Breast cancer and the black swan
Michael Retsky1, Romano Demicheli2, William Hrushesky3, Ted James4, Rick Rogers1, Michael Baum5, Jayant S Vaidya6, Osaro Erhabor7 and Patrice Forget8
1Harvard T.H. Chan School of Public Health Boston, MA 02115-6021, USA
2University of Milan, Faculty of Medicine and Surgery, Milan 20133, Italy
3University of South Carolina, Columbia, SC 292012, USA
4Harvard Medical School, Beth Israel Deaconess Medical Center, Boston, MA 02215-5400, USA
5Emeritus Prof, University College London, London N19 5LW, UK
6University College London, London N19 5NF, UK
7Usmanu Danfodiyo University, Sokoto 840004, Nigeria
8University of Aberdeen, Aberdeen AB25 2ZD, UK
Abstract
Most current research in cancer is attempting to find ways of preventing patients from dying after metastatic relapse. Driven by data and analysis, this project is an approach to solve the problem upstream, i.e., to prevent relapse.
This project started with the unexpected observation of bimodal relapse patterns in breast and a number of other cancers. This was not explainable with the current cancer paradigm that has guided cancer therapy and early detection for many years. After much analysis using computer simulation and input from a number of medical specialties, we eventually came to the conclusion that the surgery to remove the primary tumour produced systemic inflammation for a week after surgery. This systemic inflammation apparently caused exits of cancer cells and micrometastases from dormant states and resulted in relapses in the first 3 years post-surgery.
It was determined in a retrospective study that the common inexpensive perioperative non-steroidal anti-inflammatory drug (NSAID) ketorolac could curtail the early relapse events after breast cancer surgery. A second retrospective study strongly confirmed this but an apparently underpowered prospective study showed no advantage.
We are analysing these data and are now proposing to test the perioperative NSAID at Beth Israel Deaconess Medical Centre with triple-negative breast cancer (TNBC) patients, the category that could respond best to the perioperative NSAID. If this works as well as we expect, we would then transfer this technology to low- and/or middle-incomes countries (LMICs), starting with Nigeria where early onset type of TNBC is common. There is an unmet need in LMICs, especially in countries like Nigeria (190 million population), for a means to prevent surgery induced relapse that we are attempting to resolve.
This work aims, thus, to describe eventual mechanisms, and ways to test a solution addressing an unmet need. But first, we consider the context, including within an historical perspective, important to explain how and why a Kuhnian paradigm shift may be considered.
Keywords: breast cancer, bimodal relapse hazard, computer simulation, early relapse, surgery induced systemic inflammation, perioperative NSAID ketorolac, mechanisms, unmet need in Nigeria, proposed solution
Correspondence to: Michael Retsky
Email: michael.retsky@gmail.com
Published: 28/05/2020
Received: 30/10/2019
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Part A—a contextual explanation: breast cancer data challenge the cancer therapy paradigm
Introduction
This project started in 1993. Data were presented at a European conference by Romano Demicheli of the Milan National Cancer Institute. These data showed an unexpected bimodal relapse pattern for women treated with mastectomy only. At the same conference, Michael Baum described data from UK that also showed a bimodal relapse pattern.
The black swan phenomenon or how anomalous data can generate a new model
From Wikipedia: The phrase ‘black swan’ derives from a Latin expression; its oldest known occurrence is from the 2nd-century Roman poet ‘s characterisation of something being ‘rara avis in terris nigroque simillima cygno’ (‘a rare bird in the lands and very much like a black swan’). When the phrase was coined, the black swan was presumed not to exist. The importance of the metaphor lies in its analogy to the fragility of any system of thought. A set of conclusions is potentially undone once any of its fundamental postulates is disproved. In this case, the observation of a single black swan would be the undoing of the logic of any system of thought, as well as any reasoning that followed from that underlying logic.
These data from Milan will be shown in full detail but as an overview, there were 1,173 patients treated by mastectomy that have been followed-up for over 10 years. More details are shown in Table 1. The persons in Italy are known to typically stay in the same town for generations and are generally considered to be compliant with physician directives. One person—Pinuccia Valagussa has been the data base manager since the project started. These are favourable factors for a high-quality database.
The Milan data are presented separately for the post-menopausal patients and the pre-menopausal patients in hazard or rate of relapse probability. These data are presented as Figures 1 and 2 and also in the more usual disease-free survival format as Figure 3 from Bonadonna et al [1]. Data were transcribed for Figure 3.
Table 1. Findings and interpretations regarding peaks of relapses after breast cancer surgery.
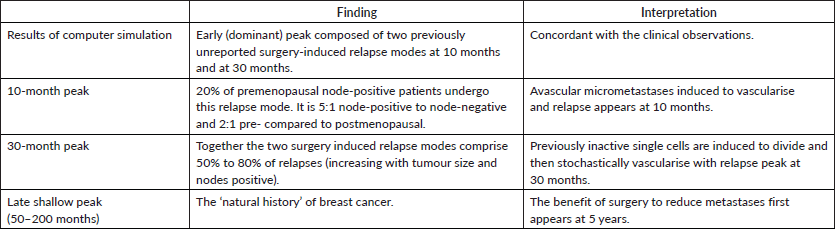
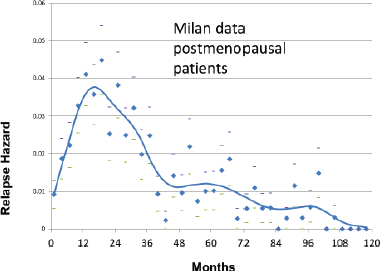
Figure 1. Relapse hazard for postmenopausal breast cancer patient vs. months since mastectomy.
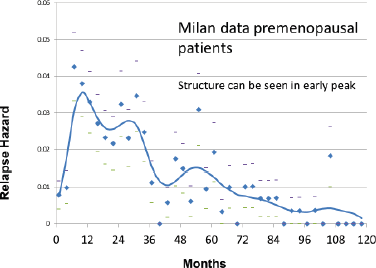
Figure 2. Relapse hazard for premenopausal breast cancer patients versus months since mastectomy.
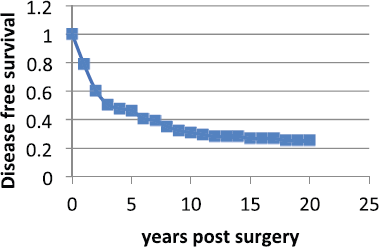
Figure 3. Disease Free Survival vs Years post-surgery from Bonadonna et al [1]. Data were transcribed from the original paper.
Retsky and Demicheli decided to collaborate. Their primary interest was to try and decipher the anomalous bimodal relapse hazard data. Demicheli checked and verified that these data were not an artefact resulting from specific periodic patient follow-up schedules that would artificially lead to such anomalies.
In a visit to Milan in 1994, Valagussa gave a computer disk with her data to Retsky. Demicheli then described what he thought to be the most basic sequence of the steps that a cancer must undergo from a single cell to a lethal size tumour.
Demicheli has MD, PhD degrees and did much experimental work on tumour growth. He described the simplest growth pattern that starts with a malignant cell that is in a non-dividing state. It can be dormant for a variable period of time. Once it starts to divide it can grow to a size of approximately 1 mm or 1 million cells. It cannot get larger than that until the host provides a blood supply or angiogenesis. After angiogenesis, the tumour can grow to be a detectable size and even achieve lethal size. This elementary growth model is considered valid now but 25 years ago in 1994 it would not have been so considered.
Figure 4 describes the collaboration. Retsky [2] had done much work in computer simulation of tumour growth data starting in 1983 and undertook the task of using Demicheli’s model and Valagussa’s data to try and understand what the bimodal relapse peaks represented. Retsky had much good and bad experiences in working with breast cancer databases. He trusts the Milan data and much of that was due to the presence of Pinuccia Valagussa. Retsky got a small grant from NIH to make a computer simulation of breast cancer using the Milan data.
A main tool that was developed in this research project was a computer simulation of tumour growth from single cells to relapse size tumours. Our concept was to build a computer simulation of tumour growth based as much as possible on published human data. A major resource was a Retsky et al paper [3] showing published data for over 100 untreated spontaneous tumours mostly in humans.
Among the data in that 1990 paper, there was one report demonstrating dormancy followed by regrowth for an untreated primary breast tumour in a 78-year-old woman [3–5]. Her husband had died recently after spending their life savings in futile treatment of cancer of the larynx. She stubbornly refused to submit to surgery but allowed the physician to take occasional roentgenograms. There were four X-rays taken over 22 months. We have seen no other untreated breast cancer data in the literature with three or more measurements taken over more than 1 year. The tumour was detected with a cross-sectional area of 4.5 sq. cm. essentially in a growth plateau (7,200–day doubling time) for almost a year and then it began to grow (180–day doubling time) before it was removed and examined after another year. It was unremarkable adenocarcinoma. A 4.5 sq. cm. tumour with a constant doubling time of 7,200 days leads to the impossible result that the tumour started growing 655 years earlier. The tumour must have grown and then gone dormant prior to diagnosis.
