Chemotherapy-induced necrotising tumour lysis and perforation of a huge gastric cancer simulating emphysematous pancreatitis
Frank S Fan1, a and Chung-Fan Yang2, b
1Section of Haematology and Oncology, Department of Medicine, Ministry of Health and Welfare Changhua Hospital, Chang-Hua County, Taiwan
2Department of Pathology, Ministry of Health and Welfare Changhua Hospital, Chang-Hua County, Taiwan
ahttps://orcid.org/0000-0002-8123-6941
bhttps://orcid.org/0000-0002-7366-4380
Abstract
A 56-year-old man was diagnosed to have a huge gastric cancer extending from the lesser curvature of the stomach to the pancreas with multiple hepatic and peritoneal metastases. Two days after completing chemotherapy with cisplatin plus high dose leucovorin and fluorouracil, drastic necrotising tumour lysis led to gastric perforation and septic shock most likely due to bacterial peritonitis. The image of tumour lysis looked like an emphysematous pancreatitis. Afterwards, immunohistochemical study of the tumour specimen confirmed moderate positivity of dihydropyrimidine dehydrogenase and absence of Bcl-2 expression. The incomplete expression of dihydropyrimidine dehydrogenase and total deficiency of Bcl-2 are considered to be the main underlying causes of such extraordinary chemosensitivity and so severe a tumour lysis phenomenon. Pre-emptive intensive survey of possible biomarkers of chemosensitivity is thus highly recommended upon treating a massive gastric cancer.
Keywords: gastric cancer, chemotherapy, dihydropyrimidine dehydrogenase, Bcl-2, emphysematous pancreatitis
Correspondence to: Frank S Fan
Email: fantast.fan@msa.hinet.net
Published: 11/06/2020
Received: 10/03/2020
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Spontaneous perforation of gastric cancer is occasionally seen with an incidence ranging from 0.39% to 9.6% of all gastric cancer patients as reported by different groups [1–4]. The outcome after emergency surgery in patients with free perforation depends on the stage and whether a curative resection could be performed [5], but is poorer than that in T3 tumour patients without perforation [6].
Although modern neoadjuvant chemotherapy makes conversion gastrectomy possible and improves survival in advanced gastric cancer patients [7] with even an exciting case report of pathological complete remission [8], gastric perforation caused by chemotherapy or sometimes chemotherapy combined with targeted therapy remains a complication incidentally seen [9–11].
We present our unusual experience in treating a patient with dramatic gastric perforation and septic peritonitis resulting from severe necrotising lysis of his massive gastric tumour shortly after systemic chemotherapy. The clinical picture and image study looked very much like an emphysematous pancreatitis.
Case presentation
A 56-year-old acute ill-looking man came to our emergency unit with the chief complaint of hiccups for four days in June 2019. General weakness, abdomen fullness, epigastric discomfort and poor intake had been noted for several weeks prior to this visit. The patient was not sure whether there was tarry stool but his daughter observed a certain weight loss of the patient. In accordance with that, his body mass index was 18.4 and his body surface area was 1.5 square meters at presentation. He did not have other major systemic diseases except a long history of type 2 diabetes mellitus with irregular control. Alcohol abuse was denied. The physical examination revealed a soft abdomen with tenderness over epigastrium and upper left quadrant. There was no rebounding pain and palpable mass.
Laboratory tests disclosed an elevated serum alkaline phosphatase level of 420 iu/L (normal 32~91) and normocytic anaemia with white cell count 9,300/mL, haemoglobin 10.6 g/dl, mean corpuscular volume 84.7 fl, platelet count 282,000/mL, segments 81.5%, lymphocytes 9.3%, and monocyte 7.6%. There were no active lung lesions in the chest X-ray routine film.
Computed tomography (CT) scan showed a huge heterogenous contrast-enhanced mass at the stomach body abutting and probably invading the duodenum and pancreas with numerous nodules in bilateral hepatic lobes and peritoneal cavity, including perigastric region (Figure 1). The picture was reasonably attributed to an advanced gastric cancer with multiple hepatic and peritoneal metastases.
