Antibiotics, cancer risk and oncologic treatment efficacy: a practical review of the literature
Moises S Martins Lopes1, Larissa M Machado1, Pedro A Ismael Amaral Silva1, Angel A Tome Uchiyama1, Cheng T Yen1, Eliza D Ricardo1, Taciana S Mutao1, Jefferson R Pimenta1, Denis S Shimba1, Rodrigo M Hanriot1 and Renata D Peixoto2
1Hospital Alemão Oswaldo Cruz, São Paulo, Brazil
2Centro Paulista de Oncologia (Grupo Oncoclínicas), São Paulo, Brazil
Abstract
Antibiotics have been extensively used to treat infectious diseases over the past century and have largely contributed to increased life expectancy over time. However, antibiotic use can impose profound and protracted changes to the diversity of the microbial ecosystem, affecting the composition of up to 30% of the bacterial species in the gut microbiome. By modifying human microbiota composition, antibiotics alter the action of several oncologic drugs, potentially leading to decreased efficacy and increased toxicities. Whether antibiotics interfere with cancer therapies or even increase the risk of cancer development has been under investigation, and no randomised trials have been conducted so far. The aim of the current review is to describe the possible effects of antibiotic therapies on different oncologic treatments, especially immunotherapies, and to explore the link between previous antibiotics use and the development of cancer.
Keywords: antibiotics, microbiome, cancer, immunotherapy, chemotherapy
Correspondence to: Renata D’Alpino Peixoto
Email: renatadalpino@gmail.com
Published: 21/09/2020
Received: 09/06/2020
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
More than 100 years ago, infectious diseases accounted for the highest morbidity and mortality worldwide [1]. The discovery of penicillin in 1928 by Alexander Fleming marked the beginning of the antibiotic revolution, which led to its wide use since 1945 and changed the landscape of infectious disease in many countries [2]. During decades, we have been witnessing a continuous growth of antibiotics use, and only from 2000 to 2015, antibiotic consumption increased by 65% [3].
Antibiotic therapy has produced unquestionable advances in the management of patients, especially those with cancer, a population with an intrinsically higher risk of bacterial infection as a result of malignancy or treatment-related immune suppression. Amongst oncologic patients, antibiotic consumption has also escalated over time, and whilst contributing to reduced mortality from several infections, it certainly impacts negatively on commensal bacterial species which live on or in individuals, also known as microbiota [4]. These organisms and their genes, metabolites and interactions with one another, as well as with their host collectively, represent the microbiome [4].
Changes in the microbiome as a result of antibiotic treatment, especially gut microbiota dysbiosis, may result in the dysregulation of host immune homeostasis and increased susceptibility to several diseases, including cancer [5]. Whether antibiotics interfere with cancer therapies or even increase the risk of cancer development has been under investigation, and no randomised trials have been conducted so far.
The aim of the current review is to describe the possible effects of antibiotic therapies on different oncologic treatments, especially immunotherapies, and to explore the link between previous antibiotics use and the development of cancer. For this purpose, a PubMed search was conducted, and the articles exploring the effects of antibiotics on human microbiome as well as those including the potential risk of cancer development with antibiotics’ use and their influence on cancer therapeutics were selected and revised.
Influence of antibiotic therapy on the microbiome
The microbiome has been denominated the second genome and is composed by bacteria, archaea, fungi and viruses, reaching a much larger number of cells and genes than those derived from the human gametes [6]. Typically, there are three categories of gut bacteria based on their functions in the host: symbionts, with mutual benefit to the host; conditioned pathobionts, normally harmless but producing disease due to fortuitous events that affect the host; and pathobionts, disease-causing organisms [7–9]. The human microbiomes are divided into four major phyla: Firmicutes (65%), Bacteroidetes (16%), Actinobacteria (9%) and Proteobacteria (5%) [10].
This complex ecosystem integrates gut microbiota and has a profound effect on all aspects of human health, such as protecting the host against pathogenic bacteria, promoting digestion, absorption, drug and carcinogens’ metabolism, regulating energy metabolism [11–17] and exerting a key role in inflammation pathways and the regulation of innate and adaptive immunological processes [18]. A healthy child’s microbiome increases in diversity in parallel with the maturation of the immune system until the age of two or three, resembling an adult microbiome [19]. Indeed, aberrant neonatal microbiota composition is associated with disease during childhood and later in life [20]. The proper function of the gut microbiome depends on a delicate homeostasis that can be disturbed by the action of many factors, such as age, diet composition, antibiotic therapies, lifestyle and physical activity, exerting an impact on gut microbiome and equilibrium [21, 22]. The disruption of this equilibrium is known as dysbiosis.
Antibiotics have been extensively used to treat infectious diseases over the past century and largely contributed to increased life expectancy over time. However, high doses and frequent use of antibiotics, particularly against anaerobes (such as vancomycin), can disrupt and destabilise the orderly bowel microbiome with complex repercussions to the tumour–host–microbe interface [23]. Antibiotics impose profound and protracted changes to the diversity of the host–microbial ecosystem, affecting the composition of up to 30% of the bacterial species in the gut microbiome, consequently leading to the loss of microbial functions that are protective for the host [24]. By modifying human microbiota composition, antibiotics alter the action of several oncologic drugs, potentially leading to decreased efficacy and increased toxicities [25].
Drug metabolism by intestinal microorganisms has been well recognised since the 1960s’[26]. However, we are still lacking a complete map of microbiota–host–drug interactions in cancer therapy [25]. Indeed, chemotherapeutic agents may exacerbate dysbiosis instead of ameliorating it, with potentially serious implications for drug tolerability [25]. In addition, oncologic drugs are known to induce changes in the diversity of the mucosal and faecal microbiota through altered biliary excretion and secondary metabolism [25].
Immunomodulation is also an important mechanism which occurs in the gut microbiota and leads to several treatment‐induced immune and inflammatory responses [25]. Lactobacillus and segmented filamentous bacteria, for instance, mediate the accumulation of type 17 T-helper (TH17) cell and type 1 T-helper (TH1) cell responses [27]. As a consequence, pathologic species might predominate, leading to deleterious diarrhoea and/or colitis [25]. This is the main physiopathology mechanism involved in the case of immunotherapy, in which the direct effect of antibiotics could induce selective pressure within the host microbiome and transform microbiota by the downregulation of major histocompatibility complex class I/II genes and impaired effector T-cell responses, which are implicated in reduced responsiveness to immunotherapy [25, 28, 29].
It has also been recently suggested that some species of bacteria provide intrinsic immune-modulating properties [29]. Bacteroidetes phylum, for example, appears to have a protective effect against checkpoint inhibitor-induced colitis [30]. Overall, CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) inhibition requires the presence of specific bacteria to work, whereas anti-PD-1 drugs seem to interact only partially with gut microbiota [31].
