Systemic therapy in pancreatic ductal adenocarcinomas (PDACs)—basis and current status
Anant Ramaswamy1, Sujay Srinivas1, Vikram Chaudhari2, Prabhat Bhargava1, Manish Bhandare2, Shailesh V Shrikhande2 and Vikas Ostwal1
1Department of Medical Oncology, Tata Memorial Hospital, Dr E Borges Road, Parel, Mumbai 400012, India
2GI and HPB Services, Tata Memorial Hospital, Homi Bhabha National Institute, Dr E Borges Road, Parel, Mumbai 400012, India
Abstract
A major shift in the approach to the management of pancreatic ductal adenocarcinomas (PDACs) has been the recognition of the systemic nature of the disease even in clinically and radiologically limited disease stages. The recalcitrant nature of PDAC is intrinsically related to the lack of therapeutic targets and dense surrounding stroma that limits the activity of currently available chemotherapeutic options. However, research is increasingly focusing on intensifying systemic management options in PDAC, resulting in gradual improvements in survival. Currently effective chemotherapeutic regimens like modified 5-fluorouracil-leucovorin-irinotecan-oxaliplatin and gemcitabine-nab-paclitaxel have improved outcomes in resectable and advanced PDAC. An increasing use of these regimens has also resulted in greater conversion of borderline resectable and locally advanced cancers to resection, though the most effective approach in this subgroup is yet to be identified. The current review presents an outline of the basic systemic nature of PDAC and current options of systemic therapy, predominantly chemotherapy .
Keywords: pancreatic cancer, systemic therapy, India, review, chemotherapy
Correspondence to: Vikas Ostwal
Email: dr.vikas.ostwal@gmail.com
Published: 24/03/2022
Received: 11/10/2021
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Pancreatic ductal adenocarcinomas (PDACs) are the twelfth most common cancer across the world in terms of incidence, but cause a disproportionate number of cancer related death in terms of proportions as per data from GLOBOCAN 2020 [1]. While there appears to be a greater incidence of PDAC in Europe and North America, there is a trend towards an increasing incidence across geographic regions [2]. A similar pattern has been noted in mortality rates, though 5-year survival rates across stages of PDAC have marginally improved from 6% to 9% over the course of the last two decades [1]. A corollary to this finding is that a majority of patients are diagnosed with PDAC in advanced or metastatic stage, where overall survivals (OSs) are in the region of approximately 9–12 months [3].
A major shift in the approach to the management of PDAC has been the recognition of the systemic nature of the disease even in clinically and radiologically limited disease stages [4]. While high-quality surgery forms an important backbone of the management of limited stage PDAC, current research is increasingly focusing on adequate control of systemic PDAC combined with local methods of disease control. This has resulted in gradual improvements in survival for patients with PDAC, though the quantum of improvement lags behind that seen in a number of other malignancies. The basis for systemic management of PDAC remains multi-agent chemotherapy, with options like immunotherapy and vaccine approaches yet to bear fruit in the treatment of PDAC [5].
Molecular landscape of PDAC
Genomic studies and analyses of copy number variations have identified four major mutations in the genomic landscape that drive the oncogenic process of PDAC – activating mutations causing telomeric shortening in KRAS (≥90%, near ubiquitous in PDAC), inactivating mutations in TP53 (approximately 74%), SMAD4 (approximately 31%) and CDKN2A (approximately 35%). Other less common alterations include changes in KDM6A and PREX2, as well as certain hereditary pathogenic mutations in BRCA, BRCA2 and PALB2.
Mutations in KRAS occur early in the pathogenesis of PDAC, while SMAD4 mutations tend to occur late and facilitate increased aggressiveness and progression of PDAC [6]. While mutations in KRAS and TP53 and abnormal CDKN2A have known to correlate with poorer prognosis, there have been disappointing results when attempts to target these genes have been made, e.g. the failure of farnesyl-transferase inhibitors in targeting KRAS [7, 8]. This lack of useful targets has resulted in shifting focus from genomics to transcriptomics.
While individual mutations give information of drivers of PDAC, the lack of firm clinical correlates has resulted in numerous attempts to evaluate the transcriptomic data analysis with clinical features and outcomes [9]. In as early as 2011, Collisson et al [10] used hybridisation array-based mRNA expression on only the epithelial component of patient samples with untreated resected PDAC to identify three subtypes: Classical, Quasi-mesenchymal (QM-PDA) and Exocrine-like. Along with correlation with specific genomic and pathological features, the QM-PDA cohort also had decreased survival compared to the other subtypes [10]. Several other classifications have emerged over the last decade, with a recent one by Puleo et al [11] subtyping 309 cases of PDAC into basal like, stroma activated, desmoplastic, pure classical and immune classical subtypes. This built upon a previous study classifying PDAC into basal and classical subtypes, while additionally identifying additional sub-classifications. Besides being identified with particular molecular signatures, the subtypes also showed different survival outcomes: the basal-like subtype had the worst outcome (median OS (mOS) – 10.3 months), whereas the pure classical and immune classical subtypes showed good prognosis (mOS values of 43.1 and 37.4 months, respectively). The stroma activated subtype was associated with a poor prognosis, but better as compared to the pure basal-like subtype (mOS – 20.2 months). An important aspect of this classification was its recognition and inclusion of the pancreatic stroma in determining PDAC biology and outcomes [11].
Increasing evidence also suggests that available transcriptomic classifications have firm translational correlates in terms of predicting responses to available systemic chemotherapeutic options [12]. Early results from the COMPASS study, which entailed performing whole genome sequencing and RNA sequencing in patients with advanced PDAC being treated with 5-fluorouracil-leucovorin-irinotecan-oxaliplatin (FOLFIRINOX) or gemcitabine with nab-paclitaxel (GN), divided patients into ‘basal’ and ‘classical’ subtypes as is commonly used in PDAC. They clearly showed that radiological responses as well as survivals with first-line chemotherapy were markedly inferior in the basal subtypes as compared to the classical subtypes [13].
Why is PDAC difficult to treat?
PDACs are notoriously difficult to treat malignancies, with limited increments in survival over the last two decades. Major reasons for this recalcitrant nature of PDAC are related to the inherent biology of the cancer. While a complete analysis of pancreatic tumour biology is beyond the scope of this review, some of the important aspects as to why the PDACs are resistant to treatment are discussed.
Systemic nature of PDAC
Elegant mouse models have shown that pancreatic cancer progression is a model in which the seeding of distant organs occurs before, and in parallel to, tumour formation at the primary site [4]. Most patients with pancreatic cancer have metastatic disease at the time of diagnosis, even if not radiologically evident. Epithelial mesenchymal transition (EMT) is considered as one of the key features of metastases acquired by epithelial cells wherein, they lose their epithelial cell like properties and gain invasive properties and stem cell-like features, allowing for metastases. In their mouse model studies using Cre-lox-based mouse model of PDACs, Rhim et al [14] have clearly shown that EMT along with bloodstream entry of transformed cells and seeding of distant organs occurs at a stage of PDAC progression previously thought to be preinvasive based on standard histological examination. Even when tumours were at the precancerous stage (pancreatic intraepithelial neoplasia), they were associated with EMT, suggestive of metastatic potential [14].
Tumour microenvironment (TME) of PDAC
Besides various genomic alterations seen in PDAC, ongoing research has increasingly identified the effect of the tumour microenvironment (TME) on the nature of PDAC. The neoplastic cells in PDAC comprise a minority of the entire tumour tissue, with the vast majority of tumour tissue being contributed by the TME, predominantly comprising dense desmoplastic stroma. The effect of stroma on tumour genotype and phenotype has been captured in the molecular classifications of PDAC as previously mentioned, thereby reiterating the footprint of the stroma on PDAC biology [15].
There are three fundamental components of the pancreatic stroma – extracellular matrix (ECM), vasculature and cancer associated fibroblasts (CAFs). Desmoplasia is a fibrotic reaction caused by an excess of fibroblasts and the deposition of ECM, and this is a hallmark of PDAC. High stromal concentrations of hyaluronic acid have directly correlated with inferior survivals in PDAC. Besides direct correlation with outcomes, ECM purportedly acts as a barrier to chemotherapy. Studies in genetically engineered mouse models have shown that tumour stroma remodelling via inhibition of small molecules such as STAT3 may improve the efficacy of standard gemcitabine chemotherapy and improve outcomes. The ECM also acts as a barrier to the delivery of chemotherapeutic agents by possibly mediating increased interstitial fluid pressure within the tumour, thereby impairing vascular function and tumour permeability [16] . Available evidence suggests that CAFs are active players during the process of cancer initiation, progression and metastasis. Besides being part of bi-directional signalling between neoplastic cells and themselves in terms of oncogenesis, a subset of CAFs called inflammatory CAF may be pro-tumorigenic themselves. IL-6, secreted by CAFs, may also contribute to immune evasion in PDAC. Numerous therapeutic targets against CAFs or CAF receptors in PDAC are under development with some of the more prominent ones under evaluation being insulin-like growth factor 1 (IGF1) receptor, Vitamin D receptor and inhibition of β-catenin nuclear localisation and invasion of cancer cells by all-trans retinoic acid [17].
