Adjuvant chemotherapy after curative D2 gastrectomy in Latin American patients with gastric cancer
Mariana Serrano1, Jhajaira M Araujo2, Cristian Pacheco1, Jackeline Macetas1, Mariella A Blum3, Alfredo Carrato4, Eloy Ruiz5, Francisco Berrospi5, Carlos Luque5, Ivan Chavez5, Eduardo Payet1, Luis Taxa6 and Paola Montenegro1
1Departamento de Medicina Oncológica, Instituto Nacional de Enfermedades Neoplásicas, Lima 15038, Peru
2Escuela Profesional de Medicina Humana, Universidad Privada San Juan Bautista, Lima 15067, Peru
3Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA
4Department of Medical Oncology, Hospital Ramón y Cajal, Madrid 28034, Spain
5Departamento de Cirugía en Abdomen, Instituto Nacional de Enfermedades Neoplásicas, Lima 15038, Peru
6Departamento de Patología, Instituto Nacional de Enfermedades Neoplásicas, Lima 15038, Peru
Abstract
Background: Gastric cancer (GC) is the fourth most common cause of cancer deaths around the world and the first cause of cancer deaths in Peru; however, there are no prospective trials for adjuvant chemotherapy in GC after curative gastrectomy in this country. The objective of this study was to evaluate the effectiveness of adjuvant chemotherapy in stage II–III gastric cancer patients who underwent D2 gastrectomy.
Methods: We included patients with stage II–III gastric cancer who underwent radical gastrectomy and D2 dissection between 2014 and 2016 at our institution. Patients received 3-week cycles of capecitabine (1,000 mg/m2 twice daily on days 1–14) plus oxaliplatin (130 mg/m2 on day 1) for 6 months. Survival curves were estimated with the Kaplan–Meier method, and the Cox proportional hazards model was used to identify prognostic factors for survival.
Results: In total, 173 patients were included: 100 (57.8%) patients received adjuvant chemotherapy and surgery (AChS) and 73 (42.2%) surgery alone (SA). Three-year disease-free survival (DFS) was higher in the AChS groups (69%) than in the SA group (52.6%) (p = 0.034). Regarding overall survival (OS), 31 patients (31%) died in the AChS group compared with 34 (46.6%) in the SA group (p = 0.027). In the multivariate analysis, adjuvant chemotherapy was an independent prognostic factor for DFS (HR = 0.60; 95% CI = 0.37–0.97; p = 0.036) and OS (HR = 0.58; 95% CI = 0.36–0.95; p = 0.029). ACh showed consistent benefit in DFS and OS for patients with albumin >3.5 g/dL, lymphovascular and perineural invasion, pT4, pN2–3, pathologic stage (PS) IIIA and IIIB and lymph node ratio (LNR) > 13.1.
Conclusion: These data suggest that adjuvant capecitabine and oxaliplatin reduce the recurrence and mortality in patients with stage II–III gastric cancer who underwent D2 gastrectomy. PS IIIA and IIIB and LNR > 13.1 benefited more from receiving adjuvant chemotherapy and poorly cohesive gastric carcinoma did not significantly reduce the rates of survival.
Keywords: gastric cancer, adjuvant chemotherapy, survival
Correspondence to: Paola Montenegro
Email: paolacmb@yahoo.es
Published: 12/05/2022
Received: 27/01/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Gastric cancer (GC) is the fourth most common cause of cancer deaths around the world and the first cause of cancer deaths in Peru [1]. GC mostly affects older people with an average age at diagnosis around 68 years old. The risk to develop this cancer is about 1 in 95 for men and 1 in 154 for women [2, 3]. Helicobacter pylori is a known and important carcinogenic factor for gastric cancer in our country. According to pathological studies, it has been reported that 54.76% of gastric cancer patients had H. pylori; while with molecular evaluation it was found that 94% were positive for this bacterium [4]. Improved socio-economic status, hygienic practices and widespread antibiotic use have led to a decrease in infection rates [5].
The standard of care for localised gastric cancer includes surgical resection. However, there is no global consensus on the optimal treatment approach for gastric cancer due to the various surgical techniques and other practices used in different parts of the world. D2 lymph node dissection is additional removal of a second tier of lymph nodes in the extraperigastric areas, which generally fall along branches of the celiac axis, including the left gastric, splenic, common hepatic and proper hepatic arteries. This surgical technique is practiced commonly in Japan and Korea but is less common in other countries [6]. In eastern Asia, the standard approach is surgical resection with D2 lymphadenectomy, followed by adjuvant chemotherapy, whereas in Western countries there is a preference for using either perioperative chemotherapy or postoperative chemoradiation, especially in cases of inadequate (<D2) lymph node dissection [7]. In our country, adjuvant chemotherapy after D2 lymph node dissection is considered the standard treatment and the postoperative morbidity and mortality in D2 radical gastrectomy for gastric cancer in Peruvian patients are 23.3% and 3.3%, respectively [8].