On the other hand, immunotherapy can increase the number of potentially dangerous bacterial species. Specifically, it may increase the number of Clostridiales whilst decreasing the number of Bacteroidales and Burkholderiales, which could affect the response to cancer therapy [28]. With all these examples, it is not difficult to understand how antibiotic-induced changes in the microbiota may affect cancer treatment efficacy and toxicity.
Cancer risk with antibiotics
Relevant publications have raised the hypothesis that certain drugs are associated with carcinogenesis [32, 33] and that the regular use of antibacterial drugs may be associated with cancer development [34]. According to a recent meta-analysis of 25 observational case–control or cohort studies, there is moderate evidence that the prolonged or excessive use of antibiotics during a person’s life is associated with a slight increased risk of various types of cancers [35]. Besides, a nested case–control study for 15 common malignancies revealed that a recurrent exposure to certain antibiotics frequently used in the community may be associated with cancer risk in specific organ sites [35, 36]. Since antibiotics have no known direct carcinogenic effect, the main hypothesis for the increased cancer risk focuses on their influence on the composition of the human microbiome, which involves the bacteria that compose the microbiota, their genes, metabolites and interactions with one another, as well as with their host collectively, including the immune system [4, 37].
In the aforementioned meta-analysis, the primary outcome was the risk of developing cancer in ever versus non-antibiotic users amongst 7,947,270 individuals. On the primary analysis of overall cancer incidence, the previous exposure to antibiotics increased the risk of cancer by 18% (odds ratio (OR): 1.18, p < 0.001), which was particularly increased for the following primary tumours: lung cancer (OR 1.29, p = 0.02), renal cell carcinoma (OR 1.28, p = 0.001), pancreatic cancer (OR 1.28, p = 0.019), lymphomas (OR 1.31, p < 0.001) and multiple myeloma (OR 1.36, 95% CI 1.18–1.56, p < 0.001). Higher risks were found in individuals with either a long duration of antibiotic exposure or higher doses [35]. In addition, the antibiotic classes with the strongest significant association with cancer development were beta-lactams, macrolides and quinolones [35].
Aside from intestinal microbiota, the association of local microbiota and some cancer types has been investigated, linking local dysbiosis and carcinogenesis [38–41]. For lung cancer, chronic inflammation linked to altered lung microbiota could explain local carcinogenesis [38]. Although the lungs were once considered sterile organs, a low-density, the diversified microbial ecosystem is currently known to be present in bronchoalveolar lavage fluid, sputum and lung tissues. Furthermore, several bacteria species have been shown to be enriched in lung cancer patients compared with healthy individuals [38]. Modifications in lung microbiota induced by antibiotics might explain the higher incidence of lung cancer amongst the users of antibiotics in the aforementioned meta-analysis [35].
Similar data were published for genitourinary and pancreatobiliary cancer as well as for lymphomas [39–42]. The urinary tract is also a host of an array of bacteria in healthy individuals, and the changes in this microbiome have been observed in certain urologic disorders, including cancers [39, 40]. Similarly, the liver, biliary tract and pancreas may be susceptible to altered local microbiota. In addition, those tissues are constantly exposed to the gut microbiome via blood flow through the portal vein, inferring that gut microbiome may influence hepatobiliary-pancreatic diseases through this gut–liver axis [41]. For lymphomas, the close interaction between the immune system and the intestinal microbiota might explain the development of some hematologic malignancies, such as mucosal-associated lymphoid tissue lymphoma [42].
Colonic microbiota is more commonly composed of anaerobic bacteria. Therefore, differently from other tumour types, whose relationship is more intense with intravenous antibiotics, sporadic colorectal cancer has a stronger association with oral anti-anaerobic antibiotics. Meanwhile, colorectal cancer tissues are enriched with polymicrobial invasive biofilms, particularly on right-sided tumours. Thus, the greater antibiotic impact on the proximal colon may reflect the disruption of biofilm formation, which has been linked with pro-carcinogenesis [43–50]. The most common class of antibiotics related to colon cancer development is anti-anaerobic, and the main one is penicillin [48, 50].
On the contrary, the risk of rectal cancer has been curiously reduced with the use of tetracyclines [49, 51]. Several studies report their anti-inflammatory and potential antineoplastic effect [52, 53]. Possible biological mechanisms contributing to diminished neoplastic risk from antibiotic exposure include the inhibition of mitochondrial protein synthesis, matrix metalloproteinases and/or angiogenesis. In addition, antibiotics can eradicate pathogens (such as those causing sexually transmitted diseases) that may contribute to malignant transformation [53, 54].
Antibiotics and cancer treatment
In the latter years, immune checkpoint inhibitors (ICI) became incontestable breakthrough advance in cancer treatment, particularly for solid tumours. There is increasing evidence that gut dysbiosis due to antibiotics exposure has an interference with immune responsiveness [55].
In a multicentre prospective study, a total of 196 patients (119 of them with non-small cell lung cancer (NSCLC), 38 with melanoma and 39 with other tumour types) were treated with ICI [56] and had their OS and response evaluated according to the prior or concurrent use of antibiotics. Respiratory tract infection was the most common indication for antibiotics exposure and possibly relates to the majority of patients having NSCLC. Prior antibiotic therapy was significantly associated with worse median OS compared to no prior antibiotics (2 versus 26 months; HR 7.4, p < 0.001). There was also a much higher likelihood of primary disease refractory to ICI therapy (81 versus 44%, respectively; p < 0.001). Interestingly, concomitant antibiotic therapy was not associated with worse outcomes. The negative impact of prior antibiotic use with ICI was observed across all tumour types and was independent of disease burden and performance status [56].
A retrospective study with 121 renal cell carcinoma and 239 NSCLC patients, who received ICI from two academic institutions in France and USA, evaluated oncologic outcomes according to antibiotics’ use within 30 days of beginning ICI versus no antibiotics [57]. Although only 64 patients had received any antibiotic (most commonly β-lactam or quinolones for pneumonia or urinary tract infections), an increased risk of primary progressive disease was associated with antibiotics’ use (75 versus 22%, p < 0.01). In addition, both progression-free survival (PFS) (median 1.9 versus 7.4 months, HR 3.1, and p < 0.01) and OS (median 17.3 versus 30.6 months, HR 3.5, and p = 0.03) were shorter amongst antibiotics’ users, independently of the tumour type [57]. To investigate the difference in antibiotic timing, the authors conducted a subgroup analysis for patients who had received antibiotics 60 days before ICI administration. They were able to demonstrate that the impact of antibiotics 2 months before ICI was not as relevant as within 1 month before ICI. Although those data still need further confirmation, it has been hypothesised that antibiotics shift microbiota composition temporally, and its recovery increases with time.