Current concepts in management of advanced PDAC
Treatment options in advanced PDAC
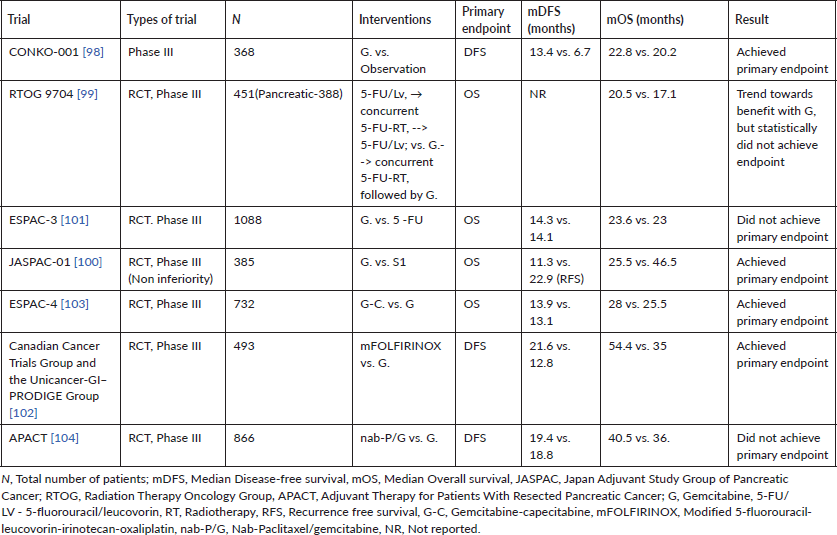
The treatment of PDACs is based on their initial presentation in terms of resectability status, with a majority of the patients having locally advanced unresectable (LAPC) or metastatic cancers with only 10%–20% having clearly resectable disease at initial presentation [18, 19] (Table 1). This implies that a majority of treatment regimens have initially been evaluated in the advanced setting, before being used in localised PDACs. Systemic chemotherapy in the form of palliative chemotherapy can improve disease related symptoms and quality of life in metastatic pancreatic cancer; while those with LAPC may be initially treated with neoadjuvant intent therapy. Multidisciplinary joint clinic decisions have become the standard for charting out the treatment plan for patients with advanced PDAC.
There have been a small number of validated regimens for the first-line treatment of advanced PDAC. The current first-line regimen has evolved over the years from bolus 5-fluorouracil (5-FU) to gemcitabine to the current era of FOLFIRINOX and GN. Table 2 summarises the major studies evaluating first-line therapy in advanced PDAC. In routine current practice, the FOLFIRINOX regimen is recommended as a standard first-line choice for advanced pancreatic cancer by the National Comprehensive Cancer Network (NCCN), the American Society of Clinical Oncology and the European Society for Medical Oncology (ESMO) for patients with an ECOG performance status score of 0 or 1 and a favourable comorbidity profile [18, 20, 21]. This is based on the results of the pivotal PRODIGE 4-ACCORD11 trial which compared FOLFIRINOX to gemcitabine in metastatic pancreatic cancer patients below 75 years of age. OS was significantly increased, with median survival of 11.1 months for FOLFIRINOX and 6.8 months for the gemcitabine regimen (Hazard ratio (HR) 0.57; 95% confidence interval (CI), 0.45–0.73; p < 0.001) [22].
Table 1. Resectability criteria in PDAC (NCCN criteria) [19].
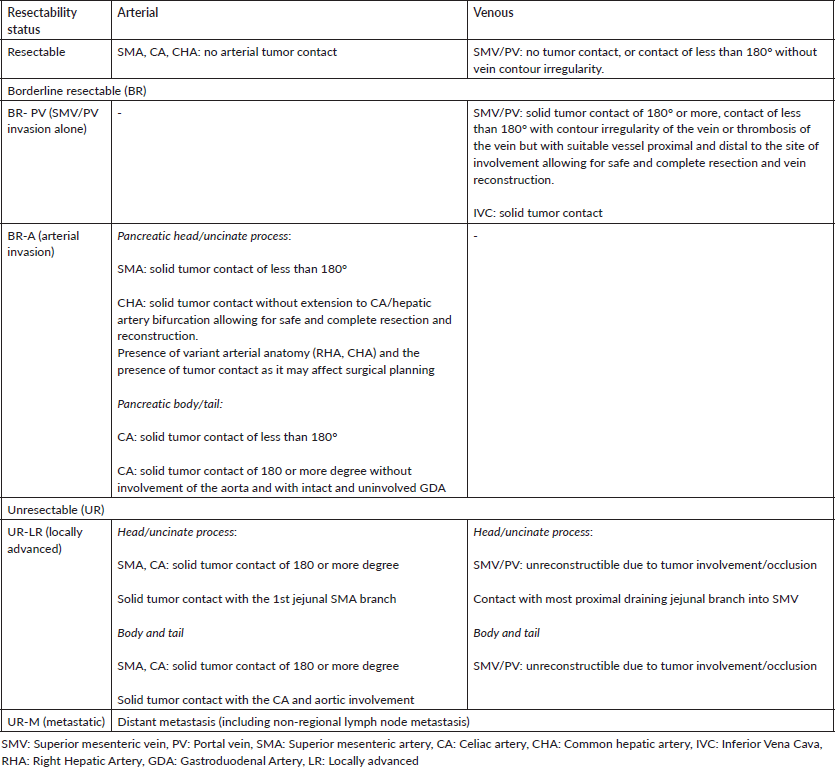
Table 2. Selected trials on first-line treatment in advanced pancreatic cancers.
The MPACT trial reported that a combination of GN was superior to gemcitabine alone as a first-line regimen for the treatment of patients with metastatic PDAC. Of 861 patients with an ECOG performance status score of 0–2 in this study, the mOS was 8.5 months in the nab-paclitaxel arm and 6.7 months in the gemcitabine arm (HR, 0.72; 95% CI, 0.62–0.83; p < 0.001). There has been no prospective randomised study comparing FOLFIRINOX to GN till date [23]. Retrospective studies comparing both have suggested greater activity for FOLFIRINOX, with a greater use of the FOLFIRINOX regimen in patients with better ECOG PS and general fitness. However, real-world evidence in the form of a metanalysis of 3,813 patients did not show a statistical OS or PFS benefit with FOLFIRINOX as opposed to GN as a first-line therapy in metastatic PDAC, underscoring the need for a prospective study to answer this question [24].
Other acceptable first-line therapy options especially in patients with ECOG PS 2 and an unfavourable co-morbidity profile are single agent gemcitabine, single agent 5-FU/capecitabine, doublet chemotherapy regimens like FOLFOX, gemcitabine plus capecitabine. Where available, S-1 monotherapy represents a reasonable alternative to gemcitabine monotherapy for patients who prefer the convenience of an oral regimen. Patients with advanced PDAC should be concurrently offered aggressive supportive treatment of cancer related symptoms like pain, etc.
Only about 40%–50% of the patients with advanced pancreatic cancer go on to receive second-line therapy [25]. A majority of trials and studies in this setting have been done in patients with good ECOG PS of 0–1, which is not the case in a real-world setting. Additionally, these were conducted in the pre-FOLFIRINOX era and evaluated oxaliplatin based chemotherapy after failure of gemcitabine which is applicable to only a very small subset of patients in the current era. Nanoliposomal irinotecan, a novel formulation of irinotecan has been evaluated in a phase III trial (NAPOLI-1) [26]. It showed that patients assigned to Nanoliposomal irinotecan plus 5-FU arm had a longer OS than patients treated with 5-FU alone (median, 6.1 versus 4.2 months; HR, 0.75; p = 0.012). Other efficacy endpoints like progression-free survival, objective response rates (RR) and time to treatment failure were also significantly superior in the Nanoliposomal irinotecan plus 5-FU arm. Two other studies with contrasting results with regard to the use of FOLFOX as a second-line regimen deserve mention in this context. The CONKO-003 trial showed a statistically significant OS benefit with FOLFOX in comparison with monotherapy with 5-FU/leucovorin (5-FU/LV) alone while the PANCREOX study did not show a benefit with using FOLFOX [27, 28]. Possible reasons for these differences, within the limitations of a cross trial comparison, included the lower doses of oxaliplatin and resulting better tolerance with FOLFOX in the CONKO-003 trial.
The data on second-line treatment options after prior FOLFIRINOX or GN and vice versa is limited to retrospective studies only. The major society guidelines suggest the choice of second line depending on the ECOG PS, co-morbidities and organ function. After first-line treatment with gemcitabine-based regimens, Nanoliposomal irinotecan plus 5-FU is the preferred option. 5-FU plus irinotecan or 5-FU plus oxaliplatin can be offered when Nanoliposomal irinotecan is not available. The choice of oxaliplatin or irinotecan depends on the pre-existing neuropa thy, though a metanalysis suggested a greater benefit for irinotecan containing combinations as compared to oxaliplatin containing combinations [29]. After first-line treatment with FOLFIRINOX, GN can be considered.
Treatment of frail and elderly patients
An aspect of the management of advanced PDAC that has been addressed to a very limited extent is the optimal management of patients who have an ECOG PS > 1 or patients who are not fit for intensive protocols like FOLFIRINOX. Additionally, this cohort of unaddressed patients increases when elderly patients with cancer are also considered. The median age at diagnosis of PDAC, as per the SEER database is approximately 70 years, yet a majority of clinical trials have very limited representation of these patients in their study populations [30]. For e.g., both the Unicancer GI PRODIGE trial evaluation FOLFIRINOX and the MPACT study evaluating nab-Paclitaxel had only 0.6% and 7% of patients with ECOG PS 2. Again, the median age of the patients in this study was 61 and 63 years, respectively. The proportion of patients aged greater than 65 was 22% and 42%, respectively [22, 23]. This is to emphasise the limited evidence available for the management of such patients in terms of clinical trials. In stark contrast, frail and elderly patients often comprise anywhere between 8% and 67% of patients in routine clinical practice and are treated with modifications of existing regimens with unclear evidence of benefit or lack, thereof [31, 32].