An upper gastrointestinal tract endoscopy led to the finding of a giant gastric ulcer over lesser curvature of the stomach (Figure 2). Pathology study of the biopsy specimen from the ulcer confirmed the nature of a poorly differentiated carcinoma (Figure 3) positive for cytokeratin AE1/AE3, with morphology patterns in favour of a gastric rather than a pancreatic origin. Immunohistochemical staining afterwards revealed only moderately positive expression of dihydropyrimidine dehydrogenase (DPD) (Rabbit monoclonal DPYD EPR8811: ab134922, Abcam, Cambridge, UK) (Figure 4) and totally negative expression of Bcl-2 (Clone bcl-2/100/D5, NovocastraTM HD, Leica Biosystems, UK) (Figure 5).
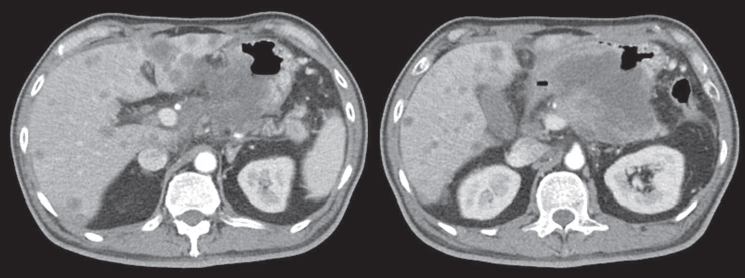
Figure 1. CT scan showing a heterogenous enhanced gastric mass abutting and probably invading the duodenum and pancreas with multiple metastatic lesions in the liver.
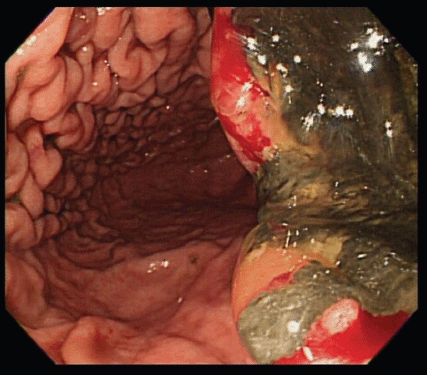
Figure 2. A giant gastric ulcer over lesser curvature of stomach (right half of the figure) revealed by upper gastrointestinal tract endoscopy.
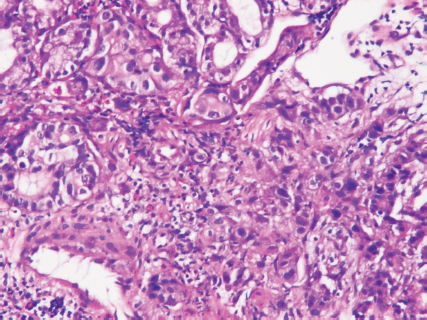
Figure 3. Poorly differentiated carcinoma of stomach, lesser curvature site, endoscopic biopsy (haematoxylin and eosin stain ×400).
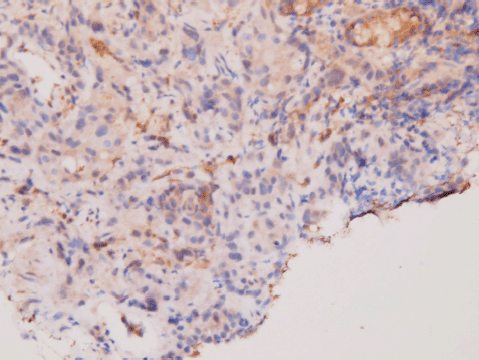
Figure 4. Immunohistochemical staining of DPD. Moderate positivity in tumour cells.
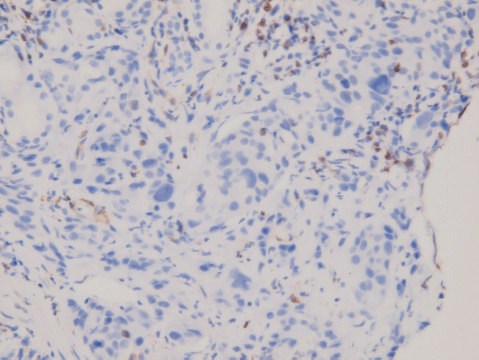
Figure 5. Immunohistochemical staining of Bcl-2. Negative in tumour cells; positive for tumour-infiltrating B-cells.
