Aspirin and cancer survival: a systematic review and meta-analyses of 118 observational studies of aspirin and 18 cancers
Peter C Elwood1, Gareth Morgan1, Christine Delon2, Majd Protty3, Julieta Galante4,5, Janet Pickering1, John Watkins1,6, Alison Weightman7 and Delyth Morris8
1Division of Population Medicine, Cardiff University, Cardiff CF14 4XN, UK
2Freelance statistician, London, UK
3Cardiff Lipidomics Group, Cardiff University, UK
4University of Cambridge, Cambridge, UK
5National Institute for Health Research (NIHR) Applied Research Collaboration East of England, Cambridge, UK
6Public Health Wales, Cardiff, UK
7Specialist Unit for Review Evidence, Cardiff University, Cardiff, UK
8University Library Service, Cardiff University, Cardiff, UK
Abstract
Background: Despite the accumulation of research papers on aspirin and cancer, there is doubt as to whether or not aspirin is an acceptable and effective adjunct treatment of cancer. The results of several randomised trials are awaited, and these should give clear evidence on three common cancers: colon, breast and prostate. The biological effects of aspirin appear likely however to be of relevance to cancer generally, and to metastatic spread, rather than just to one or a few cancers, and there is already a lot of evidence, mainly from observational studies, on the association between aspirin and survival in a wide range of cancers.
Aims: In order to test the hypothesis that aspirin taking is associated with an increase in the survival of patients with cancer, we conducted a series of systematic literature searches to identify clinical studies of patients with cancer, some of whom took aspirin after having received a diagnosis of cancer.
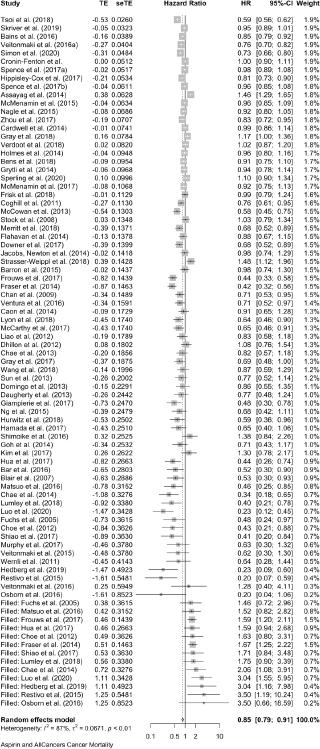
Results: Three literature searches identified 118 published observational studies in patients with 18 different cancers. Eighty-one studies report on aspirin and cancer mortality and 63 studies report on all-cause mortality. Within a total of about a quarter of a million patients with cancer who reported taking aspirin, representing 20%–25% of the total cohort, we found aspirin to be associated with a reduction of about 20% in cancer deaths (pooled hazard ratio (HR): 0.79; 95% confidence intervals: 0.73, 0.84 in 70 reports and a pooled odds ratio (OR): 0.67; 0.45, 1.00 in 11 reports) with similar reductions in all-cause mortality (HR: 0.80; 0.74, 0.86 in 56 studies and OR: 0.57; 0.36, 0.89 in seven studies). The relative safety of aspirin taking was examined in the studies and the corresponding author of every paper was written to asking for additional information on bleeding. As expected, the frequency of bleeding increased in the patients taking aspirin, but fatal bleeding was rare and no author reported a significant excess in fatal bleeds associated with aspirin. No author mentioned cerebral bleeding in the patients they had followed.
Conclusions: There is a considerable body of evidence suggestive of about a 20% reduction in mortality in patients with cancer who take aspirin, and the benefit appears not to be restricted to one or a few cancers. Aspirin, therefore, appears to deserve serious consideration as an adjuvant treatment of cancer, and patients with cancer, and their carers, have a right to be informed of the available evidence.
Keywords: aspirin, cancer, survival, mortality, bleeding, thromboembolism
Correspondence to: Gareth Morgan
Email: morganfforrdbeck@gmail.com
Published: 02/07/2021
Received: 13/10/2020
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The first suggestive evidence of benefit to patients with cancer from aspirin was reported over 50 years ago. Studies of animals with cancer showed that aspirin is associated with a reduction in the development of metastases [1, 2]. Since then, despite the reporting of much further evidence on biological effects of aspirin, and the reporting of many studies on aspirin and survival, there is still uncertainty about the role of aspirin as a possible adjuvant treatment of patients with cancer.
A number of small and inadequate randomised trials have been reported [3–6] and the pooling of results from these gives a suggestive reduction of 9% in cancer deaths in the 722 patients with cancer who had been randomised to aspirin (hazard ratio (HR): 0.91; 95% confidence interval (CI): 0.79, 1.04) [7]. While this result is only suggestive, a trial which developed within the cohort of the US Physicians Health Study of cancer prevention by aspirin is more strongly supportive. Just over 500 subjects in the cohort developed cancer, and those who had been randomised to aspirin showed a reduction in cancer deaths (HR: 0.68, 95% CI: 0.52, 0.90) [8].
Another source of evidence on the range of cancers to which aspirin may be relevant comes from opportunistic long-term follow-up studies of patients who had been involved in early randomised trials of aspirin and vascular disease. In addition to reporting a subsequent reduction in cancer incidence, Rothwell et al [9] and Mills and Wu [10] showed that deaths from a wide range of cancers were reduced in subjects who had been randomised to aspirin, and furthermore, the occurrence of metastatic spread was reduced in a range of cancers, including colon, brain, liver, lung and ‘other or multiple sites’ [11].
A number of new ad hoc randomised trial have been set up to test aspirin treatment in a few cancers and results from these are awaited [12–15]. These, however, are testing aspirin in only a very few cancers – principally colon, but also breast and prostate – while the effects of aspirin on biological mechanisms relevant to cancer lead to the possibility of benefit in most, if not all cancers [16–18]. Indeed, because of its manifold effects on biological processes, Zhang et al [19] suggest that aspirin is ‘a master regulator of the hallmarks of cancer’.
The bulk of published evidence on aspirin and the treatment of cancer comes, however, from observational studies and in this report, we present the results of 118 published observational studies to test the hypothesis that aspirin is of benefit to a wide range of cancers and not just one or a few common cancers. We also present evidence that aspirin, relative to cancer and in comparisons with other cancer treatments, is a very safe drug.
Methods
We conducted three consecutive systematic literature searches and meta-analyses of published observational studies of aspirin taken by patients with cancer. Detailed reports on the first two searches have been published [7, 20]. A description of the most recent search procedure is given in Supplementary File 1, and in Supplementary File 2 a brief description of each of the studies judged to be relevant in the most recent search is presented. Together the three searches covered up to March 2020.
Given that most of the available studies have been on the three common cancers: colon, breast and prostate, and in view of the fact that aspirin is being tested in randomised trials, we first present pooled evidence on aspirin and these three cancers. We then present evidence from 36 published reports of 15 other cancers, each of which has been examined in only one or a very few studies.
The procedures adopted followed the PRISMA guidelines throughout [21] and a full description of the search strategy is given in Supplementary File 1. In brief: each of the three systematic searches using keywords was conducted by AW and DM in MEDLINE and EMBASE. The searches were limited to human studies in peer-reviewed journals. Relevant studies were selected by two authors (PE and GM) if (a) the studied population comprised patients diagnosed with cancer; (b) aspirin appears to have been taken regularly after cancer diagnosis; (c) the studies were randomised trials, case–control studies or cohort studies. Reference lists of the relevant studies identified were searched for other relevant reports. At least one author on each selected paper in all three searches was written to and asked specifically about gastrointestinal (GI) bleeding in the patients included in their study, together with appropriate further questions.
Data on cancer deaths and deaths from all-causes in the most recent search to March 2020 are listed in Supplementary File 3, first for studies that had expressed association as HRs, followed by studies which had used odds ratios (ORs), risk ratios (RRs) or percent survival. The methodological quality of the studies was assessed and graded independently by two authors (AW and PE) using the Newcastle–Ottawa Scale [22]. We have also added to each paper listed in Supplementary File 3 a comment as to the level of certainty that aspirin had been taken – or had not been taken – throughout follow-up.
Most of the risk estimates reported by the authors were expressed as HRs, and these and their 95% CI were taken from the original articles and log-transformed to obtain the estimate of the treatment effect (TE). The standard errors (seTE) were determined by subtracting the lower log-transformed CI boundary from the upper log-transformed CI boundary and dividing this by 3.92 (2*1.96). Where HRs were not reported, ORs, RRs and their 95% CI, or number of events among patients taking aspirin and those not on aspirin, were taken from the original articles. ORs and exact 95% CIs were calculated where needed, and all were then log-transformed for meta-analysis.
Summary risk estimates of random effects models are shown as forest plots in Supplementary File 4. HR meta-analyses were conducted using the meta package, version 4.13.0 in R 4.0.2, open source. Analysis with the metagen function used sm = HR for the underlying summary method and the DerSimonian–Laird method [23] was used to estimate the between-study variance (τ and τ2). Meta-analyses of the reports as ORs were conducted using Stata/SE 16.1, and used the restricted maximum likelihood method to estimate the between-study variance and these are shown as forest plots in Supplementary File 5.
Finally, funnel plots were constructed and estimates of the probability of publication bias were derived. The forest plot added trim and fill which mirrored the studies followed by a cumulative forest plot based on decreasing standard error. This was only undertaken on a minimum of 10 papers hence there is only one examination for OR. These are all shown in Supplementary File 6.
Results
Three systematic literature searches on the topic of this report were conducted by the authors: in 2016 [7], in 2018 [20] and in 2020 up to March 2020 (Supplementary File 1). In each report, there are two outcomes, death from cancer and death from any cause, almost all of which have been presented as HRs. The new studies are described in Supplementary File 3 and their results are listed and pooled in Supplementary File 4. Some of the deaths have however been reported as OR, relative risk, etc., and all these have been converted to ORs. These ORs are presented separately from the HRs in Supplementary File 3 and are listed and pooled in Supplementary File 5. Some results however have been presented as additional survival in months or years, or during defined periods of time, such as 5 years. These are mentioned in the text, but do not appear in any table or Supplementary file.
In addition, we were concerned about undesirable side effects of the aspirin and in addition to abstracting relevant data from the published reports, following each of the three searches we wrote to an author of every report, asking for details of any unwanted side effect and in particular bleeding attributable to aspirin. A few authors supplied evidence on bleeding further to that in their published report, and these details are quoted in the text.
Figure 1 describes the findings of the three searches.
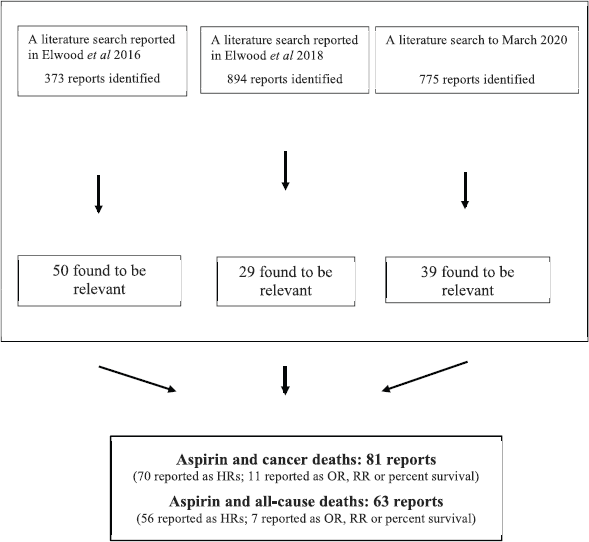
Figure 1. Flow diagram describing the findings of the three systematic literature searches.
Mortality
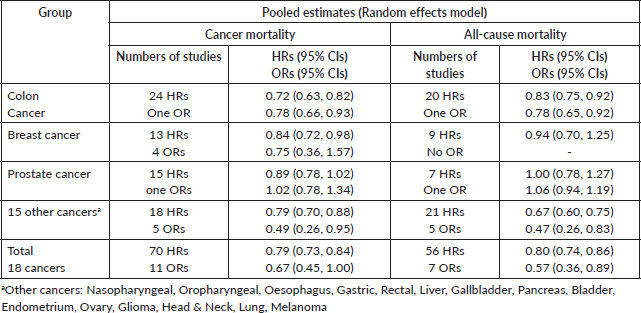
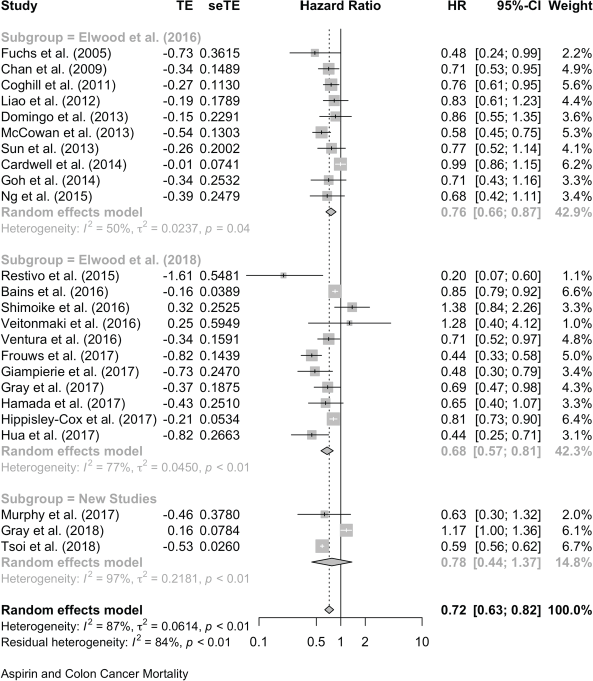
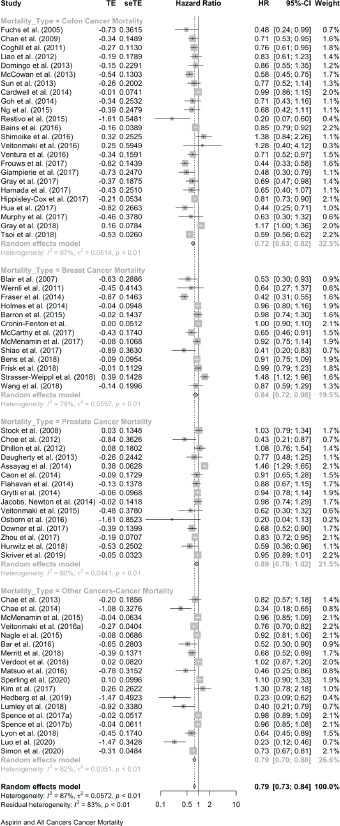
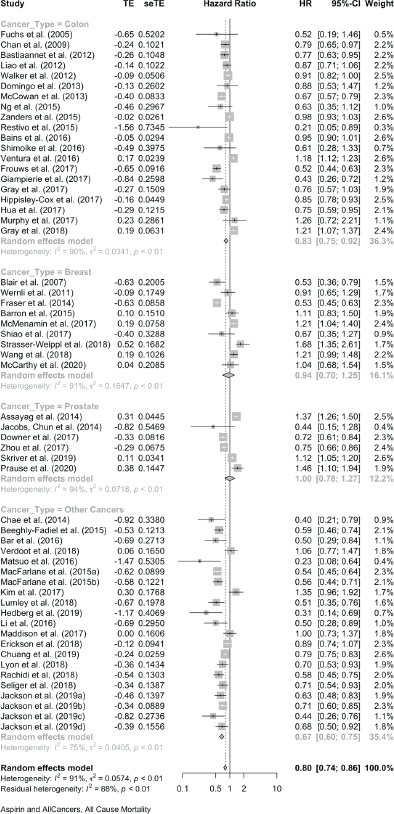
For colon cancer mortality, our three literature searches identified a total of 24 studies in which the association with aspirin was reported as HRs. Together, these give a pooled HR of 0.72 (95% CI: 0.63, 0.82), and a single report showed an OR (OR of 0.78 (0.66, 0.93) (Table 1 and Supplementary File 4). For all-cause mortality, 20 studies of colon cancer reported HRs, giving a pooled association with aspirin of 0.83 (95% CI: 0.75, 0.92) and a single HR reported an OR of 0.78 (0.65, 0.92) (Table 1 and Supplementary File 4)
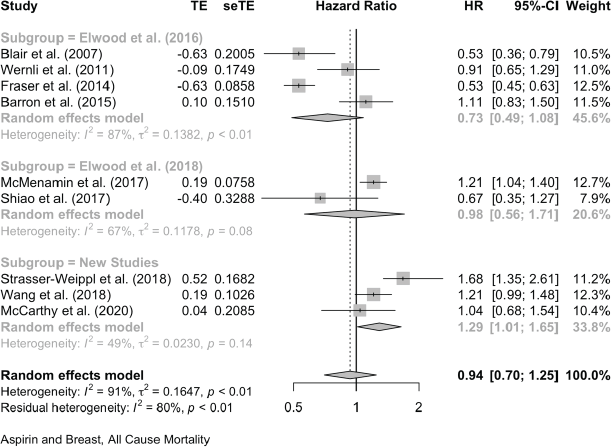
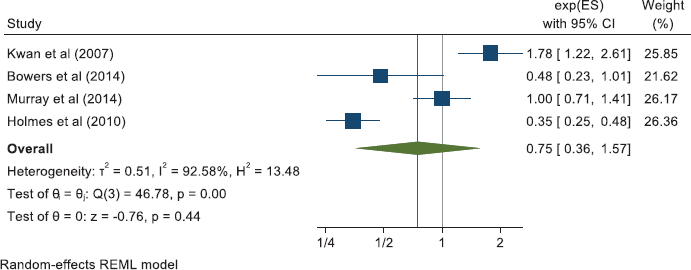
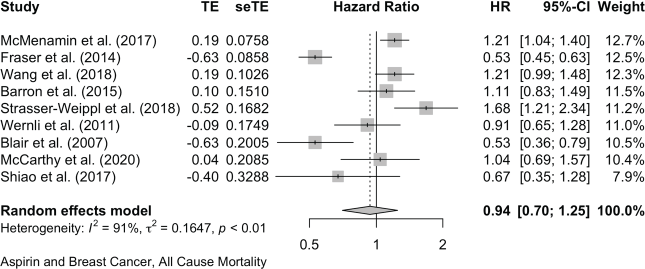
For breast cancer mortality, 13 studies reported as HRs and these give a pooled HR: 0.84 (0.72, 0.98). Four further studies give pooled OR: 0.75 (0.36, 1.57). For all-cause mortality in the breast cancer studies, nine reports give a pooled HR of 0.94 (0.70, 1.25).