In the context of this frail population, available prospective evidence comes from two well conducted studies. The first is the seminal study by Burris et al [33], which showed a benefit for palliative chemotherapy. The study evaluated gemcitabine versus 5-FU in patients with advanced PDAC and showed a relative benefit for gemcitabine in terms of palliation, tolerance and survival. Sixty-nine percent of patients in this trial had an ECOG PS of ≥2, which is why gemcitabine is still a valid option in such patients [33]. The second study evaluating such a population was the FRAGRANCE trial, a phase I/II trial evaluating different dosing and scheduling of gemcitabine and nab-paclitaxel in patients with ECOG PS 2. The conclusions of the authors were that administering nab-Paclitaxel at either 100 and 125 mg/m2 in combination with gemcitabine on a weekly schedule of 3 weeks on, 1 week off, was well tolerated with reasonable safety and efficacy in frail patients presenting with ECOG PS 2 and advanced PDAC [34].
Treatment guidelines like NCCN and ESMO are relatively ambiguous on the management of such patients [18, 35]. The ESMO guidelines state, ‘In very selected patients with ECOG performance status 2 due to heavy tumour load, gemcitabine and nab-paclitaxel can be considered for best chance of response. If the performance status of the patient is 2 and/or the bilirubin level is higher than 1.5× ULN: a monotherapy with gemcitabine should be considered’. Tumour burden is an ill-defined characteristic that has predominant subjective characteristics as well as inter-observer variability and hence may not always be a valid benchmark for selection of treatment.
Neoadjuvant therapy (NAT) in PDAC
The concept of neoadjuvant therapy (NAT) in a majority of solid tumours is based on the premise that it would increase margin negative resection rates, decrease extent of resections, provide an assessment of disease biology in terms of chemo-responsiveness and most importantly, potentially provide survival benefit. This has been proven and considered current standard practice in oesophageal, gastric and rectal cancers. However, with regard to PDAC, the biological evidence for the action of NAT has not been convincingly converted into clinically relevant data until recently. Multiple reasons exist for this. Firstly, while chemotherapy improved survivals post resection, RR with older regimens (predominantly gemcitabine, gemcitabine-platinum, 5-FU) were low in patients with advanced cancers [36]. This diluted the enthusiasm for using such regimens as NAT, though the advent of more efficacious regimens like FOLFIRINOX has overcame such lacunae in recent years [37–40]. Secondly, the negative impact of margin positive resections and significant morbidity and lack of survival benefit with extensive arterial resections has resulted in only a recent acceptance of classifying PDAC into resectable, borderline resectable pancreatic cancer (BRPC) and locally advanced PDAC (LAPC) [19, 21, 41–44]. Such a division has allowed multimodality management of BR/LA PDAC as the norm as opposed to concentration on surgical approaches alone. This approach has led to a near universal agreement on neoadjuvant strategies in BRPC with an increased intent for appropriate resections (40%–80%) while LA cancers are understood to require more conservative management with predominantly systemic therapy and resections only in a smaller proportion (1%–25%) [37, 45–47]. Thirdly, radiological responses post NAT, with chemotherapy or chemoradiotherapy, in PDAC, are mainly reflected by isovolumetric tissue replacement through fibrosis, rather than volume loss. A CT scan tends to overestimate residual tumour burden after NAT and does not accurately predict for the probability of R0 resection. This also leads to ambiguity in attempting resection post NAT [48]. Finally, most earlier studies have used long course radiotherapy (LCRT) along with gemcitabine as NAT for PDAC. Besides gemcitabine monotherapy being a weak agent against PDAC, there has been concern with regard to the development of micro-metastases during the course of LCRT as there is inadequate systemic coverage against a disease which has early systemic dissemination [49, 50]. These concerns have been mitigated to some extent by the development of techniques like stereotactic body radiation therapy (SBRT), though large-scale prospective evidence is lacking for their use.
While BRPC and to a certain extent, LAPC are treated with NAT in the current era with near universal acceptance, there are a number of studies which are also evaluating the role of NAT in resectable PDAC. Selected recent large studies using neoadjuvant chemoradiation (NACTRT) and/or neoadjuvant chemotherapy (NACT) with FOLFIRINOX and GN are discussed below.
NAT in BRPC and LAPC
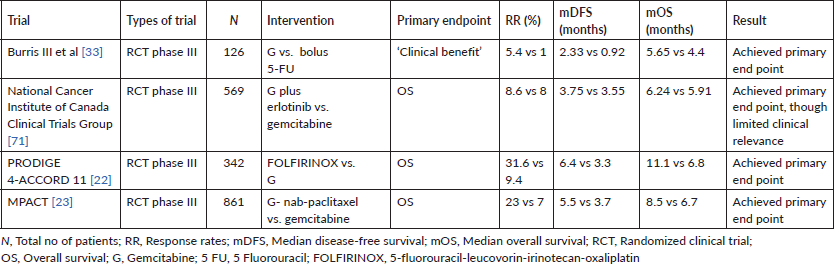
In a large prospective cohort study of 680 patients with 574 assessable patients (BRPC – 39%, LAPC – 61%) by Maggino et al [51], the most commonly used NAT was FOLFIRINOX (53%), followed by GN (21%). Complementary radiotherapy (RT) was used in 25% of patients. Resection rates were 24% and 9% for BR and LA cohorts, respectively. The median survival for the whole cohort was 12.8 months (95% CI, 11.7–13.9 months). Factors independently associated with survival included completion of chemotherapy, application of RT and resection [51].
A systematic review and patient-level meta-analysis on neoadjuvant FOLFIRINOX in patients with BRPC comprising 24 studies and 313 patients showed the feasibility and effectiveness of such an approach. The resection rate noted was 67.8% with R0-resection rates of 83.9%. The mOS varied from 11.0 to 34.2 months across studies with a patient-level mOS of 22.2 months (95% CI, 18.8–25.6 months), and patient-level median PFS of 18 months (95% CI, 14.5–21.5 months) [38]. A similar patients-level metanalysis of 689 (315 LAPC with survival data) patients treated with FOLFIRINOX reported a mOS ranging from 10 to 32.7 months with a patient-level mOS of 24·2 months. The median PFS ranged from 3 to 20.4 months with a patient-level median PFS of 15.0 months. Surgical resection rates noted were 25.9% [37].
One of the earliest prospective studies using neoadjuvant FOLFIRINOX was a feasibility phase II study by Katz et al [52], wherein 22 patients with BRPC received FOLFIRINOX followed by concurrent capecitabine-RT. Fifteen of the 22 patients (68%) underwent pancreatectomy with 14 (93%) having R0 resections. The mOS of all patients was 21.7 months (95% CI, 15.7 to not reached) [52].
The phase II LAPACT study, comprising 107 patients, evaluated the GN regimen in patients with LAPC, and showed 58% completion rate of induction treatment, a disease control rate of 78%, median PFS of 10.9 months and mOS of 18.8 months. Sixteen percent of patients underwent resection [53].
A phase II/III trial from South Korea enrolled 58 patients with BRPC and evaluated NACTRT (54 Gy RT with concurrent gemcitabine) followed by surgery versus upfront surgery followed by adjuvant chemoradiation. In the intention-to-treat analysis, the median survival and R0 resection rates were significantly better in the NACTRT arm than the upfront surgery arm (21 months, 52% versus 12 months, 26%), though it is to be noted that the trial closed prior to its planned accrual of 110 patients, thereby impacting interpretation of the results [54].
One of the largest and most important prospective studies in the space of BRPC is the recently published Phase III PREOPANC trial. In this randomised phase III trial comprising 246 patients with BRPC or resectable PDAC, patients were randomly assigned to receive NACTRT (Gemcitabine for two courses and a third course concurrent with RT) followed by surgery and adjuvant gemcitabine or to immediate surgery and six courses of adjuvant gemcitabine. The initial results of the study showed that the primary endpoint of improved OS with NACTRT (16.0 versus 14.3 months; HR, 0.78; 95% CI, 0.58–1.05; p = 0.096) was not met, though NACTRT was associated with superior disease-free survival (DFS) and R0 resection rates [55]. The subgroup analysis showed that NACTRT predominantly benefited the BPRC subgroup as opposed to limited or no benefits in group of patients who had resectable disease upfront. While the initial results appeared promising in terms of feasibility of a neoadjuvant approach, the lack of an OS benefit was disappointing. However, the long-term results post a follow-up approaching nearly 5 years suggest a definite OS benefit for using NACTRT as opposed to upfront surgery in the cohort of patients. Three- and five-year OS was 27.7% and 20.5% after NACTRT versus 16.5% and 6.5% after upfront surgery (HR, 0.73; 95% CI, 0.56–0.96; p = 0.025). Other secondary outcomes such as DFS, locoregional failure-free interval and distant metastases free interval also improved with NACTRT [54]. The PREOPANC study has set the base for further studies evaluating the role of more efficacious regimens like FOLFIRINOX in the neoadjuvant setting to improve survivals in BRPCs as well as resectable cancers [57, 58].
Preclinical data has suggested that the renin-angiotensin system (RAS) activation in fibroblasts causes tumour fibrosis and desmoplasia, a key feature in PDAC. The primary effector for the RAS system is angiotensin-II, which is inhibited by Losartan via a receptor blocker mechanism. Using this principle, a single arm phase II trial combining FOLFIRINOX and Losartan with RT (either short course using protons or long course CTRT) in patients with LAPC showed impressive R0 resection rates of 69% and mOS of 31.4 months [59]. The investigators have initiated a further phase II study using the combination of FOLFIRINOX, Losartan, SBRT and Nivolumab in localised PDAC [60].