The REGATE study was the largest international prospective registry that enrolled patients with newly diagnosed gastric cancer. In terms of adjuvant chemotherapy, the REGATE data indicated that this approach is most commonly used for stage III cancers in all regions and is more frequently practiced in the Asia-Pacific and Latin American regions than in Europe. Fluoropyrimidine-based adjuvant chemotherapy regimens are the most common globally but patients in the Asia-Pacific region are much more likely than those in other parts of the world to receive newer oral fluoropyrimidines [9].
The Japanese ACST-GC trial was the first large-scale randomised trial of adjuvant chemotherapy after curative resection with D2 gastrectomy with stage II–III gastric cancer that shows 80% of overall survival (OS) compared with surgery alone (70%) [10]. Also, the Korean Classic trial was the second largest trial of adjuvant chemotherapy after D2 gastrectomy, showing 78% in OS versus surgery alone (69%) [11]. In addition, the GASTRIC group meta-analysis suggests a 5.8% absolute OS benefit at 5 years (55.3%–49.6%) for patients treated with adjuvant chemotherapy [12].
There are no prospective trials for adjuvant chemotherapy in gastric cancer after curative gastrectomy in Peru. The objective of this study was to evaluate the effectiveness of adjuvant chemotherapy in the Peruvian population with stage II–III gastric cancer who underwent radical gastrectomy and D2 lymph node dissection, and identify prognostic factors of OS and DFS in patients treated with adjuvant chemotherapy.
Materials and methods
Study design
This was an observational and analytic study. We retrospectively reviewed data, obtained from the medical records of the National Institute of Neoplastic Diseases (INEN) in Lima-Peru, of patients diagnosed with gastric cancer between January 2014 and December 2016.
Patients and eligibility criteria
We included patients aged 18 years or older with stage II–III gastric cancer who underwent radical gastrectomy and D2 dissection. In case of patients treated with adjuvant chemotherapy, inclusion criteria were six or more courses of capecitabine plus oxaliplatin; patients who received chemotherapy as follows: 3-week cycles of oral capecitabine (1,000 mg/m2 twice daily on days 1–14 of each cycle) plus intravenous oxaliplatin (130 mg/m2 on day 1 of each cycle).
Patients were ineligible if they received preoperative therapy, adjuvant radiotherapy or death by immediate postoperative complications.
Demographic and clinical variables
Demographic data included age and gender. Neutrophil/lymphocyte ratio less than 5 [13], albumin values ≥3.5 g/dL [14] and albumin/globulin ratio greater than 1.5 [15] were considered normal values. Tumour location was evaluated by upper gastrointestinal endoscopy study and abdominal computed tomography with contrast. Lauren [16] and the WHO’s histological classifications [17] were determined by pathological anatomy of the surgical piece, as well as the evaluation of lymphovascular invasion, perineural invasion, degree of differentiation and lymph node ratio (LNR) (number of positive lymph nodes/total number of lymph nodes excised) [18]. The database also had TNM classification according to the American Joint Committee on Cancer, 8th edition [19].
Statistical analysis
Differences according to the type of treatment received (surgery alone and surgery plus chemotherapy) were evaluated with the Mann–Whitney U test in quantitative variables (after evaluating the assumption of normality), while qualitative characteristics were evaluated with the chi-square test.
DFS was estimated from the date of surgery to the date of recurrence or the date of death or the date of the last control, and OS was estimated from the date of surgery to the date of death or the date of consultation of the patient’s vital condition in the National Identification Registry (RENIEC). Survival curves were estimated with the Kaplan–Meier method and the log-rank test was used to compare them. The Cox proportional hazards model was used to calculate hazard ratios and identify prognostic factors for survival.
A p-value < 0.05 was considered statistically significant and the analysis was carried out with R Studio Software (version 1.3.959; RStudio PBC, Boston, MA, USA).
Ethics
This study was approved by the ethics research committee of INEN. The data obtained from the medical records were kept confidential.
Results
Demographics and clinical characteristics of the patients
Between January 2014 and December 2016, 2,817 patients with GC were registered at INEN. Of these, 1,541 (54.7%) had clinical stage II–III. From them, 1,325 were excluded according to the exclusion criteria described above. In total, 173 gastric cancer patients with stage II–III who underwent radical gastrectomy and D2 dissection were included in this study: 100 (57.8%) patients received adjuvant chemotherapy and surgery and 73 (42.2%) surgery alone. Table 1 shows demographics and clinical characteristics according to type of treatment.
In comparison with the surgery alone group, the average age of patients in the adjuvant chemotherapy plus surgery group was lower (64.5 (±13.7) versus 56.8 years (±12.4), respectively, (p < 0.001)). Also, the frequency of lymphovascular invasion (91%), lymph node ratio >13 (55%), nodal status (pN) 2–3 (75%) and pathologic stage III (79%) were higher in the adjuvant chemotherapy plus surgery group (p < 0.05). No statistical differences between the groups according to sex, neutrophil/lymphocyte ratio, albumin/globulin ratio, site of tumour, Lauren and OMS’ classification, perineural invasion, differentiation and tumour stage (pT) were observed (Table 1).
Table 1.Patients’ demographics and clinical characteristics according to treatment.