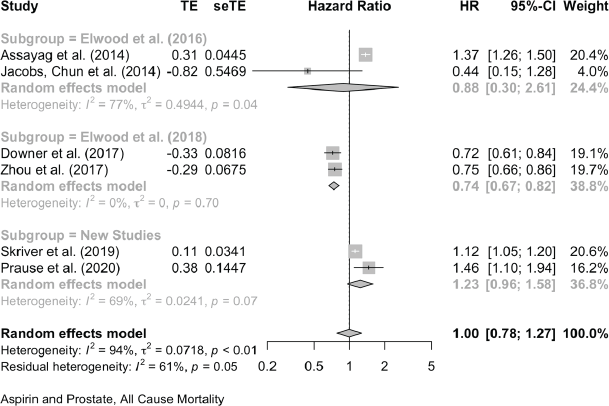
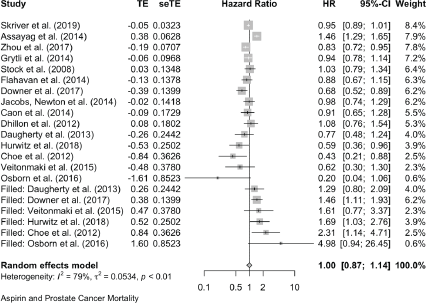
For prostate cancer mortality, the pooling of 15 studies gives an HR of 0.89 (0.78, 1.02) and there was one study with an OR of 1.02 (0.78, 1.34). For all-cause mortality in prostate cancer reports, seven studies give an HR of 1.00 (0.78, 1.27) and in one the OR is 1.06 (0.94, 1.19).
For cancers other than colon, breast and prostate, the supplementary files list ‘other’ cancers: nasopharynx [96, 102], GI cancers, including oropharynx, stomach, oesophagus and rectum, [41, 88, 97, 106, 121, 124], liver [93, 103], gallbladder in four parts [101], pancreas [125], bladder [98, 112, 114], ovary [81, 83–86, 113], endometrium [87, 89], head & neck [88, 90–92, 104, 123], lung [82, 94, 108, 122], leukaemia [79], glioma [100], melanoma [99] and two [39, 80] present a mixture of cancers.
Not all the estimates of association in these reports of ‘other’ cancers are significant at p < 0.05. However, only three are consistent with an oropharynx possible harmful effect of aspirin, having a confidence limit which includes 1, but none of the three is significant at p < 0.05 with both confidence limits above 1.
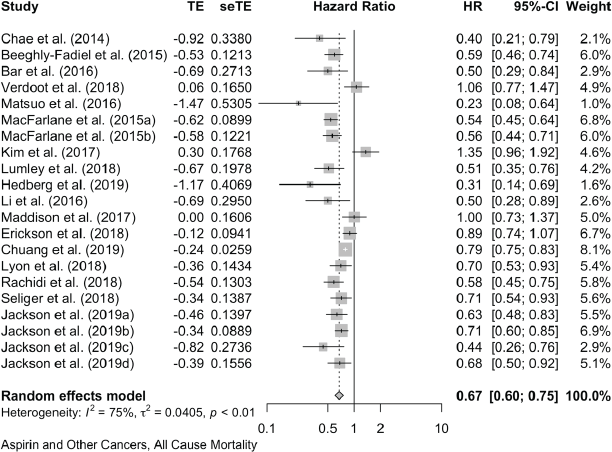
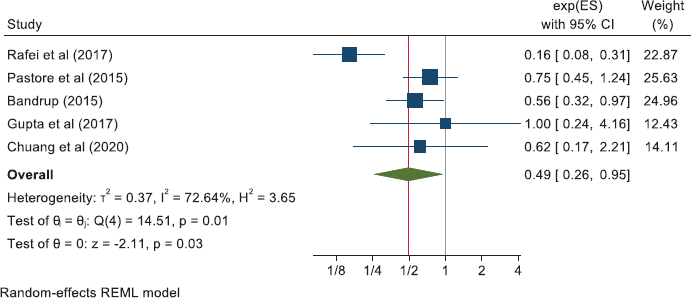
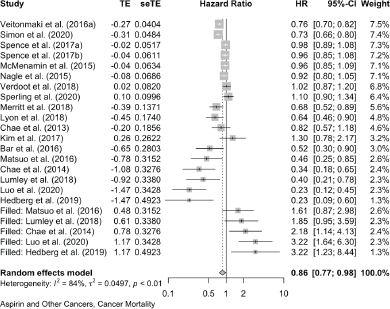
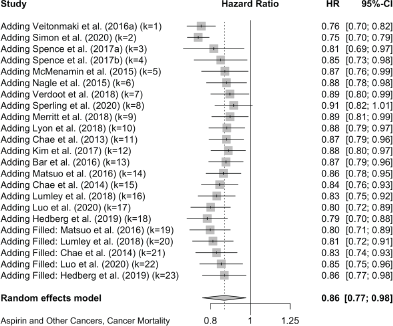
Together, these reports of ‘other’ cancers give a pooled HR for cancer mortality of 0.79 (0.70, 0.88) in 18 studies and a pooled OR of 0.49 (0.26, 0.95) in five studies. All-cause mortality in 21 of these other cancers gives a pooled HR of 0.67 (0.60, 0.75) in 21 studies and the five studies that did not report HRs give a pooled OR of 0.47 (0.26, 0.83).
The forest plots of all these data are shown in Supplementary Files 4 and 5, and Table 2 brings together all the available data on cancer deaths and on all-cause mortality.
Table 1. Summary of Eggers test for bias and of trim and fill analysis.
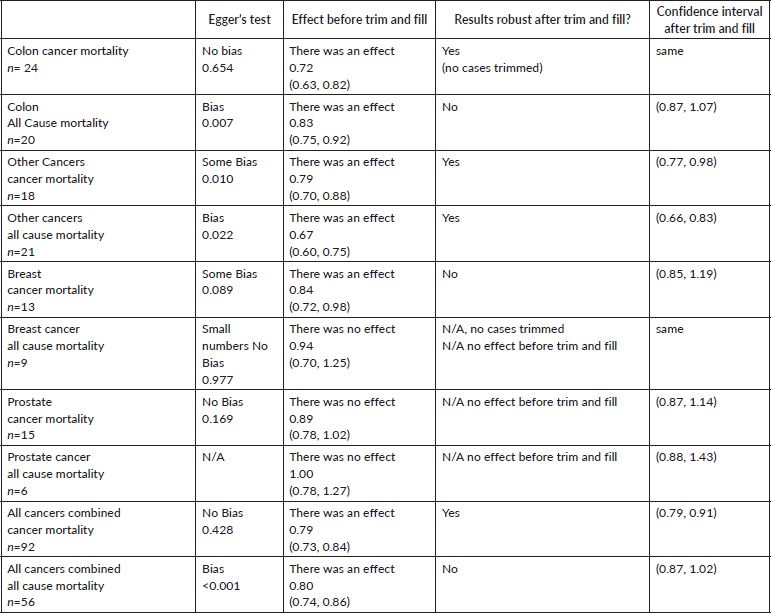
Table 2. A summary of the overall findings of the association between aspirin taking and mortality in 106 reports.
A number of authors give estimates of the association with aspirin in terms of the duration of the additional survival in patients taking the drug. Thus, Albandar [117] who followed 174 US veterans with colorectal cancer to death reported that the median survival of patients taking aspirin was 941 versus 384 days in those not taking aspirin. Several papers record an increased survival associated with aspirin taken by patients with liver cancer: in one 18 months additional survival [93]; in another 6% more patients survived 10 years with aspirin after diagnosis [103], and the median overall survival period after embolisation was longer for patients taking aspirin (57 versus 23 months) [119]. In a study of endometrial cancer, 91% of patients taking aspirin survived 10 years compared with 81% of patients not on aspirin [87]. In a study of patients with lung cancer, patients on aspirin survived 1.69 and only 1.02 years if not on aspirin [96]. In a study of pancreatic cancer, the 3-year survival was reported to be 61% in patients taking aspirin versus 26.3% in patients not taking aspirin [118], and finally, the 3-year survival of US veterans with head and neck cancer was 79% in those taking aspirin, compared with only 56% of those not taking aspirin [92].
Using a different approach, a group in Liverpool used data for over 44,000 patients with colon cancer to derive a predictive equation which relates a number of factors present at diagnosis to survival [45]. Entering the details for a non-diabetic man aged 70 with colon cancer into the predictive formula, the inclusion of aspirin taking increases the estimate of survival by about 5 years, and for a woman, about 4 years.
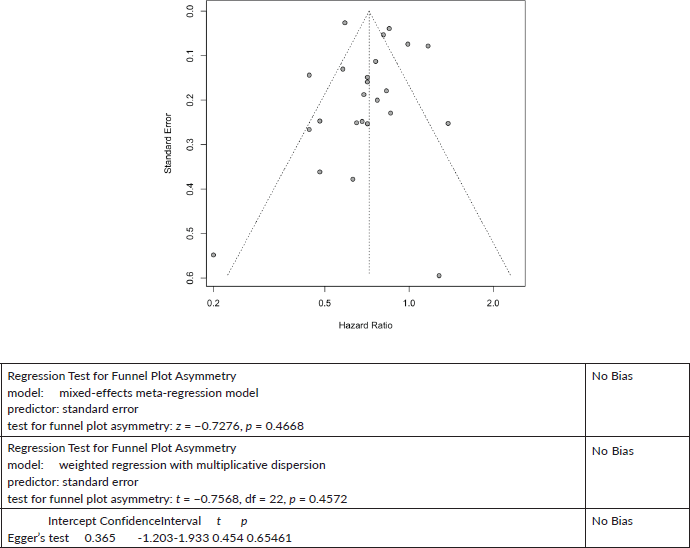
Finally, as a test of the hypothesis posed in this report, we compared the association of aspirin and cancer mortality in the 15 less common cancers with cancer mortality in colon cancer. In this comparison, we use colon cancer mortality as the ‘gold estimate’ of the effect of aspirin on the grounds that the effect of aspirin has been more thoroughly investigated in colon cancer, than in any other cancer; colon cancer is the only cancer for which the UK National Institute for Clinical Excellence has given a limited recommendation for aspirin, [120] and the U.S. Preventive Services Task Force and other professional bodies give guidance for the use of aspirin in colon cancer [121].
This comparison shows:
Colon Cancer mortality:
24 studies give a pooled HR: 0.72 (95% CI: 0.63, 0.82),
No significant publication bias z = −0.7276, p = 0.4668.
Cancer mortality in less common cancers
18 studies give a pooled HR: 0.79 (95% CI: 0.70, 0.88),
Significant publication bias z = −2.8110, p = 0.0049.
Bleeding
A search for evidence on bleeding, and fatal bleeding attributable to aspirin was made, and this included writing to the corresponding author on all the 118 papers included in the three searches. Many of the studies however had been based on recorded data, with no direct contact with the patients involved, and authors of such reports had little or no knowledge about bleeding in the patients they described.
Many of the authors reported the expected excess in GI bleeding in the patients on aspirin. However, only a very few reported fatal bleeds. In one study, 3% of the patients taking aspirin and 3.2% of those not taking aspirin had had a fatal bleed [40]. Tsoi et al [49], who studied a cohort of over 18,000 patients with colon cancer reported that deaths of aspirin users who developed GI bleeding were 0.40%, compared with 0.36% of the patients not taking aspirin. A study of patients with liver cancer treated by transarterial chemoembolisation reported that six patients in the aspirin group and seven patients in the non-aspirin group died because of upper GI bleeding [93]. One paper makes mention of the reduction in bleeding in patients who took a PPI along with the aspirin (OR: 0.85; 0.80, 0.91) [49]. All the references to bleeds relate to GI bleeds and no author made mention of cerebral bleeding.
Discussion
This report provides both confirmatory and new evidence on the benefit of aspirin in reducing mortality in patients being treated for cancer. Replication is an important procedure in science and the present study confirms the findings of our first report with 50 studies [7], and our second report with 29 studies [20]. The present study is a further replicate with 39 new observational studies.
