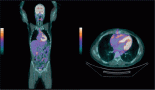Immune checkpoint inhibitors (ICIs) have increased modern anticancer armamentarium portfolios, with 15%–60% of cancer patients deriving clinical benefit while others progress, including some occurrences of accelerated progressions. ICIs have also introduced a new pattern of immune-related adverse events (irAEs). Recently, a mechanistic link was proposed in which patients who develop ICIs-related irAEs derive a survival benefit compared to those who do not, suggesting an overlap between toxicities and the treatment efficacy. Identifying predictive biomarkers to optimally identify patients who will benefit from ICIs is a contemporary research area in Oncology. However, the data remains sparse, with only several smaller studies showing a plausible direct proportionality of a therapeutic effect across tumours. In contrast, the overall survival and progression-free survival rate depend on the tumour type, degree of toxicities, duration of exposure, affected system/organs and inherent patient characteristics. Furthermore, the occurrence of irAEs appears to be more associated with a clinical benefit from programmed death 1 and programmed death-ligand 1 inhibitors than anti-cytotoxic T-lymphocyte-associated antigen 4. Several questions remain unanswered, including the association between survival benefit and specific type of organ system toxicities, toxicity grade, if the benefit is entirely due to immortal-time biases (ITBs), presence of patients confounding comorbidities like autoimmune diseases, and finally, immune heterogeneities. Considering ITB represents a key element in interpreting these studies since patients with precipitated death or with an earlier disease progresses rarely develop irAEs; in fact, such patients have not stayed in the study long enough to experience such irAEs. Conversely, patients that stayed in the study for a longer period have a higher risk of developing irAEs. Landmark analysis is key in these studies if a real association is to be found. Overall response and disease control rates are mainly higher in those who develop irAEs due to immune activation. So, this review aims to summarise the evidence from key studies that addressed this important clinical question.