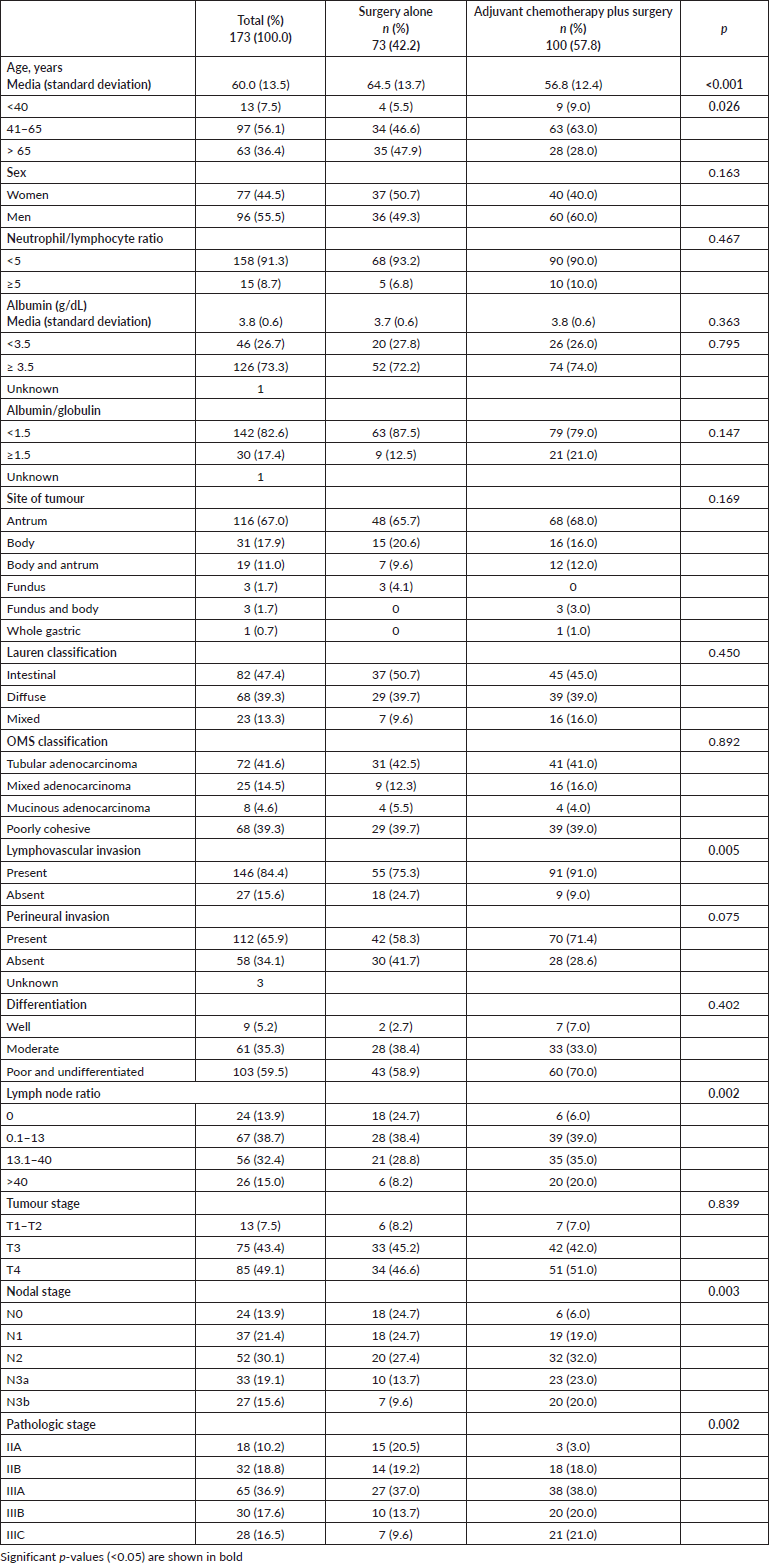
Characteristics of adjuvant chemotherapy
The median of time to adjuvant chemotherapy since surgery was 6.64 weeks (range: 3.14–19.0). Regarding treatment duration, 34.0% (n = 34) of the patients received chemotherapy for 6 months or less; 46.0% (n = 46) from 6.1 to 8 months and 20% for more than 8 months. In total, 36.0% (n = 36) received six courses, 19% (n = 19) seven courses and 45% (n = 45) eight courses of chemotherapy.
Adverse events
From patients treated with adjuvant chemotherapy, 85% (n = 85) experienced adverse effects. The most commonly reported adverse events at any grade in the chemotherapy group were neutropenia, nausea, peripheral neuropathy and diarrhoea. The most common grade 3 or 4 adverse events were neutropenia. Adverse events led to chemotherapy dose modifications in 72 (72.0%) patients; neutropenia, nausea and peripheral neuropathy were the most common reasons (Table 2).
Disease-free and overall survival
The median follow-up for DFS was 46 months (45.17–47.62) and 48 months for OS (46.22–50.96).
The 3-year disease-free survival was higher in the adjuvant chemotherapy and surgery group (69%) than in the surgery alone group (52.6%) (p = 0.034). Kaplan–Meier curves for disease-free survival show early separation between the two study groups (Figure 1a), although median disease-free survival was not reached.