The patient was initially reluctant to receive any further image examination and treatment upon knowing the diagnosis. However, he came back to our oncology clinic for helping 18 days later due to rapid downhill progress of performance status. At that time, haemoglobin concentration had dropped to 6.6 g/dL. Thereafter, red blood cells had been transfused to maintain haemoglobin level above 8 g/dl. Notable also were elevated levels of alkaline phosphatase (313 iu/l, normal 32~91), gamma-glutamyl transferase (230 iu/l, normal 7~64), lactate dehydrogenase (428 iu/l, normal 98~192), and total bilirubin (6.12 mg/dL, normal 0~1.3). The renal function was still within normal limits and no evidence of viral hepatitis B and C infection was detected. In spite of a normal value of alpha fetoprotein (3.74 ng/mL, normal 0~9), serum levels of carcinoembryonic antigen (50.99 ng/mL, normal 0~5), cancer antigen 125 (1,894.7 iu/mL, normal 0~35), and cancer antigen 19-9 (78257.6 μ/mL, normal 0~35) were abnormally high.
At this moment, he took our advice and agreed to systemic chemotherapy with a regimen composed of cisplatin 50 mg/m2 on day 1, leucovorin 500 mg/m2 followed by bolus fluorouracil 500 mg/m2 and 22-hour continuous fluorouracil 2,000 mg/m2 on day 2 and 3. This protocol, designed to be given on a bimonthly schedule, was modified from those adopted in two previous clinical trials [12, 13]. When calculating the absolute doses for the patient, we used 1 m2 as his body surface area in hope of avoiding too severe adverse effects for such a fragile patient.
Unfortunately, a catastrophic event did happen 2 days after finishing the first course of chemotherapy. The patient experienced sudden onset of intolerable abdomen pain with hypoactive bowel sounds, muscle guarding, and definitely apparent rebounding pain. An emergent non-contrast CT scan gave a picture of distended stomach fully filled with foods, irregular mottled gas collections between stomach and duodenum, ascites accumulation, and free air in the abdomen cavity, leading to an impression of gastrointestinal tract perforation with the perforation site clearly seen (Figures 6–8). In comparison with CT scan performed at initial diagnosis, it seemed that a severe necrotising tumour lysis induced by chemotherapy, morphologically resembling emphysematous pancreatitis [14–16], could explain the whole scenario logically.
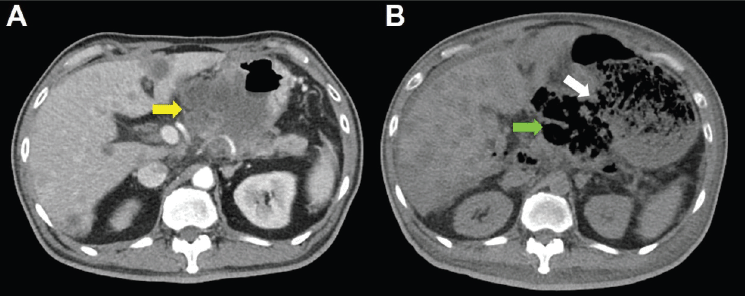
Figure 6. CT scan of the abdomen, horizontal view. A. June 8, 2019. Yellow arrow: the huge gastric tumour. B. July 3, 2019. White arrow: gastric perforation site. Green arrow: Necrotising tumour lysis simulating emphysematous pancreatitis.
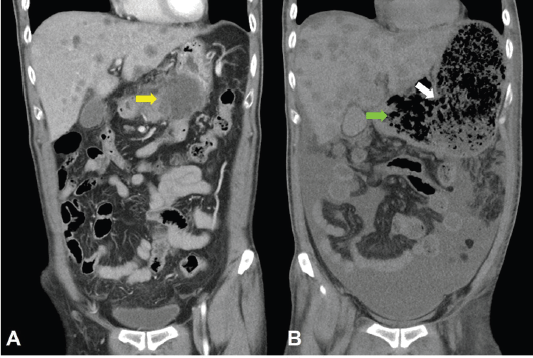
Figure 7. CT scan of the abdomen, coronal view. A. June 8, 2019. Yellow arrow: the huge gastric tumour. B. July 3, 2019. White arrow: gastric perforation site. Green arrow: Necrotising tumour lysis simulating emphysematous pancreatitis.