The meta-analyses we now present are all based on pooling of the data provided by 118 observational studies comprising about a quarter of a million patients with cancer who were recorded as taking aspirin. This reveals that aspirin taking is associated with a reduction of cancer deaths of about one fifth in a range of 18 cancers (HR: 0.79 (0.73, 0.84) in 70 observational studies and OR: 0.67 (0.45, 1.00) in 11 studies (Table 2 and Supplementary Files 4 and 5). The effect of aspirin on all-cause mortality is closely similar (HR: 0.80 (0.74, 0.86) in 56 observational studies and OR: 0.57 (0.36, 0.89) in 7 studies). A reasonable interpretation of these results is – that at any time after a diagnosis of a wide range of different cancers, about 20% more of the patients who take aspirin are likely to be alive, compared with patients not taking aspirin.
The evidence of publication bias throughout this work is a most important issue. Bias due to the selective publication of positive findings for aspirin was expected, and for some of the pooled results the magnitude of this bias is greater than could be reasonably expected in chance grounds alone (Supplementary File 6). While conclusions drawn from these 118 papers have, therefore, to be cautious, the evidence is strengthened by the absence of significant bias at p < 0.05 for the data for colon cancer. It is also encouraging that the trim and fill analysis on the less common cancers maintained the beneficial TE for both cancer specific mortality and all-cause mortality.
Bleeding
A bleed, either GI or intra-cerebral, is a crisis for a patient, but the seriousness of a bleed attributable to aspirin should be evaluated against the likely benefits attributable to its use and furthermore the severity of the additional bleeds attributable to aspirin should be considered and not just their frequency [122, 123]. In relation to the treatment of cancer, our examination of the 118 reports gives a considerable degree of reassurance on aspirin, and particularly on the most serious bleeds. It is of relevance that most of the patients appear to have been taking low-dose aspirin primarily for cardiovascular protection.
Low-dose aspirin is however associated with additional GI bleeds in between 0.8 and 5.0 patients per 1,000 person years aged 50–84 years in the general population [124]. This represents an increase above spontaneous GI bleeding of between about 50% [125] and 90% [121]. It is important to note that these increases imply that only one in every two or every three bleeds that occur in patients taking low-dose aspirin is likely to be truly attributable to the aspirin, the other bleeds being spontaneous and nothing to do with aspirin.
The most serious bleeds are those that lead to death and our concern on this led us, early in our investigations of aspirin and cancer, to conduct a careful evaluation of fatal bleeding [126] A meta-analysis based on 11 randomised trials showed that the additional bleeds attributable to aspirin are less serious than spontaneous bleeds and are seldom, if ever fatal (relative risk of death: 0.45; (0.25, 0.80), and the risk of a fatal bleed in the totality of subjects randomised to aspirin, relative to subjects randomised to placebo was RR: 0.77 (0.41, 1.42). As we reported in our overview [126], others have reported similar findings of a reduction in the proportion of fatal bleeds in patients taking aspirin [9, 127–130].
Findings on bleeding in the recent ASPREE trial of prophylactic aspirin are of interest as more than 19,000 subjects with a median age of 74 years were followed for 5 years. Eighty-nine subjects randomised to aspirin, or 1.9 in every 1,000 experienced a bleed each year, compared with 48 bleeds, or just over 1.1 per thousand per year in those not taking aspirin. Granted this was not a trial of aspirin treatment, but it is of relevance to the safety of the drug that only two fatal bleeds occurred, and neither was in a subject taking aspirin [131].
The most serious side effect of aspirin, intra-cerebral bleeding, is fortunately rare [132], and no author in our literature review mentioned cerebral bleeding within the patients they followed. The risk associated with aspirin is estimated to be around 1.39 (95% CI: 1.08, 1.78) [125] equivalent to one or two additional haemorrhagic strokes per year in every 10,000 subjects [133].
Hypertension is the major factor in haemorrhagic stroke and in one major overview of randomised trials there was a doubling of cerebral haemorrhages for a rise of 20 mmHg in blood pressure (RR: 2.18; 95% CI: 1.65, 2.87) [127]. The relevance of hypertension was further highlighted in a trial of aspirin based on 20,000 patients with hypertensive disease, all of whom were adequately treated with anti-hypertensive drugs. There were no additional cerebral bleeds attributable to aspirin: the same number of patients on aspirin experienced cerebral bleeds (19 patients) as those on placebo (20 patients) [133].
Strengths and limitations of this study
In addition to the risks of publication bias as detailed above, a most important limitation is that almost all the evidence we present are from observational studies. A number of randomised trials of therapeutic aspirin are in progress but these focus entirely on either one, or a few of the common cancers: colon [12–15], breast [12, 14] and prostate [12, 15]. Our concern, however, is for all cancers and not one or a few cancers, and as others have pointed out many of the actions of aspirin on cancer development, growth and metastatic spread, appear likely to be relevant to a wide range of cancers [6–19].
It is important to note that amongst the uncertainties in these observational studies, two uncertainties appear to stand out in their probable relevance to every observational study, and to the possible size of their effects. These are: first: uncertainties about the classification of patients with regard to continuous aspirin taking, and uncertainties about the non-taking of aspirin by the ‘controls’, and secondly, co-morbidity in the patients taking aspirin.
Few authors give reassurance about continued aspirin taking during follow-up, and no authors comment on the possibility of ‘contamination’ of control subjects starting to take ‘over the counter’ aspirin during the follow-up. An additional column in Supplementary File 3 lists quotations from the papers reviewed and these show that most authors assumed that if there is evidence of aspirin taking at the time of diagnosis, it can reasonably be assumed that aspirin taking was continuous during follow-up. Thus, ‘Low-dose aspirin use was defined as a minimum of one filled prescription after cancer diagnosis’ [89] and another: ‘the patients were receiving aspirin from diagnosis to at least 1 year after treatment initiation’ [90]. One author pointed out however that ‘the inverse association with aspirin appeared to be only among men who reported using aspirin regularly’, [76] and another noted that a reduction in mortality was ‘notably among patients filling prescriptions for a large quantity of low dose aspirin tablets during the (follow-up) period [77]. Another author found that prescribed aspirin alone was not associated with decreased mortality, but when OTC aspirin was added, a large reduction was detected [39].
A recent study by a group in Dublin examined the influence of approaching death on end-of-life aspirin use in patients with breast or colorectal cancer. They found that the use of aspirin declined ‘considerably’ during the 2 years before death, and at the time of death rates of aspirin use had dropped from around 60% to around 20% for colorectal cancer and from around 80% to around 45% for breast cancer [134].
The only comment about aspirin taking by control subjects comes from an overview of 12 studies in which the authors state that the pooled survival in patients on aspirin was only HR: 0.96 (0.88, 1.04) but if non-aspirin taking was more tightly defined as less than once per week, the HR was 0.89 (0.82, 0.98) [135].
The other important limitation is confounding by co-morbidity. Many authors mention that the aspirin takers in their study were older than the control patients not on aspirin. While this can be adjusted for statistically, the fact that a number of studies state that most of the patients who were taking aspirin were doing so because of a prior vascular event or prevalent vascular disease. Clearly, the morbidity that had led some of the patients to take aspirin can have eroded any benefit achievable by aspirin and while many of the papers mention this, few give details.
Yet a further limitation arises from possible miscoding of the causes of death in these studies. In the SEER programme on mortality in patients with cancer in the USA, it was found that 11% of cancer deaths had been attributed to vascular disease [136]. Any such miscoding will lead to an underestimate of the reduction in cancer deaths associated with aspirin.
The very broad range in the estimates of effect of aspirin leading to high heterogeneity estimates in our meta-analyses is worrying, and some of the differences between studies seem to defy any reasonable explanation. And yet, this was predicted from the beginning of the work on aspirin treatment [7]. There are many biases and sources of possible differences between the series of patients in the various studies, including differences in age and social factors, differences in other treatments and in general clinical management [41, 48]. Then there are possible differences in consistency of aspirin taking and the differences in co-morbidity already mentioned. Both poor aspirin taking and co-morbidity in patients taking aspirin will increase heterogeneity, and are probably inevitable in a series of studies such as we present. On the other hand, it seems unlikely that such differences could account for the overall benefits we find to be associated with aspirin taking.
Conclusions
We judge that the body of evidence now available on the efficacy and the safety of aspirin justifies its use as an adjunct treatment in a wide range of cancers. Clinical care includes an ethical imperative for shared decision making [137] and we, therefore, believe that doctors should present, and patients with cancer should be encouraged to raise the topic of aspirin taking with their doctors. At the same time, we stress that aspirin is not a possible alternative to any other treatment, although in poorer countries aspirin could be one of very few, or perhaps the only acceptable treatment on the grounds of cost and availability [138].
Further research into aspirin and cancer would clearly be of great value, and studies including observational and randomised trial should be encouraged, especially if focused upon one of the less common cancers.
Conflicts of interest
The author(s) declare that they have no conflict of interest. All the authors have read the paper and agree with its content.
Funding
No special funding was obtained for any of the work described in this paper. Julieta Galante was supported by the National Institute for Health Research (NIHR) Applied Research Collaboration East of England. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
References
1. Gasic GJ, Gasic TB and Stewart CC (1968) Anti-metastatic effects associated with platelet reduction Proc Natl Acad Sci USA 61 46–52 https://doi.org/10.1073/pnas.61.1.46
2. Gasic GJ, Gasic TB, and Murphy S (1972) Anti-metastatic effect of aspirin Lancet 2 932–933 https://doi.org/10.1016/S0140-6736(72)92581-0 PMID: 4116642
3. Lipton A, Scialla S, and Harvey H, et al (1982) Adjuvant antiplatelet therapy with aspirin in colorectal cancer J Med 13 419–429
4. Lebeau B, Chastang C, and Muir JF, et al (1993) No effect of an antiaggregant treatment with aspirin in small cell lung cancer treated with CCAVP16 chemotherapy. Results from a randomised clinical trial of 303 patients Cancer 71 1741–1745 https://doi.org/10.1002/1097-0142(19930301)71:5<1741::AID-CNCR2820710507>3.0.CO;2-Q PMID: 8383578
5. Cregan ET, Twito DI, and Johansson S, et al (1991) A randomised prospective assessment of recombinant leukocyte A interferon with or without aspirin in advanced renal adenocarcinoma J Clin Oncol 9 2104–2109 https://doi.org/10.1200/JCO.1991.9.12.2104
6. Liu JF, Jamieson GG, and Wu TC, et al (2009) A preliminary study on the postoperative survival of patients given aspirin after resection for squamous cell carcinoma of the esophagus or adenocarcinoma of the cardia Ann Surg Oncol 16 1397–1402 https://doi.org/10.1245/s10434-009-0382-z PMID: 19241108
7. Elwood PC, Morgan G, and Pickering JE, et al (2016) Aspirin in the treatment of cancer: reductions in metastatic spread and in mortality: a systematic review and meta-analysis of published studies PLoS One 11(4) e0152402 https://doi.org/10.1371/journal.pone.0152402 PMCID: 4838306
8. Downer MK, Allard CB, and Preston MA, et al (2017) Regular aspirin use and the risk of lethal prostate cancer in the physicians health study Eur Urol 72 821–827 https://doi.org/10.1016/j.eururo.2017.01.044 PMID: 28189429
9. Rothwell PM, Price JF, and Fowkes G, et al (2012) Short-term effects of daily aspirin on cancer incidence, mortality and non-vascular death: analysis of the time course of risks and benefits in 51 randomised trials Lancet 379 1602–1612 https://doi.org/10.1016/S0140-6736(11)61720-0 PMID: 22440946
10. Mills EJ, WU P, and Alberton M, et al (2012) Low-dose aspirin and cancer mortality: a meta-analysis of randomised trials Am J Med 125 560–567 https://doi.org/10.1016/j.amjmed.2012.01.017 PMID: 22513195
11. Rothwell PM, Fowkes FGR, and Belch JFF et al (2011) Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials Lancet 327 31–41 https://doi.org/10.1016/S0140-6736(10)62110-1
12. Coyle C, Cafferty FH, and Rowley S, et al (2016) Add-aspirin: a phase III, double-blind, placebo control randomised trial assessing the effects of aspirin on disease recurrence and survival after primary therapy in common non-metastatic solid tumours Contemp Clin Trials 51 56–64 https://doi.org/10.1016/j.cct.2016.10.004 PMID: 27777129 PMCID: 5127874
13. Ali R, Toh HC, and Toh HC, et al (2011) The utility of aspirin in Dukes C and high risk Dukes B CRC – the ASCOLT study: study protocol for a randomised controlled trial Trials 12 261 https://doi.org/10.1186/1745-6215-12-261
14. Chen WY, Winer EP, and Ballman KV, et al (2017) ABC TRIAL: a randomised double blinded placebo controlled trial of aspirin as adjuvant therapy for node positive breast cancer J Clin Oncol 37
15. Powles T (2018) PROVENT: a randomised double blind feasibility trial randomised trial to examine the clinical effectiveness of aspirin and/or vitamin D3 to prevent disease progression in men on active surveillance for prostate cancer Eur J Surg Oncol 44
16. Chan AT, Ogino S and Fuchs CS (2009) Aspirin use and survival after diagnosis of colorectal cancer JAMA 302 649–659 https://doi.org/10.1001/jama.2009.1112 PMID: 19671906 PMCID: 2848289