Regarding OS, 31 patients (31%) died in the adjuvant chemotherapy group compared with 34 (46.6%) in the surgery only group (p = 0.027); the median OS was not reached (Figure 1b). Three-year OS was 73% in the adjuvant chemotherapy plus surgery group and 57.5% in the surgery alone group.
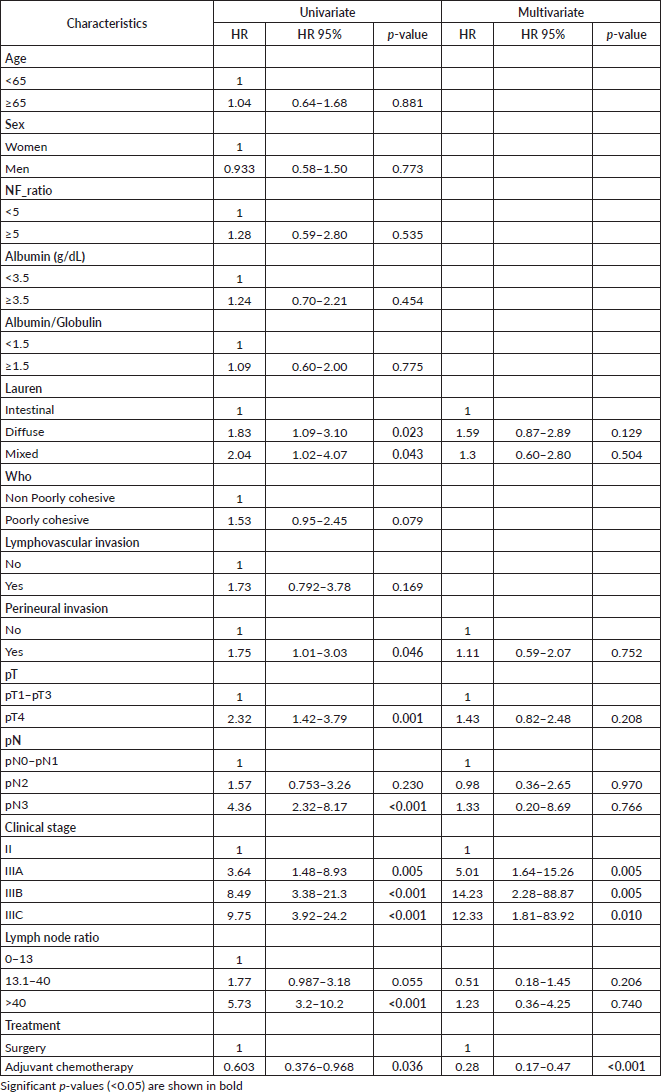
In the multivariate analysis, adjuvant chemotherapy was an independent prognostic factor for survival, for both DFS (HR = 0.28, 95% CI = 0.17–0.47; p < 0.001) and OS (HR = 0.27, 95% CI = 0.16–0.46; p < 0.001) (Tables 4 and 5).
Prognostic factors for survival according to treatment
Subgroup analysis of disease-free survival showed consistent benefits for chemotherapy plus surgery compared with surgery alone for patients with albumin >3.5 g/dL, non-poorly cohesive subtypes by the WHO’s classification, lymphovascular and perineural invasion, pT4, pN2–3, pathologic stage IIIA and IIIB and LNR > 13.1 factors (Figure 2).
Table 2. Most common adverse events (>5%) reported by patients treated with adjuvant chemotherapy (n = 85).
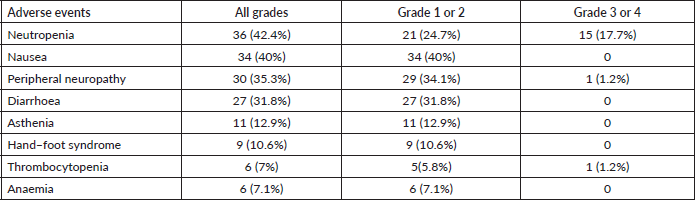
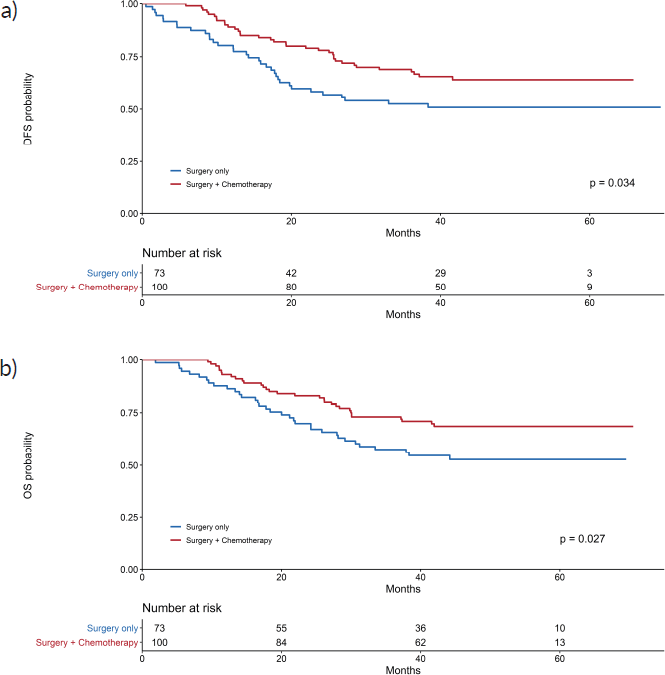
Figure 1. Kaplan–Meier curves for (a): DFS and (b): OS.