SBRT has emerged as a modality that overcomes the shortcomings of LCRT, while potentially increasing the chances of R0 resection margins when used as NAT [61]. However, the use of SBRT does not seem to improve 18-month survival rates as compared to historical controls in the Alliance A021501 trial. The phase II randomised non-comparative trial studied eight cycles of neoadjuvant modified 5-fluorouracil-leucovorin-irinotecan-oxaliplatin (mFOLFIRINOX) in one arm (Arm A) and seven cycles of mFOLFIRINOX followed by SBRT or hypo fractionated image guided RT] in the other arm (Arm B). The primary endpoint, 18-month OS rate, of each arm was compared to a historical control of 50%. The 18-month OS rate based on Kaplan–Meier estimates was 67.9% (95% CI, 54.6–78.0) in Arm A and 47.3% (95% CI, 33.7–59.7) in Arm B, with the ensuing interpretation that while neoadjuvant mFOLFIRINOX was associated with favourable OS relative to historical data, while mFOLFIRINOX with hypo fractionated RT did not compare favourably to historical data [62].
An update from the ESPAC-5F study, a prospective, international, randomised phase II trial of immediate surgery versus NACT or chemoradiotherapy has also provided some prospective evidence with regard to the benefit of NAT in BRPC. The study randomised 90 patients to either upfront surgery or NAT (either gemcitabine-capecitabine, FOLFIRINOX or concurrent capecitabine-RT). While there was no difference in R0 resection rates (surgery upfront – 44% versus NAT – 41%, p = 0.668), there was a significant OS difference at 12 months (surgery upfront – 42% versus NAT – 77%, p < 0.001) in favour of NAT. While the numbers are small, the maximum 12-month OS in any cohort was seen with neoadjuvant FOLFIRINOX (84%) [63].
NAT in resectable PDAC
The rationale for using NAT in BRPC/LAPC has also been used to push the case for NAT in resectable PDAC. The major concerns with using such an approach include delaying resection inordinately, thereby resulting in local progression of the tumour to unresectability, chemotherapy related complications resulting in inoperability, e.g., liver sinusoidal injury due to agents like oxaliplatin, and as previously mentioned, difficulties in assessing resectability by imaging post NAT [64, 65].
A number of studies using gemcitabine or gemcitabine-based combinations with or without RT have shown feasibility of NAT in resectable PDAC, though large-scale comparative prospective data is yet to emerge [66]. Besides the PREOPANC data, one of the largest studies evaluating NAT in resectable PDAC is the Prep-02/JSAP-05 trial from Japan. Patients received two cycles of gemcitabine plus S1 followed by resection and adjuvant S1 in one arm while in the other arm, patients underwent upfront resection followed by adjuvant S1. The study enrolled 364 patients and achieved its primary endpoint of improved OS in the NAT arm (mOS, 36.7 versus 26.6 months; HR, 0.72; 95% CI, 0.55–0.94; p = 0.015). There were no differences in the resection rate, R0 resection rate and perioperative morbidity, though an increased incidence of neutropenia was noted in the NAT arm [67].
A smaller prospective ‘pick the winner design’ phase II randomised study, looking at outcomes with either FOLFIRINOX (Arm 1) or GN (Arm 2) as compared to historical cohorts, did not suggest an improvement in OS in patients with resectable PDAC. In the 102 evaluable patients, 73% in Arm A and 70% in Arm B underwent resection. Two-year OS was 47% (95% CI, 31%–61%) for Arm 1 and 48% (95% CI, 31%–63%) for Arm 2. As per the authors, neither arm’s 2-year OS estimate was significantly higher than the a priori threshold of 40% [68]. The study is important in the context of highlighting the need for precise radiological assessment of resectability in PDAC as well as the fact that there were no significant differences between the two arms of the study in terms of tolerance and efficacy when used as NAT. However, the lack of a significant improvement in 2-year OS highlights the need for further clarification on the role of NAT in resectable PDAC. Table 3 highlights key features of select studies which have evaluated NAT in PDAC.
A systematic review and metanalysis comprising eight studies and approximately 1,300 patients (predominantly BRPC/LAPC, though with a cohort of resectable PDAC) receiving either FOLFIRINOX or GN suggested that FOLFIRINOX showed improved 1-, 2- and 3-year survival rates compared to GN. However, R0 resection rates and 4- and 5-year survival rates showed similar results [69].
Surgical challenges in pancreatic resections post NAT
Assessment of tumour response for resection post NAT
Contrast enhanced CT scan remains the primary tool in initial categorisation of pancreatic cancer into resectable, BRPC or LAPC. However, as previously described, CT imaging frequently underestimates tumour response post NAT. It fails to discriminate between viable tumour and fibrotic tissue [70]. ‘Halo sign’ and ‘string sign’, respectively, suggest actual vessel wall involvement or periadventitial involvement of superior mesenteric artery (SMA) by the tumour which may help in assessing operability. Functional imaging via PET scans and changes in CA 19.9 may also help guide management decisions. Though there is no definite recommendation, in absence of disease progression on reassessment imaging all patients of pancreatic cancer should be explored for surgery post NAT [48].
Table 3. Selected studies evaluating neoadjuvant treatment (NAT) in PDAC.
Options of systemic therapy beyond chemotherapy
Precision medicine has led to the identification of specific targets in various cancers and has led to some success in the management of advanced cancers. As previously described, targets have been identified in PDAC as well, though disappointingly, very few ‘druggable’ targets have achieved relevance in clinical trials and practice. Commonly used monoclonal antibodies that are active in other cancers and target EGFR (e.g. cetuximab, erlotinib, etc.) and VEGF (e.g. bevacizumab), besides others, have shown disappointing results in PDAC [71–74].
Olaparib, a Poly (ADP-Ribose) Polymerase inhibitor, targets cancer cells with a homologous recombination repair deficiency, such as due to BRCA gene mutation, by synthetic lethality. Germline loss-of-function mutations in BRCA1, BRCA2 or both (BRCA) genes occur in approximately 4%–7% of patients with PDAC. The phase III Pancreas Cancer Olaparib Ongoing trial randomised patients with advanced PDAC post 16 weeks of continuous first-line platinum-based chemotherapy to receive either olaparib (600 mg/day) or placebo as a form of maintenance therapy. The study achieved its primary endpoint of showing improved PFS (7.4 versus 3.8 months; HR, 0.53; 95% CI, 0.35–0.82; p = 0.004) with olaparib and is the first instance of a targeted therapy showing benefit in advanced PDAC [75].
KRAS is the most common genetic alteration in PDAC and has long been considered as ‘non-druggable’. However, two molecules (AMG 510 and MRTX849) which use covalent allosteric inhibition to target a shallow pocket on the KRAS surface, specifically against the KRASG12C codon, have shown promising activity in early phase studies. Approximately 1%–4% of PDACs have KRASG12C codon mutation and these drugs may be considered in the future for treatment [76].
Gene fusions involving neurotrophic tyrosine receptor kinases (NTRKs) have been identified in approximately 1% of solid tumours and inhibitors of these kinases have been shown to have activity in a tumour agnostic manner [77, 78]. These fusions appear to be even rarer in PDAC, though case reports showing activity for NTRK inhibitors in advanced PDAC have been noted [79, 80]. Immune checkpoint inhibition by using antibodies against cytotoxic T-lymphocyte-associated antigen 4, programmed cell death protein-1 and programmed cell death protein ligand-1 has revolutionised the management of non-small cell lung carcinoma, melanoma, urothelial cancers, renal cell carcinomas amongst other cancers [81]. The strongest biomarker for the use of immune checkpoint inhibitors is a deficient mismatch repair (dMMR) protein status. The MMR proteins are key in error repair during DNA replication, with a defective system leading to random mutations occurring in small repetitive elements called microsatellites, i.e. microsatellite instability (MSI). Available evidence shows that a dMMR (MSI-H) status is rarely seen in PDAC, approximating a prevalence of 1%–2% [82]. While this is rare, immune checkpoint inhibitors may be considered in patients with PDAC and a dMMR status, though the limited available evidence suggests lesser responses with immunotherapy in PDACs as compared to responses seen in other tumours [83]. Other forms of immunotherapy like vaccines and adoptive cell transfer have not proven to be successful in PDAC, though there is emerging data for combination therapy, e.g. chemotherapy and pembrolizumab, ipilimumab plus GVAX vaccine, etc. [5].
Vascular resections and ‘Artery first approach’
With the advent of effective NAT regimens (FOLFIRINOX) and surgeons advancing their limits of resection, a proportion of initially unresectable (UR) patients now undergo successful resection.
Survival and perioperative outcomes of patients undergoing vein resection during pancreatectomy are similar to patients undergoing standard pancreatectomy. Porto-mesenteric vein resection has been accepted as a standard of care in surgery for BRPC [70, 84]. Tumour encasement resulting in complete collateralisation and cavernous transformation of porto-mesenteric vein complex and absence of reconstructible venous stumps are the only venous criteria for inoperability [85].
Except for celiac artery (CA) involvement in pancreatic body cancers and short segment common hepatic or gastroduodenal artery involvement in case of right-sided pancreatic cancers, arterial resection is considered a contraindication for surgery. This recommendation stems from the experience of high morbidity, mortality in excess of 10% and limited survival benefit with major arterial resection, in most historical series [86, 87].
Unless an arterial resection is planned, early intraoperative assessment of tumour-artery interphase is important before committing irreversible steps of resection while exploring patients with BRPC or LAPC for possible resection. SMA first approach involves systematic dissection of pancreas off SMA to achieve negative margins of resection and assess operability before performing other major steps of resection. It allows surgeon to abandon the procedure in cases where complete resection seems unlikely. In addition to early assessment of operability, this approach has been shown to improve perioperative outcomes such as blood loss, fistula rate and delayed gastric emptying and long-term survival [88–90].