17. Langley RW, Burdett S, and Tierney JF, et al (2011) Aspirin and cancer: has aspirin been overlooked as an adjuvant therapy Br J Cancer 105 1107–1113 https://doi.org/10.1038/bjc.2011.289 PMID: 21847126 PMCID: 3208483
18. Langley RE (2013) Clinical evidence for the use of aspirin in the treatment of cancer Ecancermedicalscience 7 297 https://doi.org/10.3332/ecancer.2013.297 PMID: 23589726 PMCID: 3622409
19. Zhang X, Feng Y, and Liu X, et al (2019) Beyond a chemopreventive reagent, aspirin is a master regulator of the hallmarks of cancer J Cancer Res Clin Oncol 145 1387–1403 https://doi.org/10.1007/s00432-019-02902-6 PMID: 31037399
20. Elwood PC, Pickering JE, and Morgan G, et al (2018) Systematic review update of observational studies further supports aspirin role in cancer treatment: time to share evidence and decision-making with patients PLoS One 13(9) e0203957 https://doi.org/10.1371/journal.pone.0203957 PMCID: 6155524
21. Moher D, Liberati A, and Tetzlaff J, et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement PLoS One 6(7) e1000097
22. Wells G, Shea B, and O’Connell D, et al (2011) The Newcastle-Ottaway scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis
23. DerSimonian R and Laird N (1986) Meta-analysis in clinical trials Control Clin Trials 7 177–188 https://doi.org/10.1016/0197-2456(86)90046-2 PMID: 3802833
24. Fuchs C, Meyerhardt JA, and Heseltine DL, et al (2005) Influence of regular aspirin on survival for patients with stage III colon cancer. Findings from intergroup trial CALGB 89803 J Clin Oncol 23(16) 3530 https://doi.org/10.1200/jco.2005.23.16_suppl.3530
25. Bastiaannet E, Sampieri K, and Dekkers OM, et al (2012) Use of aspirin postdiagnosis improves survival for colon cancer patients Br J Cancer 106 1564–1570 https://doi.org/10.1038/bjc.2012.101 PMID: 22454078 PMCID: 3341868
26. Coghill AE, Newcomb PA, and Campbell PT, et al (2011) Prediagnostic NSAIDs use and survival after diagnosis of colorectal cancer Gut 60 491–498 https://doi.org/10.1136/gut.2010.221143
27. Liao X, Lockhead P, and Nishara R, et al (2012) Aspirin use, tumour PIK3CA mutation and colorectal cancer survival N Engl J Med 367 1597–1606 https://doi.org/10.1056/NEJMoa1207756
28. Walker AJ, Grainge MJ, and Card TR (2012) Aspirin and other NSAID drug use and colorectal cancer survival: a cohort study Br J Cancer 107 1602–1607 https://doi.org/10.1038/bjc.2012.427 PMID: 23011483 PMCID: 3493766
29. Domingo E, Church DN, and Sieber O, et al (2013) Evaluation of PIK3CA mutation as a predictor of benefit from NSAID drug therapy in colorectal cancer J Clin Oncol 34 4297–4305 https://doi.org/10.1200/JCO.2013.50.0322
30. McCowan C, Munro AJ, and Donnan PT, et al (2013) Use of aspirin post-diagnosis in a cohort of patients with colorectal cancer and its association with all-cause and colorectal specific mortality Eur J Cancer 49 1049–1057 https://doi.org/10.1016/j.ejca.2012.10.024
31. Sun Nishihara QR, Chan AT, and Ogino S (2013) Aspirin and colorectal cancer incidence and mortality by ctnnb1 expression: a molecular pathological epidemiology (MPE) study Cancer Epidemiol Biomarkers Prev 22 472–473
32. Cardwell CR, Kunzmann AT, and Cantwell MM, et al (2014) Low-dose aspirin after diagnosis of colorectal cancer does not increase survival: a case-control analysis of a population-based cohort Gastroenterol 146 700–708 https://doi.org/10.1053/j.gastro.2013.11.005
33. Goh HH, Leong WQ, and Chew MH, et al (2014) Post-operative aspirin use and colorectal cancer-specific survival in patients with stage I-III colorectal cancer Anticancer Res 34 7407–7414 PMID: 25503181
34. Ng K, Meyerhardt JA, and Chan AT, et al (2015) Aspirin and COX-2 inhibitor use in patients with stage III colon cancer J Natl Cancer Inst 107 345 https://doi.org/10.1093/jnci/dju345
35. Zanders MM, van Herk-Sukel MP, and Vissers PA, et al (2015) Are metformin, statin and aspirin use still associated with overall mortality among colorectal cancer patients with diabetes if adjusted for one another? Br J Cancer 113 401–410 https://doi.org/10.1038/bjc.2015.259
36. Restivo A, Cocco IMF, and Casula G, et al (2015) Aspirin as a neoadjuvant agent during preoperative chemoradiation for rectal cancer Br J Cancer 113 1133–1139 https://doi.org/10.1038/bjc.2015.336 PMID: 26372700 PMCID: 4647877
37. Bains SJ, Mahic M, and Myklebust TA, et al (2016) Aspirin as secondary prevention in patients with colorectal cancer: an unselected population-based study J Clin Oncol 34 2501–2508 https://doi.org/10.1200/JCO.2015.65.3519 PMID: 27247217
38. Shimoike N, Fujikawa T, and Yoshimoto Y, et al (2016) Does antiplatelet therapy affect short-term and long-term outcomes of patients undergoing surgery for colorectal cancer? – surgical radicality versus perioperative antiplatelet-related morbidity risks J Gastroenterol Hepatol Res 5 1962–1969 https://doi.org/10.17554/j.issn.2224-3992.2016.05.605
39. Veitonmaki T, Murtola TJ, and Maattanen L, et al (2015) Use of non-steroidal anti-inflammatory drugs and prostate cancer survival in the finnish prostate cancer screening trial Prostate 75 1394–1402 https://doi.org/10.1002/pros.23020 PMID: 26073992
40. Ventura L, Miccinesi G, and Barchielli A, et al (2016) Does low-dose aspirin use for cardiovascular disease prevention reduce colorectal cancer deaths? A comparison of two cohorts in the Florence district, Italy Eur J Cancer Prev 27 134–139 https://doi.org/10.1097/CEJ.0000000000000319 PMID: 27845951
41. Frouws MA, Bastiaannet E, and LangleyMadis RE, et al (2017) Effect of low-dose aspirin use on survival of patients with gastrointestinal malignancies; an observational study Br J Cancer 116 405–413 https://doi.org/10.1038/bjc.2016.425 PMID: 28072768 PMCID: 5294482
42. Giampieri R, Restivo A, and Pusceddu V, et al (2017) The role of aspirin as antitumoral agent for heavily pretreated patients with metastatic colorectal cancer receiving capecitabine monotherapy Clin Colorectal Cancer 16 38–43 https://doi.org/10.1016/j.clcc.2016.07.011
43. Gray RT, Cantwell MM, and Coleman HG, et al (2017) Evaluation of PTGS2 expression, PIK3CA mutation, aspirin use and colon cancer survival in a population-based cohort study Clin Transl Gastroenterol 8 e91 https://doi.org/10.1038/ctg.2017.18 PMID: 28448072 PMCID: 5543466
44. Hamada T, Cao Y, and Qian ZR, et al (2017). Aspirin use and colorectal cancer survival according to tumor CD274 (programmed cell death 1 ligand 1) expression status J Clin Oncol 35 1836–1844 https://doi.org/10.1200/JCO.2016.70.7547 PMID: 28406723 PMCID: 5455595
45. Hippisley-Cox J and Coupland C (2017) Development and validation of risk prediction equations to estimate survival in patients with colorectal cancer: cohort study Br Med J 357 2497–2522 https://doi.org/10.1136/bmj.j2497
46. Hua X, Phipps AI, and Burnett-Hartman AN, et al (2017) Timing of aspirin and other nonsteroidal anti-inflammatory drug use among patients with colorectal cancer in relation to tumor markers and survival J Clin Oncol 35 2806–2813 https://doi.org/10.1200/JCO.2017.72.3569 PMID: 28617623 PMCID: 5562174
47. Murphy C, Turner N, and Wong HI, et al (2017) Examining the impact of regular aspirin use and PIK3CA on survival in stage 2 colon cancer Intern Med J 47 88–98 https://doi.org/10.1111/imj.13312
48. Gray RT, Coleman HG, and Hughes CH, et al (2018) Low-dose aspirin use and survival in colorectal cancer: results from a population-based cohort study BMC Cancer 18 228 https://doi.org/10.1186/s12885-018-4142-y PMID: 29486728 PMCID: 6389196
49. Tsoi KF, Chan FC, and Hiral HW et al (2018) Risk of gastrointestinal bleeding and benefit from colorectal cancer reduction from long-term use of low-dose aspirin: a retrospective study of 612 509 patients J Gastroenterol Hepatol 33 1728–1736 https://doi.org/10.1111/jgh.14261 PMID: 29665624
50. Blair CK, Sweeney C, and Anderson KE et al (2007) NSAID use and survival after breast cancer diagnosis in post-menopausal women Breast cancer Res Treat 101 191–197 https://doi.org/10.1007/s10549-006-9277-x
51. Wernli KJ, HamptonJM, and Trentham-Dietz A et al (2011) Use of antidepressants and NSAIDs in relation to mortality in long-term breast cancer survivors Pharmacoepidemiol Drug Saf 20 131–137 https://doi.org/10.1002/pds.2064 PMID: 21254283 PMCID: 3074001
52. Fraser DM, Sullivan FM, and Thompson AM, et al (2014) Aspirin use and survival after the diagnosis of breast cancer: a population-based cohort study Br J Cancer 111 623–627 https://doi.org/10.1038/bjc.2014.264 PMID: 24945997 PMCID: 4119969
53. Holmes MD, Chen WY, and Li L, et al (2014) Aspirin intake and survival after breast cancer J Clin Oncol 28 1467–1472 https://doi.org/10.1200/JCO.2009.22.7918
54. Barron TI, Murphy LM, and Brown C, et al (2015) De-novo post-diagnostic aspirin use and mortality in women with stage I-III breast cancer Cancer Epidemiol Biomarkers Prev 24 898–904 https://doi.org/10.1158/1055-9965.EPI-14-1415 PMID: 25791705
55. Cronin-Fenton D, Heide-Jorgensen U, and Ahern TP, et al (2016) Low-dose non-steroidal antiinflammatory drugs, selective COX-2 inhibitor prescriptions and breast cancer recurrence. A Danish population based cohort study Proceedings of the Pharmacoepidemiology and Drug Safety Conference, Boston
56. McCarthy AM, He W, and Regan S, et al (2017) Impact of PIK3CA tumor mutation on the association of aspirin or NSAID use and time to breast cancer recurrence J Clin Oncol 35(15_Suppl) 1521 https://doi.org/10.1200/JCO.2017.35.15_suppl.1521
57. Mc Menamin UC, Cardwell CR, and Hughes CM et al (2017) Low-dose aspirin use and survival in breast cancer patients: a nationwide cohort study Cancer Epidemiol 47 20–27 https://doi.org/10.1016/j.canep.2016.12.008 PMID: 28088656
58. Shiao J, Thomas KM, and RaHuanhimi AS, et al (2017) Aspirin/antiplatelet agent use improves disease-free survival and reduces the risk of distant metastases in Stage II and III triple-negative breast cancer patients Breast Cancer Res Treat 161 463–471 https://doi.org/10.1007/s10549-016-4081-8
59. Bens A, Friis S, and Dehlendorff C, et al (2018) Low-dose aspirin use and risk of contralateral breast cancer: a Danish nationwide cohort study Prev Med 115 186–193 https://doi.org/10.1016/j.ypmed.2018.09.015
60. Frisk G, Ekberg S, and Lidbrink E, et al (2018) No association between low-dose aspirin use and breast cancer outcomes overall: a Swedish population-based study Breast Cancer Res 20 142 https://doi.org/10.1186/s13058-018-1065-0 PMID: 30458873 PMCID: 6247765
61. Strasser-Wippl K, Higgins MJ, and Chapman JAW, et al (2018). Effects of celecoxib and low-dose aspirin on outcomes in adjuvant aromatase inhibitor-treated patients: CCTG MA.27 J Natl Cancer Inst 110 1003–1008 https://doi.org/10.1093/jnci/djy017
62. Wang T, Parada H, and McClain KM, et al (2018) Pre-diagnostic aspirin and mortality after breast cancer Cancer Causes Control 29 417–425 https://doi.org/10.1007/s10552-018-1020-5 PMID: 29516320
63. McCarthy AM, KumarNP, and Wei H, et al (2020) Different associations of tumor PIK3CA mutations and clinical outcomes according to aspirin use among women with metastatic hormone receptor positive breast cancer BMC Cancer 20 347 https://doi.org/10.1186/s12885-020-06810-8 PMID: 32326897 PMCID: 7181475
64. Stock DC, Groome PA, and Siemens DR, et al (2008) Effects of non-selective NSAIDs on the aggressiveness of prostate cancer Prostate 68 1655–1665 https://doi.org/10.1002/pros.20834 PMID: 18698582
65. Choe KS, Cowan JE, and Chan JM, et al (2012) Aspirin use and the risk of prostate cancer mortality in men treated with prostatectomy or radiotherapy J Clin Oncol 30 3540–3544 https://doi.org/10.1200/JCO.2011.41.0308 PMID: 22927523 PMCID: 3454771
66. Dhillon PK, Kenfield SA, and Stampfer MJ, et al (2012) Aspirin use after a prostate cancer diagnosis and cancer survival in a prospective cohort Cancer Prev Res 5 1223–1228 https://doi.org/10.1158/1940-6207.CAPR-12-0171
67. Daugherty SER, Pfeiffer R, and Ghazarian A, et al (2013) Frequency of aspirin use and prostate cancer mortality among prostate cancer cases in the control arm of the prostate, lung, colorectal, and ovarian (PLCO) cancer screening trial Am Assoc Cancer Res 73(8) 15
68. Assayag J, Pollak MN and Azoulay L (2014) The use of aspirin and the rosk of mortality in patients with prostate cancer J Urol 193 1220–1225 https://doi.org/10.1016/j.juro.2014.11.018
69. Caon J, Paquette M, and Hamm J, et al (2014). Does statin or ASA affect survival when prostate cancer is treated with external beam radiation therapy? Prostate Cancer 2014 184297 https://doi.org/10.1155/2014/184297 PMID: 24729876 PMCID: 3960556
70. Flahavan EM, Bennett K, and Sharp L, et al (2014) A cohort study investigating aspirin use and survival in men with prostate cancer Ann Oncol 25 154–159 https://doi.org/10.1093/annonc/mdt428
71. Grytli HH, Fagerland MW, and Fossa SD et al (2014) Associaton between use of beta-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high risk or metastatic disease Eur Urol 65 635–641 https://doi.org/10.1016/j.eururo.2013.01.007
72. Jacobs, EJ, Newton CC, and Stevens VJ, et al (2014) Daily aspirin use and prostate cancer-specific mortality in a large cohort of men with nonmetastatic prostate cancer J Clin Oncol 32 3716–3722 https://doi.org/10.1200/JCO.2013.54.8875 PMID: 25332245
73. Jacobs CD, Chun SG, and Yan J, et al (2014) Aspirin improves outcome in high risk prostate cancer patients treated with radiation therapy Cancer Biol Ther 15 699–706 https://doi.org/10.4161/cbt.28554 PMID: 24658086 PMCID: 4049786
74. Osborn VW, Chen SC, and Weiner J, et al (2016) Impact of aspirin on clinical outcomes for African American men with prostate cancer undergoing radiation Tumori 102 65–70 https://doi.org/10.5301/tj.5000424
75. Zhou CK, Daugherty SE, and Liao LM, et al (2017) Do aspirin and other NSAIDs confer a survival benefit in men diagnosed with prostate cancer? A pooled analysis of NIH-AARP and PLCO cohorts Cancer Prev Res 10 410–420 https://doi.org/10.1158/1940-6207.CAPR-17-0033
76. Hurwitz LM, Joshu CE, and Barber JR, et al (2018) Aspirin use and risk of lethal prostatic cancer in the atherosclerosis risk in communities cohort Cancer Research. Proceedings of the AACR Annual Meeting
77. Skriver C, Dehlendorff C, and Borre M, et al (2019) Use of low-dose aspirin and mortality after prostate cancer diagnosis. A nationwide cohort study Ann Intern Med 170 443–452 https://doi.org/10.7326/M17-3085 PMID: 30831581
78. Prause LW, Manka L, and Millan C, et al (2019) Influence of regular aspirin intake on PSA values, prostate cancer incidence and overall survival in a prospective screening trial (ERSPC Aarau) World J Urol 38(10) 2485–2491 https://doi.org/10.1007/s00345-019-03054-5 PMID: 31865534
79. Chae YK, Trinh L, and Jain P, et al (2014) Statin and aspirin use is associated with improved outcome of FCR therapy in relapsed/refractory chronic lymphocytic leukaemia Blood 22 1424–1426 https://doi.org/10.1182/blood-2013-07-517102
80. Chae Y, Hong DS, and Kim KH (2013) PIK3CA mutations, aspirin use and mortality in patients with women’s cancers or colorectal cancers treted in early phase clinical trials. POSTER Epidemiology Primary and Secondary Prevention. Abstract No 1454.