Pooled data from the phase II POPLAR and phase III OAK trials with 1,515 NSCLC patients randomised to either atezolizumab or docetaxel revealed that one-quarter of them had received prior antibiotics. For those who were in the atezolizumab arms, antibiotics’ use was associated with decreased median OS (8.5 versus 14.1 months, HR 1.32, 95% CI: 1.06–1.63, and p = 0.01). On the other hand, within the docetaxel population, there was no association between the use of antibiotics and OS [58]. Despite those results suggest that the use of antibiotics in patients with metastatic NSCLC is associated with poor outcome and may influence the efficacy of ICI, those analyses were all unplanned and retrospective in nature.
In Switzerland, 218 patients with advanced NSCLC had their outcomes evaluated according to prior use (15.1% of the cohort) or not of antibiotics within 2 months before ICI (59). Compared to non-users, prior antibiotic use was significantly associated with lower response rate (18.2% versus 28.3%, p = 0.02), shorter PFS (median 1.4 versus 5.5 months, HR 2.22, and p < 0.01) and worse OS (median 1.8 versus 15.4 months, HR 2.61, and p < 0.01). In a sensitivity analysis, the use of ATB either during ICI treatment or within 1 month after ICI discontinuation had no deleterious effect on outcomes.
Similar results were described amongst melanoma patients. In a retrospective analysis of a group of 568 stage III or IV melanoma patients, the use of antibiotics up to 90 days before the beginning of ICI led to an impairment in the 2-year melanoma-specific mortality rate (47 versus 37.4%, HR 1.95, and p = 0.03). The incidence of immune-mediated colitis was also higher in antibiotics’ exposed patients, who also required more use of steroids (9.8% in exposed versus 4.6% in non-exposed patients, HR 2.14, and p = 0.03) [60]. A possible explanation to the increased immune-mediated colitis is that dysbiosis caused by antibiotics may reduce the population of microorganisms that promote regulatory T-cells in mucosa with the consequent growth of pro-inflammation bacterial [61, 62]. A small prospective study with 26 metastatic melanoma patients treated with the anti-CTLA4 ipilimumab showed that patients with gut microbiota rich in Bacterioidetes and microorganisms known to be involved in regulatory T-cell differentiation developed no immune-related colitis [63].
The way the antibiotic is administered may also impact on outcomes when ICI is concerned. In a retrospective analysis with 291 metastatic cancer patients (melanoma, renal cell carcinoma or NSCLC) who were treated with ICI, antibiotic users during ICI therapy had a significant reduction on median PFS (3.1 versus 6.3 months, HR 1.56, and p = 0.003) and OS (10.4 versus 21.7 months, HR 1.69, p =0.002) [64]. In addition, when the authors looked at the antibiotic use itself, they found that when only a single course of antibiotics was administrated, no detrimental effect was seen on PFS or OS, whereas patients who had received the cumulative courses of antibiotics (duration of therapy longer than 7 days and/or more than one intravenous or oral antibiotic or sequential antibiotic use for multiple sources of infection) had significantly worse PFS (median PFS 2.8 months, HR 2.625, and p = 0.026) and OS (median OS, 6.3 months, HR 1.904, and p = 0.009) [64]. Similarly, a retrospective analysis involving 157 patients with metastatic NSCLC treated with ICI found that the length of antibiotic therapy in relation to ICI mattered, with multiple or prolonged cycles of antibiotics correlating negatively with PFS and OS [65]. Other retrospective analyses also pointed out to the negative effects of antibiotics on ICI outcomes [66, 67].
Meta-analyses also tried to address the question whether antibiotics interfere with ICI efficacy. The first meta-analysis to evaluate the association between the use of antibiotics and ICI in cancer patients included 19 eligible studies with 2,740 patients. The authors showed that the use of antibiotics was associated with worse OS (HR = 2.37 and p < 0.001), without heterogeneity. Furthermore, antibiotic exposure also significantly reduced PFS in cancer patients treated with ICIs (HR = 1.84 and p < 0.001). An unfavourable impact on outcomes was also demonstrated irrespective of the time of the use of antibiotics and type of cancer. Subgroup analysis on the type of ICI revealed a negative impact on all drugs and combinations [68].
In another meta-analysis including 18 studies with multiple tumour types and 2889 patients, 826 of whom had been exposed to antibiotics, and OS with ICI was prolonged amongst those without antibiotic exposure (HR 1.92 and p < 0.0001) (69). The effect of antibiotics on OS was more pronounced amongst patients exposed to antibiotics 42 days prior to ICI treatment (HR 3.43 and p < 0.0001), indicating that the timing of antibiotic exposure in relation to ICI is also relevant [69]. Other meta-analysis that pointed out to the same detrimental effects of antibiotics on ICI therapy was performed with 5,745 NSCLC patients from 23 studies [70]. Both PFS and OS were shorter amongst antibiotics’ users, and median OS was reduced on average by 6.7 months (70). In line with the other studies, the authors of this meta-analysis also noted an influence of the time window of exposure to antibiotics on the ICI treatment effect, with a greater impact when antibiotics were taken shortly before or after ICI initiation.
Nonetheless, not all studies found a negative impact on the use of antibiotics in the efficacy of ICI. A small retrospective study with 74 NSCLC patients treated with nivolumab could not find a detrimental effect of the use of antibiotics [71]. However, only 15 (20.3%) patients had been exposed to antibiotic medication in the 3 months before the first nivolumab injection or during treatment. Therefore, the small number of patients could have influenced the negative results in this study. Most data reported so far indicate that ICI treatment may be influenced by the use of antibiotics.
Antibiotics are frequently prescribed during the course of chemotherapy although their effect on cancer treatment outcomes is poorly described, and most of the scarce data come from animal models. It has been well known that chemotherapy can cause profound dysbiosis and affect multiple metabolic pathways [72, 73]. Meanwhile, the majority of antibiotics prescribed in this setting have broad-spectrum activity with a high potential to alter the normal microbiota [74].
A study conducted in mice suggested that antibiotic administration disrupted the gut microbiota, which contributed to the reduction of antitumour efficacy of 5-fluorouracil [75]. In a similar fashion, cyclophosphamide has been shown to alter the microbiota composition in mice small bowel and induce the translocation of selected species of Gram-positive bacteria into secondary lymphoid organs, where the generation of a pathogenic subset of T-helper 17 cells and memory T-helper 1 immune responses occurred [27]. Interestingly, germ-free or antibiotics-treated tumour-bearing mice had a reduction in T-helper 17 responses, whereas their tumours were resistant to cyclophosphamide [27]. In this mouse model, the reduction in the antitumour effect of cyclophosphamide was more pronounced after exposure to Gram-positive spectrum antibiotics, as opposed to antibiotics with Gram-negative spectrum.
Another study conducted in mice showed the disruption of the microbiota impaired the response of tumours to platinum chemotherapy [76]. In antibiotics-treated or germ-free mice, tumour-infiltrating myeloid-derived cells had a less response to therapy, resulting in the deficient production of reactive oxygen species through Toll-like receptors and lower cytotoxicity after platinum agents. Thus, the authors concluded that optimal responses to cancer therapy in mice require an intact commensal microbiota that modulates myeloid-derived cell functions in the tumour microenvironment.