The first version of the simulation was based on the growth model described in Figure 4. It turned out to be well suited to simulate the relapses from years 4 to 10 for the Milan data shown in Figures 1 and 2. However, it was unable to simulate the early relapses in years 1–3. The early relapse peaks were too sharp for that original model.
We were also aware of human data showing sudden growth of pulmonary metastases after amputation of osteosarcoma in a young boy in a 1968 paper by Smithers [3,6]. Thus, it was reasonable to include possible surgery induced exits from dormancy. With that addition, the computer model was well able to simulate all relapses in years 1–10 of Figures 1 and 2.
Based on the simulation that was fit to the Milan data, we hypothesised that the relapses at 10 months were surgery induced angiogenesis of avascular micrometastases. The relapses at 30 months were surgery induced single cell division followed by stochastic angiogenesis. The relapses that had a shallow peak at 60–70 months with a long tail extending to 10 and more years were not stimulated by surgery. The differences between premenopausal and postmenopausal patients are described in Table 1.
There were few relapses at 10 months for postmenopausal patients so the 10-month peak and the 30-month peak were indistinguishable in the data, thus appearing as a single 18-month peak.
The results of the simulation are shown in Figure 5 superimposed on the relapse hazard for the premenopausal patients.
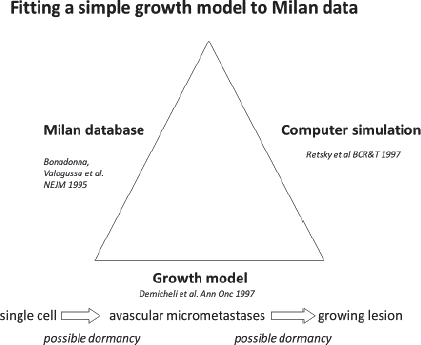
Figure 4. Description of the collaboration between Retsky, Demicheli and Valagussa.
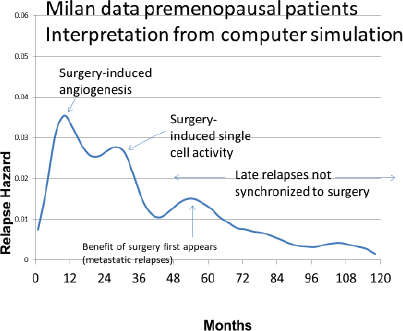
Figure 5. Simulation of breast cancer using the Milan data.
According to the simulation, the relapses in the first 3 years after surgery are iatrogenic or precipitated somehow by an intervention at or near the time of surgery. The relapses at the 10th month are dormant avascular micrometastases that were induced somehow into angiogenesis by the surgery and appeared as detectable metastases at 10 months. The 30-month events were single dormant cells that were induced into division by the surgery and then underwent angiogenesis stochastically and showed up as relapses at approximately 30 months.
The small shallow peak at 60 or 70 months represents the more or less natural history of breast cancer. The point at which the benefit of removal of the primary tumour to reduce metastases first appears at about 60 months. The peaks at 60–70 months and 100 months are currently under investigation. The quantitative results are listed in Table 1.
Ironically, Retsky was treated for Stage IIIc colon cancer during 1995–1997 while doing the computer simulation. This became the first use of metronomic chemotherapy for early stage cancer and was documented by Bernstein in Propublica [7]. The therapy was designed by W. Hrushesky.
Retsky and Demicheli later edited a book on this project that was published by Springer-Nature in July 2017 [5]. Much of the information presented in this paper was published in the book. Since the book is available online at Retsky’s Harvard website (https://www.researchgate.net/publication/321146437_Perioperative_inflammation_as_triggering_origin_of_metastasis_development), we can go over some of this information in an abbreviated fashion.
How this new model can challenge the current paradigm
The Foreword to the book was written by Robert Weinberg of MIT. There are two provocative comments at the end of Weinberg’s Foreword that need to be presented here.
He notes that if demonstrated definitively, the findings in this book lead to a notion that is likely to be accepted only reluctantly by many in the clinical oncology community: primary tumour resection does not provide an undiluted benefit to the breast cancer patient in terms of long-term survival. He also notes that the findings reported in the book are likely to profoundly influence future surgical oncology and post-surgical treatment protocols.
A clear bimodal relapse pattern can be identified in figure from a 1984 paper by Fisher et al [8]. This figure is transcribed from the original. The original Fisher, Sass and Fisher figure can be seen in the Springer-Nature book on page 24. Likewise, the original Bonadonna, Valagussa NEJM 1995 figure can be seen on page 23.
These are disease free survival data for patients treated only with mastectomy and grouped by the number of axillary nodes positive. The number of nodes positive is well known to be the most important prognostic factor for relapse in breast cancer. The curve on top is for patients with zero nodes and the curve on the bottom is for patients with more than 12 nodes positive for cancer.
Consider the zero node patients. Mastectomy alone will cure about 80% of these patients. These 80% will never have a relapse. Of the 20% that relapse, 10% or half of the relapses occur in the first 3 years and then there is a period from 3 years to 5 or 6 years with few relapses and then the remaining 10% of relapses occur between 6 and 10 years.
Now looking at the patients with more than 12 nodes positive, essentially all the patients relapsed. The prognosis for >12 nodes was very poor, especially prior to the time of adjuvant chemotherapy in the 1980s. Of the essentially 100% that relapse, about 85% relapse in the first 3 years and then there are few relapses until 6 years when the remaining 15% relapse.
It is very interesting that while the magnitudes differ greatly, the timing of the early and late relapses for N = 0 patients is virtually the same as for the >12 node patients. This suggested to us that something happens to about 10% of patients with zero nodes that causes them to relapse within 3 years of surgery. Something similar seems to occur to 85% of patients with >12 nodes that causes them to relapse within 3 years of surgery. That apparently is the major difference between the zero node and the >12 node populations.
This finding may not be such a major surprise to the medical community. National Institutes of Health has long denied that surgery can cause cancer to spread ‘The chance that surgery will cause cancer to spread to other parts of the body is extremely low’ [9]. However, as noted by Komaroff in NEJM June 14, 2018, ‘Clinical lore says that resection of a primary tumour can cause previously inapparent metastatic deposits to flare’ [10]. Therefore, overlooking any mendacity, the possibility that primary surgery can cause metastatic activity may not be as revolutionary as we originally thought.
Taking a clue from the computer simulation shown in Figure 5 and the Fisher Sass and Fisher data in Figure 6, there appears to be a connection between the number of nodes positive and the probability of surgery-initiated relapses within 3 years of surgery.
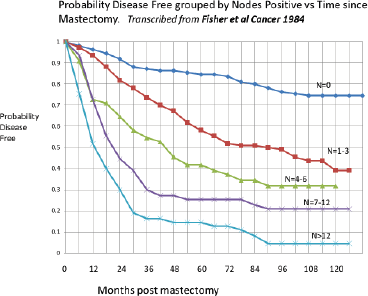
Figure 6. Data from Fisher, Sass and Fisher [8]. This figure has been transcribed from the original. The relapses in the first 3 years and after 6 years can be seen especially for the N = 0 and the N > 12 data. The magnitudes vary but the timing is quite similar from N = 0 to N > 12 data.
Figure 6 has relevance to the early detection of breast cancer. Mammography was initiated to detect cancer in a most curable state—early in the disease and thus mostly with zero or few nodes positive and small tumours. It is clear from Figure 6 that such patients are easiest to cure with surgery only. Adjuvant therapy would provide additional benefit. Patients diagnosed with many nodes positive are considered a failure of early detection. Numerically, with mammography the percentage of patients age 40–49 diagnosed with positive nodes is 22.4% while without mammography the percentage is 43.4% [11]. The speculative question arises: Would early detection be nearly as important if the surgery-induced relapses can somehow be prevented?
After becoming familiar with relapse hazard and DFS data in breast cancer, reasonably similar patterns can be identified in other cancers (Table 2).
Was this problem known 2,000 years ago?
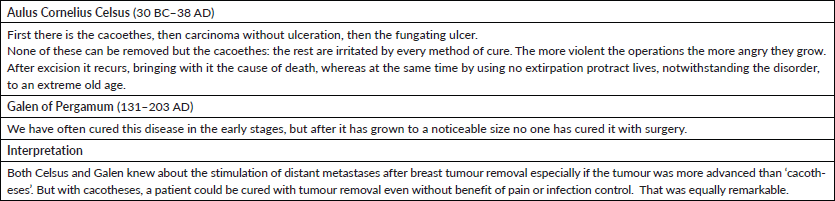
A paper by Baum [29] described his comment about the bimodal relapse pattern. In that same book, a paper by Irving Ariel [30] provides a deep historical perspective of the treatment of breast cancer. It was most interesting in that Ariel presented information written by Galen of Pergamum and Aulus Cornelius Celsus. These were two famous historic Greek and Roman scientists and physicians. Their statements are shown in Table 3. These statements have been translated several times so the exact wording may not be perfect.
