2022) Primary care and cancer: an analysis of the impact and inequalities of the COVID-19 pandemic on patient pathways BMJ Open [Internet] 12(3) e059374 [https://bmjopen.bmj.com/content/12/3/e059374] Date accessed: 01/03/22 https://doi.org/10.1136/bmjopen-2021-059374 PMID: 35332047 PMCID: 8948073
14. Richards M, Anderson M, and Carter P, et al (2020) The impact of the COVID-19 pandemic on cancer care Nat Cancer [Internet] 1(6) 565–567 [https://www.nature.com/articles/s43018-020-0074-y] Date accessed: 19/07/22 https://doi.org/10.1038/s43018-020-0074-y PMID: 35121972 PMCID: 7238956
15. Greenwood E and Swanton C (2020) Consequences of COVID-19 for cancer care – a CRUK perspective Nat Rev Clin Oncol [Internet] 18(1) 3–4 [https://www.nature.com/articles/s41571-020-00446-0] Date accessed: 19/07/22 https://doi.org/10.1038/s41571-020-00446-0 PMID: 33097915 PMCID: 7582444
16. Nnaji CA and Moodley J (2021) Impact of the COVID-19 pandemic on cancer diagnosis, treatment and research in African health systems: a review of current evidence and contextual perspectives Ecancermedicalscience [Internet] 15 Date accessed: 30/07/22 https://doi.org/10.3332/ecancer.2021.1170 PMID: 33680084 PMCID: 7929764
17. Levac D, Colquhoun H, and O’Brien KK (2010) Scoping studies: advancing the methodology Implement Sci [Internet] 5(1) 1–9 [https://implementationscience.biomedcentral.com/articles/10.1186/1748-5908-5-69] Date accessed: 09/07/21 https://doi.org/10.1186/1748-5908-5-69
18. Tricco AC, Lillie E, and Zarin W, et al (2018) PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation Ann Intern Med [Internet] 169(7) 467–473 Date accessed: 09/07/21 https://doi.org/10.7326/M18-0850 PMID: 30178033
19. Bramer WM, Jonge GB, and Rethlefsen ML, et al (2018) A systematic approach to searching: an efficient and complete method to develop literature searches J Med Libr Assoc JMLA [Internet] 106(4) 531 Date accessed: 09/07/21 PMID: 30271302 PMCID: 6148622
20. El Salih I, Widjajanto PH, and Njuguna F, et al (2022) Impact of COVID-19 measures on a paediatric oncology outreach-program Psychooncology [Internet] 31(5) 860–864 [https://onlinelibrary.wiley.com/doi/full/10.1002/pon.5934] Date accessed: 01/05/22 https://doi.org/10.1002/pon.5934 PMID: 35403292 PMCID: 9088594
21. Umar S, Chybisov A, and McComb K, et al (2022) COVID-19 and access to cancer care in Kenya: patient perspective Int J Cancer [Internet] 150(9) 1497–1503 [https://pubmed.ncbi.nlm.nih.gov/34927724/] Date accessed: 27/06/22 https://doi.org/10.1002/ijc.33910 PMCID: 9303218
22. Grossheim L, Ruff P, and Ngoma T, et al (2021) Cancer and COVID-19 experiences at African cancer centers: the silver lining JCO Glob Oncol 7 410–415 https://doi.org/10.1200/GO.20.00564 PMID: 33760639 PMCID: 8081519
23. van Wyk AC, de Jager LJ, and Razack R, et al (2021) The initial impact of the COVID-19 pandemic on the diagnosis of new cancers at a large pathology laboratory in the public health sector, Western Cape Province, South Africa SAMJ S Afr Med J [Internet] 111(6) 570–574 [http://www.scielo.org.za/scielo.php?script=sci_arttext&pid=S0256-95742021000600020&lng=en&nrm=iso&tlng=en] Date accessed: 27/06/22 PMID: 34382569
24. van Wyngaard T, Cairncross L, and Maswime S, et al (2022) Impact of COVID-19 on breast cancer diagnostic and surgical services at a South African academic hospital S Afr J Surg [Internet] 60(2) 119–123 [http://www.scielo.org.za/scielo.php?script=sci_arttext&pid=S0038-23612022000200009&lng=en&nrm=iso&tlng=en] Date accessed: 20/07/22 https://doi.org/10.17159/2078-5151/SAJS3750 PMID: 35851366