Arterial Divestment, Periadventitial dissection and triangle operation
To minimise morbidity and to avoid arterial resection when disease does not directly involve the artery, periadventitial SMA dissection technique (also called as arterial divestment) has been described. This dissection technique also labelled as ‘level 3’ mesopancreas dissection, by Inoue et al [91], involves en block mesopancreas resection with right hemi or complete circumferential SMA dissection in periadventitial layer and complete clearance of tumour, lymphatics and perineural tissue around SMA. Such a procedure offers the possibility of achieving radical resection in LAPCs post NAT without arterial resection, thereby avoiding the morbidity and mortality associated with arterial resections. Long-term outcomes and further prospective evidence for such procedures should be the aim of future surgical studies [92].
Adjuvant therapy in PDAC
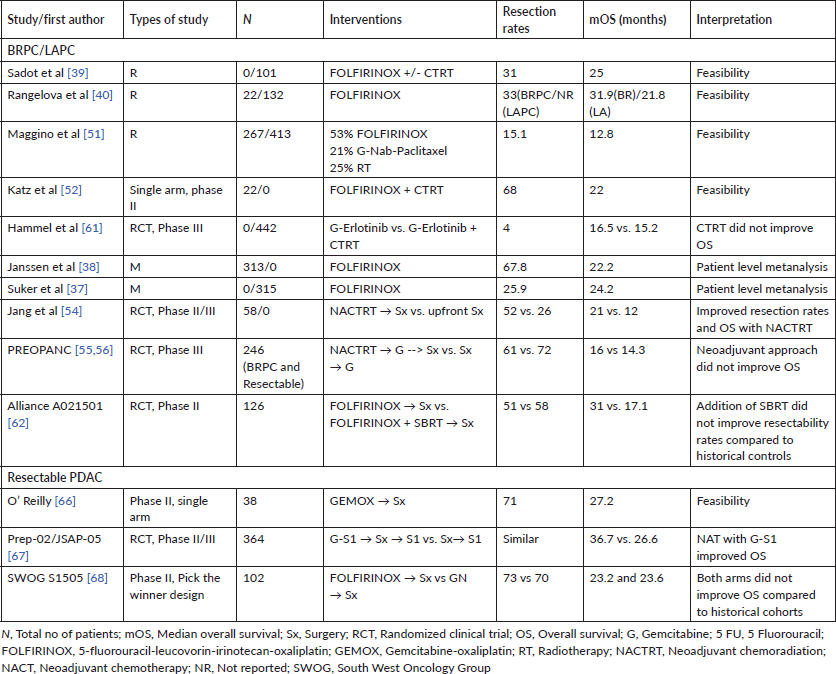
Approximately 10%–15% of patients with PDAC are resectable at presentation. The important determinants of outcomes in resectable PDAC are the presence or absence of involved lymph nodes, resection margins status (beyond scope of current review) and administration of effective adjuvant chemotherapy (AT) [93, 94]. Lymph nodal involvement, number of lymph nodes involved as well as parameters like lymph node ratios have been repeatedly shown to be prognostic in major staging systems as well prospective studies evaluating adjuvant therapy [93, 95]. The major reason for the development of AT in resected PDAC was the early recurrence and dismal OS (approximately 10–12 months) post a major procedure like classical Whipple resection or pylorus preserving pancreaticoduodenectomy [96]. The first study to show randomised evidence for the benefit of AT was the Gastrointestinal Tumor Study Group study published in 1985. The investigators found a near doubling of OS (21 versus 10.9 months; p = 0.03) when combining 5-FU with RT post resection as opposed to surveillance alone [97]. Since the advent of this trial, a number of prospective trials have shown the importance of AT in improving survival. Studies over the last two decades have concentrated on intensifying adjuvant chemotherapeutic regimens, while adjuvant RT has fallen out of favour due to lack of survival benefit and issues with tolerance [98–101]. The current standard of care for patients with resected PDAC is adjuvant FOLFIRINOX biweekly for 6 months. This is based on a Phase III randomised control trial, which compared modified FOLFIRINOX with gemcitabine monotherapy in 493 patients and showed a statistically significant improvement in the primary endpoint of DFS (21.6 versus 12.8 months; stratified HR, 0.58; 95% CI, 0.46–0.73; p < 0.001). A significant difference in OS was also noted in favour of the modified FOLFIRINOX arm (54.4 versus 35 months; stratified HR, 0.64; 95% CI, 0.48–0.86; p = 0.003) [102]. While the modified FOLFIRINOX regimen has shown the best survivals in this setting, the regimen also entails a significant proportion of patients having grade 3 or grade 4 adverse events (75.9%). This coupled with delayed recovery and nutritional depletion post pancreatic resections means a significant proportion of patients may be unable to receive mFOLFIRINOX. In such a cohort of patients, regimens for which evidence exists include combination gemcitabine-capecitabine, S1 or gemcitabine monotherapy [100, 103, 104]. Table 4 lists the major large randomised adjuvant therapy trials and the significant results associated with these trials.
PDAC – Data from India
Epidemiological data
As per the GLOBOCAN 2020 India factsheet, PDAC ranks 24th in terms of incidence with 12,642 new cases (0.95%) and 19th in terms of mortality with 12,153 cases (1.4%) [1]. The data published from 28 population-based cancer registries (PBCRs) of India, puts the cumulative risk of pancreatic cancer for males and females at 1 in 429 and 1 in 519, respectively, for the year 2020 [105]. The north-eastern region of India, especially Mizoram, has higher incidence rates as compared to other regions of the country. The age-adjusted incidence rates of PDAC have increased by more than two-fold as per the data from four PBCR in India over a period spanning from 1982 to 2010 [106]. The peak incidence rates are a decade earlier in India (sixth decade) as compared to that of the United States (seventh decade) [107].
Practice patterns and survival
The practice of pancreatic surgeries and perioperative management has evolved over the last few decades in India, just like in the developed countries. This has led to a significant decrease in the post-operative surgical complications. In one of the largest series on perioperative outcomes for pancreaticoduodenectomy from a tertiary care centre in India, the reported rates of morbidity and mortality were 33% and 5.4%, respectively [108]. The authors from this series reported a significant drop in the overall morbidity rates for surgeries done post 2003 as compared to the ones done a decade earlier. There was no statistically significant difference in the mortality rates, though. Such improvements in volume and safety indicators were noted despite the significant increase in the volume of cases per year (from 16 to 60; p < 0.0001). The 2-year DFS rates for those who underwent surgery post 2003 were 75% in this series.
Table 4. Randomised large-scale trials for adjuvant therapy of resected PDAC in the modern era.
Limited retrospective studies have suggested that patients with advanced PDAC in India have similar outcomes compared to published data [3, 46]. The systemic treatment for UR locally advanced and metastatic pancreatic cancer has also evolved from single agent gemcitabine to combination regimens like GN, and mFOLFIRINOX, thereby improving the survival and outcomes. A retrospective analysis of metastatic pancreatic cancer patients treated at a tertiary centre between 2013 and 2016 showed the most commonly used first-line regimens to be GN (39.2%), gemcitabine–erlotinib (16.3%) and mFOLFIRINOX (13.7%) [3]. The proportion of patients receiving mFOLFIRINOX (6.9% to 13.7%) and GN (5.9% to 39.2%) had increased, when compared to earlier published data from the same institution. Nearly 44% of patients received chemotherapy with dose modifications, out of which more than a half were upfront dose-modifications due to poor nutrition, co-morbidities and ECOG PS 2. With a median follow-up of approximately 9 months, the reported mOS was 7 months [3, 109]. A prospective study (published in abstract form only till date) comparing FOLFIRINOX with gemcitabine showed improved OS with FOLFIRINOX, at the cost of an increased toxicity profile. The OS in both arms of the study was in line with the data from the 4-ACCORD11 trial [110]
Approximately 40% of patients with inoperable pancreatic cancer receive second-line chemotherapy [111]. Similar rates have been reported from Indian literature too, with the most common regimens used being modified FOLFIRI (used without bolus 5FU) and GN [112]. The choice of regimen in later lines depends heavily on the ECOG PS and the regimen used prior. With a median follow-up of 7.57 months, a retrospective series reported an mOS of approximately 8 months.
Conclusions
A greater understanding of the biology of PDAC has resulted in a gradual shift towards intensifying systemic management options in PDAC, with accompanying standardisation of surgical approaches. The recalcitrant nature of PDAC is intrinsically related to the lack of therapeutic targets and dense surrounding stroma that hampers effectiveness of currently available chemotherapeutic options. An additional issue is the presentation of these cancers in predominantly advanced stages of disease. Despite these constraints, gradual improvements in survival are being seen with more effective chemotherapeutic regimens like mFOLFIRINOX and GN, whether in resectable or advanced PDAC. An increasing use of these effective chemotherapeutic regimens has also resulted in greater conversion of BR and locally advanced cancers to resection, though the most effective approach in this subgroup is yet to be identified. Available data from India shows similar outcomes across the spectrum of PDAC when compared to published data.
Acknowledgments
None.
Funding sources and grants
None.
Conflicts of interest
None.