To investigate the impact of antibiotic treatment on antineoplastic treatment outcomes in humans, a German group identified 800 patients treated with a cyclophosphamide containing first-line therapy for chronic lymphocytic leukaemia (CLL) as well as 122 patients treated with a cisplatin containing regimen for relapsed lymphoma (RL). Potential associations between anti-Gram-positive antibiotic treatment and patient outcome were retrospectively analysed [77]. For both CLL and RL cohorts, treatment with anti-Gram-positive antibiotics was significantly associated with worse response rates, PFS and OS, indicating a potential negative impact of anti-Gram-positive antibiotics on the anticancer activity of cyclophosphamide and cisplatin. The prospective studies are urgently needed to confirm those findings. Data on the deleterious effect of microbiota disruption by early exposure to broad-spectrum antibiotics also exist amongst 621 patients undergoing allogeneic stem cell transplantation (ASCT). Antibiotic administration before ASCT was significantly associated with a higher transplant-related mortality compared to post-ASCT or no antibiotics (34% versus 21% versus 7%, respectively) [78].
Similar findings have been reported amongst patients with solid malignancies. In a recent retrospective study, the impact of antibiotic therapy on treatment outcomes following curative-intent chemotherapy and RT in patients with locally advanced head and neck cancer (LAHNC) was analysed. Patients who had received antibiotics progressed significantly earlier compared with patients in the non-antibiotic group (median PFS 147.8 months versus not reached). Overall survival (OS) was also significantly lower in patients who had received antibiotics (71.9 versus 132 months, p = 0.0007). Antibiotics were an independent prognostic factor for PFS and OS in this study. Furthermore, the use of more than two antibiotics was related to an even worse negative impact [74].
Not only efficacy but also chemotherapy-induced toxicity may be altered with antimicrobial agents. A recent analysis from the MPACT trial revealed that patients with metastatic pancreatic adenocarcinoma treated with gemcitabine had an increased rate of gemcitabine-associated toxicity during and after antibiotic therapy. This observation comes in line with preclinical evidence that intratumour bacteria metabolise gemcitabine to an inactive form [79].
The interaction of radiotherapy with anti-neoplastic drugs is already reasonably well known, with positive interactions, such as sensitisation (concurrent and supra-additive), protective interactions (sub-additive, inhibitory or antagonistic) or inert interactions, both for anti-neoplastic efficacy and side effects, as well as anachronistic interactions, the so-called ‘recall effect’ [80].
More recently, the interaction between nutritional supplements, more specifically anti-oxidants (pentoxifylline, tocopherol and ascorbic acid in high doses, glucosamines, beta-carotenes and so on) have brought conflicting data, either by potentially reducing effectiveness or not showing any interference with chemotherapy or radiation therapy. As such, the current recommendation is a temporary suspension of those agents for irradiation treatment, unless the antioxidant is considered essential [81].
When antibiotics are concerned, the modifications of the intestinal microbiome induced by those drugs may lead to greater toxicity when concomitant or sequential pelvic radiotherapy is used [82, 83]. Animal studies have shown that vancomycin use, an antibiotic that acts against Gram-positive bacteria and is restricted to the intestinal tract, promotes an increase in the antitumour potential of radiotherapy despite greater toxicity [84]. However, scarce information is available on a possible interaction between antibiotics and radiation therapy in humans.
For LAHNC, a retrospective analysis of 272 patients found a negative association with antibiotics when used between 1 week before the start and 2 weeks after the end of the combined chemoradiation therapy. Interestingly, 45.6% of patients had received antibiotics during the course of their anticancer treatment. Of the 272 patients in the total sample, 233 of them (85%) received induction chemotherapy (cisplatin or carboplatin with 5-fluorouracil) followed by chemoradiotherapy (with cisplatin or carboplatin), 12 were treated with induction chemotherapy followed by radiation alone, only 6 with chemoradiotherapy alone, 17 with surgery followed by chemoradiotherapy, 3 with radiotherapy alone and only one with radiotherapy associated with cetuximab. A negative impact was observed on PFS, disease-specific survival (DSS) and overall survival (OS), with HRs of 1.98 (p = 0.001), 1.95 (p = 0.004) and 1.85 (p = 0.001), respectively. The use of two or more courses of antibiotic therapy had the greatest negative impact on PFS, DSS and OS, whereas the use of probiotics had a reducing effect on this risk. Given the negative influence of antibiotics on outcomes, the authors recommended avoiding the use of broad-spectrum antibiotics whenever possible, especially for prophylactic purposes (74). A prospective randomised phase II trial with 95 LAHCNC patients evaluated the role of prophylactic antibiotic therapy with amoxicillin/clavulanic acid versus standard care without prophylaxis during chemoradiotherapy (85). The authors found no difference in the primary endpoint (reduction in pneumonia) although a lower rate of hospitalisation and febrile episodes were reported in the prophylactic group, with a better impact on the final cost and quality of life. However, there is no long-term evaluation of PFS, DFS or OS (85,86), and those results are eagerly awaited.
Briefly, the concomitant use of antibiotics and radiotherapy has yet little evidence of safety or interaction. However, given the few clinical studies, the recommendation is to minimise antimicrobial use as much as possible, adopting preventive measures and limiting prophylactic use.
What could be done to minimise the potential risks of antibiotics
The composition of gut microbiome is known to be influenced by genetics, lifestyle, immune conditions of the patient, comorbidities and previous treatment including the previous use of antibiotics. All of these factors may have a favourable influence or not on the cancer treatment of patients [87].
As previously mentioned, several studies have shown that the use of antibiotics close to or concurrent with immunotherapy is associated with reduced OS and response to treatment [57, 88], whereas the interaction of antibiotics with other oncologic therapies is also being investigated. It has been a consensus that the use of broad-spectrum antibiotics should be avoided during the use of immunotherapy whenever possible. In addition, antibiotics should be prescribed only when properly indicated. On the other hand, one may argue that the use of antibiotics could turn a bad microbiome, for example, a microbiome rich in bacteria that promote immunosuppression through the expansion of FoxP3 Tregs, into a proper gut microbiome [89]. Furthermore, the clinical studies are needed to better understand the role of antibiotics and their interactions with the microbiome.
Since antibiotics kill bacteria based on broad features, such as Gram-positive or negative staining, they do not seem the best alternative to eliminate specific pathogens from the microbiome. Probably, a better approach would be to modulate the existing commensal community via prebiotics or dietary changes to favour the expansion of beneficial bacteria or, more specifically, to identify metabolic pathways utilised by bacteria and target them [90].