We are apparently latecomers to a 2000 year old problem in oncology.
Cacotheses is a term frequently used up to the 1800s and refers to small but apparent tumours. According to the theory presented in this document, primary surgery causes metastatic activity from dormancy and adjuvant chemotherapy is administered to partially offset this metastatic activity. If we could prevent metastatic activity from becoming stimulated after primary surgery, would adjuvant chemotherapy be needed and would it be effective? Theoretically the answer is no to both questions. Further, according to data from Agresti et al [31], Her2 patients have significant surgery induced exit from dormancy. What might that mean? These are very serious issues and need to be examined in careful detail before any clinical changes are considered.
Table 2. Similar bimodal relapse pattern seen in other solid cancers.
Table 3. Comments from Celsus and Galen on treating breast cancer 2000 years ago.
Can this explain important clinical observations in breast cancer?
These surgery-induced effects are so large that they should be observable in clinical data if we knew what to look for. Following are four such effects that can be explained by our theory.
Adjuvant chemotherapy
Adjuvant chemotherapy works particularly well for premenopausal N patients. The curative effect of adjuvant chemotherapy is mostly confined to premenopausal node positive patients in which case approximately 12% are cured. For other categories, the curative rate is in the lower single digits [32]. This is reasonable since surgery induced angiogenesis produces the most rapidly growing cancer deposits possible just post-surgery when it was empirically found that maximum tolerated chemotherapy works best [33].
Notice that the coauthors in this 2004 paper [33] include Gianna Bonadonna with 30 years experience in developing adjuvant chemotherapy protocols and Judah Folkman with 30 years experience in developing the science that a tumour needs angiogenesis in order to grow larger than a mm or so.
Mammography screening
Mammography works better for women age 50–59 than age 40–49 years.
Mammography researchers knew that the early detection would be beneficial but they did not know the quantitative benefit so they conducted clinical trials in US, UK and Sweden. When examining the results of early detection for women age 50–59, they found 20% survival advantage that appeared early and was similar across the various trials. However, when they looked at trials for women age 40–49, there was an anomaly. Early in all trials (US, UK and Swedish overview), there was excess mortality for the intervention arm compared to controls. This caused great confusion and consternation and precipitated what came to be known as the mammography wars [34–36].
We could explain this. For women age 40–49 years, who are mostly premenopausal, there are some women who have breast cancer at detectable size and some of these who also have avascular microdeposits. When these cancers are detected, they are surgically removed which activates the dormant metastatic deposits. These appear as detectable relapses at about 10 months post-surgery. Since survival after relapse is approximately 2 years, if this is true, there would be a surge in mortality starting 3 years after the screening trial begins. That is precisely what was found in US, UK and Swedish overview data [37]. The magnitude was also calculated from the Milan data and agreed with mammography trial data. We published several papers explaining this.
Before publication, the 2005 paper [37] happened to become known to Amy Marcus, a Pulitzer Prize winning reporter at Wall Street Journal, since she was a guest at several of the Folkman lab meetings. On the day the paper came out (September 13, 2005), Harvard issued a press release and Amy Marcus published a 1,200-word report in the Wall Street Journal [38]. This drew much attention and we received a number of letters from readers. One in particular from Dr. Isaac Gukas led us into a new direction of our research.
Dr. Gukas was a surgeon in UK. He was originally from Nigeria where he practiced oncology for about 15 years. He wrote in his letter that our paper could explain what happens in Nigeria. Breast cancer in Nigeria is typically detectable as a lump at age mid 40 years. Relapses occur very soon after mastectomy and this became well known among the population. The word Gukas used to describe this effect was that the surgery ‘provoked’ the cancer. As a result, women who find breast lumps would usually see an herbalist rather than a surgeon and then go back to their village where they eventually die from untreated breast cancer.
Baum, Gukas and Retsky met at the Royal Medical Society in London in 2006. Gukas described a time in Nigeria when he had a televised debate with an herbalist on how to best treat breast cancer.
Racial disparity in outcome (inversion at age 57 years)
In the US, there is 1.5 excess mortality from breast cancer for African Americans (AA) compared to European Americans (EA) Retsky et al [39]. However, there is an inversion at age 57 years [40]. AA who are diagnosed under age 57 years have excess mortality compared to EA but if diagnosed over age 57 years have superior mortality outcome compared to EA. This means that we cannot explain the excess mortality of AA on reduced access to quality medical care. It must be biological. This was considered good news since it is far easier to fix a biological problem than to fix a socio-economic problem. Demicheli et al [41] is particularly important.
‘Aggressiveness’ of breast cancer in young women
Breast cancer is often referred to by clinicians as aggressive in young women. From the clinicians’ viewpoint, premenopausal breast cancer appears as aggressive since young patients often relapse soon after primary surgery, while from our perspective, it is clockwork relapse at 10 months from surgery.
At this point, it was apparent that something happens at or about the time of surgery to initiate exit from dormancy and result in relapses within 3 years of surgery. These relapses within 3 years of surgery comprise 50% to 80% of all relapses. (Reminder that we are still speaking of breast cancer data from patients treated before the routine use of adjuvant chemotherapy and hormone therapy.) We did not know what that process was or how to stop it. However, it was clear that something had to be done before or at the time of surgery.
Part B—mechanisms, testing a solution and addressing an unmet need
Two events occurred that led us to consider a mechanism based on inflammation as a driving force for metastatic activity. Could inflammatory processes be therapeutic targets?
El Saghir et al paper 2005 [42]
First, a paper was submitted by El Saghir et al to BMC Cancer and Retsky was asked to review it. This was a case report of a 50-year-old Lebanese male who was diagnosed with inoperable non-small-cell-lung-cancer. The patient was treated with radiation and released from the hospital. His prognosis was very poor but at least for a while he could lead a normal life. While driving his car, he bumped his head on the sun visor and within 30 days a 7 cm tumour grew there. El Saghir suggested this could be an example of surgery-induced growth that Retsky and Demicheli had been describing.
Having a computer simulation allowed us to numerically consider if the El Saghir et al patient’s tumour growth could be what we have been writing about. It was clearly numerically not possible. But then we did not know what could have caused this sudden growth. Taturo Udagawa suggested that we look at a paper from Mina Bissel’s group on inflammation seen in an Avian Roux Sarcoma model. The paper was Martins-Greens et al [43]. Also, see Dolberg 1985 [44]. The inflammation is responsible for the development of wound-induced tumours in chickens infected with Rous sarcoma virus and described a virus model in which tumour would grow at any point where a wound was produced but the interesting part was that this was controllable with inflammation. If inflammation was allowed, the tumour would grow but if inflammation was prevented, no tumour would grow at the wound site.
Retsky’s published commentary to the El Saghir et al [45] paper stated: ‘The unusual isolated and exaggerated situation allowed El Saghir et al to observe what may be a new and possibly important hematologic pathway: inflammation as a facilitating precursor to tumour’. However, a virus particle is 1/100 of the size of a cancer cell so the mechanism reported in the Martins-Green paper was not a clear link to explain the El Saghir report but we were alerted to the possible importance of inflammation as a facilitating mechanism for wounding leading to cancer activity. http://www.biomedcentral.com/1471-2407/5/94/comments
Forget et al paper 2010 [46]
Second and much more important, a paper [46] was published by a Brussels anaesthesiology group that reported perioperative use of non-steroidal anti-inflammatory drug (NSAID) ketorolac significantly reduced early relapses in a retrospective study. This group is based at a teaching hospital so they need to expose the anaesthesiology residents to all the drugs used in anaesthesia. There were six anaesthesia drugs considered. There are now three NSAIDs that are available as iv but just one—ketorolac—was significantly used in this centre at that time. Their data are shown as Figure 7. There was a clear strong reduction in relapses for perioperative ketorolac in a retrospective study of 320 consecutive breast cancer patients given mastectomy by one surgeon and treated with conventional adjuvant therapy.
After a visit to Brussels by Demicheli and Retsky, data were updated by Sarah Amar and analysed by Demicheli. This is shown in Figure 8.
Mathematically, in chaos theory a minute change in a complex system can have large effects elsewhere. Perhaps cancer can be considered a system in chaos [47].
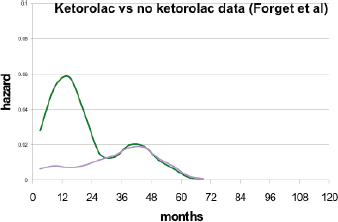
Figure 7. Breast cancer relapses after breast cancer surgery, observed by Forget et al in 2010. Purple is for patients having received ketorolac vs. no ketorolac in blue.