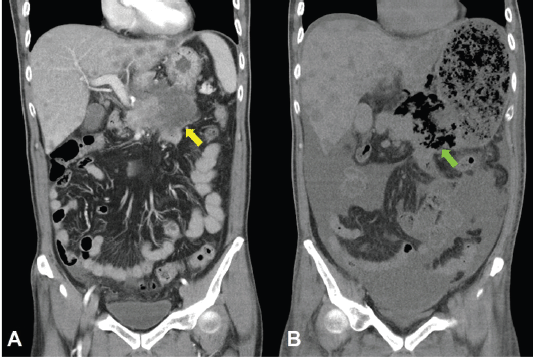
Figure 8. CT scan of the abdomen, coronal view. A. June 8, 2019. Yellow arrow: the huge gastric tumour. B. July 3, 2019. Green arrow: Necrotising tumour lysis simulating emphysematous pancreatitis.
The patient’s family understood the nature of the disease and the dismal prognosis of this subsequent emergent condition very well. Although we planned to sample blood for confirming features characteristic for tumour lysis syndrome, such as serum potassium, uric acid and lactate dehydrogenase levels, they declined further laboratory examination, decided not to let the patient be operated and requested only supportive care with strong pain control. Air hunger with blood oxygen desaturation, drowsy consciousness and blood pressure dropping as the signs of septic shock were observed about 4 hours after abdomen pain attacked. Vital signs became undetectable 2 more hours later.
Discussion
Clinical outcomes of gastrointestinal perforation in metastatic gastric cancer patients undergoing palliative chemotherapy were very poor, especially for undifferentiated carcinoma and patients with septic shock [17]. Peculiarly, gastric perforation due to severe necrotising tumour lysis of a massive stomach lesion, simulating emphysematous pancreatitis, leading to rapidly progressive septic peritonitis like what was seen in this patient, so far as we know, has been hardly reported in the English literature.
Although the risk is low [18], tumour lysis of gastric cancer, either spontaneous [19, 20] or induced by chemotherapy [21, 22], occurred sporadically. Nevertheless, traditional concern about tumour lysis chiefly focus on life-threatening metabolic complications, including hyperuricemia, hyperkalaemia, hyperphosphatemia and hypocalcaemia, which result in renal failure, cardiac arrhythmia, and neurologic deficit [23, 24]. The patient reported here, on the contrast, faced mainly a rare mechanic complication of tumour lysis syndrome. Accordingly, the underlying causes of this unusual tumour lysis phenomenon deserve careful investigation.
The quantity and activity of cellular DPD, the rate-limiting metabolic enzyme of fluoropyrimidine, are significantly correlated with toxicity of fluropyrimidine [25] and the intratumoural DPD expression level is directly linked to chemosenstivity of fluropyrimidine [26, 27]. Therefore, the not so strong DPD expression in our patient’s tumour specimen might be considered as a possible cause of so severe a tumour lysis on receiving fluorouracil therapy. In support of this view, pharmacogenetic variants of DPD and its deficiency have been suggested to guide individualisation of fluropyrimidine dosage [28] and pre-therapeutic survey of DPD activity was recommended for prevention of fluorouracil-induced early severe toxicity [29].
On the other hand, the apoptosis pathway could also play an important role in determining chemotherapy sensitivity. Bcl-2, an anti-apoptosis protein, has been identified as a hopeful major target in cancer therapy [30, 31]. Increasing Bcl-2 expression in gastric cancer cells led to fluorouracil resistance [32], while interference of Bcl-2 expression made gastric adenocarcinoma cells more sensitive to fluorouracil [33]. Hence, the loss of Bcl-2 in our patient’s gastric cancer most likely contributes to such an extreme necrotising tumour lysis causally.