A thought-provoking study published in 2014 showed that short-term consumption of diets composed entirely of animal products, which means a diet rich in meats, eggs and cheeses or plant products, mainly grains, legumes, fruits and vegetables, alters microbial community structure and overwhelms interindividual differences in microbial gene expression. The animal-based diet increased the abundance of bile-tolerant microorganisms (Alistipes, Bilophila and Bacteroides) and decreased the levels of Firmicutes that metabolise dietary plant polysaccharides (Roseburia, Eubacterium rectale and Ruminococcus bromii). The animal-based diet supports a link between dietary fat, bile acids and the outgrowth of microorganisms capable of triggering inflammatory bowel disease [91]. The process of T-cell differentiation utilises the short-chain fatty acid butyrate, which allows one to hypothesise the possibility of bypass gut microbiota by the use of short-chain fatty acid supplements [92–94]. These studies reveal the importance that the research on the microbiome needs to be comprehensive, including interactions with metabolism and immunology in order to develop specific intervention strategies to target the host microbiota [95].
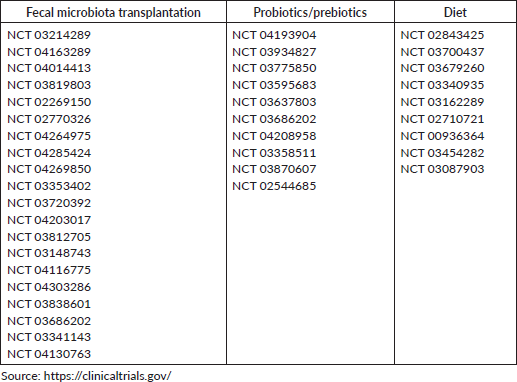
Another potential approach to alter gut microbiome is faecal microbiota transplantation (FMT). Despite the encouraging results of FMT in the treatment of refractory Clostridium difficile diarrhoea, it requires a consideration of several key factors such as the definition of a favourable microbiota, the possibility of delivering immune-regulatory bacteria and the potential to transfer disease-promoting bacteria (89,96). A small pilot study of four FMT post-hematopoietic stem cell transplants with steroid-refractory graft-versus-host disease (GVHD) showed a remarkable resolution of GVHD in 75% of patients [97]. However, the development of invasive infection caused by multidrug-resistant organisms has been reported in two patients receiving FMT treatment, and one of the patients died. Furthermore, the clinical investigation should focus on both FMT-induced adverse events and its oncologic efficacy [98]. Currently, many studies are being conducted, and their results are expected to provide a better understanding of the intestinal microbiome as a therapeutic target in cancer treatment (Table 1).
Table 1. Current clinical trials with interventions in the gut microbiota
Conclusion
The composition of gut microbiome is influenced by genetics, lifestyle, immune conditions of the patient, comorbidities and previous treatment including the previous use of antibiotics. Several studies have linked dysbiosis to carcinogenesis, whereas others have shown that the use of antibiotics close to or concurrent with cancer therapy may be associated with worse outcomes. Therefore, the use of antibiotics should be avoided whenever possible, and physician education on the possible harms of antibiotics is urgently needed.
Conflicts of interest
The authors declare no conflicts of interest.
Funding source
There was no financial funding for this manuscript.
References
1. National Centre for Health Statistics [https:www.cdc.gov/nchs/fastats/life-expectancy.htm]. Date accessed: 05/02/20
2. Fleming A (1929) On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzæ Br J Exp Pathol 10(3) 226–236
3. Klein EY, Levin SA, and Laxminarayan R (2018) Reply to Abat et al.: Improved policies necessary to ensure an effective future for antibiotics Proc Natl Acad Sci 115(35) E8111–E8112 https://doi.org/10.1073/pnas.1811245115 PMID: 30087188 PMCID: 6126734
4. Blaser MJ (2016) Antibiotic use and its consequences for the normal microbiome Science 352(6285) 544–545 https://doi.org/10.1126/science.aad9358 PMID: 27126037 PMCID: 4939477
5. Bp W, Sl R, and Bb F (2011) Shifting the balance: antibiotic effects on host-microbiota mutualism Nat Rev Microbiol 9(4) 233–243 https://doi.org/10.1038/nrmicro2536
6. Neu J (2015) Developmental aspects of maternal-fetal, and infant gut microbiota and implications for long-term health Matern Health Neonatol Perinatol 1 6 https://doi.org/10.1186/s40748-015-0007-4 PMID: 27057323 PMCID: 4772751
7. Weinstock GM (2012) Genomic approaches to studying the human microbiota Nature 489(7415) 250–256 https://doi.org/10.1038/nature11553 PMID: 22972298 PMCID: 3665339
8. Ventura M, O’Toole PW, and de Vos WM, et al (2018) Selected aspects of the human gut microbiota Cell Mol Life Sci 75(1) 81–82 https://doi.org/10.1007/s00018-017-2669-8
9. Bik EM (2009) Composition and function of the human-associated microbiota Nutr Rev 67(Suppl 2) S164–S171 https://doi.org/10.1111/j.1753-4887.2009.00237.x PMID: 19906220
10. Arumugam M, Raes J, and Pelletier E, et al (2011) Enterotypes of the human gut microbiome Nature 473(7346) 174–180 https://doi.org/10.1038/nature09944 PMID: 21508958 PMCID: 3728647
11. Sekirov I, Russell SL, and Antunes LCM, et al (2010) Gut microbiota in health and disease Physiol Rev 90(3) 859–904 https://doi.org/10.1152/physrev.00045.2009 PMID: 20664075
12. Clemente JC, Ursell LK, and Parfrey LW, et al (2012) The impact of the gut microbiota on human health: an integrative view Cell 148(6) 1258–1270 https://doi.org/10.1016/j.cell.2012.01.035 PMID: 22424233 PMCID: 5050011
13. Virta L, Auvinen A, and Helenius H, et al (2012) Association of repeated exposure to antibiotics with the development of pediatric Crohn’s disease--a nationwide, register-based finnish case-control study Am J Epidemiol 175(8) 775–784 https://doi.org/10.1093/aje/kwr400 PMID: 22366379
14. Chai G, Governale L, and McMahon AW, et al (2012) Trends of outpatient prescription drug utilization in US children, 2002–2010 Pediatrics 130(1) 23–31 https://doi.org/10.1542/peds.2011-2879 PMID: 22711728
15. Aloisio I, Quagliariello A, and De Fanti S, et al (2016) Evaluation of the effects of intrapartum antibiotic prophylaxis on newborn intestinal microbiota using a sequencing approach targeted to multi hypervariable 16S rDNA regions Appl Microbiol Biotechnol 100(12) 5537–5546 https://doi.org/10.1007/s00253-016-7410-2 PMID: 26971496