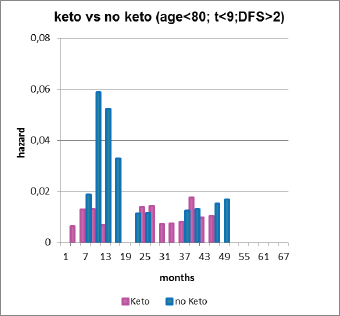
Figure 8. Forget et al data updated by Sarah Amar and analyzed by Demicheli. Note the five-fold reduction in relapses months 9–18 (3 versus 15 events). This histogram is useful to visually show the large reduction in early relapses.
Considering Figure 9, it may be a coincidence but there has been a steady small reduction in breast cancer mortality starting in 1990. It has been claimed that this is due to improvements in early detection and also in effect of therapy. Is it also possible that the use of ketorolac is another reason?
What mechanisms could explain these data and our computations?
We naturally suspected something to do with inflammation. At this point, we undertook the task of looking for correlations among the fields of surgery, dormancy, inflammation, immunology, oncology, circulating tumour cells and wounding. We of course knew these were very large and complex fields (Table 4).
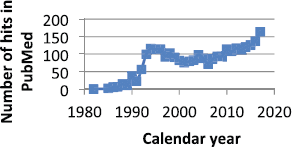
Figure 9. Number of times ketorolac is mentioned in PubMed since 1980. It was apparently brought into significant use starting in 1990.
Table 4. Survey of observations and hypotheses among the fields of surgery, dormancy, inflammation, circulating tumour cells, wounding and immunology.
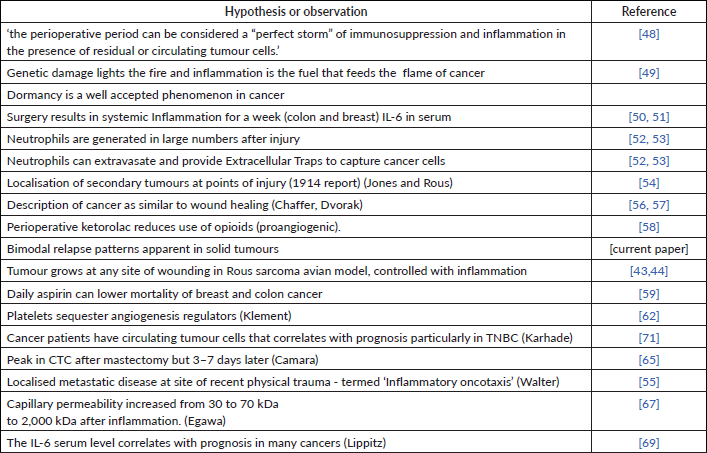
Platelets
Platelets actively sequester angioactive factors and degranulate in the presence of inflammation [62]. Platelet count drops by about 10% and VEGF increases also by 10% for a week after surgery. This could account for the relapses 10 months after surgery that happen for all categories of patients but most pronounced for premenopausal node positive patients.
Neutrophils
Neutrophil extracellular traps can capture circulating cancer cells [52, 53] and since there is increased capillary permeability after surgery [67] and neutrophils have the general ability to extravasate, it is possible for this to cause sudden increase in CTCs to become trapped in distant organs within a week of surgery and cause relapses 30 months later.
The Bonnelykke—Behrndtz Chapter in the Springer book [69] describes an experiment in zebrafish where neutrophils are attracted to a wound. That is reasonable since the main function of neutrophils is to aid in wound healing. However, the neutrophils are diverted to nearby preneoplastic cells that then start to divide. Zebrafish are semitransparent and neutrophils can be colour labelled allowing visual tracking of their paths. Fish are a far older species than humans but this could be a mechanism to explain 30 month relapse. That it remains a possibility is also addressed by Dillekas and Straum [65].
Krall et al protumoural immunological balance (2018)
In a major project at MIT in Dr. Weinberg’s lab, Krall et al [70] developed an animal model for dormancy of single cancer cells. In mice with an intact immune system Krall et al were able to inject cancer cells that became dormant and stayed viable. If the immune system was compromised this did not happen. Any surgery would cause activated monocytes from the marrow to travel to the sites of the pseudo-metastases and become tumour-associated macrophages and suppress the immune system locally causing exit from dormancy.
From our particular perspective this was very important since they could do an experiment. We are skilled in analyzing clinical data but cannot do an experiment. Krall et al were able to show that they could operate on the left side of a mouse and tumour grew on the right side (and vice versa). This process could be controlled with a perioperative NSAID. MIT issued a press release that received national attention.
Panigraphy et al (2019)
Another very important paper by Panigrahy et al [84] reports in animal models that Ketorolac and resolvins exhibited synergistic antitumour activity and prevented surgery or chemotherapy-induced dormancy escape.
Are there specific clinical data that support the hypothesis that post-op inflammation provokes metastases?
Reference to data from Kita et al [85]—Does Postoperative Serum Interleukin-6 (IL-6) Influence Early Recurrence after Curative Pulmonary Resection of Lung Cancer?
Kita et al [85] examined the influence of inflammatory cytokine levels on postoperative early recurrence in patients who underwent curative lung cancer surgery. In 107 patients who underwent curative pulmonary resections for non-small cell lung cancer from November 2007 to June 2008, they measured IL-6 levels preoperatively, and on postoperative day (POD) 0, 1, and 2.
One year after the date of enrolment of the last patient, they investigated survival status of each patient and identified a group with recurrence.
Among the 107 patients, 29 patients developed recurrence with a mean follow-up of 18.1 months (range 14 to 21). Clinical stage was significantly more advanced in the recurrence group than in the non-recurrence group (p = 0.005). Serum IL-6 levels on POD 1 were significantly higher in the recurrence group than in the non-recurrence group (p = 0.007). Stage and serum IL-6 levels on POD 1 were significant independent predicting factors for postoperative early recurrence (p = 0.006, p = 0.003). They concluded that the higher the serum IL-6 levels on POD 1, the higher the risk of early postoperative recurrence.
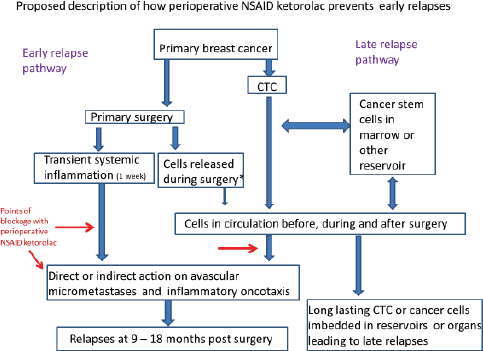
Figure 10. Proposed mechanism along with the conventional mechanisms.
On the right is presented the usually accepted relapse mechanism. Cancer cells leave the primary and get deposited in reservoirs where they can reside for variable times up to a number of years. Eventually, some escape and ultimately lead to relapses. On the left is our proposal. As a result of the primary surgery, there is period lasting a week during which systemic inflammation exists and this can cause increased capillary permeability leading to circulating cancer cells getting trapped or deposited in distant organs. Also, platelets degranulate releasing VEGF causing angiogenesis of dormant avascular deposits. Resulting relapses in the first three years are a result. We do not think cancer cells are often released during surgery.
Are there other clinical data to show that ketorolac prevents early relapses?
In addition to the initial Forget et al [46] report, there are retrospective data for breast (Desmedt et al [79]) and for ovarian (Hudson et al [86]). As reported by Hudson:
‘At the 60‐month follow‐up, 3/17 ketorolac‐treated (18%) and 40/92 non‐ treated patients (43%) had died of ovarian cancer (log‐rank test p‐value = 0.09). Stratified log‐rank tests for categorical factors such as age group, American Joint Committee on Cancer (AJCC) stage, completion of chemotherapy as planned, and receipt of neoadjuvant chemotherapy showed a consistent ketorolac survival benefit in each stratum. The survival benefit of ketorolac was also evident in the proportional hazards analysis when adjusted for age at diagnosis, AJCC stage, completion of chemotherapy as planned and receipt of neoadjuvant chemotherapy. The adjusted HR for ovarian cancer–specific mortality associated with peri‐operative ketorolac (yes versus no) was 0.30 (95% confidence interval (CI), 0.11–0.88). While these findings must be interpreted cautiously because they are only partially controlled for the propensity to receive ketorolac, they suggest that, similar to the breast cancer data, peri‐operative ketorolac reduces ovarian cancer‐specific mortality.’
How to test the ketorolac prevention of early postoperative relapses?