81. Beeghly-Fadiel A, Holman G, and Stansel SP, et al (2015) Aspirin, NSAIDs and ovarian cancer survival from electronic medical records Clinical Cancer Research. Proceedings of the 10th Biennual Ovarian Cancer Research Symposium, United States
82. McMenamin UC, Cardwell CR, and Hughes CM, et al (2015) Low-dose aspirin and survival from lung cancer: a population-based cohort study BMC Cancer 15 911 https://doi.org/10.1186/s12885-015-1910-9
83. Nagle CM, Ibiebele TI, and DeFrazio A, et al (2015) Aspirin, non-aspirin nonsteroidal anti-inflammatory drugs, acetaminophen and ovarian cancer survival Cancer Epidemiol 39 196–199 https://doi.org/10.1016/j.canep.2014.12.010 PMID: 25666512
84. Bar D, Lavie O, and Stein N, et al (2016) The effect of metabolic co-morbidities and commonly used drugs on the prognosis of patients with ovarian cancer Eur J Obstet Gynaecol Reprod Biol 207 227–231 https://doi.org/10.1016/j.ejogrb.2016.09.005
85. Merritt MA, Rice MS, and Barnard ME, et al (2018) Pre-diagnosis and post-diagnosis use of common analgesics and ovarian cancer prognosis (NHS/NHSII): a cohort study Lancet Oncol 19 1107–1116 https://doi.org/10.1016/S1470-2045(18)30373-5 PMID: 30029888 PMCID: 6242286
86. Verdoot F, Kjaer SK, and Dehlendorff C, et al (2018) Aspirin use and ovarian cancer mortality in a Danish nationwide cohort study Br J Cancer 118 611–615 https://doi.org/10.1038/bjc.2017.449
87. Matuso K, Cahoon SS, and Yoshihara K, et al (2016) Association of low-dose aspirin and survival of women with endometrial cancer Obstet Gynecol 128 127–137 https://doi.org/10.1097/AOG.0000000000001491
88. MacFarlane TV, Murchie P and Watson MC (2015) Aspirin and other non-steroidal anti-inflammatory drug prescriptions and survival after the diagnosis of head and neck and oesophageal cancer Cancer Epidemiol 39 1015–1022 https://doi.org/10.1016/j.canep.2015.10.030 PMID: 26590503
89. Sperling CD, Verdoodt F, and Aalborg G, et al (2019) Low-dose aspirin use and endometrial cancer mortality – a nationwide cohort study Pharmacoepidemiol Drug Saf 2 194–195
90. Kim SA, Roh JL, and Kim SB, et al (2017) Aspirin use and head and neck cancer survival: an observational study of 11,623 person-years follow-up Int J Clin Oncol 23(1) 52–58 https://doi.org/10.1007/s10147-017-1165-3
91. Hedberg ML, Peyser ND, and Bauman JE, et al (2019) Use of nonsteroidal anti-inflammatory drugs predicts improved patients survival for PIC3CAaltered head and neck cancer J Exp Med 216 419–427 https://doi.org/10.1084/jem.20181936 PMID: 30683736 PMCID: 6363423
92. Lumley CJ, Kaffenberger TM, and Desale S, et al (2018) Post-diagnostic aspirin use and survival in veterans with head and neck cancer Head Neck 41 1220–1226 https://doi.org/10.1002/hed.25518
93. Li JH, Wang Y, and Xie XY, et al (2019) Aspirin in combination with TACE in treatment of unresectable HCC: a matched-pair analysis Am J Cancer Res 6 2109–2116
94. Maddison P (2017) Effects of aspirin on small-cell lung cancer mortality and metastatic presentation Lung Cancer 106 67–69 https://doi.org/10.1016/j.lungcan.2017.01.018 PMID: 28285696
95. Erickson P, Gardner LD, and Loffredo CA, et al (2018) Racial and ethnic differences in the relationship between aspirin use and non-small cell lung cancer risk and survival Cancer Epidemiol Biomarkers Prev 27 1518–1526 https://doi.org/10.1158/1055-9965.EPI-18-0366 PMID: 30171037 PMCID: 6279562
96. Chuang, Hsieh M, and Tsai Y et al (2018) Survival benefit associated with aspirin use in inoperable non-small cell lung cancer patients: a population-based retrospective cohort study Am J Respir Crit Care Med 197 A6420
97. Spence AD, Busby J, and Johnson BT, et al (2017) Low-dose aspirin use does not increase survival in 2 independent population based cohort of patients with Esophageal or gastric cancer Gastroenterol 154 849–860 https://doi.org/10.1053/j.gastro.2017.10.044
98. Lyon TD, Frank I, and Shah PH, et al (2018) The association of aspirin use with survival following radical cystoscopy Urology 200 1014–1021 https://doi.org/10.1016/j.juro.2018.05.119
99. Rachidi S, Wallace K, and Li H, et al (2018) Postdiagnosis aspirin use and overall survival in patients with melanoma. J Am Acad Dermatol 78 949–956 https://doi.org/10.1016/j.jaad.2017.12.076 PMID: 29317280
100. Seliger C, Schaertl J, and Gerken M, et al (2018) Use of statins or NSAIDs and survival of patients with high grade glioma PLoS One 13 e0207858 https://doi.org/10.1371/journal.pone.0207858
101. Jackson S, Pleiffer RM, and Liu Z, et al (2019) Association between aspirin use and biliary tract cancer survival JAMA Oncol 5 1802–1804 https://doi.org/10.1001/jamaoncol.2019.4328 PMID: 31621809 PMCID: 6802421
102. Luo SD, Chen WC, and Wu CN, et al (2020) Low-dose aspirin use significantly improves the survival of late-stage NPC: a propensity score-matched conort study in Taiwan Cancers 12 1551 https://doi.org/10.3390/cancers12061551
103. Simon TG, Duberg AS, and Aleman S et al (2020) Association of aspirin with hepatocellular carcinoma and liver-related mortality N Engl J Med 382 1018–1028 https://doi.org/10.1056/NEJMoa1912035 PMID: 32160663 PMCID: 7317648
104. Rafei H, LumleyC, and Han J et al (2017) Aspirin use and survival in head and neck squamous cell cancer (HNC) patients J Clin Oncol 35 (15 suppl) 6042 https://doi.org/10.1200/JCO.2017.35.15_suppl.6042
105. Reimers MS, Bastiaannet E, and Langley RE, et al (2014) Expression of HLA class 1 antigen, aspirin use and survival after a diagnosis of colon cancer JAMA Intern Med 174 732–739 https://doi.org/10.1001/jamainternmed.2014.511 PMID: 24687028
106. van Staald-uinen J, Frouws M, and Reimers M, et al (2016) The effect of aspirin and non-steroidal anti-inflammatory drug use after diagnosis on survival of eosophageal cancer patients Br J Cancer 114 1053–1059 https://doi.org/10.1038/bjc.2016.65
107. Cardwell CR, Flahavan EM, and Hughes CM, et al (2014) Low-dose aspirin and survival in men with prostate cancer: a study using the UK clinical practice research datalink Cancer Causes Control 25 33–43 https://doi.org/10.1007/s10552-013-0306-x
108. Fontaine E, McShane J, and Page R, et al (2010) Aspirin and non-small cell lung cancer resections: effect on long-term survival Eur J Cardiothorac Surg 38 21–26 https://doi.org/10.1016/j.ejcts.2010.01.015 PMID: 20359903
109. Din FVN, Theodoratou E, and Farrington SM, et al (2010) Effect of aspirin and NSIADs on risk and survival from colorectal cancer Gut 59 1670–1679 https://doi.org/10.1136/gut.2009.203000 PMID: 20844293
110. Bowers LW, Maximo LXF, and Brenner AJ, et al (2014) NSAID use reduces breast cancer recurrence in overweight and obese women: role of prostaglandin-aromatase interactions Cancer Res 74 4446–4457 https://doi.org/10.1158/0008-5472.CAN-13-3603 PMID: 25125682
111. Murray LJ, Cooper JA, and Hughes CM, et al (2014) Post-diagnostic prescriptions for low-dose aspirin and breast cancer-specific survival: a nested case-control study in a breast cancer cohort from the UK clinical practice research datalink Breast Cancer Res 16 R34 https://doi.org/10.1186/bcr3638 PMID: 24708725 PMCID: 4053148
112. Pastore AL, Palleschi G, and Fuschi A, et al (2015) Can daily intake of aspirin and /or statins influence the behaviour of non-muscle invasive bladder cancer? A retrospective study on a cohort of patients undergoing transurethral bladder resection BMC Cancer 15 120 https://doi.org/10.1186/s12885-015-1152-x
113. Bandrup L. (2015) Drugs with potential chemopreventive properties in relations to epithelial ovarian cancer – a nationwide case-control study Dan Med J 62(7) B5117
114. Gupta R, Das RK, and Gupta S, et al (2017) Role of aspirin in patients with bladder cancer receiving intravesical BCG: a prospective observational study J Clin Diagn Res 11 PC01–PC04
115. Holmes MD, Olsson H, and Pawitan Y, et al (2014) Aspirin intake and breast cancer survival – a nationwide study using prospectively recorded data in Sweden BMC Cancer 14 391–398 https://doi.org/10.1186/1471-2407-14-391
116. Kwan ML, Habel LA, and Slattery ML, et al (2007) NSAIDs and breast cancer recurrence in a prospective cohort study Cancer Causes Control 18 613–612 https://doi.org/10.1007/s10552-007-9003-y PMID: 17404892 PMCID: 3461348
117. Albandar HJ, Markert R, and Agarawal S (2018) The relationship between aspirin use and mortality in colorectal cancer J Gastroentest Oncol 9 133–137
118. Pretzsch E, d’Haese JG, and Renz B, et al (2021) Effect of platelet inhibition with perioperative aspirin on survival in patients undergoing curative resection for pancreatic cancer: a propensity score matched analysis BMC Surg 21 98 https://doi.org/10.1186/s12893-021-01083-9 PMID: 33618686 PMCID: 7901208
119. Boas FE, Brown KT, and Ziv E, et al (2019) Aspirin is associated with improved liver function after embolization of hepatocellular carcinoma Am J Roentgenol 213 1–7 https://doi.org/10.2214/AJR.18.20846
120. National institute for Clinical Excellence (2019) Consider daily aspirin, to be taken for more than 2 years years, to prevent colorectal cancer in people with the Lynch syndrome [https://www.nice.org.uk/search?q=Aspirin+and+the+Lynch+Syndrome]
121. Chubak J, Kaminebi A, and Buist DSM et al (2015) Aspirin use for the prevention of colorectal cancer: an updated systematic evidence review for the U.S. preventive services task force. Evidence syntheses, No. 133 Kaiser Permanente Research Affiliates Evidence-based Practice Center, Rockville (MD): Agency for Healthcare Research and Quality; Report No.: 15-05228-EF-1); USSep 2015
122. Morgan G (2009) Aspirin for primary prevention of vascular events Public Health 123 787–788 https://doi.org/10.1016/j.puhe.2009.10.007 PMID: 19914670
123. Elwood P (2014) Aspirin prophylaxis: putting gut bleeds into perspective Gastroenterol Hepatol 10 61–62
124. Thorat MA and Cuzick J (2015) Prophylactic use of aspirin: systematic review of harms and approaches to mitigation in the general population Eur J Epidemiol 30 5–18 https://doi.org/10.1007/s10654-014-9971-7
125. Patrono C, Garcia Rodriguez LA, and Landolfi R, et al (2005) Low dose aspirin for the prevention of atherosclerosis N Engl J Med 353 2373–2383 https://doi.org/10.1056/NEJMra052717 PMID: 16319386
126. Elwood PC, Morgan G, and Galante J, et al (2016) Systematic review and meta-analysis of randomised trials to ascertain fatal gastrointestinal bleeding events attributable to preventive low-dose aspirin: no evidence of increased risk PLoS One 11 e0166166 https://doi.org/10.1371/journal.pone.0166166 PMID: 27846246 PMCID: 5113022
127. Antithrombotic Trialists’ Collaboration (2009) Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials Lancet 373 1849–1860 https://doi.org/10.1016/S0140-6736(09)60503-1 PMID: 19482214 PMCID: 2715005
128. Lanas A, Wu P, and Medin J, et al (2011) Low doses of acetylsalicylic acid increase risk of gastrointestinal bleeding in a meta-analysis Clin Gastroenterol Hepatol 9 762–768 https://doi.org/10.1016/j.cgh.2011.05.020 PMID: 21699808
129. McQuaid KR and Laine L (2006) Systematic review and meta-analysis of adverse events of low-dose aspirin and clopidogrel in randomised controlled trials Am J Med 119 624–638 https://doi.org/10.1016/j.amjmed.2005.10.039 PMID: 16887404
130. Wu C, Alotaibi GS, and Alsaleh K, et al (2015) Case-fatality of recurrent venous thromboenbolism and major bleeding associated with aspirin, warfarin, and direct oral anticoagulants for secondary prevention Thromb Res 135 243–248 https://doi.org/10.1016/j.thromres.2014.10.033
131. Mahady SE, Margolis KL, and Chan A, et al (2020) Major gastrointestinal bleeding in older persons using aspirin: incidence and risk factors in the ASPREE randomised controlled trial Gut 70(4) 8
132. Cuzick J, Thorat MA, and Bosetti C, et al (2015) Estimates of benefits and harms of prophylactic use of aspirin in the general population Ann Oncol 26(1) 47–57 https://doi.org/10.1093/annonc/mdu225 PMCID: 4269341
133. Hansson L, Zanchetti A, and Carruthers SG, et al (1998) Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the hypertension optimal treatment (HOT) trial. HOT study group Lancet 351 1755–1762 https://doi.org/10.1016/S0140-6736(98)04311-6 PMID: 9635947
134. Murphy L, Brown C, & Smith A, et al (2019) End-of-life prescribing of aspirin in patients with breast ot colorectal cancer Support Palliat Care 9 e6 https://doi.org/10.1136/bmjspcare-2017-001370
135. Dixon SC, Nagle CM, and Wentzensen N, et al (2017) Use of common analgesic medications and ovarian cancer survival: results for a pooled analysis in the overian cancer association consortium Br J Cancer 116 1223–1228 https://doi.org/10.1038/bjc.2017.68 PMID: 28350790 PMCID: 5418444
136. Sturgeon KM, Deng L, and Bluethmann SM, et al (2019) A population based study of cardiovascular disease mortality risk in US cancer patients Eur Heart J 40 3889–3897 https://doi.org/10.1093/eurheartj/ehz766 PMID: 31761945 PMCID: 6925383
137. Elwyn G, Tilburt J and Montori VM (2012) The ethical imperative for shared decision making Eur J Person Centred Healthc 1 129–131 https://doi.org/10.5750/ejpch.v1i1.645
138. Chan M (2010) Cancer in developing countries: facing the challenge [https://www.who.int/dg/speeches/2010/iaea_forum_20100921/en/]
Supplementary materials
Supplementary File 1. Search strategy.
SUPPLEMENTARY FILE 1
ASPIRIN AND CANCER SURVIVAL IA systematic review and meta-analyses of 118 observational studies of aspirin and 18 cancers
Peter C Elwood, Gareth Morgan, Christine Delon, Majd Protty, Julieta Galante, Janet Pickering, John Watkins, Alison Weightman, Delyth Morris
Search strategy
Search strategy developed using the following search filters for study design.
• Observational studies: SIGN filter (http://www.sign.ac.uk/methodology/filters.html#obs)
• Randomised controlled trials: Cochrane highly sensitive search filter (http://handbook.cochrane.org/chapter_6/box_6_4_c_cochrane_hsss_2008_sensmax_ovid.htm)
MEDLINE and Medline in Process (searched 11 March 2020)
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. randomized.ab.