16. Bäumler AJ and Sperandio V (2016) Interactions between the microbiota and pathogenic bacteria in the gut Nature 535(7610) 85–93 https://doi.org/10.1038/nature18849 PMID: 27383983 PMCID: 5114849
17. Thaiss CA, Zmora N, and Levy M, et al (2016) The microbiome and innate immunity Nature 535(7610) 65–74 https://doi.org/10.1038/nature18847 PMID: 27383981
18. Belizário JE and Faintuch J (2018) Microbiome and gut dysbiosis Exp Suppl 109 459–476 PMID: 30535609
19. Yatsunenko T, Rey FE, and Manary MJ, et al (2012) Human gut microbiome viewed across age and geography Nature 486(7402) 222–227 https://doi.org/10.1038/nature11053 PMID: 22699611 PMCID: 3376388
20. Boggess KA, Watts DH, and Hillier SL, et al (1996) Bacteremia shortly after placental separation during cesarean delivery Obstet Gynecol 87(5) 779–784 https://doi.org/10.1016/0029-7844(96)00037-3 PMID: 8677085
21. Mondot S, de Wouters T, and Doré J, et al (2013) The human gut microbiome and its dysfunctions Dig Dis Basel Switz 31(3–4) 278–285 https://doi.org/10.1159/000354678
22. Tetel MJ, de Vries GJ, and Melcangi RC, et al (2018) Steroids, stress and the gut microbiome-brain axis J Neuroendocrinol 30(2) https://doi.org/10.1111/jne.12548
23. Andersson DI and Hughes D (2014) Microbiological effects of sublethal levels of antibiotics Nat Rev Microbiol 12(7) 465–478 https://doi.org/10.1038/nrmicro3270 PMID: 24861036
24. Francino MP (2015) Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances Front Microbiol 6 1543
25. Alexander JL, Wilson ID, and Teare J, et al (2017) Gut microbiota modulation of chemotherapy efficacy and toxicity Nat Rev Gastroenterol Hepatol 14(6) 356–365 https://doi.org/10.1038/nrgastro.2017.20 PMID: 28270698
26. Siegel RL, Miller KD, and Jemal A (2016) Cancer statistics, 2016 CA Cancer J Clin 66(1) 7–30 https://doi.org/10.3322/caac.21332 PMID: 26742998
27. Viaud S, Saccheri F, and Mignot G, et al (2013) The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide Science 342(6161) 971–976 https://doi.org/10.1126/science.1240537 PMID: 24264990 PMCID: 4048947
28. Cianci R, Franza L, and Schinzari G, et al (2019) The interplay between immunity and microbiota at intestinal immunological Niche: the case of cancer Int J Mol Sci 20(3) 501 https://doi.org/10.3390/ijms20030501 PMCID: 6387318
29. Pinato DJ, Gramenitskaya D, and Altmann DM, et al (2019) Antibiotic therapy and outcome from immune-checkpoint inhibitors J Immunother Cancer 7(1) 287 https://doi.org/10.1186/s40425-019-0775-x PMID: 31694714 PMCID: 6836427
30. Dubin K, Callahan MK, and Ren B, et al (2016) Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis Nat Commun 7 10391. https://doi.org/10.1038/ncomms10391 PMID: 26837003 PMCID: 4740747
31. Sivan A, Corrales L, and Hubert N, et al (2015) Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy Science 350(6264) 1084–1089 https://doi.org/10.1126/science.aac4255 PMID: 26541606 PMCID: 4873287
32. Anthony HM, Kenny TE, and Practitioners FYG, et al (1982) Drugs in the aetiology of cancer: a retrospective study Int J Epidemiol 11(4) 336–344 https://doi.org/10.1093/ije/11.4.336 PMID: 6130048
33. Hoover R and Fraumeni JF (1981) Drug-induced cancer Cancer 47(S5) 1071–1080 https://doi.org/10.1002/1097-0142(19810301)47:5 <1071::AID-CNCR2820471304>3.0.CO;2-7 PMID: 7016298
34. Friedman GD, Udaltsova N, and Chan J, et al (2009) Screening pharmaceuticals for possible carcinogenic effects: initial positive results for drugs not previously screened Cancer Causes Control 20(10) 1821–1835 https://doi.org/10.1007/s10552-009-9375-2 PMID: 19582585 PMCID: 3010483
35. Petrelli F, Ghidini M, and Ghidini A, et al (2019) Use of antibiotics and risk of cancer: a systematic review and meta-analysis of observational studies Cancers 11(8) 1174 https://doi.org/10.3390/cancers11081174 PMCID: 6721461
36. Boursi B, Mamtani R, and Haynes K, et al (2015) Recurrent antibiotic exposure may promote cancer formation – another step in understanding the role of the human microbiota? Eur J Cancer Oxf Engl 51(17) 2655–2664 https://doi.org/10.1016/j.ejca.2015.08.015
37. Blaser MJ (2016) Antibiotic use and its consequences for the normal microbiome Science 352(6285) 544–545 https://doi.org/10.1126/science.aad9358 PMID: 27126037 PMCID: 4939477
38. Mao Q, Jiang F, and Yin R, et al (2018) Interplay between the lung microbiome and lung cancer Cancer Lett 415 40–48 https://doi.org/10.1016/j.canlet.2017.11.036
39. Aragón IM, Herrera-Imbroda B, and Queipo-Ortuño MI, et al (2018) The urinary tract microbiome in health and disease Eur Urol Focus 4(1) 128–138 https://doi.org/10.1016/j.euf.2016.11.001
40. Whiteside SA, Razvi H, and Dave S, et al (2015) The microbiome of the urinary tract—a role beyond infection Nat Rev Urol 12(2) 81–90 https://doi.org/10.1038/nrurol.2014.361 PMID: 25600098
41. Mima K, Nakagawa S, and Sawayama H, et al (2017) The microbiome and hepatobiliary-pancreatic cancers Cancer Lett 402 9–15 https://doi.org/10.1016/j.canlet.2017.05.001 PMID: 28527946
42. Yamamoto ML, Schiestl RH. Intestinal microbiome and lymphoma development. Cancer J Sudbury Mass. June 2014;20(3):190–4. https://doi.org/10.1097/PPO.0000000000000047
43. Dejea CM, Fathi P, and Craig JM, et al (2018) Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria Science 359(6375) 592–597 https://doi.org/10.1126/science.aah3648 PMID: 29420293 PMCID: 5881113
44. Drewes JL, White JR, and Dejea CM, et al (2017) High-resolution bacterial 16S rRNA gene profile meta-analysis and biofilm status reveal common colorectal cancer consortia. NPJ Biofilms Microbiomes 3(1) 1–12 https://doi.org/10.1038/s41522-017-0040-3
45. Dejea CM, Wick EC, and Hechenbleikner EM, et al (2014) Microbiota organization is a distinct feature of proximal colorectal cancers Proc Natl Acad Sci USA 111(51) 18321–18326 https://doi.org/10.1073/pnas.1406199111 PMID: 25489084 PMCID: 4280621