A chance meeting with some Nigerian physicians and scientists at a conference led to three trips to Nigeria and intense collaboration. Our main contact is co-author Prof. Osaro Erhabor, who is on Faculty of Medical Laboratory Science, Usmanu Danfodiyo University, Sokoto, Nigeria. Retsky spoke a number of times on the breast cancer perioperative NSAID project and why it might be very effective at low cost in sub-Saharan Africa where early onset (mid age 40s) and triple negative is typical. We also learned about the many potential and real problems that would be encountered doing a clinical trial in Nigeria.
Women in Nigeria typically avoid mastectomy since it is well known to them that the surgery ‘provokes’ the cancer to recur and results in death shortly thereafter. Instead they go to an herbalist for soothing ointments and then go back to their village where they die from untreated breast cancer. There is an unmet need, especially in Nigeria (190 million population) for a means to prevent surgery induced relapse that we are attempting to resolve.
After extensive discussions with our Nigerian, European and US colleagues, it was determined that all these problems are serious but solvable and that a trial could be conducted in Nigeria. Several grant proposals were submitted but rejected.
There were several significant resolutions of potential problems. For one, we had a meeting on email about possible use of mammography to detect breast cancer. Our colleague Michael Baum is a world authority on the benefits and harms from mammography [29]. He wrote the first chapter in the Springer Nature book that discusses the history of breast cancer. Baum argued that in order to use mammography, Nigeria would have to train a generation of radiologists, mammography operators and technicians to calibrate the devices that would be highly expensive and take much time. Other solutions are possible. He suggested downstaging as has been done successfully in Malaysia and India. After examining published results from Malaysia [77, 78], we agreed that downstaging would be a very reasonable method of finding breast cancer in a state treatable with perioperative NSAID ketorolac. Our Nigerian colleagues agreed.
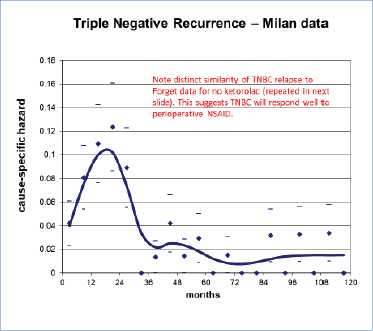
Figure 11. TNBC recurrence data from Milan as seen in Figure 11 is quite similar to the no-ketorolac arm of Forget et al data (Figure 7). This strongly suggested to us that perioperative ketorolac will be effective in sub-Saharan Africa where TNBC is common (See chapter 6.) The low cost will be another enhancing factor. Low and Middle Income Countries have 70% of the world’s cancer burden but 5% of the financial resources [75].
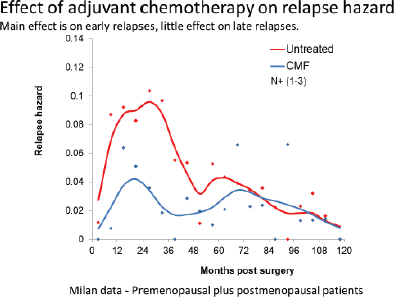
Figure 12. Data from Milan show that the benefit of adjuvant chemotherapy is mainly to reduce the early relapses. This is consistent with the analysis based on the computer simulation.
Malaysia was able to reduce locally advanced breast cancer at presentation from 60% to 35% in 5 years with an expenditure of $35,000. They trained several hundred staff to talk to groups at churches and other gatherings. They would tell women that they should contact their staff if a breast lump was detected. They would send a nurse to determine if the lump was a cyst or possibly breast cancer. If we were to do this, and the lump was suspicious for breast cancer, at no cost the person would be taken to our facility for a diagnostic mammogram (not a screening mammogram) for confirmation. If breast cancer was confirmed and the situation was appropriate, a lumpectomy (not a mastectomy) would be conducted and intra-operative radiation therapy would be conducted. Jayant S. Vaidya, one of our colleagues originated this technology. There is potential benefit. (See chapter 11 by Vaidya in the Springer book.) Among other things, that intervention would minimise the travel for patients which would be very important in a country the size of Nigeria with few treatment centres.
We would need to provide a stand-alone facility in Abuja, Nigeria with reliable power and clean water where these interventions can be done. The total cost for all equipment and resources is under $2 million.
Chapter 6 in the Springer-Nature book was written by our Nigerian colleagues. They openly listed all the potential and real problems that would be encountered in a clinical trial in Nigeria.
We concluded that a clinical trial in Nigeria would be possible and would be an opportunity for the Western world to learn a simpler, far less expensive and more effective way to treat TNBC. All would benefit. However, after considering the legitimate concerns about liability from conducting a clinical trial in Nigeria, we came up with a better plan. As the result of a series of meetings with Dr. Ted James, Chief of Breast Cancer Surgical Oncology at Beth Israel Deaconess Medical Center (Harvard), we now propose to conduct a clinical trial of TNBC at Beth Israel Deaconess Medical Center under control of Ted James.
If this trial works as well as we expect, we would transfer the technology to Nigeria and provide all necessary equipment and resources to pursue the therapy there. We have agreement on this strategy from our Nigerian colleagues.
There is an unmet need in sub-Saharan Africa and particularly Nigeria with population of 190 million. The purpose of this paper is to document this situation that seems to be solvable with perioperative ketorolac.
What about postoperative bleeding with use of perioperative NSAID? An important known adverse effect from perioperative ketorolac or any NSAID is increased post-op bleeding. Others include renal dysfunction, that may be prevented by the avoidance in patients with preexisting renal insufficiency, and gastrointestinal ulceration, typically not seen with short term administration (and eventually prevented by anti-acid medications in case of long terms needs). Orthopedic surgeons have used tranexamic acid (TXA) for years to prevent post-op bleeding (A Knopf, MD). A clinical trial of TXA in mammoplasty showed 39% reduced bleeding and no other adverse effects [72]. Moreover, the Belgian randomised controlled trial did not identify any serious bleeding linked to the intraoperative use of ketorolac.
Retrospective and prospective studies of perioperative ketorolac to prevent early relapses in breast cancer
As of this time, there have been two retrospective studies and one prospective study of perioperative NSAID ketorolac. The Forget et al retrospective study was impressively verified in another retrospective study also from Belgium (JNCI 2019 Desmedt, Demicheli et al and accompanying editorial by Ben-Eliahu et al) [79, 80].
A recent, modest sized, randomised controlled trial, testing a single administration of 30 mg of ketorolac, versus placebo, in patients undergoing breast cancer surgery apparently did not confirm retrospective findings [94]. Besides a possible under-powering and quite short follow-up of the trial, patients were randomised according to a not soundly standardised factor, which could have introduced confounding unbalance between the trial arms of the frequency of usual prognostic factors (e.g., TNBC, treatment modalities, BMI etc.). The possible gains from perioperative ketorolac may be so clinically rich that the question deserves a well planned and carried out randomised clinical trial leaving no queries.
The early results are summarised in a blog published online by Ralph Moss [81]. After careful analysis of the prospective trial by Forget et al, additional details will be published while abbreviated summaries are presented in this current document.
The black swan metaphor
The black swan metaphor provided an appropriate way to present these data, challenge the existing treatment and explore new ideas. We have shown that there is a serious weakness in the cancer treatment paradigm and a new paradigm is needed. We propose that exploring the perioperative window will be a good place to start this process [80, 81]. Other relevant papers have been published [82–99].
Conclusions
1. Bimodal relapse patterns are identified in breast and other cancers.
2. The unexpected observations of bimodal relapse patterns are not explainable with the current cancer paradigm that has guided cancer therapy and early detection for many years. Using computer simulation and input from a number of medical specialties, we came to the conclusion that the surgery to remove the primary tumour produces systemic inflammation for a week after surgery. This systemic inflammation apparently caused exits of cancer cells and micrometastases from dormant states and resulted in relapses in the first 3 years post-surgery.
3. Retrospective data suggest perioperative NSAID ketorolac reduces early relapses 5-fold. This still needs to be confirmed. Perhaps more than a single administration may be helpful (Forget et al, 2019). This may reduce breast cancer mortality by 25% to 50% at low cost and toxicity. (There are mixed reports of excessive bleeding but that is known to be reduced by 39% with Tranexamic acid [2018] and Forget et al 2019 data are reassuring.)
4. These data suggest transient systemic inflammation is the precipitating factor resulting in angiogenesis and single cell growth from dormancy.
5. Breast cancer runs its course in over a decade but most of the events leading to relapse seem to occur in the week after surgery.
6. This suggests metastatic progression is amplified 100-fold during the week post primary surgery.
7. After two retrospective studies, extensive clinical data analysis, and three animal models (Krall et al [70], Bonnelykke-Behrndtz et al chapter-8 [4], and Panigrahy et al [81]), we suggest it is time to conduct one or more prospective clinical trials. A trial is in planning stage at Beth Israel Deaconess Medical Center. This may permit us to resolve an unmet need in sub-Saharan Africa.
8. What about the cost? Drugs cost $5 per patient and there are 2 million new breast cancers per year in the world.
9. These surgery-induced effects are apparently host responses to surgery with unknown relationship with the cancer cell genotype and definitely not the fault of the surgeons.