References
1. 13-Pancreas-Fact-Sheet.pdf [https://gco.iarc.fr/today/data/factsheets/cancers/13-Pancreas-fact-sheet.pdf] Date accessed: 4/02/21
2. Klein AP (2019) Pancreatic cancer: a growing burden Lancet Gastroenterol Hepatol 4(12) 895–896 https://doi.org/10.1016/S2468-1253(19)30323-1 PMID: 31648975 PMCID: 7376745
3. Ramaswamy A, Ostwal V, and Goel A, et al (2018) Treatment practices for metastatic pancreatic cancer: Can we deliver an appropriately efficacious and safe regimen in Indian patients? Indian J Cancer 55(2) 138 https://doi.org/10.4103/ijc.IJC_552_17
4. Sohal DPS, Walsh RM, and Ramanathan RK, et al (2014) Pancreatic adenocarcinoma: treating a systemic disease with systemic therapy J Natl Cancer Inst 106(3) dju011 https://doi.org/10.1093/jnci/dju011 PMID: 24563516
5. Schizas D, Charalampakis N, and Kole C, et al (2020) Immunotherapy for pancreatic cancer: a 2020 update Cancer Treat Rev 86 102016 https://doi.org/10.1016/j.ctrv.2020.102016 PMID: 32247999
6. Waddell N, Pajic M, and Patch AM, et al (2015) Whole genomes redefine the mutational landscape of pancreatic cancer Nature 518(7540) 495–501 https://doi.org/10.1038/nature14169 PMID: 25719666 PMCID: 4523082
7. Hayashi A, Hong J, and Iacobuzio-Donahue CA (2021) The pancreatic cancer genome revisited Nat Rev Gastroenterol Hepatol 18(7) 469–481 [ https://doi.org/10.1038/s41575-021-00463-z PMID: 34089011
8. Appels NMGM, Beijnen JH, and Schellens JHM (2005) Development of farnesyl transferase inhibitors: a review Oncologist 10(8) 565–578 https://doi.org/10.1634/theoncologist.10-8-565 PMID: 16177281
9. Pompella L, Tirino G, and Pappalardo A, et al (2020) Pancreatic cancer molecular classifications: from bulk genomics to single cell analysis Int J Mol Sci 21(8) https://doi.org/10.3390/ijms21082814 PMID: 32316602 PMCID: 7215357
10. Collisson EA, Sadanandam A, and Olson P, et al (2011) Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy Nature Med 17(4) 500–503 https://doi.org/10.1038/nm.2344 PMID: 21460848 PMCID: 3755490
11. Puleo F, Nicolle R, and Blum Y, et al (2018) Stratification of pancreatic ductal adenocarcinomas based on tumor and microenvironment features Gastroenterology 155(6) 1999–2013.e3 https://doi.org/10.1053/j.gastro.2018.08.033 PMID: 30165049
12. Wang S, Zheng Y, and Yang F, et al (2021) The molecular biology of pancreatic adenocarcinoma: translational challenges and clinical perspectives Sig Transduct Target Ther 6(1) 1–23 https://doi.org/10.1038/s41392-021-00659-4
13. Aung KL, Fischer SE, and Denroche RE, et al (2018) Genomics-Driven precision medicine for advanced pancreatic cancer: early results from the COMPASS trial Clin Cancer Res 24(6) 1344–1354 https://doi.org/10.1158/1078-0432.CCR-17-2994 PMCID: 5968824
14. Rhim AD, Mirek ET, and Aiello NM, et al (2012) EMT and dissemination precede pancreatic tumor formation Cell 148(1) 349–361 https://doi.org/10.1016/j.cell.2011.11.025 PMID: 22265420 PMCID: 3266542
15. Ho WJ, Jaffee EM, and Zheng L (2020) The tumour microenvironment in pancreatic cancer — clinical challenges and opportunities Nat Rev Clin Oncol 17(9) 527–540 https://doi.org/10.1038/s41571-020-0363-5 PMID: 32398706 PMCID: 7442729
16. Thomas D and Radhakrishnan P (2019) Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis Mol Cancer 18(1) 14 https://doi.org/10.1186/s12943-018-0927-5 PMID: 30665410 PMCID: 6341551
17. Bolm L, Cigolla S, and Wittel UA, et al (2017) The role of fibroblasts in pancreatic cancer: extracellular matrix versus paracrine factors Translat Oncol 10(4) 578–588 https://doi.org/10.1016/j.tranon.2017.04.009
18. Ducreux M, Cuhna AS, and Caramella C, et al (2015) Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up† Ann Oncol 26 v56–v68 https://doi.org/10.1093/annonc/mdv295
19. Isaji S, Murata Y, and Kishiwada M (2018) New Japanese classification of pancreatic cancer Pancreatic Cancer eds JP Neoptolemos, R Urrutia, and JL Abbruzzese, et al (Springer) pp 1021–1037 https://doi.org/10.1007/978-1-4939-7193-0_84
20. Sohal DPS, Kennedy EB, and Cinar P, et al (2020) Metastatic pancreatic cancer: ASCO guideline update JCO 38(27) 3217–3230 https://doi.org/10.1200/JCO.20.01364
21. NCCN (2021) Pancreatic.pdf [https://www.nccn.org/professionals/physician_gls/PDF/pancreatic.pdf]
22. Conroy T, Desseigne F, and Ychou M, et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer N Engl J Med 364(19) 1817–1825 https://doi.org/10.1056/NEJMoa1011923 PMID: 21561347
23. Von Hoff DD, Ervin T, and Arena FP, et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine N Engl J Med 369(18) 1691–1703 https://doi.org/10.1056/NEJMoa1304369 PMID: 24131140 PMCID: 4631139
24. Pusceddu S, Ghidini M, and Torchio M, et al (2019) Comparative effectiveness of gemcitabine plus nab-paclitaxel and FOLFIRINOX in the first-line setting of metastatic pancreatic cancer: a systematic review and meta-analysis Cancers 11(4) 484 https://doi.org/10.3390/cancers11040484 PMCID: 6520876
25. Walker EJ and Ko AH (2014) Beyond first-line chemotherapy for advanced pancreatic cancer: an expanding array of therapeutic options? World J Gastroenterol 20(9) 2224–2236 https://doi.org/10.3748/wjg.v20.i9.2224 PMID: 24605022 PMCID: 3942828
26. Wang-Gillam A, Li CP, and Bodoky G, et al (2016) Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial Lancet 387(10018) 545–557 https://doi.org/10.1016/S0140-6736(15)00986-1
27. Oettle H, Riess H, and Stieler JM, et al (2014) Second-Line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial JCO 32(23) 2423–2429 https://doi.org/10.1200/JCO.2013.53.6995
28. Gill S, Ko YJ, and Cripps C, et al (2016) PANCREOX: a randomized phase III study of fluorouracil/leucovorin with or without oxaliplatin for second-line advanced pancreatic cancer in patients who have received gemcitabine-based chemotherapy JCO 34(32) 3914–3920 https://doi.org/10.1200/JCO.2016.68.5776
29. Sonbol MB, Firwana B, and Wang Z, et al (2017) Second-line treatment in patients with pancreatic ductal adenocarcinoma: a meta-analysis Cancer 123(23) 4680–4686 https://doi.org/10.1002/cncr.30927
30. Saad AM, Turk T, and Al-Husseini MJ, et al (2018) Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study BMC Cancer 18 https://doi.org/10.1186/s12885-018-4610-4 PMID: 29940910 PMCID: 6020186
31. Blomstrand H, Scheibling U, and Bratthäll C, et al (2019) Real world evidence on gemcitabine and nab-paclitaxel combination chemotherapy in advanced pancreatic cancer BMC Cancer 19(1) 40 https://doi.org/10.1186/s12885-018-5244-2 PMID: 30621618 PMCID: 6325739
32. Yamamoto T, Yagi S, and Kinoshita H, et al (2015) Long-term survival after resection of pancreatic cancer: a single-center retrospective analysis World J Gastroenterol 21(1) 262–268 https://doi.org/10.3748/wjg.v21.i1.262 PMID: 25574100 PMCID: 4284344
33. Burris HA, Moore MJ, and Andersen J, et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial JCO 15(6) 2403–2413 https://doi.org/10.1200/JCO.1997.15.6.2403
34. Macarulla T, Pazo-Cid R, and Guillén-Ponce C, et al (2019) Phase I/II trial to evaluate the efficacy and safety of nanoparticle albumin-bound paclitaxel in combination with gemcitabine in patients with pancreatic cancer and an ECOG performance status of 2 JCO 37(3) 230–238 https://doi.org/10.1200/JCO.18.00089
35. Tempero MA, Malafa MP, and Al-Hawary M, et al (2021) Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology J Nat Comprehen Cancer Netw 19(4) 439–457 https://doi.org/10.6004/jnccn.2021.0017
36. Andriulli A, Festa V, and Botteri E, et al (2012) Neoadjuvant/preoperative gemcitabine for patients with localized pancreatic cancer: a meta-analysis of prospective studies Ann Surg Oncol 19(5) 1644–1662 https://doi.org/10.1245/s10434-011-2110-8
37. Suker M, Beumer BR, and Sadot E, et al (2016) A patient-level meta-analysis of FOLFIRINOX for locally advanced pancreatic cancer Lancet Oncol 17(6) 801–810 https://doi.org/10.1016/S1470-2045(16)00172-8 PMID: 27160474 PMCID: 5527756
38. Janssen QP, Buettner S, and Suker M, et al (2019) Neoadjuvant FOLFIRINOX in patients with borderline resectable pancreatic cancer: a systematic review and patient-level meta-analysis J Natl Cancer Inst 111(8) 782–794 https://doi.org/10.1093/jnci/djz073 PMID: 31086963 PMCID: 6695305
39. Sadot E, Doussot A, and O’Reilly EM, et al (2015) FOLFIRINOX induction therapy for stage 3 pancreatic adenocarcinoma Ann Surg Oncol 22(11) 3512–3521 https://doi.org/10.1245/s10434-015-4647-4