46. Tomkovich S, Dejea CM, and Winglee K, et al (2019) Human colon mucosal biofilms from healthy or colon cancer hosts are carcinogenic J Clin Invest 130 1699–1712 https://doi.org/10.1172/JCI124196
47. Kilkkinen A, Rissanen H, and Klaukka T, et al (2008) Antibiotic use predicts an increased risk of cancer Int J Cancer 123(9) 2152–2155 https://doi.org/10.1002/ijc.23622 PMID: 18704945
48. Dik VK, van Oijen MGH, and Smeets HM, et al (2016) Frequent use of antibiotics is associated with colorectal cancer risk: results of a nested case-control study Dig Dis Sci 61(1) 255–264 https://doi.org/10.1007/s10620-015-3828-0
49. Cao Y, Wu K, and Mehta R, et al (2018) Long-term use of antibiotics and risk of colorectal adenoma Gut 67(4) 672–678
50. Boursi B, Haynes K, and Mamtani R, et al (2015) Impact of antibiotic exposure on the risk of colorectal cancer Pharmacoepidemiol Drug Saf 24(5) 534–542 https://doi.org/10.1002/pds.3765 PMID: 25808540
51. Wang J-L, Chang C-H, and Lin J-W, et al (2014) Infection, antibiotic therapy and risk of colorectal cancer: a nationwide nested case-control study in patients with Type 2 diabetes mellitus Int J Cancer 135(4) 956–967 https://doi.org/10.1002/ijc.28738 PMID: 24470385
52. Tang X, Wang X, and Zhao YY, et al (2017) Doxycycline attenuates breast cancer related inflammation by decreasing plasma lysophosphatidate concentrations and inhibiting nF-κB activation Mol Cancer 16 36 https://doi.org/10.1186/s12943-017-0607-x
53. Sapadin AN and Fleischmajer R (2006) Tetracyclines: nonantibiotic properties and their clinical implications J Am Acad Dermatol 54(2) 258–265 https://doi.org/10.1016/j.jaad.2005.10.004 PMID: 16443056
54. Koltai T (2015) Tetracyclines against cancer. A review
55. Reed JP, Devkota S, and Figlin RA (2019) Gut microbiome, antibiotic use, and immunotherapy responsiveness in cancer Ann Transl Med 7(Suppl 8) S309 https://doi.org/10.21037/atm.2019.10.27
56. Pinato DJ, Howlett S, and Ottaviani D, et al (2019) Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer JAMA Oncol 5(12) 1774–1778 https://doi.org/10.1001/jamaoncol.2019.2785
57. Derosa L, Hellmann MD, and Spaziano M, et al (2018) Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer Ann Oncol Off J Eur Soc Med Oncol 29(6) 1437–1444 https://doi.org/10.1093/annonc/mdy103
58. Chalabi M, Cardona A, and Nagarkar DR, et al (2020) Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials Ann Oncol 31(4) 525–531 https://doi.org/10.1016/j.annonc.2020.01.006 PMID: 32115349
59. Schett A, Rothschild SI, and Curioni-Fontecedro A, et al (2020) Predictive impact of antibiotics in patients with advanced non small-cell lung cancer receiving immune checkpoint inhibitors: Antibiotics immune checkpoint inhibitors in advanced NSCLC Cancer Chemother Pharmacol 85(1) 121–131 https://doi.org/10.1007/s00280-019-03993-1
60. Mohiuddin JJ, Chu B, and Facciabene A, et al (2020) Association of antibiotic exposure with survival and toxicity in patients with melanoma receiving immunotherapy J Natl Cancer Inst djaa057 https://doi.org/10.1093/jnci/djaa057 PMID: 32294209
61. Belkaid Y and Hand TW (2014) Role of the microbiota in immunity and inflammation Cell 157(1) 121–141 https://doi.org/10.1016/j.cell.2014.03.011 PMID: 24679531 PMCID: 4056765
62. Samaan MA, Pavlidis P, and Papa S, et al (2018) Gastrointestinal toxicity of immune checkpoint inhibitors: from mechanisms to management Nat Rev Gastroenterol Hepatol 15(4) 222–234 https://doi.org/10.1038/nrgastro.2018.14 PMID: 29512649
63. Chaput N, Lepage P, and Coutzac C, et al (2017) Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab Ann Oncol Off J Eur Soc Med Oncol 28(6) 1368–1379 https://doi.org/10.1093/annonc/mdx108
64. Tinsley N, Zhou C, and Tan G, et al (2020) Cumulative antibiotic use significantly decreases efficacy of checkpoint inhibitors in patients with advanced cancer Oncologist 25(1) 55–63 https://doi.org/10.1634/theoncologist.2019-0160 PMCID: 6964118
65. Galli G, Triulzi T, and Proto C, et al (2019) Association between antibiotic-immunotherapy exposure ratio and outcome in metastatic non small cell lung cancer Lung Cancer 132 72–78 https://doi.org/10.1016/j.lungcan.2019.04.008 PMID: 31097097
66. Ahmed J, Kumar A, and Parikh K, et al (2018) Use of broad-spectrum antibiotics impacts outcome in patients treated with immune checkpoint inhibitors Oncoimmunology 7(11) e1507670 https://doi.org/10.1080/2162402X.2018.1507670 PMID: 30377571 PMCID: 6205076
67. Elkrief A, Raichani LE, and Richard C, et al (2019) Antibiotics are associated with decreased progression-free survival of advanced melanoma patients treated with immune checkpoint inhibitors OncoImmunology 8(4) e1568812 https://doi.org/10.1080/2162402X.2019.1568812 PMID: 30906663 PMCID: 6422373
68. Huang X-Z, Gao P, and Song Y-X, et al (2019) Antibiotic use and the efficacy of immune checkpoint inhibitors in cancer patients: a pooled analysis of 2740 cancer patients Oncoimmunology [Internet] 8(12) e1665973 https://doi.org/10.1080/2162402X.2019.1665973 PMID: 31741763 PMCID: 6844307
69. Wilson BE, Routy B, and Nagrial A, et al (2020) The effect of antibiotics on clinical outcomes in immune-checkpoint blockade: a systematic review and meta-analysis of observational studies Cancer Immunol Immunother 69(3) 343–354 https://doi.org/10.1007/s00262-019-02453-2
70. Lurienne L, Cervesi J, and Duhalde L, et al (2020) NSCLC immunotherapy efficacy and antibiotic use: a systematic review and meta-analysis J Thorac Oncol 15(7) 1147–1159 https://doi.org/10.1016/j.jtho.2020.03.002 PMID: 32173463
71. Kaderbhai C, Richard C, and Fumet JD, et al (2017) Antibiotic use does not appear to influence response to nivolumab Anticancer Res 37(6) 3195–3200 PMID: 28551664
72. Alexander JL, Wilson ID, and Teare J, et al (2017) Gut microbiota modulation of chemotherapy efficacy and toxicity Nat Rev Gastroenterol Hepatol 14(6) 356–365 https://doi.org/10.1038/nrgastro.2017.20 PMID: 28270698