10. This seems to be a general effect not just limited to breast cancer.
We are analysing these data and are now proposing to test the perioperative NSAID at Beth Israel Deaconess Medical Center with Triple Negative Breast Cancer patients, the category that could respond best to the perioperative NSAID. If this works as well as we expect, we would then transfer this technology to Nigeria where an early onset type of TNBC is common. Women in Nigeria typically avoid mastectomy since it is well known to them that the surgery ‘provokes’ the cancer to recur and results in death shortly thereafter. Instead they go to an herbalist for soothing ointments and then go back to their village where they die from untreated breast cancer. There is an unmet need especially in Nigeria (190 million population) for a means to prevent surgery induced relapse that we are attempting to resolve.
Funding declaration
This project was supported by grant 1R43CA65314 from NIH (PI: M Retsky) and 100484 from The Komen Foundation (PI: Rick Rogers).
Conflicts of interest
M Retsky has three patents pending. No other conflicts of interest are reported.
References
1. Bonadonna G, Valagussa P, and Moliterni A, et al (1995) Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up N Engl J Med 332(14) 901–906 https://doi.org/10.1056/NEJM199504063321401 PMID: 7877646
2. Speer J, Petrosky V, and Retsky M, et al (1984) A stochastic numerical model of breast cancer growth that simulates clinical data Cancer Res 44 4124–4130 PMID: 6744323
3. Retsky MW, Swartzendruber DE, and Wardwell RH, et al (1990) Is Gompertzian or exponential kinetics a valid description of individual human cancer growth? Med Hypotheses 33(2) 95–106 https://doi.org/10.1016/0306-9877(90)90186-I PMID: 2259298
4. Ingleby H and Moore L (1956) Periodic roentgenographic studies of a growing human mammary cancer Cancer 9(4) 749–752 https://doi.org/10.1002/1097-0142(195607/08)9:4<749::AID-CNCR2820090420>3.0.CO;2-M PMID: 13356257
5. Retsky M and Demicheli R (2017) Springer book [Available online] [https://www.researchgate.net/publication/321146437_Perioperative_inflammation_as_triggering_origin_of_metastasis_development]
6. Smithers DW (1968) Clinical assessment of growth-rate in human tumours Clin Radiol 19(2) 113–119 https://doi.org/10.1016/S0009-9260(68)80048-0 PMID: 4296751
7. Bernstein J MIA in the war on cancer: where are the low-cost treatments? [http://www.propublica.org/article/where-are-the-low-cost-cancer-treatments] [Online] Date accessed: 16/05/19
8. Fisher ER, Sass R, and Fisher B (1984) Pathologic findings from the National Surgical Adjuvant Project for breast cancers (protocol no. 4). X. Discriminants for tenth year treatment failure Cancer 53 712–723 https://doi.org/10.1002/1097-0142(19840201)53:3 <712::AID-CNCR2820531320>3.0.CO;2-I PMID: 6692274
9. Cancer.gov [https://www.cancer.gov/about-cancer/causes-prevention/risk/myths] [Online] Date accessed: 16/05/19
10. Komaroff (2018) Surgery might promote growth metastases [https://www.jwatch.org/na46860/2018/06/14/surgery-might-promote-growth-metastases] [Online] Date accessed: 16/05/19
11. James TA, Wade JE, and Sprague BL (2016) The impact of mammographic screening on the surgical management of breast cancer J Surg Oncol 113(5) 496–500 https://doi.org/10.1002/jso.24184 PMID: 26799535 PMCID: 4844800
12. Deylgat B, Van Rooy F, and Vansteenkiste F, et al (2011) Postsurgery activation of dormant liver micrometastasis: a case report and review of literature J Gastrointest Cancer 42(1) 1–4 https://doi.org/10.1007/s12029-010-9182-8 PMID: 20602181
13. Tseng WW, Doyle JA, and Maguiness S, et al (2009) Giant cutaneous melanomas: evidence for primary tumour induced dormancy in metastatic sites? BMJ Case Rep https://doi.org/10.1136/bcr.07.2009.2073 PMID: 21977058 PMCID: 3027359
14. Demicheli R, Fornili M, and Biganzoli E (2014) Bimodal mortality dynamics for uveal melanoma: a cue for metastasis development traits? BMC Cancer 14 https://doi.org/10.1186/1471-2407-14-392 PMID: 24890689 PMCID: 4047778
15. Demicheli R, Fornili M, and Ambrogi F, et al (2012) Recurrence dynamics for non-small-cell lung cancer: effect of surgery on the development of metastases J Thorac Oncol 7(4) 723–730 https://doi.org/10.1097/JTO.0b013e31824a9022 PMID: 22425921
16. Kelsey CR, Fornili M, and Ambrogi F, et al (2013) Metastasis dynamics for non-small-cell lung cancer: effect of patient and tumor-related factors Clin Lung Cancer 14(4) 425–432 Epub 2013 Mar 15. https://doi.org/10.1016/j.cllc.2013.01.002 PMID: 23499299
17. Maniwa Y, Okada M, and Ishii N, et al (1998) Vascular endothelial growth factor increased by pulmonary surgery accelerates the growth of micrometastases in metastatic lung cancer Chest 114(6) 1668–1675 https://doi.org/10.1378/chest.114.6.1668 PMID: 9872204
18. Hanin L and Zaider M (2011) Effects of surgery and chemotherapy on metastatic progression of prostate cancer: evidence from the natural history of the disease reconstructed through mathematical modeling Cancers (Basel) 3(3) 3632–3660 https://doi.org/10.3390/cancers3033632 PMID: 24212971 PMCID: 3759214
19. Weckermann D, Polzer B, and Ragg T, et al (2009) Perioperative activation of disseminated tumor cells in bone marrow of patients with prostate cancer J Clin Oncol 27(10) 1549–1556 https://doi.org/10.1200/JCO.2008.17.0563 PMID: 19237635
20. Tsunemi T, Nagoya S, and Kaya M, et al (2003) Postoperative progression of pulmonary metastasis in osteosarcoma Clin Orthop Relat Res (407) 159–166 https://doi.org/10.1097/00003086-200302000-00024 PMID: 12567143
21. Zhu J-F, Feng X-Y, and Zhang X-W, et al (2015) Time distribution of recurrence risk of oesophageal squamous cell carcinoma with complete resection (R0) in a Chinese population Eur J Cardiothorac Surg 48 899–905 https://doi.org/10.1093/ejcts/ezv147 PMID: 25899517
22. Lama N, Boracchi P, and Biganzoli E (2011) Partial logistic relevance vector machines in survival analysis J Appl Stat 38(11) 2445–2458 https://doi.org/10.1080/02664763.2011.559204
23. Xia WX, Ye YF, and Lu X, et al (2013) The impact of baseline serum C-reactive protein and C-reactive protein kinetics on the prognosis of metastatic nasopharyngeal carcinoma patients treated with palliative chemotherapy PLoS One 8(10) e76958 eCollection 2013. https://doi.org/10.1371/journal.pone.0076958 PMID: 24130817 PMCID: 3794978
24. Lange PH, Hekmat K, and Bosl G, et al (1980) Acclerated growth of testicular cancer after cytoreductive surgery Cancer 45(6) 1498–1506 https://doi.org/10.1002/1097-0142(19800315)45:6<1498::AID-CNCR2820450633>3.0.CO;2-7 PMID: 6153570
25. Schack A, Fransgaard T, and Klein MF, et al (2019) Perioperative use of nonsteroidal anti-inflammatory drugs decreases the risk of recurrence of cancer after colorectal resection: a cohort study based on prospective data Ann Surg Oncol [Epub ahead of print] https://doi.org/10.1245/s10434-019-07600-8 PMID: 31313040
26. Guo Y, Kenney SR, and Cook L, et al (2015) A novel pharmacologic activity of ketorolac for therapeutic benefit in ovarian cancer patients Clin Cancer Res 21(22) 5064–5072 Epub 2015 Jun 12. https://doi.org/10.1158/1078-0432.CCR-15-0461 PMID: 26071482 PMCID: 4644688
27. Hamard L, Ratel D, and Selek L, et al (2016) The brain tissue response to surgical injury and its possible contribution to glioma recurrence J Neurooncol 128(1) 1–8Epub 2016 Mar 9. Review. https://doi.org/10.1007/s11060-016-2096-y PMID: 26961772
28. Ratel D, van der Sanden B, and Wion D (2016) Glioma resection and tumor recurrence: back to Semmelweis Neuro Oncol 18(12) 1688–1689 Epub 2016 Oct 7. https://doi.org/10.1093/neuonc/now201 PMID: 27765836 PMCID: 5744249
29. Baum M (1994) Breast Cancer: Controversies in Management Leslie W and Houston J eds. (Armonk, NY: Futura Publishing Co.)