On the other hand, OS was significantly higher in the chemotherapy plus surgery group than in the surgery alone group for patients with albumin >3.5 g/dL, lymphovascular and perineural invasion, pT4, pN2–3, pathologic stage IIIA and IIIB and LNR > 13.1 factors (Figure 3).
Table 3. Prognostic factor for DFS, univariate and multivariate analyses.
Discussion
In this study, we compared the effectiveness of adjuvant chemotherapy plus surgery versus only surgery in Peruvian patients with stage II–III gastric cancer who underwent radical gastrectomy and D2 lymph node dissection. Although patients in the AChs group were younger and had poor prognostic characteristics, the OS and DFS were longer in patients with gastric cancer who received adjuvant chemotherapy with CAPOX compared to those with only surgery treatment. In the multivariate analysis where possible confounders were included in the model, adjuvant chemotherapy was an independent prognostic factor for survival, with DFS (HR = 0.28, 95% CI = 0.17–0.47; p < 0.001) and OS (HR = 0.27, 95% CI = 0.16–0.46; p < 0.001). The decreased risk of death and recurrence was greater than those published in the GASTRIC group meta-analysis of 17 trials, which showed a reduction of 18% for both disease-free survival and OS in patients with resectable disease [12].
Furthermore, the 3-year DFS and OS were higher in the CAPOX adjuvant chemotherapy and surgery group than in the surgery alone group (69% versus 52.6%, p = 0.034; 31% versus 46.6%, p = 0.027, respectively). These results are similar to other studies that investigated postoperative adjuvant chemotherapy regimens after D2 lymph node dissection without radiotherapy or neoadjuvant therapy [10, 11]. In addition, the main guidelines of management of gastric cancer recommend a multidisciplinary approach in treatment planning, whether to use neoadjuvant or adjuvant CHT, both with clinical benefits in DFS and OS.
In subgroup analysis of disease-free survival and OS according to treatment, age >65 years showed benefits for chemotherapy plus surgery compared with surgery alone (HR = 0.46 p = 0.006 and HR = 0.43 p = 0.004, respectively). Some studies confirm these results, the adjuvant chemotherapy in elderly patients with gastric cancer has same effectiveness as non-elderly patients, with median survival rates around 20.8 months in patients younger than 65 years and 19.5 months in patients aged 65 years or older [20, 21]. Based on the available data, it seems clear that adjuvant chemotherapy is as effective in elderly patients with GC as younger patients if it is administered with more caution under careful monitoring for severe toxicities. However, many oncologists hesitate to recommend elderly patients to receive chemotherapy because comorbidities or age-related changes, pharmacokinetics and pharmacodynamics may lead to higher toxicity.
About 40% of our patients had poorly cohesive gastric cancer (PCGC), a distinct type of gastric cancer that is persistently increasing in Asia, Europe and the United States, and accounts for 35%–45% of new adenocarcinoma cases [22, 23]. PCGC is frequently and highly infiltrative and resistant to chemotherapy [24, 25]. Although radical gastrectomy is a standard treatment for PCGC, recurrence is a critical issue for long-term survival of patients, and so far, the role of the adjuvant chemotherapy in these patients is controversial [26]. Our data suggest that adjuvant chemotherapy did not significantly reduce DFS (51.7% versus 43.6%, surgery alone and surgery plus adjuvant chemotherapy group, respectively) and OS (51.7% versus 38.5%, surgery alone and surgery plus adjuvant chemotherapy group, respectively) after curative resection and D2 lymph node dissection in poorly cohesive gastric carcinoma. Therefore, understanding its molecular mechanisms and effective therapeutic options remains a challenge.
Table 4. Prognostic factor for OS, univariate and multivariate analyses.