The current therapeutic approach for stage IV gastric cancer with incurable metastasis aims at volume reduction surgery after adequate chemotherapy probably in combination with targeted or anti-angiogenesis agents [34]. It is exciting to see so many novel therapeutic modalities in development for advanced-stage gastric cancer [35, 36]. Nonetheless, based on our experience in this patient, tumour lysis syndrome with severe adverse complications must be taken into consideration when more and more efficient agents are approved for clinical use. Among them, in our opinion, anti-angiogenic monoclonal antibody ramucirumab, with its potential risk of wound healing impairment and gastrointestinal perforation [37, 38], is an example in case of treating a massive gastric tumour like our patient’s.
Conclusions
Despite reducing chemotherapy dose in advance, disastrous tumour lysis with gastric perforation still occurred during treatment of this patient’s huge cancer lesion. Based on this painful experience, we recommend that pre-emptive survey of key metabolic enzyme deficiency and other relative molecules like anti-apoptotic Bcl-2 appears be a necessary step in cancer patient receiving fluropyrimidine therapy. In addition to that, well prepared rescue plan including surgical intervention seems mandatory in treating patients with heavy gastrointestinal tumour burdens like ours. Regarding the risk of gastrointestinal tract perforation, how to get a balance between astonishing efficacy and too severe adverse effects remains an issue to be intelligently solved.
Conflicts of interest
The authors declare that they have no competing interests regarding publication of this case report.
Funding
There is no financial support for this report.
Consent for publication
Written informed consent was obtained from the patient’s daughter for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
1. Roviello F, Rossi S, and Marrelli D, et al (2006) Perforated gastric carcinoma: a report of 10 cases and review of the literature World J Surg Oncol 4 19 https://doi.org/10.1186/1477-7819-4-19 PMID: 16573818 PMCID: 1524767
2. Jwo SC, Chien RN, and Chao TC, et al (2005) Clinicopathological features, surgical management, and disease outcome of perforated gastric cancer J Surg Oncol 91 219–225 https://doi.org/10.1002/jso.20307 PMID: 16121341
3. Ignjatovic N, Stojanov D, and Djordjevic M, et al (2016) Perforation of gastric cancer - What should the surgeon do? Bosn J Basic Med Sci 16 222–226 PMID: 27131023 PMCID: 4978115
4. Kotan C, Sumer A, and Baser M, et al (2008) An analysis of 13 patients with perforated gastric carcinoma: A surgeon’s nightmare? World J Emerg Surg 3 17 https://doi.org/10.1186/1749-7922-3-17 PMID: 18471321 PMCID: 2397385
5. Tsujimoto H, Hiraki S, and Sakamoto N, et al (2010) Outcome after emergency surgery in patients with a free perforation caused by gastric cancer Exp Ther Med 1 199–203 https://doi.org/10.3892/etm_00000032 PMID: 23136615 PMCID: 3490384
6. Lee HJ, Park DJ, and Yang HK, et al (2006) Outcome after emergency surgery in gastric cancer patients with free perforation or severe bleeding Dig Surg 23 217–223 https://doi.org/10.1159/000094753 PMID: 16874002
7. Kinoshita J, Fushida S, and Tsukada T, et al (2015) Efficacy of conversion gastrectomy following docetaxel, cisplatin, and S-1 therapy in potentially resectable stage IV gastric cancer Eur J Surg Oncol 41 1354–1360 https://doi.org/10.1016/j.ejso.2015.04.021 PMID: 26028256
8. Tada K, Etoh T, and Shitomi Y, et al (2017) A case of advanced gastric cancer achieved a pathological complete response by chemotherapy Surg Case Rep 3 68 https://doi.org/10.1186/s40792-017-0344-9 PMID: 28500392 PMCID: 5429316