30. Ariel I (1994) A historical review of breast cancer treatment Leslie W and Houston J eds. Breast Cancer: Controversies in Management (Armonk, NY: Futura Publishing Co)
31. Agresti R, Triulzi T, and Sasso M, et al (2019) Wound healing fluid reflects the inflammatory nature and aggressiveness of breast tumors Cells 8(2) https://doi.org/10.3390/cells8020181 PMID: 30791501 PMCID: 6406730
32. Bergh J, Jönsson PE, and Glimelius B, et al (2001) Swedish council of technology assessment in health care a systematic overview of chemotherapy effects in breast cancer Acta Oncol 40 253–281 https://doi.org/10.1080/02841860151116349
33. Retsky M, Bonadonna G, and Demicheli R, et al (2004) Hypothesis: induced angiogenesis after surgery in premenopausal node-positive breast cancer patients is a major underlying reason why adjuvant chemotherapy works particularly well for those patients Breast Cancer Res 6(4) R372–R374 https://doi.org/10.1186/bcr804 PMID: 15217504 PMCID: 468653
34. Godlee F (2011) Mammography wars BMJ 343 d7623 https://doi.org/10.1136/bmj.d7623
35. Fletcher SW (1997) Whither scientific deliberation in health policy recommendations? Alice in the wonderland of breast-cancer screening N Engl J Med 336(16) 1180–1183 https://doi.org/10.1056/NEJM199704173361612 PMID: 9099666
36. Narod SA, Sun P, and Wall C, et al (2014) Impact of screening mammography on mortality from breast cancer before age 60 in women 40 to 49 years of age Curr Oncol 21(5) 217–221 https://doi.org/10.3747/co.21.2067 PMID: 25302030 PMCID: 4189562
37. Retsky M, Demicheli R, and Hrushesky WJ (2005) Does surgery induce angiogenesis in breast cancer? Indirect evidence from relapse pattern and mammography paradox Int J Surg 3(3) 179–187 https://doi.org/10.1016/j.ijsu.2005.08.002 PMID: 17462282
38. Marcus AD The Wall Street Journal, September 13, 2005; Page D1 probing surgery’s link to cancer recurrence. Some researchers say removing a tumor can trigger a process that leads to new growth [https://www.wsj.com/articles/SB112657348401238852*]
39. Retsky MW, Demicheli R, and Hrushesky WJ, et al (2008) Dormancy and surgery-driven escape from dormancy help explain some clinical features of breast cancer APMIS 116(7–8) 730–741 https://doi.org/10.1111/j.1600-0463.2008.00990.x PMID: 18834415
40. Jatoi I, Anderson WF, and Rao SR, et al (2005) Breast cancer trends among black and white women in the United States J Clin Oncol 23 7836–7841 https://doi.org/10.1200/JCO.2004.01.0421 PMID: 16258086
41. Demicheli R, Retsky MW, and Hrushesky WJ, et al (2007) Racial disparities in breast cancer outcome: insights into host-tumor interactions Cancer 110 1880–1888 https://doi.org/10.1002/cncr.22998 PMID: 17876835
42. El Saghir NS, Elhajj II, and Geara FB, et al (2005) Trauma associated growth of suspected dormant micrometastasis BMC Cancer 5 94 https://doi.org/10.1186/1471-2407-5-94
43. Martins-Green M, Boudreau N, and Bissell MJ (1994) Inflammation is responsible for the development of wound-induced tumors in chickens infected with Rous sarcoma virus Cancer Res 54(16) 4334–4341 PMID: 7519120
44. Dolberg DS, Hollingsworth R, and Hertle M, et al (1985) Wounding and its role in RSV-mediated tumor formation Science 230(4726) 676–678 https://doi.org/10.1126/science.2996144 PMID: 2996144
45. Retsky M (2005) Comment—what would explain the sudden growth of lung cancer after minor trauma reported by El Saghir et al? BMC Cancer 5 94
46. Forget P, Vandenhende J, and Berliere M, et al (2010) Do intraoperative analgesics influence breast cancer recurrences after mastectomy? A retrospective analysis Anesth Analg 110(6) 1630–1635 https://doi.org/10.1213/ANE.0b013e3181d2ad07 PMID: 20435950
47. Baum M (2015) Why does the weeping willow weep? Reconceptualizing oncogenesis in breast cancer N Engl J Med 373(13) 1267–1269 https://doi.org/10.1056/NEJMms1505722 PMID: 26398075
48. Hiller J, Brodner G, and Gottschalk A (2013) Understanding clinical strategies that may impact tumour growth and metastatic spread at the time of cancer surgery Best Pract Res Clin Anaesthesiol 27(4) 427–439 https://doi.org/10.1016/j.bpa.2013.10.003 PMID: 24267549
49. Balkwill F and Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357(9255) 539–545 https://doi.org/10.1016/S0140-6736(00)04046-0 PMID: 11229684
50. Perez-Rivas LG, Jerez JM, and Fernandez-De Sousa CE, et al (2012) Serum protein levels following surgery in breast cancer patients: a protein microarray approach Int J Oncol 41(6) 2200–2206 https://doi.org/10.3892/ijo.2012.1667 PMID: 23076779
51. Pascual M, Alonso S, and Parés D, et al (2011) Randomized clinical trial comparing inflammatory and angiogenic response after open versus laparoscopic curative resection for colonic cancer Br J Surg 98(1) 50–59 https://doi.org/10.1002/bjs.7258
52. Hiller JG, Perry NJ, and Poulogiannis G, et al (2018) Perioperative events influence cancer recurrence risk after surgery Nat Rev Clin Oncol 15(4) 205–218 https://doi.org/10.1038/nrclinonc.2017.194 PMID: 29283170
53. Najmeh S, Cools-Lartigue J, and Rayes RF, et al (2017) Neutrophil extracellular traps sequester circulating tumor cells via β1-integrin mediated interactions Int J Cancer 140(10) 2321–2330 https://doi.org/10.1002/ijc.30635 PMID: 28177522
54. Jones FS and Rous P (1914) On the cause of the localization of secondary tumors at points of injury J Exp Med 20(4) 404–412 https://doi.org/10.1084/jem.20.4.404 PMID: 19867830 PMCID: 2125216
55. Walter ND, Rice PL, and Redente EF, et al (2011) Wound healing after trauma may predispose to lung cancer metastasis: review of potential mechanisms Am J Respir Cell Mol Biol 44(5) 591–596 https://doi.org/10.1165/rcmb.2010-0187RT
56. Dvorak HF (1986) Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing N Engl J Med 315(26) 1650–1659 https://doi.org/10.1056/NEJM198612253152606 PMID: 3537791
57. Chaffer CL, San Juan BP, and Lim E, et al (2016) EMT, cell plasticity and metastasis Cancer Metastasis Rev 35(4) 645–654 Review. https://doi.org/10.1007/s10555-016-9648-7 PMID: 27878502
58. Burke JP, Pestotnik SL, and Classen DC, et al (1996) Evaluation of the financial impact of ketorolac tromethamine therapy in hospitalized patients Clin Ther 18(1) 197–211 https://doi.org/10.1016/S0149-2918(96)80190-1 PMID: 8851463
59. Chen WY and Holmes MD (2017) Role of aspirin in breast cancer survival Curr Oncol Rep 19(7) 48 Review. https://doi.org/10.1007/s11912-017-0605-6 PMID: 28597105
60. McAllister SS and Weinberg RA (2014) The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis Nat Cell Biol 16(8) 717–727 Review. https://doi.org/10.1038/ncb3015 PMID: 25082194 PMCID: 6220424
61. Pakneshan P, Birsner AE, and Adini I, et al (2008) Differential suppression of vascular permeability and corneal angiogenesis by nonsteroidal anti-inflammatory drugs Invest Ophthalmol Vis Sci 49(9) 3909 https://doi.org/10.1167/iovs.07-1527 PMID: 18487370
62. Klement GL, Yip TT, and Cassiola F, et al (2009) Platelets actively sequester angiogenesis regulators Blood 113(12) 2835–2842 https://doi.org/10.1182/blood-2008-06-159541
63. Krebs MG, Hou JM, and Ward TH, et al (2010) Circulating tumour cells: their utility in cancer management and predicting outcomes Ther Adv Med Oncol 2(6) 351–365 https://doi.org/10.1177/1758834010378414
64. Zhe X, Cher ML, and Bonfil RD (2011) Circulating tumor cells: finding the needle in the haystack Am J Can Res 1(6) 740–751
65. Camara O, Kavallaris A, and Nöschel H, et al (2006) Seeding of epithelial cells into circulation during surgery for breast cancer: the fate of malignant and benign mobilized cells World J Surg Oncol 4 67 https://doi.org/10.1186/1477-7819-4-67 PMID: 17002789 PMCID: 1599731