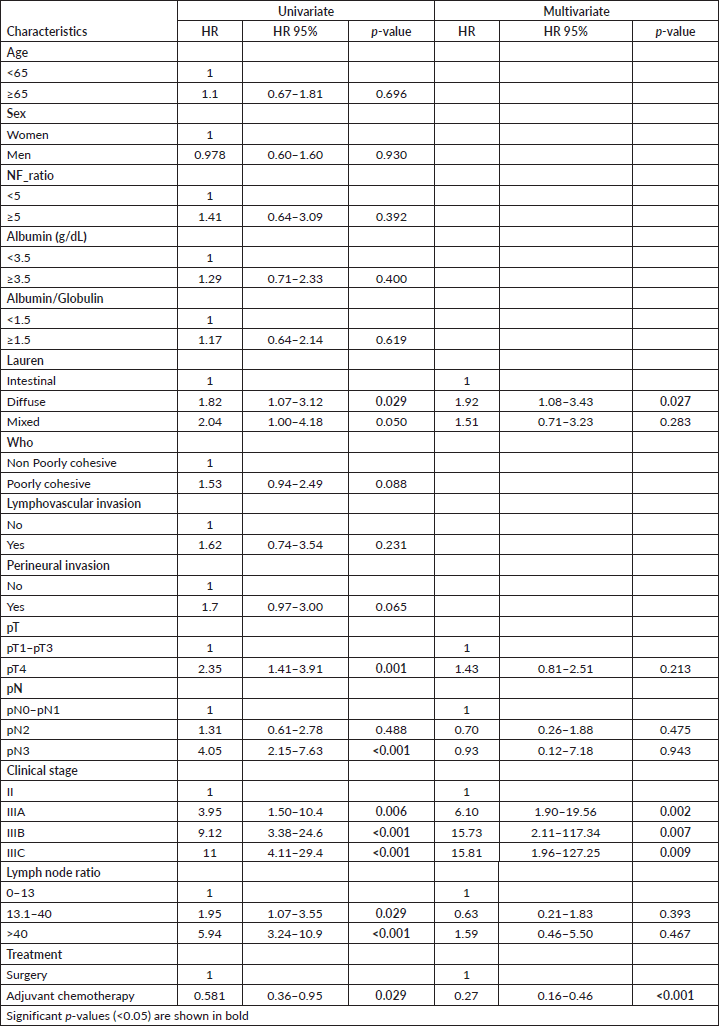
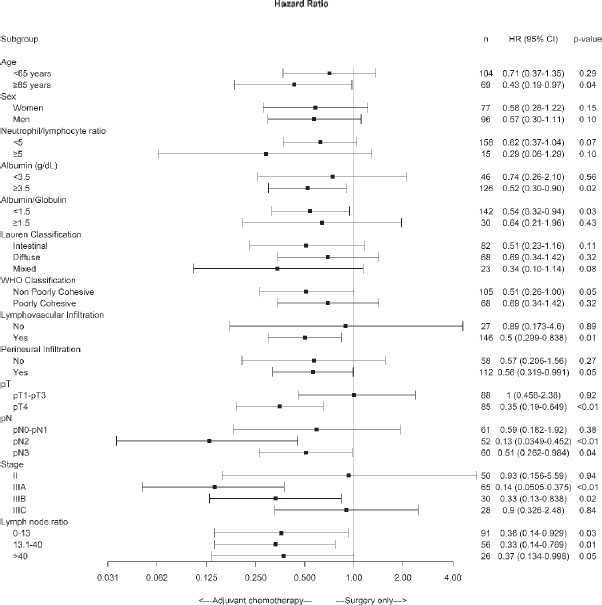
Figure 2. Forest plot of the treatment effect on disease-free survival in specific subgroups.
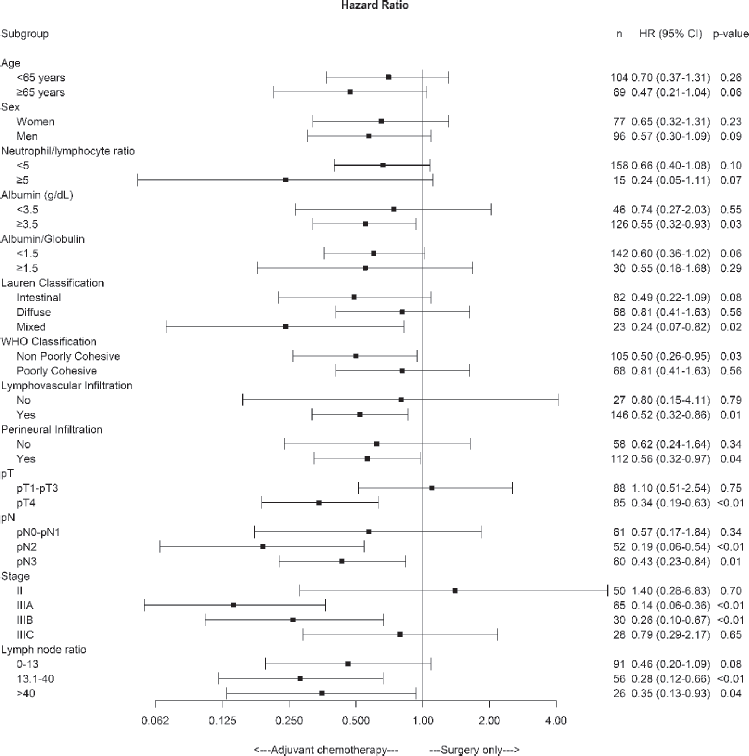
Figure 3. Forest plot of the treatment effect on overall survival in specific subgroups.
LNR remains as an important independent prognostic factor in patients undergoing radical gastrectomy and D2 lymph node dissection for gastric cancer, despite the various cut-off points used in its classification [18, 27, 28]. In our study, a subgroup analysis of disease-free survival and OS showed consistent benefits for chemotherapy plus surgery compared with surgery alone for lymph node ratio of 13.1 to >40, which is a good finding because different studies have found that this group of patients has a poor prognosis. Guevara et al [29] found LNR as an important prognostic factor to explain the time of death (LNR = 13.1–40; HR = 6.77; 95% CI = 3.34–13.70, p < 0.05) and recurrence time (LNR = 13.1–40; 95% CI = 2.10–13.43; p < 0.05) in gastric cancer patients who underwent radical gastrectomy D2 treated at INEN [29]. Likewise, Kim et al [30] have demonstrated that adjuvant treatment in patients with D2 lymphadenectomy and with a high LNR (≥0.25) had greater benefit with better disease-free survival compared to those who did not receive.