25. Muli E, Waithanji R, and Kamita M, et al (2021) Leveraging technology for health services continuity in times of COVID-19 pandemic: patient follow-up, and mitigation of worse patient outcomes J Glob Health [Internet] 11 [https://pubmed.ncbi.nlm.nih.gov/35047184/] Date accessed: 27/06/22 https://doi.org/10.7189/jogh.11.05024
26. Puricelli Perin DM, Elfström KM, and Bulliard JL, et al (2021) Early assessment of the first wave of the COVID-19 pandemic on cancer screening services: the international cancer screening network COVID-19 survey Prev Med [Internet] 151 106642 Date accessed: 01/10/21 https://doi.org/10.1016/j.ypmed.2021.106642 PMID: 34217420 PMCID: 8241661
27. Villain P, Carvalho AL, and Lucas E, et al (2021) Cross-sectional survey of the impact of the COVID-19 pandemic on cancer screening programs in selected low- and middle-income countries: study from the IARC COVID-19 impact study group Int J Cancer 149(1) 97–107 https://doi.org/10.1002/ijc.33500 PMID: 33533501 PMCID: 8014228
28. Chu KM, Marco J, and Bougard H, et al (2021) Estimating the surgical backlog from the COVID-19 lockdown in South Africa: a retrospective analysis of six government hospitals S Afr Med J [Internet] 111(7) 685–688 [http://www.samj.org.za/index.php/samj/article/view/13302] Date accessed: 27/06/22 https://doi.org/10.7196/SAMJ.2021.v111i7.15686 PMID: 34382554
29. Chu KM, Smith M, and Steyn E, et al (2020) Changes in surgical practice in 85 South African hospitals during COVID-19 hard lockdown S Afr Med J [Internet] 110(9) 916–919 [http://www.samj.org.za/index.php/samj/article/view/13024] Date accessed: 27/06/22 https://doi.org/10.7196/SAMJ.2020.v110i9.15014 PMID: 32880278
30. Joseph A, Olatosi B, and Haider MR, et al (2022) Patient’s perspective on the impact of COVID-19 on cancer treatment in Nigeria JCO Glob Oncolo [Internet] 8(8) [https://pubmed.ncbi.nlm.nih.gov/35157511/] Date accessed: 27/06/22
31. Martei YM, Rick TJ, and Fadelu T, et al (2021) Impact of COVID-19 on cancer care delivery in Africa: a cross-sectional survey of oncology providers in Africa JCO Glob Oncol [Internet] 7(7) 368–377 [https://pubmed.ncbi.nlm.nih.gov//] Date accessed: 27/06/22 https://doi.org/10.1200/GO.20.00569 PMID: 33689484 PMCID: 8081536
32. Olabumuyi AA, Ali-Gombe M, and Biyi-Olutunde OA, et al (2020) Oncology practice in the COVID-19 pandemic: a report of a Nigerian expert panel discussion (oncology care in Nigeria during the COVID-19 pandemic) Pan Afr Med J [Internet] 36 1–9 [/pmc/articles/PMC7436648/] Date accessed: 27/06/22 https://doi.org/10.11604/pamj.2020.36.153.23662
33. Belsky JA, Tullius BP, and Lamb MG, et al (2021) COVID-19 in immunocompromised patients: a systematic review of cancer, hematopoietic cell and solid organ transplant patients J Infect [Internet] 82(3) 329 Date accessed: 01/08/22 https://doi.org/10.1016/j.jinf.2021.01.022 PMID: 33549624 PMCID: 7859698
34. Lenihan D, Carver J, and Porter C, et al (2020) Cardio-oncology care in the era of the coronavirus disease 2019 (COVID-19) pandemic: an international cardio-oncology society (ICOS) statement CA Cancer J Clin [Internet] 70(6) 480–504 [https://onlinelibrary.wiley.com/doi/full/10.3322/caac.21635] Date accessed: 01/08/22 https://doi.org/10.3322/caac.21635 PMID: 32910493 PMCID: 7934086
35. Madan A, Siglin J, and Khan A (2020) Comprehensive review of implications of COVID-19 on clinical outcomes of cancer patients and management of solid tumors during the pandemic Cancer Med [Internet] 9(24) 9205–9218 [https://onlinelibrary.wiley.com/doi/full/10.1002/cam4.3534] Date accessed: 01/08/22 https://doi.org/10.1002/cam4.3534 PMID: 33078903 PMCID: 7774721