66. Komi DEA, Khomtchouk K, and Santa Maria PL (2019) A review of the contribution of mast cells in wound healing: involved molecular and cellular mechanisms Clin Rev Allergy Immunol [Epub ahead of print] Review. https://doi.org/10.1007/s12016-019-08729-w PMID: 30729428
67. Egawa G, Nakamizo S, and Natsuaki Y, et al (2013) Intravital analysis of vascular permeability in mice using two-photon microscopy Nature Sci Rep 3 1932 doi:10.1038/srep01932 PMID: 23732999 PMCID: 3671357
68. Dillekås H, Demicheli R, and Ardoino I, et al (2016) The recurrence pattern following delayed breast reconstruction after mastectomy for breast cancer suggests a systemic effect of surgery on occult dormant micrometastases Breast Cancer Res Treat 158 169–178 https://doi.org/10.1007/s10549-016-3857-1 PMID: 27306422 PMCID: 4937089
69. Dillekås H and Straume O (2019) The link between wound healing and escape from tumor dormancy Surg Oncol 28 50–56 Epub 2018 Nov 9. Review. https://doi.org/10.1016/j.suronc.2018.11.009 PMID: 30851911
70. Lippitz BE and Harris RA (2016) Cytokine patterns in cancer patients: a review of the correlation between interleukin 6 and prognosis OncoImmunology 5(5) e1093722 https://doi.org/10.1080/2162402X.2015.1093722 PMID: 27467926 PMCID: 4910721
71. Karhade M, Hall C, and Mishra P, et al (2014) Circulating tumor cells in non-metastatic triple-negative breast cancer Breast Cancer Res Treat 147(2) 325–333 https://doi.org/10.1007/s10549-014-3103-7 PMID: 25164970 PMCID: 4149877
72. Ausen K, Fossmark R, and Spigset O, et al (2015) Randomized clinical trial of topical tranexamic acid after reduction mammoplasty Br J Surg 102(11) 1348–1353 https://doi.org/10.1002/bjs.9878 PMID: 26349843 PMCID: 4600231
73. Retsky M and Demicheli R (2017) Springer Book 2017 Chapter 8 by Bonnelykke—Behrndtz et al [https://www.researchgate.net/publication/321146437_Perioperative_inflammation_as_triggering_origin_of_metastasis_development] [Available online]
74. Krall JA, Reinhardt F, and Mercury OA, et al (2018) The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy Sci Transl Med 10(436) https://doi.org/10.1126/scitranslmed.aan3464 PMID: 29643230 PMCID: 6364295
75. Sikora K (1999) Developing a global strategy for cancer Eur J Cancer 35 1870–1877 https://doi.org/10.1016/S0959-8049(99)00290-7
76. Berry DA, Baines CJ, and Baum M (2004) Flawed inferences about screening mammography's benefit based on observational data. J Clin Oncol 27(4) 639–640 https://doi.org/10.1200/JCO.2008.17.9341
77. Corbex M, Burton R, and Sancho-Garnier H (2012) Breast cancer early detection methods for low and middle income countries, a review of the evidence Breast 21(4) 428–434 https://doi.org/10.1016/j.breast.2012.01.002 PMID: 22289154
78. Devi BC, Tang TS, and Corbex M (2007) Reducing by half the percentage of late-stage presentation for breast and cervix cancer over 4 years: a pilot study of clinical downstaging in Sarawak, Malaysia Ann Oncol 18(7) 1172–1176 Epub 2007 Apr 13 https://doi.org/10.1093/annonc/mdm105 PMID: 17434897
79. Desmedt C, Demicheli R, and Fornili M, et al (2018) Potential benefit of intra-operative administration of ketorolac on breast cancer recurrence according to the patient's body mass index J Natl Cancer Inst 110(10) 1115–1122 https://doi.org/10.1093/jnci/djy042 PMID: 29718396
80. Ben-Eliyahu S and Golan T (2018) Harnessing the perioperative period to improve long-term cancer outcomes J Natl Cancer Inst 110(10) 1137–1138 https://doi.org/10.1093/jnci/djy055 PMID: 29718287
81. Moss R [https://www.mossreports.com/breast-cancer-pill-ketorolac/] [Online] Date accessed: 16/05/19
82. Chow LW, Yuen KY, and Woo PC, et al (2000) Clarithromycin attenuates mastectomy- induced acute inflammatory response Clin Diagn Lab Immunol 7(6) 925–931 https://doi.org/10.1128/CDLI.7.6.925-931.2000 PMID: 11063500 PMCID: 95987
83. Forget P, Aguirre JA, and Bencic I, et al (2019) How anesthetic, analgesic and other non-surgical techniques during cancer surgery might affect postoperative oncologic outcomes: a summary of current state of evidence Cancers (Basel) 11(5) pii: E592 doi:10.3390/cancers11050592 PMID: 31035321 PMCID: 6563034
84. Panigrahy D, Gartung A, and Yang J, et al (2019) Preoperative stimulation of resolution and inflammation blockade eradicates micrometastases J Clin Invest 129(7) 2964–2979eCollection 2019 Jun 17. https://doi.org/10.1172/JCI127282 PMID: 31205032 PMCID: 6597207
85. Kita H, Shiraishi Y, and Watanabe K, et al (2011) Does postoperative serum interleukin-6 influence early recurrence after curative pulmonary resection of lung cancer? Ann Thorac Cardiovasc Surg 17(5) 454–460 Epub 2011 Jul 13. https://doi.org/10.5761/atcs.oa.10.01627 PMID: 21881374
86. Hudson LG, Cook LS, and Grimes MM, et al (2019) Dual actions of ketorolac in metastatic ovarian cancer Cancers (Basel) 11(8) pii: E1049 Review. https://doi.org/10.3390/cancers11081049 PMID: 31344967
87. O’Reilly MS, Holmgren L, and Shing Y, et al (1994) Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma Cell 79 315–328 https://doi.org/10.1016/0092-8674(94)90200-3
88. Holmgren L, O'Reilly MS, and Folkman J (1995) Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression Nat Med 1 149–153 https://doi.org/10.1038/nm0295-149 PMID: 7585012
89. Guba M, Cernaianu G, and Koehl G, et al (2001) A primary tumor promotes dormancy of solitary tumor cells before inhibiting angiogenesis Cancer Res 61 5575–5579 PMID: 11454710
90. Kuhn TS (1962) The Structure of Scientific Revolutions (Chicago, IL: University of Chicago Press) ISBN 0-226-45808-3
91. Barber B (1961) Resistance by scientists to scientific discovery Science 134 596–602 https://doi.org/10.1126/science.134.3479.596 PMID: 13686762
92. Fleck L (1979) Genesis and Development of a Scientific Fact eds. Trenn TJ and Merton RK foreword by Thomas Kuhn (Chicago, IL: University of Chicago Press)
93. Grzelak CA and Ghajar CM (2017) Metastasis ‘systems’ biology: how are macro-environmental signals transmitted into microenvironmental cues for disseminated tumor cells? Curr Opin Cell Biol 48 79–86 https://doi.org/10.1016/j.ceb.2017.06.002 PMID: 28715713
94. Forget P, Bouche G, and Duhoux FP, et al (2019) Intraoperative ketorolac in high-risk breast cancer patients. A prospective, randomized, placebo-controlled clinical trial PLoS One 14(12) e0225748 eCollection 2019. https://doi.org/10.1371/journal.pone.0225748 PMID: 31800611 PMCID: 6892544
95. Chaffer CL, San Juan BP, and Lim E, et al (2016) EMT, cell plasticity and metastasis Cancer Metastasis Rev 35(4) 645–654 Review. https://doi.org/10.1007/s10555-016-9648-7 PMID: 27878502
96. Pakneshan P, Birsner AE, and Adini I, et al (2008) Differential suppression of vascular permeability and corneal angiogenesis by nonsteroidal anti-inflammatory drugs Invest Ophthalmol Vis Sci 49(9) 3909–3913 Epub 2008 May 16. https://doi.org/10.1167/iovs.07-1527 PMID: 18487370
97. Vaidya J (2017) Springer Nature Book eds. Retsky M and Demicheli R Chapter 11 [https://www.researchgate.net/publication/321146437_Perioperative_inflammation_as_triggering_origin_of_metastasis_development] [Available online]
98. Retsky MW, Demicheli R, and Swartzendruber DE, et al (1997) Computer simulation of a breast cancer metastasis model Breast Cancer Res Treat 45(2) 193–https://doi.org/10.1023/A:1005849301420 PMID: 9342444
99. Rojas KE, Fortes TA, and Flom P, et al (2019) Intraoperative ketorolac use does not increase the risk of bleeding in breast surgery Ann Surg Oncol 26(10) 3368–3373 Epub 2019 Jul 24. https://doi.org/10.1245/s10434-019-07557-8 PMID: 31342387