4. placebo.ab.
5. drug therapy.fs.
6. randomly.ab.
7. trial.ab.
8. groups.ab.
9. or/1-8
10. Epidemiologic studies/
11. exp case control studies/
12. exp cohort studies/
13. Case control.tw.
14. (cohort adj (study or studies)).tw.
15. Cohort analy*.tw.
16. (Follow up adj (study or studies)).tw.
17. (observational adj (study or studies)).tw.
18. Longitudinal.tw.
19. Retrospective.tw.
20. Cross sectional.tw.
21. Cross-sectional studies/
22. or/10-21
23. 9 or 22
24. exp animals/ not humans.sh.
25. 23 not 24
26. Exp neoplasms/
27. (cancer* or malign* or tumour* or tumor*).tw
28. 26 or 27
29. Aspirin/
30. (aspirin* or “acetylsalicylic acid”).tw
31. 29 or 30
32. 25 and 28 and 31
33. Limit 32 to ed”20180529-20200311”
EMBASE (searched 11 March 2020)
1. Random*.tw
2. Clinical trial*.mp
3. Exp health care quality/
4. placebo.ab.
5. or/1-4
6. clinical study/
7. case control study/
8. family study/
9. longitudinal study/
10. retrospective study/
11. prospective study/
12. Cohort analysis/
13. (Cohort adj (study or studies)).mp.
14. (Case control adj (study or studies)).tw.
15. (follow up adj (study or studies)).tw.
16. (observational adj (study or studies)).tw.
17. (epidemiologic* adj (study or studies)).tw.
18. (cross sectional adj (study or studies)).tw.
19. or/6-18
20. 5 or 19
21. exp animal/ not human.sh.
22. 20 not 21
23. Exp neoplasms/
24. (cancer* or malign* or tumour* or tumor*).tw
25. 23 or 24
26. Aspirin/
27. (aspirin* or “acetylsalicylic acid”).tw
28. 26 or 27
29. 22 and 25 and 28
30. Limit 29 to “year = Aug 2017 to March 2020”
Supplementary File 2. Details of the new studies included in the review.
A systematic review and meta-analyses of 118 observational studies of aspirin and 18 cancers
Peter C Elwood, Gareth Morgan, Christine Delon, Majd Protty, Julieta Galante, Janet Pickering, John Watkins, Alison Weightman, Delyth Morris
SUPPLEMENTARY FILE 2
Description of papers identified in the 2021 literature search.
Those which reported Hazard Ratios (HRs) are shown first, followed by those which reported RRs, OR, etc.
Details of papers identified in the literature searches in 2016 are given in Elwood et al [7] and those identified in the 2018 search are given in Elwood et al [20].
1. Studies in which results are reported as hazard ratios
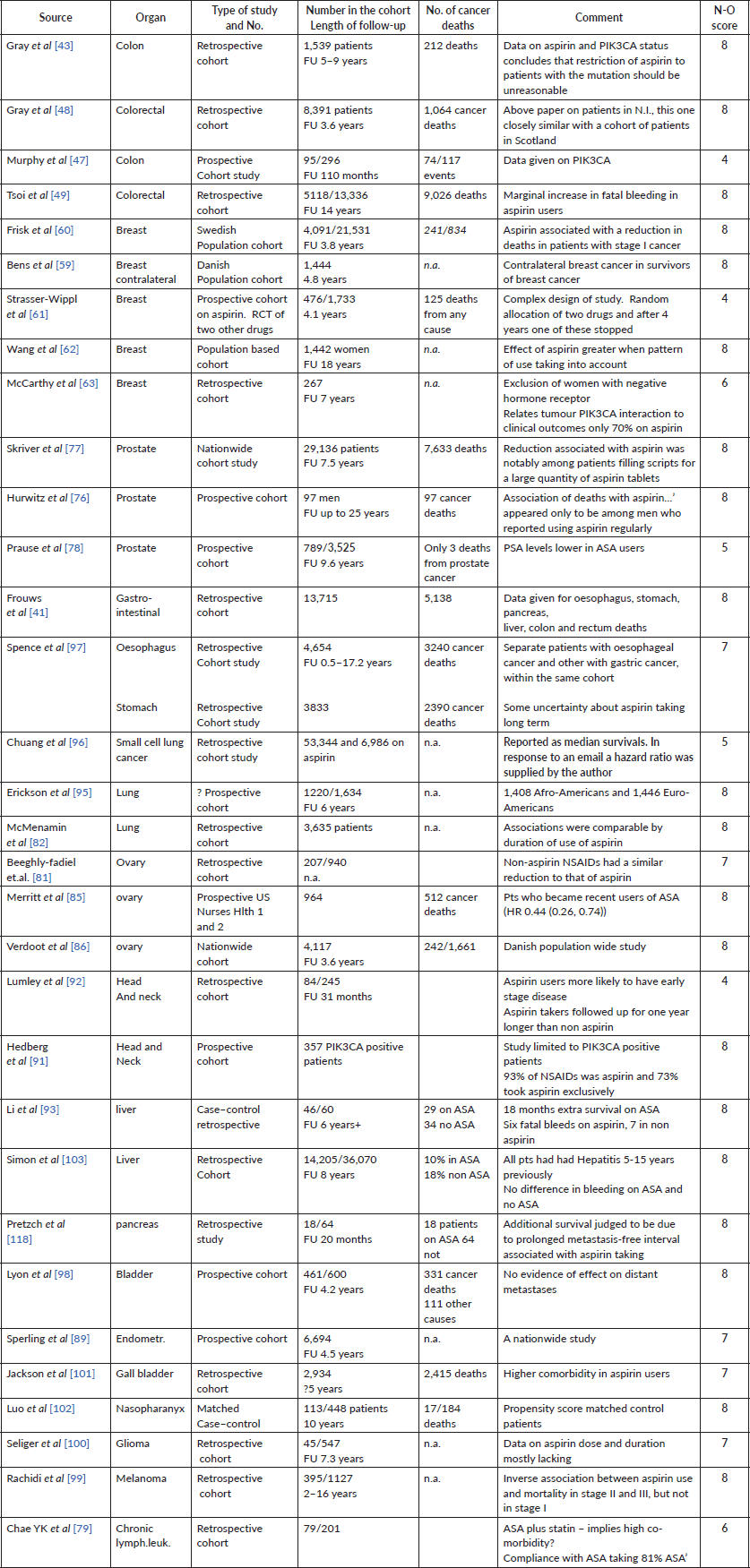
Reports on ‘other’ cancers, described in our report published in 2016 (Elwood et al [7]):
[83] Nagle et al (2015) Ovarian cancer
[108] Fontaine et al (2010) Lung cancer
[112] Pastore et al (2015) Bladder cancer
[80] Chae et al (2013) Mix of female cancers
[79] Chae et al (2014) Lymphocytic cancer
[88] McFarlaine et al (2015) Head and neck
Reports on ‘other’ cancers, described in our report published in 2018 (Elwood et al [20]):
[84] Bar et al (2016) Ovarian cancer
[87] Matuso et al (2016) Endometrium
[93] Li et al (2016) Liver cancer
[39] Veitonmaki et al (2016) Lung
[94] Maddison et al (2017 Lung
[90] Kim et al (2018) Head and neck
2. Studies in which results are reported as RRs, ORs, etc.
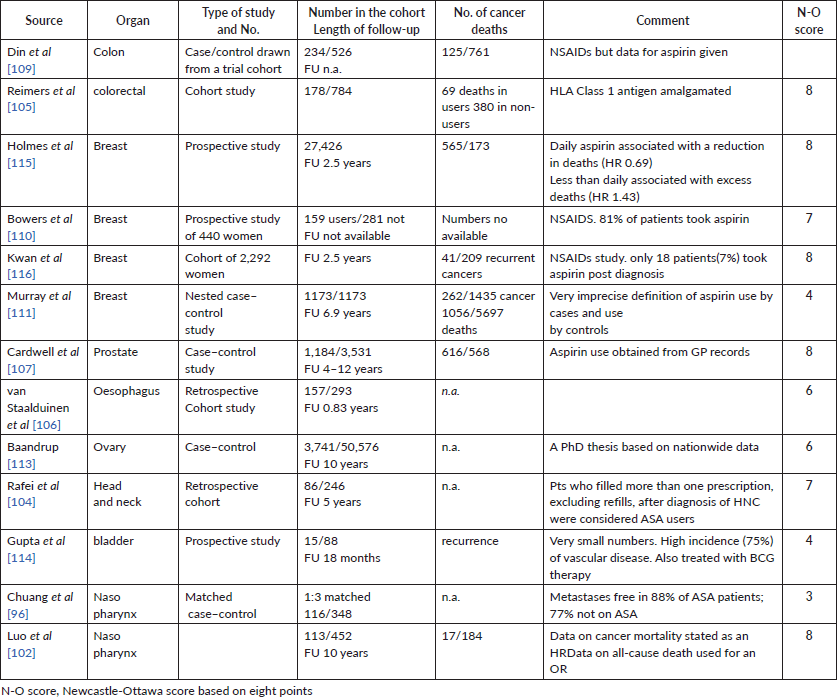
Supplementary File 3. Results: estimates of association: aspirin and mortality.
A systematic review and meta-analyses of 118 observational studies of aspirin and 18 cancers
Peter C Elwood, Gareth Morgan, Christine Delon, Majd Protty, Julieta Galante, Janet Pickering, John Watkins, Alison Weightman, Delyth Morris
SUPPLEMENTARY FILE 3
Results of studies identified in the 2021 literature search.
Those which reported Hazard Ratios (HRs) are shown first, followed by those which reported RRs , OR, etc.
Results of studies identified in the literature searches in 2016 are given in Elwood et al [7] and those identified in the 2018 search are given in Elwood et al [20]
1. Studies in which results are reported as hazard ratios
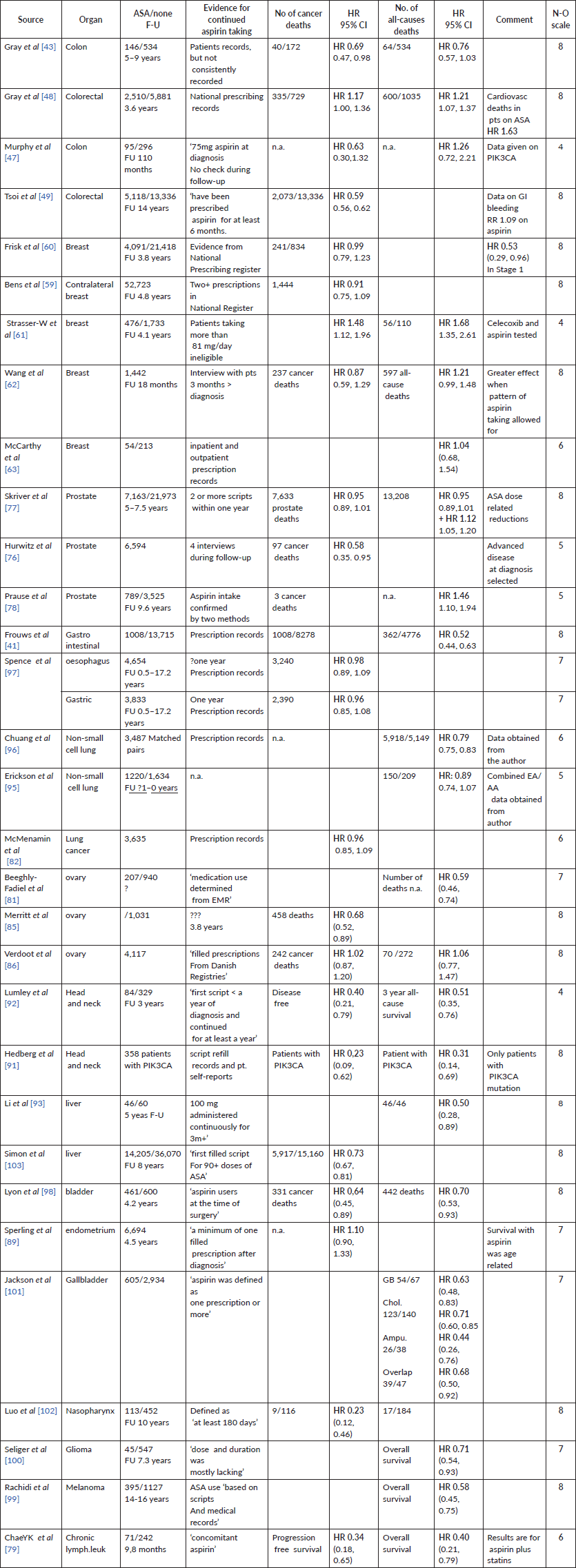
Papers on ‘other’ cancers, described in our report published in 2016 (Elwood et al [7]):
Nagel et al [83] (2015)
Fontaine et al [108] (2010)
Pastore et al [112] (2015)
Chae et al [80] (2013)
Chae et al [79] (2014)MacFarlane et al [88] (2015)
Papers on ‘other’ cancers, described in our report published in 2018 (Elwood et al [20]):
Bar et al [84] (2016)
Matuso et al [87] (2016)
Li et al [93] (2016)
Veitonmaki et al [39] (2015)
Maddison et al [94] (2017)
Kim et al [90] (2017)
2. Studies in which results are reported as RRs, ORs, etc.
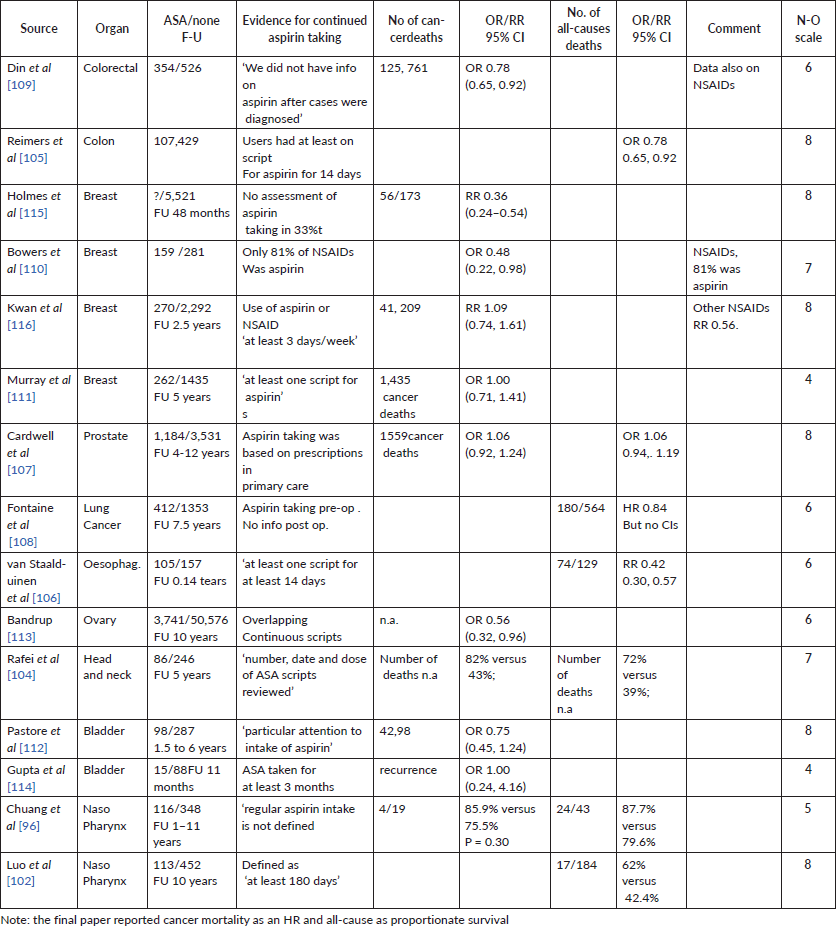
Supplementary File 4. Forest plots for estimates of aspirin and mortality as HRs.