Several studies have noted that postoperative recurrence is associated with factors such as pT, extent of lymph node invasion and clinical stage [31–34], which is consistent with this study. The majority of our patients were diagnosed as advanced gastric cancer, 50% as pT4, more than half as pN2/N3 and 65% as IIIA/IIIB clinical stage. For these patient groups, disease-free survival and OS were significantly improved with oxaliplatin and capecitabine after curative surgery compared with surgery only. These data are consistent with that reported in the CLASSIC trial [11].
This study has some limitations. The information was obtained retrospectively from the review of medical records and, in some cases, there were missing data. Likewise, the time of follow-up, although long, was insufficient since the median OS had not yet been reached. However, the source used is representative and allows to characterise our reality. Our future direction is to carry out a prospective and comparative analysis with others types of systemic treatment.
Conclusion
These data suggest that adjuvant capecitabine and oxaliplatin reduce the recurrence and mortality in patients with stage II–III gastric cancer who underwent radical gastrectomy and D2 dissection. Patients with pathologic stage IIIA and IIIB and lymph node ratio >13.1 benefited more from receiving adjuvant chemotherapy. The addition of adjuvant chemotherapy in patients with poorly cohesive gastric carcinoma did not significantly reduce the rate of recurrence and mortality after D2 gastrectomy. However, prospective studies are required to confirm these findings.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
1. Sung H, Ferlay J, and Siegel RL, et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
2. Pisani P, Parkin DM, and Ferlay J (1993) Estimates of the worldwide mortality from eighteen major cancers in 1985. Implications for prevention and projections of future burden Int J Cancer 55(6) 891–903 https://doi.org/10.1002/ijc.2910550604 PMID: 8253525
3. Howlader N, Noone AM, and Krapcho M (eds) (2021) SEER Cancer Statistics Review, 1975–2018 (Bethesda: National Cancer Institute) [https://seer.cancer.gov/csr/1975_2018/] based on November 2020 SEER data submission, posted to the SEER web site
4. Bernabé-Monsalve L (2017) Prevalencia de Helicobacter pylori en el agua de consumo humano de pacientes diagnosticados con cáncer gástrico Helicobacter pylori positivo en el Instituto Nacional de Enfermedades Neoplásicas 2015–2016 (Univ Nac Mayor San Marcos Lima-Perú)
5. Payet E, et al (2016) Registro de cáncer de Lima Metropolitana: Incidencia y mortalidad 2010–2012
6. Quiros RM and Desai DC (2011) Multidisciplinary approach for the treatment of gastric cancer Minerva Gastroenterol Dietol 57(1) 53–68 PMID: 21372770
7. Kovoor PA and Hwang J (2009) Treatment of resectable gastric cancer: current standards of care Expert Rev Anticancer Ther 9(1) 135–142 https://doi.org/10.1586/14737140.9.1.135
8. Paredes Torres O, Prado Cucho S, and Taxa Rojas L, et al (2021) Clinicopathological factors associated with the presence of tumor deposits in resected gastric cancer patients Heliyon 7(6) e07185 https://doi.org/10.1016/j.heliyon.2021.e07185 PMID: 34141939 PMCID: 8188374
9. Ter-Ovanesov M, Yalcin S, and Zalcberg J, et al (2013) Registry of gastric cancer treatment evaluation (REGATE): II treatment practice: REGATE: gastric cancer treatment Asia Pac J Clin Oncol 9(4) 373–380 https://doi.org/10.1111/ajco.12089 PMID: 23909998
10. Sasako M, Sakuramoto S, and Katai H, et al (2011) Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with s-1 versus surgery alone in stage II or III gastric cancer J Clin Oncol 29(33) 4387–4393 https://doi.org/10.1200/JCO.2011.36.5908 PMID: 22010012
11. Noh SH, Park SR, and Yang H-K, et al (2014) Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial Lancet Oncol 15(12) 1389–1396 https://doi.org/10.1016/S1470-2045(14)70473-5 PMID: 25439693
12. Paoletti X, Oba K, and Burzykowski T (2010) Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis JAMA 303(17) 1729 https://doi.org/10.1001/jama.2010.534 PMID: 20442389
13. Szor D, Dias A, and Pereira M, et al (2018) Prognostic role of neutrophil/lymphocyte ratio in resected gastric cancer: a systematic review and meta-analysis Clinics [Internet] 73 [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5996440/?report=classic] Date accessed: 13 May 2021 https://doi.org/10.6061/clinics/2018/e360
14. Kudsk K, Tolley E, and DeWitt R, et al (2003) Preoperative albumin and surgical site identify surgical risk for major postoperative complications J Parenter Enter Nutr 27(1) 1–9 https://doi.org/10.1177/014860710302700101
15. He J, Pan H, and Liang W, et al (2017) Prognostic effect of albumin-to-globulin ratio in patients with solid tumors: a systematic review and meta-analysis J Cancer 8(19) 4002–4010 https://doi.org/10.7150/jca.21141 PMID: 29187875 PMCID: 5706002
16. Laurén P (1965) The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma: an attempt at a histo-clinical classification Acta Pathol Microbiol Scand 64(1) 31–49 https://doi.org/10.1111/apm.1965.64.1.31
17. Gonzalez RS (2021) WHO Classification PathologyOutlines.com website. [https://www.pathologyoutlines.com/topic/stomachWHOclassification.html]
18. Chen S, Zhao B-W, and Li Y-F, et al (2012) The prognostic value of harvested lymph nodes and the metastatic lymph node ratio for gastric cancer patients: results of a study of 1,101 patients PLoS One 7(11) e49424 https://doi.org/10.1371/journal.pone.0049424 PMID: 23166665 PMCID: 3499537