40. Rangelova E, Wefer A, and Persson S, et al (2021) Surgery improves survival after neoadjuvant therapy for borderline and locally advanced pancreatic cancer: a single institution experience Ann Surg 273(3) 579–586 https://doi.org/10.1097/SLA.0000000000003301
41. Verbeke CS (2008) Resection margins and R1 rates in pancreatic cancer – are we there yet? Histopathology 52(7) 787–796 https://doi.org/10.1111/j.1365-2559.2007.02935.x
42. Kim KS, Kwon J, and Kim K, et al (2016) Impact of resection margin distance on survival of pancreatic cancer: a systematic review and meta-analysis Cancer Res Treat 49(3) 824–833 https://doi.org/10.4143/crt.2016.336 PMID: 27561314 PMCID: 5512376
43. Klaiber U, Mihaljevic A, and Hackert T (2019) Radical pancreatic cancer surgery – with arterial resection Transl Gastroenterol Hepatol 4 https://doi.org/10.21037/tgh.2019.01.07
44. Małczak P, Sierżęga M, and Stefura T, et al (2020) Arterial resections in pancreatic cancer – systematic review and meta-analysis HPB 22(7) 961–968 https://doi.org/10.1016/j.hpb.2020.04.005
45. Toesca DAS, Koong AJ, and Poultsides GA, et al (2018) Management of borderline resectable pancreatic cancer Int J Radiat Oncol Biol Phys 100(5) 1155–1174 https://doi.org/10.1016/j.ijrobp.2017.12.287 PMID: 29722658
46. Ramaswamy A, Jandyal S, and Ostwal V, et al (2017) Nontrial, real-world outcomes in unresectable locally advanced pancreatic cancer: chemotherapy and chemoradiation is the standard while surgery is uncommon Indian J Cancer 54(3) 530 https://doi.org/10.4103/ijc.IJC_377_17
47. Gemenetzis G, Groot VP, and Blair AB, et al (2019) Survival in locally advanced pancreatic cancer after neoadjuvant therapy and surgical resection Ann Surg 270(2) 340–347 https://doi.org/10.1097/SLA.0000000000002753
48. Cassinotto C, Sa-Cunha A, and Trillaud H (2016) Radiological evaluation of response to neoadjuvant treatment in pancreatic cancer Diagn Interventional Imaging 97(12) 1225–1232 https://doi.org/10.1016/j.diii.2016.07.011
49. Shridhar R, Takahashi C, and Huston J, et al (2019) Neoadjuvant therapy and pancreatic cancer: a national cancer database analysis J Gastrointest Oncol 10(4) 663–673 https://doi.org/10.21037/jgo.2019.02.09 PMID: 31392047 PMCID: 6657333
50. Cloyd JM, Heh V, and Pawlik TM, et al (2020) Neoadjuvant therapy for resectable and borderline resectable pancreatic cancer: a meta-analysis of randomized controlled trials J Clin Med 9(4) https://doi.org/10.3390/jcm9041129 PMID: 32326559 PMCID: 7231310
51. Maggino L, Malleo G, and Marchegiani G, et al (2019) Outcomes of primary chemotherapy for borderline resectable and locally advanced pancreatic ductal adenocarcinoma JAMA Surg 154(10) 932–942 https://doi.org/10.1001/jamasurg.2019.2277 PMID: 31339530 PMCID: 6659151
52. Katz MHG, Shi Q, and Ahmad SA, et al (2016) Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: alliance for clinical trials in oncology trial A021101 JAMA Surg 151(8) e161137 https://doi.org/10.1001/jamasurg.2016.1137 PMID: 27275632 PMCID: 5210022
53. Philip PA, Lacy J, and Portales F, et al (2020) Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): a multicentre, open-label phase 2 study Lancet Gastroenterol Hepatol 5(3) 285–294 https://doi.org/10.1016/S2468-1253(19)30327-9 PMID: 31953079
54. Jang JY, Han Y, and Lee H, et al (2018) Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a prospective, randomized, open-label, multicenter phase 2/3 trial Ann Surg 268(2) 215–222 https://doi.org/10.1097/SLA.0000000000002705 PMID: 29462005
55. Versteijne E, Suker M, and Groothuis K, et al (2020) Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial JCO 38(16) 1763–1773 https://doi.org/10.1200/JCO.19.02274
56. Van Eijck CHJ, Versteijne E, and Suker M, et al (2021) Preoperative chemoradiotherapy to improve overall survival in pancreatic cancer: long-term results of the multicenter randomized phase III PREOPANC trial JCO 39(15_suppl) 4016–4016 https://doi.org/10.1200/JCO.2021.39.15_suppl.4016
57. Janssen QP, van Dam JL, and Bonsing BA, et al (2021) Total neoadjuvant FOLFIRINOX versus neoadjuvant gemcitabine-based chemoradiotherapy and adjuvant gemcitabine for resectable and borderline resectable pancreatic cancer (PREOPANC-2 trial): study protocol for a nationwide multicenter randomized controlled trial BMC Cancer 21(1) 300 https://doi.org/10.1186/s12885-021-08031-z PMID: 33757440 PMCID: 7989075
58. Koerkamp BG (2022) Perioperative Versus Adjuvant FOLFIRINOX for Resectable Pancreatic Cancer: The PREOPANC-3 Study [https://clinicaltrials.gov/ct2/show/NCT04927780] Date accessed: 16/02/22
59. Murphy JE, Wo JY, and Ryan DP, et al (2019) Total neoadjuvant therapy with FOLFIRINOX in combination with Losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: a phase 2 clinical trial JAMA Oncol 5(7) 1020–1027 https://doi.org/10.1001/jamaoncol.2019.0892 PMID: 31145418 PMCID: 6547247
60. Hong TS (2021) A Randomized Phase 2 Study of Losartan and Nivolumab in Combination With FOLFIRINOX and SBRT in Localized Pancreatic Cancer [https://clinicaltrials.gov/ct2/show/NCT03563248] Date accessed: 3/02/21
61. Hammel P, Huguet F, and van Laethem JL, et al (2016) Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial JAMA 315(17) 1844–1853 https://doi.org/10.1001/jama.2016.4324
62. Meeting Library Alliance A021501: Preoperative mFOLFIRINOX or mFOLFIRINOX plus hypofractionated radiation therapy (RT) for borderline resectable (BR) adenocarcinoma of the pancreas [https://meetinglibrary.asco.org/record/194235/abstract] Date accessed: 5/02/21
63. Ghaneh P, Palmer DH, and Cicconi S, et al (2020) ESPAC-5F: four-arm, prospective, multicenter, international randomized phase II trial of immediate surgery compared with neoadjuvant gemcitabine plus capecitabine (GEMCAP) or FOLFIRINOX or chemoradiotherapy (CRT) in patients with borderline resectable pancreatic cancer JCO 38(15_suppl) 4505–4505 https://doi.org/10.1200/JCO.2020.38.15_suppl.4505
64. Oba A, Ho F, and Bao QR, et al (2020) Neoadjuvant treatment in pancreatic cancer Front Oncol 10 https://doi.org/10.3389/fonc.2020.00245 PMID: 32185128 PMCID: 7058791
65. Zhan HX, Xu JW, and Wu D, et al (2017) Neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of prospective studies Cancer Med 6(6) 1201–1219 https://doi.org/10.1002/cam4.1071 PMID: 28544758 PMCID: 5463082
66. O’Reilly EM, Perelshteyn A, and Jarnagin WR, et al (2014) A single-arm, non-randomized phase ii trial of neoadjuvant gemcitabine and oxaliplatin in patients with resectable pancreas adenocarcinoma Ann Surg 260(1) 142–148 https://doi.org/10.1097/SLA.0000000000000251
67. Unno M, Motoi F, and Matsuyama Y, et al (2019) Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP-05) JCO 37(4_suppl) 189–189 https://doi.org/10.1200/JCO.2019.37.4_suppl.189
68. Ahmad SA, Duong M, and Sohal DPS, et al (2020) Surgical outcome results from SWOG S1505: a randomized clinical trial of mFOLFIRINOX versus gemcitabine/nab-paclitaxel for perioperative treatment of resectable pancreatic ductal adenocarcinoma Ann Surg 272(3) 481–486 https://doi.org/10.1097/SLA.0000000000004155 PMID: 32740235 PMCID: 7856053
69. Tang R, Meng Q, and Wang W, et al (2021) Head-to-head comparison between FOLFIRINOX and gemcitabine plus nab-paclitaxel in the neoadjuvant chemotherapy of localized pancreatic cancer: a systematic review and meta-analysis Gland Surg 10(5) 1564575–1561575 https://doi.org/10.21037/gs-21-16
70. Tamburrino D, Riviere D, and Yaghoobi M, et al (2016) Diagnostic accuracy of different imaging modalities following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer Cochrane Database Syst Rev 2016(9)
71. Moore MJ, Goldstein D, and Hamm J, et al (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group JCO 25(15) 1960–1966 https://doi.org/10.1200/JCO.2006.07.9525
72. Faloppi L, Andrikou K, and Cascinu S (2013) Cetuximab: still an option in the treatment of pancreatic cancer? Expert Opin Biol Ther 13(5) 791–801 https://doi.org/10.1517/14712598.2013.786697 PMID: 23560505
73. Van Cutsem E, Vervenne WL, and Bennouna J, et al (2009) Phase III Trial of Bevacizumab in Combination With Gemcitabine and Erlotinib in Patients With Metastatic Pancreatic Cancer JCO 27(13) 2231–2237 https://doi.org/10.1200/JCO.2008.20.0238
74. Stoica AF, Chang CH, and Pauklin S (2020) Molecular therapeutics of pancreatic ductal adenocarcinoma: targeted pathways and the role of cancer stem cells Trends Pharmacol Sci 41(12) 977–993 https://doi.org/10.1016/j.tips.2020.09.008 PMID: 33092892