Supplementary file 4
ASPIRIN AND CANCER SURVIVAL I.
A systematic review and meta-analyses of 117 observational studies of aspirin and 18 cancers
Peter C Elwood, Gareth Morgan, Christine Delon, Majd Protty, Julieta Galante, Janet Pickering, John Watkins, Alison Weightman, Delyth Morris
Five forest plots of aspirin and deaths from cancer: Colon, breast, prostate, other cancers, all cancers
Five forest plots of aspirin and deaths from all-causes: Colon, breast, prostate, other cancers, all cancers
Aspirin and Colon Cancer Mortality
Aspirin and Breast Cancer Mortality
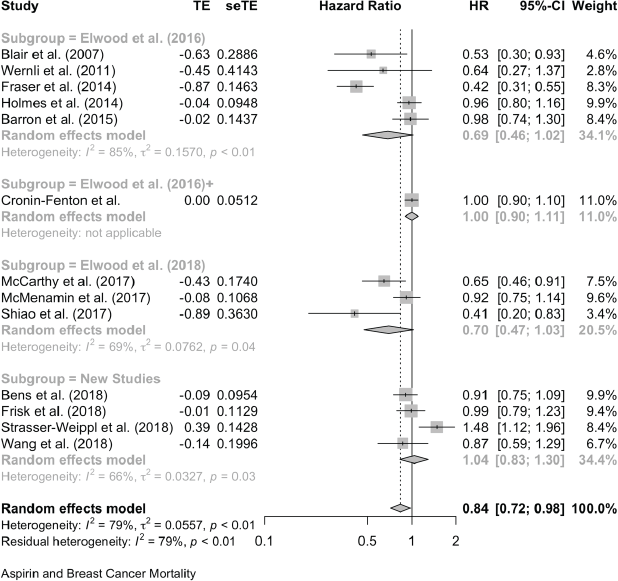
Aspirin and Prostate Cancer Mortality
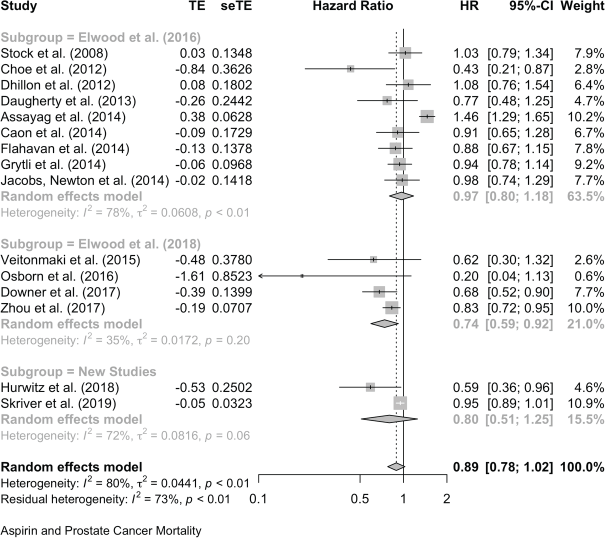
Aspirin and Cancer Mortality in Other Cancers
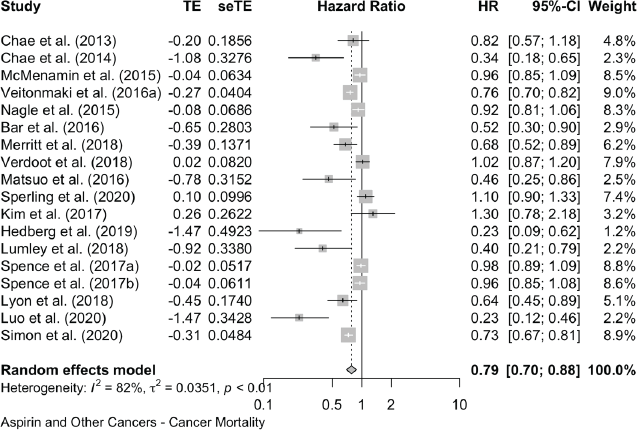
Aspirin and Cancer Mortality in All Cancers Combined
Aspirin and Colon Cancer, All-Cause Mortality
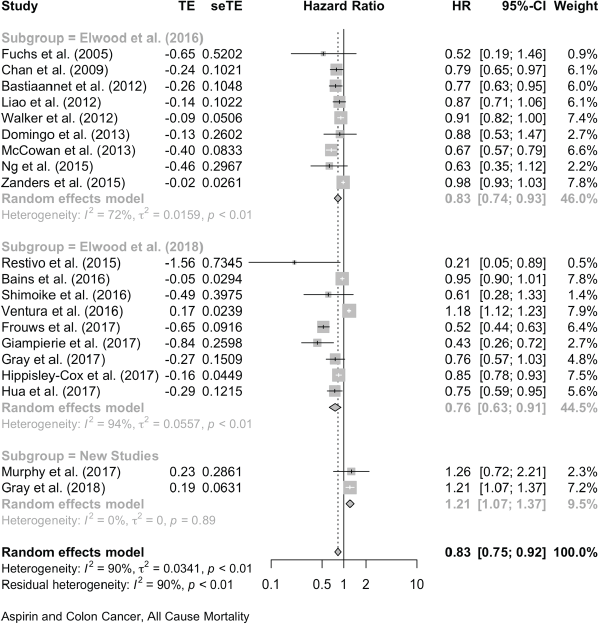
Aspirin and Breast Cancer, All-Cause Mortality
Aspirin and Prostate Cancer, All-Cause Mortality
Aspirin and All-Cause Mortality in Other Cancers
Aspirin and All-Cause Mortality in All Cancers
Supplementary File 5. Forest plots for aspirin and mortality as ratios other than HR.
ASPIRIN AND CANCER SURVIVAL I.
A systematic review and meta-analyses of 114 observational studies of aspirin and 18 cancers
Peter C Elwood, Gareth Morgan, Christine Delon, Majd Protty, Julieta Galante, Janet Pickering, John Watkins, Alison Weightman, Delyth Morris
Supplementary file 5
Associations reported a ORs RRs and percentage survival all converted to ORs
Cancer-specific mortality
All cancers
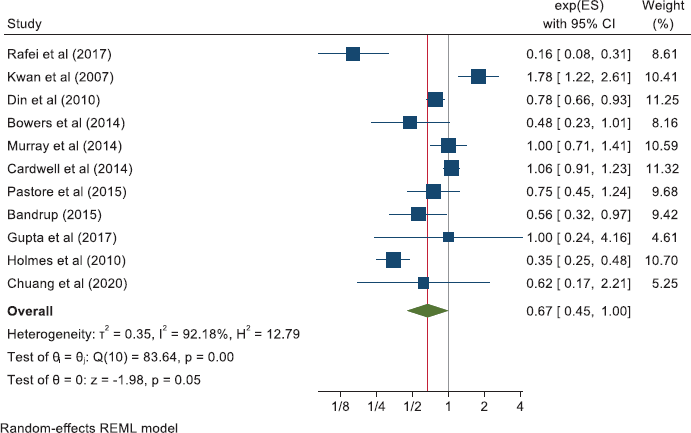
Other cancers
Breast cancer
All-cause mortality
All cancers
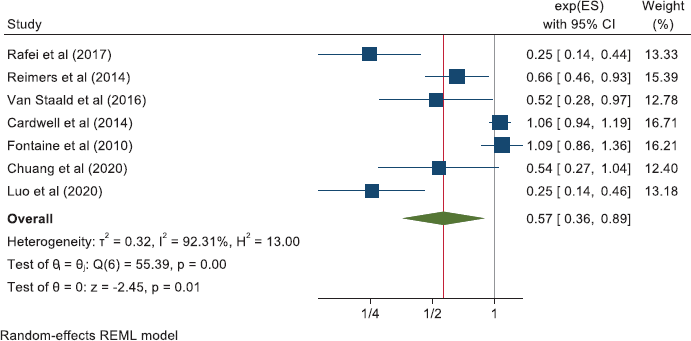
Other cancers

Supplementary File 6. Estimates of publication bias.
Publication Bias, Funnel plots
All cancers combined
All cancers combined mortality
13 cases added with Trim and fill
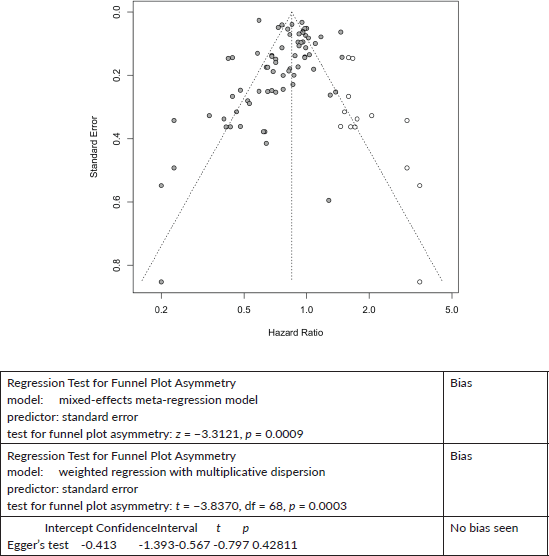
All Cancers Combined Cancer mortality:
Trim and Fill and Cumulative forest plot ranked by SE with trim and fill.
Forest plot in order of SE with trim and fill mirrored studies added below.
Cumulative forest plot ranked by SE with Trim and fill at end.

Published value 0.79 (0.73, 0.84)
Results with trim and fill 0.85 (0.79, 0.91)
Results are robust with trim and fill.
All Cancers Combined All-Cause Mortality
19 cases added with Trim and fill
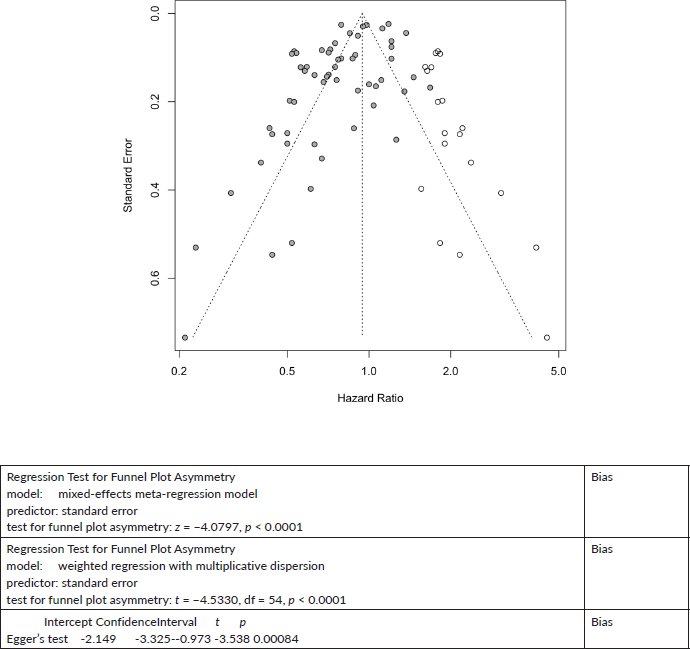
All Cancers Cancer All-Cause mortality:
Trim and Fill and Cumulative forest plot ranked by SE with trim fill
Forest plot ranked by SE with trim and fill mirrored studies added below.
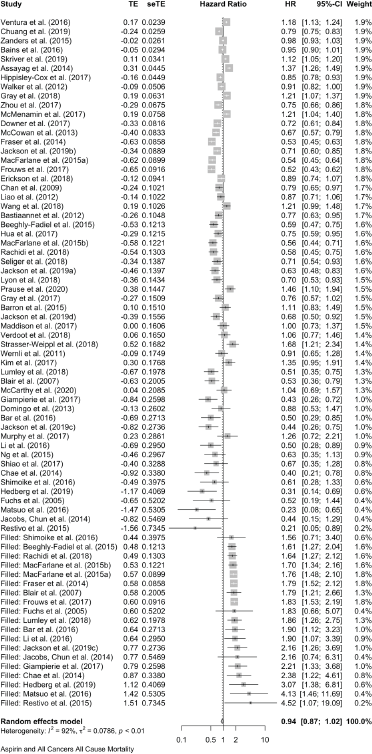
Cumulative forest plot ranked by SE with Trim and fill at end.

Published value 0.80 (0.74, 0.86)
Results with trim and fill 0.94 (0.87, 1.02)
Results are not robust with trim and fill.
Breast Cancer
Breast Cancer Mortality
Six cases added with Trim and fill
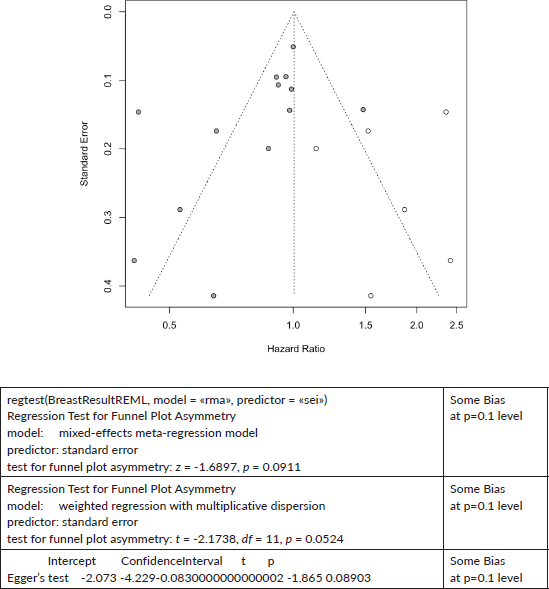
Breast Cancer Mortality:
Trim and Fill and Cumulative forest plot ranked by SE with trim fill
Forest plot ranked by SE with trim and fill mirrored studies added below.
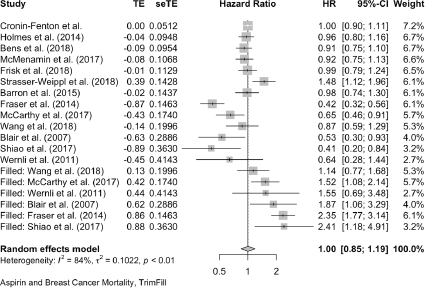
Cumulative forest plot ranked by SE with Trim and fill at end.
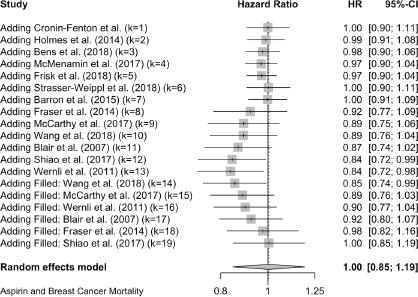
Published value 0.84 (0.72, 0.98)
Results with trim and fill 1.00 (0.85, 1.19)
Results are not robust with trim and fill.