36. Al‐Shamsi HO, Alhazzani W, and Alhuraiji A, et al (2020) A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID‐19) pandemic: an international collaborative group Oncologist [Internet] 25(6) e936–e945 [https://academic.oup.com/oncolo/article/25/6/e936/6443401] Date accessed: 01/08/22 https://doi.org/10.1634/theoncologist.2020-0213
37. Hanna TP, King WD, and Thibodeau S, et al (2020) Mortality due to cancer treatment delay: systematic review and meta-analysis BMJ [Internet] 371 m4087 [https://www.bmj.com/content/371/bmj.m4087] Date accessed: 01/09/22 https://doi.org/10.1136/bmj.m4087 PMID: 33148535 PMCID: 7610021
38. Korde LA, Somerfield MR, and Carey LA, et al (2021) Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline J Clin Oncol 39(13) 1485–1505 https://doi.org/10.1200/JCO.20.03399 PMID: 33507815 PMCID: 8274745
39. Dhada S, Stewart D, and Cheema E, et al (2021) Cancer services during the COVID-19 pandemic: systematic review of patient’s and caregiver’s experiences Cancer Manag Res [Internet] 13 5875–5887 Date accessed: 25/07/22 https://doi.org/10.2147/CMAR.S318115 PMID: 34349561 PMCID: 8328387
40. Stout NL, Santa Mina D, and Lyons KD, et al (2021) A systematic review of rehabilitation and exercise recommendations in oncology guidelines CA Cancer J Clin 71(2) 149–175 https://doi.org/10.3322/caac.21639 PMCID: 7988887
41. Ezenwankwo EF, Motsoeneng P, and Atterbury EM, et al (2022) Plausible conditions and mechanisms for increasing physical activity behavior in men with prostate cancer using patient education interventions: sequential explanatory mixed studies synthesis Support Care Cancer [Internet] 30(6) 4617–4633 [https://link.springer.com/article/10.1007/s00520-021-06693-w] Date accessed: 25/07/22 https://doi.org/10.1007/s00520-021-06693-w PMID: 35064329
42. Ezenwankwo EF, Nnate DA, and Usoro GD, et al (2022) A scoping review examining the integration of exercise services in clinical oncology settings BMC Health Serv Res [Internet] 22(1) 1–15 [https://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-022-07598-y] Date accessed: 25/07/22 https://doi.org/10.1186/s12913-022-07598-y
43. Osborn RL, Demoncada AC, and Feuerstein M (2006) Psychosocial interventions for depression, anxiety, and quality of life in cancer survivors: meta-analyses Int J Psychiatry Med 36(1) 13–34 https://doi.org/10.2190/EUFN-RV1K-Y3TR-FK0L PMID: 16927576
44. Mewes JC, Steuten LMG, and IJzerman MJ, et al (2012) Effectiveness of multidimensional cancer survivor rehabilitation and cost-effectiveness of cancer rehabilitation in general: a systematic review Oncologist 17(12) 1581–1593 https://doi.org/10.1634/theoncologist.2012-0151 PMID: 22982580 PMCID: 3528391
45. Duncan M, Moschopoulou E, and Herrington E, et al (2017) Review of systematic reviews of non-pharmacological interventions to improve quality of life in cancer survivors BMJ Open [Internet] 7(11) e015860 [https://bmjopen.bmj.com/content/7/11/e015860] Date accessed: 25/07/22 https://doi.org/10.1136/bmjopen-2017-015860 PMID: 29187408 PMCID: 5719270
46. Ezenwankwo EF, Ezeukwu AO, and Abaraogu UO (2021) Effects of physical activity changes induced by behaviour change interventions on inflammation and patient-centred outcomes in breast cancer survivors: a systematic review Eur J Physiother https://doi.org/10.1080/21679169.2021.1933586
47. Buck C, Pini S, and Lally P, et al (2022) The impact of the COVID-19 pandemic on the health behaviours of people living with and beyond breast, prostate, and colorectal cancer – a qualitative study J Cancer Surviv [Internet] 1–11 [https://link.springer.com/article/10.1007/s11764-022-01234-8] Date accessed: 25/07/22
Supplementary Material
Supplementary Table 1. Provisional search strategy — to be optimised in PubMed.