9. Blanke CD, Chansky K, and Christman KL, et al (2010) S9511: a Southwest Oncology Group phase II study of trimetrexate, 5-fluorouracil, and leucovorin in unresectable or metastatic adenocarcinoma of the stomach Am J Clin Oncol 33 117–120 PMCID: 2967385
10. Yamada T, Kanazawa Y, and Yokoi K, et al (2013) A case of gastric cancer with perforation caused by chemotherapy with docetaxel and S-1 J Nippon Med Sch 80 451–455 https://doi.org/10.1272/jnms.80.451
11. Matsueda K, Toyokawa T, and Ueda Y, et al (2017) Two cases of unresectable advanced HER2-positive gastric cancer perforation during chemotherapy with trastuzumab Nihon Shokakibyo Gakkai Zasshi 114 59–68 PMID: 28070095
12. Ott K, Sendler A, and Becker K, et al (2003) Neoadjuvant chemotherapy with cisplatin, 5-FU, and leucovorin (PLF) in locally advanced gastric cancer: a prospective phase II study Gastric Cancer 6 159–167 https://doi.org/10.1007/s10120-003-0245-4 PMID: 14520529
13. Bamias A, Syrigos K, and Fountzilas G, et al (2004) Intensified bimonthly cisplatin with bolus 5-fluorouracil, continuous 5-fluorouracil and high-dose leucovorin (LV5FU2) in Patients with advanced gastrointestinal carcinomas: a phase I dose-finding and pharmacokinetic study Am J Clin Oncol 27 465–471 https://doi.org/10.1097/01.coc.0000128870.72525.c7 PMID: 15596912
14. Wig JD, Kochhar R, and Bharathy KG, et al (2008) Emphysematous pancreatitis. Radiological curiosity or a cause for concern? JOP 9 160–166 PMID: 18326923
15. Balani A, Dey AK, and Sarjare S, et al (2016) Emphysematous pancreatitis: classic findings BMJ Case Rep 2016 pii: bcr2016217445 [doi: 10.1136/bcr-2016-217445] PMID: 27979845 PMCID: 5174763
16. Bul V, Yazici C, and Staudacher JJ, et al (2017) Multiorgan failure predicts mortality in emphysematous pancreatitis: a case report and systematic analysis of the literature Pancreas 46 825–830 https://doi.org/10.1097/MPA.0000000000000834 PMID: 28609373 PMCID: 5470594
17. Kang MH, Kim SN, and Kim NK, et al (2010) Clinical outcomes and prognostic factors of metastatic gastric carcinoma patients who experience gastrointestinal perforation during palliative chemotherapy Ann Surg Oncol 17 3163–3172 https://doi.org/10.1245/s10434-010-1164-3 PMID: 20585878
18. Mika D, Ahmad S, and Guruvayoorappan C (2012) Tumour lysis syndrome: implications for cancer therapy Asian Pac J Cancer Prev 13 3555–3560 https://doi.org/10.7314/APJCP.2012.13.8.3555 PMID: 23098434
19. Goyal H, Sawhney H, and Bekara S, et al (2014) Spontaneous acute tumour lysis syndrome in gastric adenocarcinoma: a case report and literature review J Gastrointest Cancer 45(Suppl 1) 208–211 https://doi.org/10.1007/s12029-014-9632-9 PMID: 24952154
20. Salmón-González Z, Vieitez-Santiago M, and Martino-González M, et al (2019) Spontaneous tumor lysis syndrome occurring in untreated gastric adenocarcinoma QJM 112 39–40 https://doi.org/10.1093/qjmed/hcy232
21. Han HS, Park SR, and Kim SY, et al (2008) Tumor lysis syndrome after capecitabine plus cisplatin treatment in advanced gastric cancer J Clin Oncol 26 1006–1008 https://doi.org/10.1200/JCO.2007.14.7231 PMID: 18281674
22. Vodopivec DM, Rubio JE, and Fornoni A, et al (2012) An unusual presentation of tumor lysis syndrome in a patient with advanced gastric adenocarcinoma: case report and literature review Case Rep Med 2012 468452 https://doi.org/10.1155/2012/468452 PMID: 22685470 PMCID: 3368228
23. Firwana BM, Hasan R, and Hasan N, et al (2012) Tumor lysis syndrome: a systematic review of case series and case reports Postgrad Med 124 92–101 https://doi.org/10.3810/pgm.2012.03.2540 PMID: 22437219