Breast Cancer All-Cause Mortality
Zero cases added with Trim and Fill
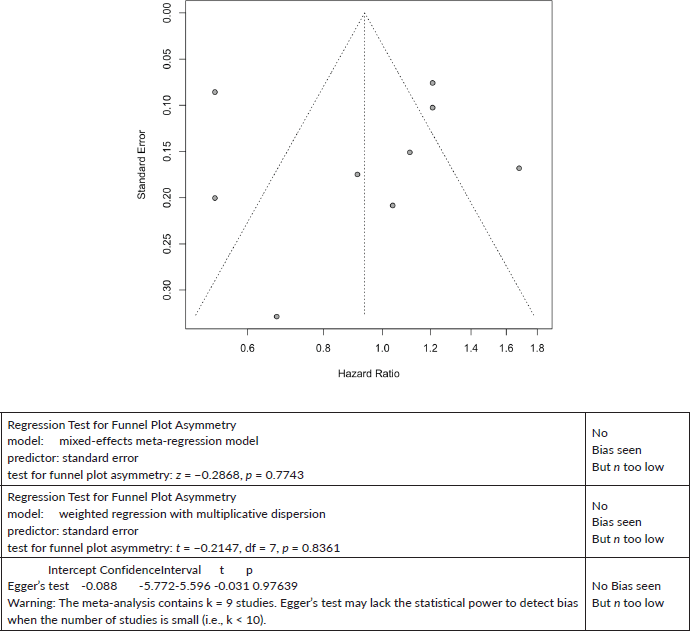
Breast Cancer All-Cause mortality:
Trim and Fill and Cumulative forest plot ranked by SE with trim fill
Forest plot ranked by SE.
No Trim and fill as no cases added.
Cumulative forest plot ranked by SE. No Trim and fill.
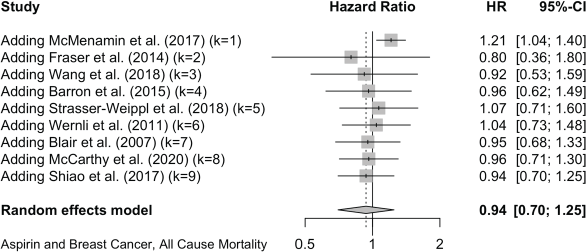
Published value 0.94 (0.70, 1.25)
The same
Non-effect is robust with trim and fill.
Colon Cancer
Colon Cancer Mortality,
No cases added with Trim and fill
Colon Cancer mortality: Cumulative forest plot ranked by SE.
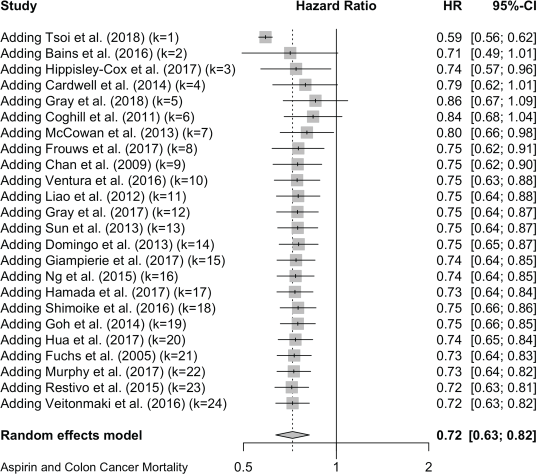
Published value 0.72 (0.63, 0.82)
Colon results are robust.
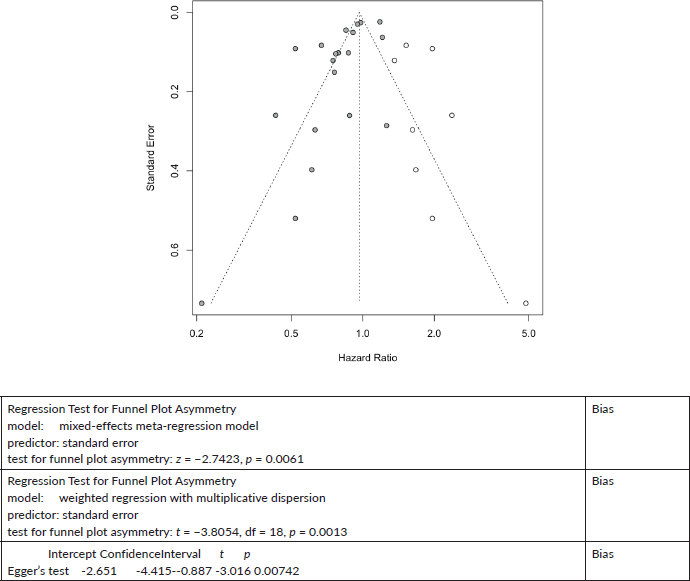
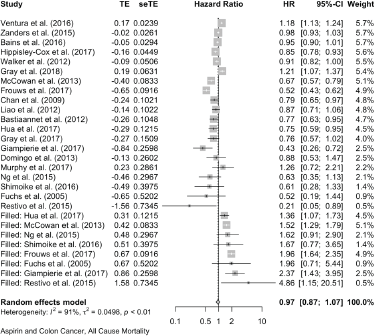
Colon Cancer All-Cause Mortality
Eight cases added with Trim and fill
Colon Cancer All-Cause mortality:
Trim and Fill and Cumulative forest plot ranked by SE with trim fill
Forest plot ranked by SE with trim and fill mirrored studies added below.
Cumulative forest plot ranked by SE with Trim and fill at end.
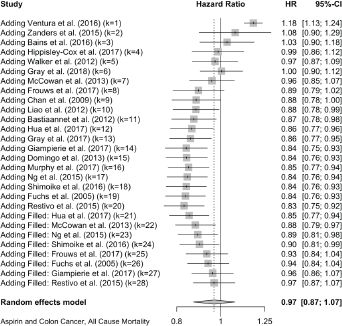
Published value 0.83 (0.75, 0.92)
Results with trim and fill 0.97 (0.87, 1.07)
Results are not robust with trim and fill.
Other Cancers
Other Cancers Mortality
Five cases added with Trim and fill
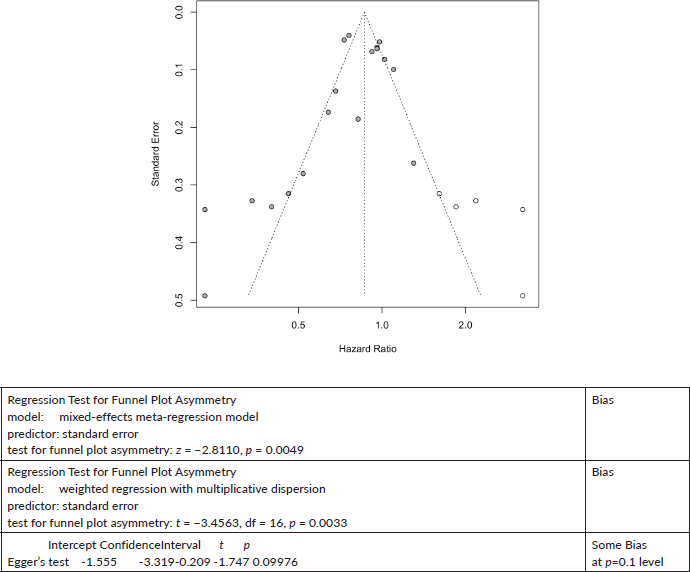
Other Cancers Cancer mortality:
Trim and Fill and Cumulative forest plot ranked by SE with trim fill
Forest plot ranked by SE with trim and fill mirrored studies added below.
Cumulative forest plot ranked by SE with Trim and fill at end.
Published value 0.79 (0.70, 0.88)
Results with trim and fill 0.86 (0.77, 0.98)
Results are robust with trim and fill.
Other Cancers All-Cause Mortality
Seven cases added with Trim and fill.
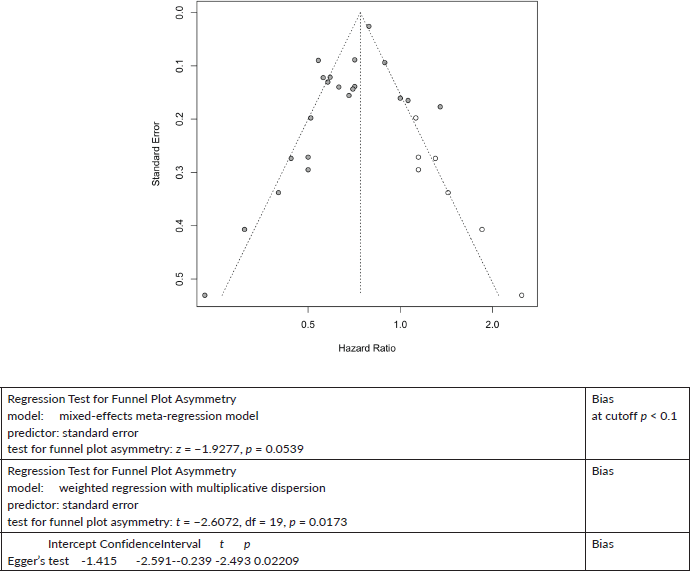
Other Cancers All-Cause Mortality
Trim and Fill and Cumulative forest plot ranked by SE with trim fill
Forest plot ranked by SE with trim and fill mirrored studies added below.
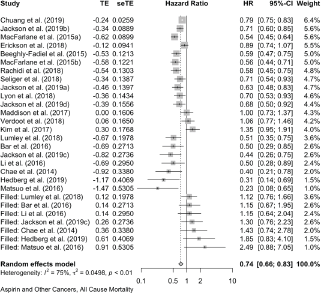
Cumulative forest plot ranked by SE with Trim and fill at end.
Published value 0.67 (0.60, 0.75)
Results with trim and fill 0.74 (0.66, 0.83)
Results are robust with trim and fill.
Prostate Cancer
Prostate Cancer Mortality
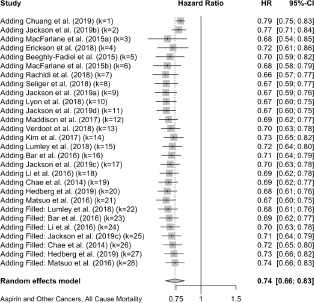
Six cases added with Trim and fill.
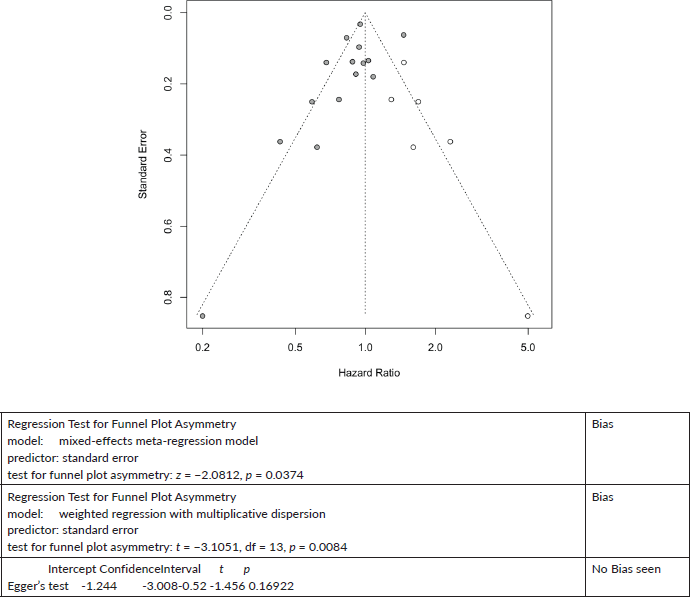
Prostate Cancer mortality
Trim and Fill and Cumulative forest plot ranked by SE with trim fill
Forest plot ranked by SE with trim and fill mirrored studies added below.
Cumulative forest plot ranked by SE with Trim and fill at end.
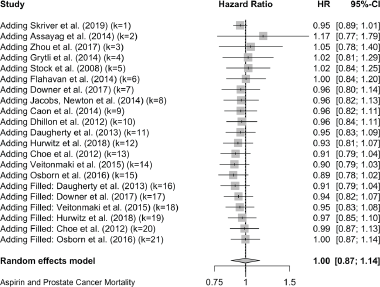
Published value 0.89 (0.78, 1.02)
Results with trim and fill 1.00 (0.87, 1.14)
Non-effect is robust with trim and fill.
Prostate Cancer All-Cause Mortality
Two cases added with Trim and Fill
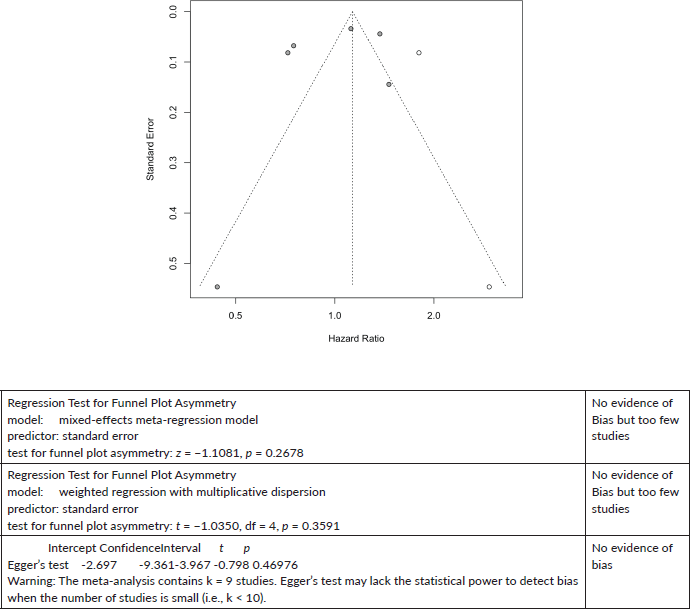
Prostate Cancer All-Cause mortality:
Trim and Fill and Cumulative forest plot ranked by SE with trim fill
Forest plot ranked by SE with trim and fill mirrored studies added below.
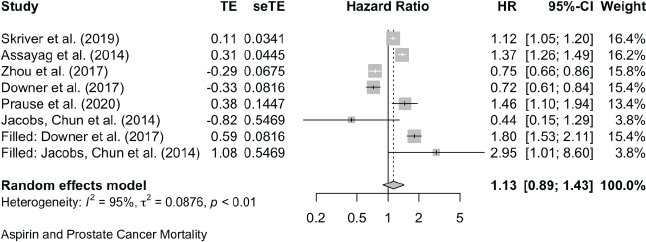
Cumulative forest plot ranked by SE with Trim and fill at end.
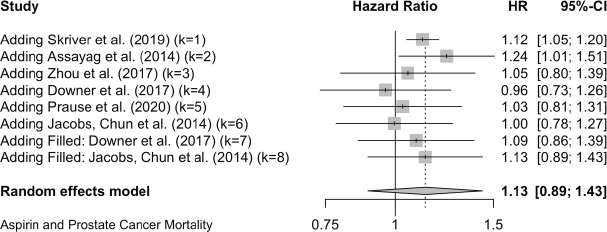
Published value 1.00 (0.78, 1.27)
Results with trim and fill 1.13 (0.88, 1.43)
Non-effect is robust with trim and fill.
Egger’s test
On ORs for 10+ studies of cancer-specific mortality meta-analysis for all cancers
Regression-based Egger test for small-study effects
Random-effects model
Method: REML
H0: beta1 = 0; no small-study effects
beta1 = -0.79
SE of beta1 = 1.180
z = -0.67
Prob > |z| = 0.5011 (this is the p value)
No evidence of publication bias.