19. Amin MB, Edge SB, and Greene FL, et al AJCC Cancer Staging Manual (Springer) 1032 p. 2018.
20. Kim HS, Kim JH, and Kim JW, et al (2016) Chemotherapy in elderly patients with gastric cancer J Cancer 7(1) 88–94 https://doi.org/10.7150/jca.13248 PMID: 26722364 PMCID: 4679385
21. Karaca M, Tural D, and Kocoglu H, et al (2018) Adjuvant chemotherapy for gastric cancer in elderly patients has same benefits as in younger patients J Cancer Res Ther 14(3) 593 https://doi.org/10.4103/0973-1482.172588 PMID: 29893324
22. Pernot S (2015) Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge World J Gastroenterol 21(40) 11428 https://doi.org/10.3748/wjg.v21.i40.11428 PMID: 26523107 PMCID: 4616218
23. European Chapter of International Gastric Cancer Association, Mariette C, and Carneiro F, et al (2019) Consensus on the pathological definition and classification of poorly cohesive gastric carcinoma Gastric Cancer 22(1) 1–9 https://doi.org/10.1007/s10120-018-0868-0
24. Voron T, Messager M, and Duhamel A, et al (2016) Is signet-ring cell carcinoma a specific entity among gastric cancers? Gastric Cancer 19(4) 1027–1040 https://doi.org/10.1007/s10120-015-0564-2
25. Lemoine N, Adenis A, and Bouche O, et al (2016) Signet ring cells and efficacy of first-line chemotherapy in advanced gastric or oesogastric junction adenocarcinoma Anticancer Res 36(10) 5543–5550 https://doi.org/10.21873/anticanres.11138 PMID: 27798928
26. Charalampakis N, Nogueras González GM, and Elimova E, et al (2016) The proportion of signet ring cell component in patients with localized gastric adenocarcinoma correlates with the degree of response to pre-operative chemoradiation Oncology 90(5) 239–247 https://doi.org/10.1159/000443506 PMID: 27046280 PMCID: 4870109
27. Zhao L-Y, Li C-C, and Jia L-Y, et al (2016) Superiority of lymph node ratio-based staging system for prognostic prediction in 2575 patients with gastric cancer: validation analysis in a large single center Oncotarget 7(32) 51069–51081 https://doi.org/10.18632/oncotarget.9714 PMID: 27363014 PMCID: 5239459
28. Saito H, Fukumoto Y, and Osaki T, et al (2008) Prognostic significance of the ratio between metastatic and dissected lymph nodes (n ratio) in patients with advanced gastric cancer J Surg Oncol 97(2) 132–135 https://doi.org/10.1002/jso.20929
29. Guevara Jabiles A, Ruiz Figueroa E, and Berrospi Espinoza F, et al (2018) Prognostic value of lymph node ratio (LNR) in patients who underwent radical gastrectomy] Rev Gastroenterol Peru 38(3) 253–260 PMID: 30540729
30. Kim Y, Squires MH, and Poultsides GA, et al Impact of lymph node ratio in selecting patients with resected gastric cancer for adjuvant therapy Surgery 162(2) 285–294 PMID: 28578142 PMCID: 6036903
31. Spolverato G, Ejaz A, and Kim Y, et al (2014) Rates and patterns of recurrence after curative intent resection for gastric cancer: a United States multi-institutional analysis J Am Coll Surg 219(4) 664–675 https://doi.org/10.1016/j.jamcollsurg.2014.03.062 PMID: 25154671
32. Eom BW, Yoon H, and Ryu KW, et al (2010) Predictors of timing and patterns of recurrence after curative resection for gastric cancer Dig Surg 27(6) 481–486 https://doi.org/10.1159/000320691 PMID: 21063125
33. Choi JY, Ha TK, and Kwon SJ (2011) Clinicopathologic characteristics of gastric cancer patients according to the timing of the recurrence after curative surgery J Gastric Cancer 11(1) 46 https://doi.org/10.5230/jgc.2011.11.1.46 PMID: 22076201 PMCID: 3204481
34. Kang W-M, Meng Q-B, and Yu J-C, et al (2015) Factors associated with early recurrence after curative surgery for gastric cancer World J Gastroenterol 21(19) 5934–5940 https://doi.org/10.3748/wjg.v21.i19.5934 PMID: 26019458 PMCID: 4438028