24. Dubbs SB (2018) Rapid fire: tumor lysis syndrome Emerg Med Clin North Am 36 517–525 https://doi.org/10.1016/j.emc.2018.04.003 PMID: 30037438
25. Del Re M, Di Paolo A, and van Schaik RH, et al (2010) Dihydropyrimidine dehydrogenase polymorphisms and fluoropyrimidine toxicity: ready for routine clinical application within personalized medicine? EPMA J 1 495–502 https://doi.org/10.1007/s13167-010-0041-2 PMID: 23199091 PMCID: 3405332
26. Henricks LM, Opdam FL, and Beijnen JH, et al (2017) DPYD genotype-guided dose individualization to improve patient safety of fluoropyrimidine therapy: call for a drug label update Ann Oncol 28 2915–2922 https://doi.org/10.1093/annonc/mdx411 PMID: 29045513
27. Ishikawa Y, Kubota T, and Otani Y, et al (2000) Dihydropyrimidine dehydrogenase and messenger RNA levels in gastric cancer: possible predictor for sensitivity to 5-fluorouracil Jpn J Cancer Res 91 105–112 https://doi.org/10.1111/j.1349-7006.2000.tb00866.x PMID: 10744051 PMCID: 5926227
28. Zhang C, Liu H, and Ma B, et al (2017) The impact of the expression level of intratumoral dihydropyrimidine dehydrogenase on chemotherapy sensitivity and survival of patients in gastric cancer: a meta-analysis Dis Markers 2017 9202676 https://doi.org/10.1155/2017/9202676 PMID: 28255193 PMCID: 5307138
29. Boisdron-Celle M, Capitain O, and Faroux R, et al (2017) Prevention of 5-fluorouracil-induced early severe toxicity by pre-therapeutic dihydropyrimidine dehydrogenase deficiency screening: Assessment of a multiparametric approach Semin Oncol 44 13–23 https://doi.org/10.1053/j.seminoncol.2017.02.008 PMID: 28395758
30. Labi V, Grespi F, and Baumgartner F, et al (2008) Targeting the Bcl-2-regulated apoptosis pathway by BH3 mimetics: a breakthrough in anticancer therapy? Cell Death Differ 15 977–987 https://doi.org/10.1038/cdd.2008.37 PMID: 18369371 PMCID: 4563920
31. Adams JM and Cory S (2018) The BCL-2 arbiters of apoptosis and their growing role as cancer targets Cell Death Differ 25 27–36 https://doi.org/10.1038/cdd.2017.161
32. Du P, Hu C, and Qin Y, et al (2019) LncRNA PVT1 mediates antiapoptosis and 5-fluorouracil resistance via increasing Bcl2 expression in gastric cancer J Oncol 2019 9325407 https://doi.org/10.1155/2019/9325407 PMID: 31205469 PMCID: 6530232
33. Yu DF, Wu FR, and Liu Y, et al (2013) Bcl-2 gene silence enhances the sensitivity toward 5-Fluorouracil in gastric adenocarcinoma cells Biomed Pharmacother 67 615–619 https://doi.org/10.1016/j.biopha.2013.03.007 PMID: 23684481
34. Yoshida K, Yamaguchi K, and Okumura N, et al (2016) Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification Gastric Cancer 19 329–338 https://doi.org/10.1007/s10120-015-0575-z PMCID: 4824831
35. Kumar V, Soni P, and Garg M, et al (2018) Emerging therapies in the management of advanced-stage gastric cancer Front Pharmacol 9 404 https://doi.org/10.3389/fphar.2018.00404 PMID: 30271341 PMCID: 6146175
36. Fontana E and Smyth EC (2016) Novel targets in the treatment of advanced gastric cancer: a perspective review Ther Adv Med Oncol 8 113–125 https://doi.org/10.1177/1758834015616935 PMID: 26929787 PMCID: 4753351
37. Mandai K, Shirakawa A, and Uno K, et al (2017) Endoscopic ultrasound-guided drainage of intra-abdominal abscess after gastric perforation in a patient receiving ramucirumab and paclitaxel for advanced gastric cancer Case Rep Oncol 10 15–20 https://doi.org/10.1159/000455226 PMID: 28203161 PMCID: 5301121
38. Urakawa S, Sakai D, and Miyazaki Y, et al (2017) A case of ramucirumab-related gastrointestinal perforation in gastric cancer with small bowel metastasis Surg Case Rep 3 127 https://doi.org/10.1186/s40792-017-0399-7 PMID: 29260338 PMCID: 5736507