75. Golan T, Hammel P, and Reni M, et al (2019) Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer N Engl J Med 381(4) 317–327 https://doi.org/10.1056/NEJMoa1903387 PMID: 31157963 PMCID: 6810605
76. Nagasaka M, Li Y, and Sukari A, et al (2020) KRAS G12C Game of Thrones, which direct KRAS inhibitor will claim the iron throne? Cancer Treat Rev 84 101974 https://doi.org/10.1016/j.ctrv.2020.101974 PMID: 32014824 PMCID: 7041424
77. Demetri GD, Paz-Ares L, and Farago AF, et al (2018) Efficacy and safety of entrectinib in patients with NTRK fusion-positive (NTRK-fp) tumors: pooled analysis of STARTRK-2, STARTRK-1 and ALKA-372-001 Ann Oncol 29 viii713 https://doi.org/10.1093/annonc/mdy424.017
78. Forsythe A, Zhang W, and Phillip Strauss U, et al (2020) A systematic review and meta-analysis of neurotrophic tyrosine receptor kinase gene fusion frequencies in solid tumors Ther Adv Med Oncol 12 1758835920975613 https://doi.org/10.1177/1758835920975613
79. Demols A, Perez-Casanova L, and Rocq L, et al (2020) NTRK gene fusions in bilio-pancreatic cancers JCO 38(15_suppl) e16664 https://doi.org/10.1200/JCO.2020.38.15_suppl.e16664
80. Pishvaian MJ, Rolfo CD, and Liu SV, et al (2018) Clinical benefit of entrectinib for patients with metastatic pancreatic cancer who harbor NTRK and ROS1 fusions JCO 36(4_suppl) 521 https://doi.org/10.1200/JCO.2018.36.4_suppl.521
81. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun 11(1) 3801
82. Luchini C, Brosens LAA, and Wood LD, et al (2021) Comprehensive characterisation of pancreatic ductal adenocarcinoma with microsatellite instability: histology, molecular pathology and clinical implications Gut 70(1) 148–156 https://doi.org/10.1136/gutjnl-2020-320726
83. Henriksen A, Dyhl-Polk A, and Chen I, et al (2019) Checkpoint inhibitors in pancreatic cancer Cancer Treat Rev 78 17–30 https://doi.org/10.1016/j.ctrv.2019.06.005 PMID: 31325788
84. Habib JR, Kinny-Köster B, and van Oosten F, et al (2020) Periadventitial dissection of the superior mesenteric artery for locally advanced pancreatic cancer: Surgical planning with the “halo sign” and “string sign.” Surgery Published online October 6, 2020
85. Hackert T, Schneider L, and Büchler MW (2015) Current state of vascular resections in pancreatic cancer surgery Gastroenterol Res Pract 2015 120207 https://doi.org/10.1155/2015/120207 PMID: 26609306 PMCID: 4644845
86. Bockhorn M, Uzunoglu FG, and Adham M, et al (2014) Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS) Surgery 155(6) 977–988 https://doi.org/10.1016/j.surg.2014.02.001 PMID: 24856119
87. Jegatheeswaran S, Baltatzis M, and Jamdar S, et al (2017) Superior mesenteric artery (SMA) resection during pancreatectomy for malignant disease of the pancreas: a systematic review HPB 19(6) 483–490 https://doi.org/10.1016/j.hpb.2017.02.437 PMID: 28410913
88. Jiang X, Yu Z, and Ma Z, et al (2020) Superior mesenteric artery first approach can improve the clinical outcomes of pancreaticoduodenectomy: a meta-analysis Int J Surg 73 14–24 https://doi.org/10.1016/j.ijsu.2019.11.007
89. Pal S, George J, and Singh AN, et al (2018) Posterior superior mesenteric artery (SMA) first approach vs. standard pancreaticoduodenectomy in patients with resectable periampullary cancers: a prospective comparison focusing on circumferential resection margins J Gastrointest Cancer 49(3) 252–259 https://doi.org/10.1007/s12029-017-9933-x
90. Negoi I, Hostiuc S, and Runcanu A, et al (2017) Superior mesenteric artery first approach versus standard pancreaticoduodenectomy: a systematic review and meta-analysis Hepatobiliary & Pancreat Dis Int 16(2) 127–138 https://doi.org/10.1016/S1499-3872(16)60134-0
91. Inoue Y, Saiura A, and Yoshioka R, et al (2015) Pancreatoduodenectomy with systematic mesopancreas dissection using a supracolic anterior artery-first approach Ann Surg 262(6) 1092–1101 https://doi.org/10.1097/SLA.0000000000001065 PMID: 25587814
92. Hackert T, Strobel O, and Michalski CW, et al (2017) The TRIANGLE operation – radical surgery after neoadjuvant treatment for advanced pancreatic cancer: a single arm observational study HPB 19(11) 1001–1007 https://doi.org/10.1016/j.hpb.2017.07.007 PMID: 28838632
93. Morales-Oyarvide V, Rubinson DA, and Dunne RF, et al (2017) Lymph node metastases in resected pancreatic ductal adenocarcinoma: predictors of disease recurrence and survival Br J Cancer 117(12) 1874–1882 https://doi.org/10.1038/bjc.2017.349 PMID: 28982112 PMCID: 5729468
94. Klaiber U, Hackert T, and Neoptolemos JP (2019) Adjuvant treatment for pancreatic cancer Transl Gastroenterol Hepatol 4(0) [https://tgh.amegroups.com/article/view/5029] Date accessed: 5/02/21 https://doi.org/10.21037/tgh.2019.04.04
95. Baldwin S, Kukar M, and Gabriel E (2016) Pancreatic cancer metastatic to a limited number of lymph nodes has no impact on outcome HPB 18(6) https://doi.org/10.1016/j.hpb.2016.02.004 PMID: 27317957 PMCID: 4913131
96. Conroy T and Ducreux M (2019) Adjuvant treatment of pancreatic cancer Curr Opin Oncol 31(4) 346–353 https://doi.org/10.1097/CCO.0000000000000546 PMID: 30994497
97. Kalser MH and Ellenberg SS (1985) Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection Arch Surg 120(8) 899–903 https://doi.org/10.1001/archsurg.1985.01390320023003 PMID: 4015380
98. Oettle H, Neuhaus P, and Hochhaus A, et al (2013) Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial JAMA 310(14) 1473–1481 https://doi.org/10.1001/jama.2013.279201
99. Regine WF, Winter KA, and Abrams R, et al (2011) Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial Ann Surg Oncol 18(5) 1319–1326 https://doi.org/10.1245/s10434-011-1630-6
100. Uesaka K, Boku N, and Fukutomi A, et al Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01) Lancet 388(10041) 248–257 PMID: 27265347
101. Neoptolemos JP, Stocken DD, and Bassi C, et al (2010) Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial JAMA 304(10) 1073–1081https://doi.org/10.1001/jama.2010.1275
102. Conroy T, Hammel P, and Hebbar M, et al (2018) FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer N Engl J Med 379(25) 2395–2406 https://doi.org/10.1056/NEJMoa1809775 PMID: 30575490
103. Neoptolemos JP, Palmer DH, and Ghaneh P, et al (2017) Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial Lancet 389(10073) 1011–1024 https://doi.org/10.1016/S0140-6736(16)32409-6 PMID: 28129987
104. Reni M, Riess H, and O’Reilly EM, et al (2020) Phase III APACT trial of adjuvant nab-paclitaxel plus gemcitabine (nab-P + Gem) versus gemcitabine (Gem) alone for patients with resected pancreatic cancer (PC): outcomes by geographic region JCO 38(15_suppl) 4515–4515 https://doi.org/10.1200/JCO.2020.38.15_suppl.4515
105. Mathur P, Sathishkumar K, and Chaturvedi M, et al (2020) Cancer Statistics, 2020: report from National Cancer Registry Programme, India JCO Glob Oncol (6) 1063–1075 https://doi.org/10.1200/GO.20.00122 PMID: 32673076 PMCID: 7392737
106. NCRP Annual Reports [https://ncdirindia.org/ncrp/Annual_Reports.aspx] Date accessed: 5/02/21
107. Midha S, Chawla S, and Garg PK (2016) Modifiable and non-modifiable risk factors for pancreatic cancer: a review Cancer Lett 381(1) 269–277 https://doi.org/10.1016/j.canlet.2016.07.022 PMID: 27461582
108. Shrikhande SV, Barreto SG, and Somashekar BA, et al (2013) Evolution of pancreatoduodenectomy in a tertiary cancer center in India: improved results from service reconfiguration Pancreatology 13(1) 63–71 https://doi.org/10.1016/j.pan.2012.11.302 PMID: 23395572
109. Sirohi B, Dawood S, and Rastogi S, et al (2015) Treatment of patients with metastatic pancreatic cancer: experience from a tertiary Indian cancer center Indian J Cancer 52(3) 449 https://doi.org/10.4103/0019-509X.176732
110. Singhal MK, Kapoor A, and Bagri PK, et al 617PD – a phase III trial comparing folfirinox versus gemcitabine for metastatic pancreatic cancer Ann Oncol 25 iv210
111. Nagrial AM, Chin VT, and Sjoquist KM, et al (2015) Second-line treatment in inoperable pancreatic adenocarcinoma: a systematic review and synthesis of all clinical trials Crit Rev Oncol Hematol 96(3) 483–497 https://doi.org/10.1016/j.critrevonc.2015.07.007 PMID: 26481952
112. Ramaswamy A, Parthiban S, and Malhotra M, et al (2018) Outcomes with second-line chemotherapy in advanced pancreatic cancers: a retrospective study from a tertiary cancer center in India Indian J Cancer 55(2) 144 https://doi.org/10.4103/ijc.IJC_553_17