73. Montassier E, Gastinne T, and Vangay P, et al (2015) Chemotherapy-driven dysbiosis in the intestinal microbiome Aliment Pharmacol Ther 42(5) 515–528 https://doi.org/10.1111/apt.13302 PMID: 26147207
74. Nenclares P, Bhide SA, and Sandoval-Insausti H (2020) Impact of antibiotic use during curative treatment of locally advanced head and neck cancers with chemotherapy and radiotherapy Eur J Cancer 131 9–15 https://doi.org/10.1016/j.ejca.2020.02.047 PMID: 32248073
75. Yuan L, Zhang S, and Li H, et al (2018) The influence of gut microbiota dysbiosis to the efficacy of 5-Fluorouracil treatment on colorectal cancer Biomed Pharmacother Biomedecine Pharmacother 108 184–193 https://doi.org/10.1016/j.biopha.2018.08.165
76. Iida N, Dzutsev A, and Stewart CA, et al (2013) Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment Science 342(6161) 967–970 https://doi.org/10.1126/science.1240527 PMID: 24264989 PMCID: 6709532
77. Pflug N, Kluth S, and Vehreschild JJ, et al (2016) Efficacy of antineoplastic treatment is associated with the use of antibiotics that modulate intestinal microbiota Oncoimmunology 5(6) e1150399 https://doi.org/10.1080/2162402X.2016.1150399 PMID: 27471619 PMCID: 4938364
78. Weber D, Jenq RR, and Peled JU, et al (2017) Microbiota disruption induced by early use of broad-spectrum antibiotics is an independent risk factor of outcome after allogeneic stem cell transplantation Biol Blood Marrow Transplant 23(5) 845–852 https://doi.org/10.1016/j.bbmt.2017.02.006 PMID: 28232086 PMCID: 5546237
79. Corty RW, Langworthy BW, and Fine JP, et al (2020) Antibacterial use is associated with an increased risk of hematologic and gastrointestinal adverse events in patients treated with gemcitabine for stage IV pancreatic cancer Oncologist 25(7) 579–584 https://doi.org/10.1634/theoncologist.2019-0570 PMID: 32181968 PMCID: 7356778
80. Cox J and Ang KK (2010) Radiation Oncology: Rationate, Technique, Results 9th edn (Mosby) pp 3–49
81. Yasueda A, Urushima H, and Ito T (2016) Efficacy and interaction of antioxidant supplements as adjuvant therapy in cancer treatment: a systematic review Integr Cancer Ther 15(1) 17–39 https://doi.org/10.1177/1534735415610427
82. Wang A, Ling Z, and Yang Z, et al (2015) Gut microbial dysbiosis may predict diarrhea and fatigue in patients undergoing pelvic cancer radiotherapy: a pilot study PLoS One 10(5) e0126312 https://doi.org/10.1371/journal.pone.0126312 PMID: 25955845 PMCID: 4425680
83. Xavier JB, Young VB, and Skufca J, et al (2020) The cancer microbiome: distinguishing direct and indirect effects requires a systemic view Trends Cancer 6(3) 192–204 https://doi.org/10.1016/j.trecan.2020.01.004 PMID: 32101723 PMCID: 7098063
84. Uribe-Herranz M, Rafail S, and Beghi S, et al (2020) Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response J Clin Invest 130(1) 466–479 https://doi.org/10.1172/JCI124332 PMCID: 6934221
85. Ham JC, Driessen CM, and Hendriks MP, et al (1990) Prophylactic antibiotics reduce hospitalisations and cost in locally advanced head and neck cancer patients treated with chemoradiotherapy: a randomised phase 2 study Eur J Cancer Oxf Engl 113 32–40 https://doi.org/10.1016/j.ejca.2019.02.013
86. Ham JC, van Herpen CML, and Driessen CML, et al (2019) Health-related quality of life of patients treated with chemoradiotherapy plus or minus prophylactic antibiotics to reduce the number of pneumonias for locally advanced head and neck cancer, the PANTAP study Oral Oncol 96 105–112 https://doi.org/10.1016/j.oraloncology.2019.07.014 PMID: 31422201
87. Vetizou M and Trinchieri G (2018) Anti-PD1 in the wonder-gut-land Cell Res 28(3) 263–264 https://doi.org/10.1038/cr.2018.12 PMID: 29336431 PMCID: 5835771
88. Routy B, Le Chatelier E, and Derosa L, et al (2018) Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors Science 359(6371) 91–97 https://doi.org/10.1126/science.aan3706
89. Fessler J, Matson V, and Gajewski TF (2019) Exploring the emerging role of the microbiome in cancer immunotherapy J Immunother Cancer 7(1) 108 https://doi.org/10.1186/s40425-019-0574-4 PMID: 30995949 PMCID: 6471869
90. Strouse C, Mangalam A, and Zhang J (2019) Bugs in the system: bringing the human microbiome to bear in cancer immunotherapy Gut Microbes 10(2) 109–112 https://doi.org/10.1080/19490976.2018.1511665 PMCID: 6546317
91. David LA, Maurice CF, and Carmody RN, et al (2014) Diet rapidly and reproducibly alters the human gut microbiome Nature 505(7484) 559–563 https://doi.org/10.1038/nature12820 PMCID: 3957428
92. Arpaia N, Campbell C, and Fan X, et al (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation Nature 504(7480) 451–455 https://doi.org/10.1038/nature12726 PMID: 24226773 PMCID: 3869884
93. Furusawa Y, Obata Y, and Fukuda S, et al (2013) Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells Nature 504(7480) 446–450 https://doi.org/10.1038/nature12721 PMID: 24226770
94. Smith PM, Howitt MR, and Panikov N, et al (2013) The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis Science 341(6145) 569–573 https://doi.org/10.1126/science.1241165 PMID: 23828891 PMCID: 3807819
95. Galloway-Peña JR, Jenq RR, and Shelburne SA (2017) Can consideration of the microbiome improve antimicrobial utilization and treatment outcomes in the oncology patient? Clin Cancer Res Off J Am Assoc Cancer Res 23(13) 3263–3268 https://doi.org/10.1158/1078-0432.CCR-16-3173
96. Nood EV, Vrieze A, and Nieuwdorp M, et al (2013) Duodenal infusion of donor feces for recurrent clostridium difficile N Engl J Med 368 407–415 https://doi.org/10.1056/NEJMoa1205037 PMID: 23323867
97. Kakihana K, Fujioka Y, and Suda W, et al (2016) Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut Blood 128(16) 2083–2088 https://doi.org/10.1182/blood-2016-05-717652 PMID: 27461930 PMCID: 5085256
98. Yi M, Jiao D, and Qin S, et al (2019) Manipulating gut microbiota composition to enhance the therapeutic effect of cancer immunotherapy Integr Cancer Ther 18 1534735419876351 https://doi.org/10.1177/1534735419876351 PMID: 31517538 PMCID: 7242797






