Non-hormonal strategies for managing menopausal symptoms in cancer survivors: an update
Nicoletta Biglia1, Valentina E Bounous1, Francesco De Seta2, Stefano Lello3, Rossella E Nappi4 and Anna Maria Paoletti5
1Division of Gynecology and Obstetrics, Department of Surgical Sciences, School of Medicine, University of Torino, Largo Turati 62, 10128 Torino, Italy
2Institute for Maternal and Child Health-IRCCS ‘Burlo Garofolo’, University of Trieste, via dell’Istria 65/1, 34137 Trieste, Italy
3Department of Woman and Child Health, Policlinico Gemelli Foundation, Largo Gemelli 1, 00168 Rome, Italy
4Research Center for Reproductive Medicine, Gynecological Endocrinology and Menopause, IRCCS S Matteo Foundation, Department of Clinical, Surgical, Diagnostic and Paediatric Sciences, University of Pavia, Viale Camillo Golgi 19, 27100 Pavia, Italy
5Department of Obstetrics and Gynecology, Department of Surgical Sciences, University of Cagliari, University Hospital of Cagliari, SS 554 km 4,500, 09042 Monserrato, Italy
Abstract
Vasomotor symptoms, particularly hot flushes (HFs), are the most frequently reported symptom by menopausal women. In particular, for young women diagnosed with breast cancer, who experience premature ovarian failure due to cancer treatments, severe HFs are an unsolved problem that strongly impacts on quality of life. The optimal management of HFs requires a personalised approach to identify the treatment with the best benefit/risk profile for each woman. Hormonal replacement therapy (HRT) is effective in managing HFs but it is contraindicated in women with previous hormone-dependent cancer. Moreover, many healthy women are reluctant to take HRT and prefer to manage symptoms with non-hormonal strategies. In this narrative review, we provide an update on the current available non-oestrogenic strategies for HFs management for women who cannot, or do not wish to, take oestrogens. Since isoflavones have oestrogenic properties and it is not known if they can be safely consumed by women with previous hormone-dependent cancer, they were excluded. Selective serotonin reuptake inhibitors/selective serotonin-norepinephrine reuptake inhibitors, as well as other neuroactive agents, some herbal remedies and behavioural strategies are considered.
Keywords: vasomotor symptoms, hot flushes, menopause, non-oestrogenic therapies, non-hormonal therapies
Correspondence to: Nicoletta Biglia
Email: nicoletta.biglia@unito.it
Published: 11/03/2019
Received: 09/10/2018
Publication costs for this article were supported by the ecancer Global Foundation.
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Hot flushes (HFs) are the most bothersome menopause-related symptom, affecting up to 85% of menopausal women with various severity, frequency and duration [1, 2]. They first begin during the menopausal transition and they last around 7–10 years, although it is reported that some women can experience HFs for longer periods of time [3, 4]. Hormone replacement therapy (HRT) is considered the most effective treatment, but it is not indicated for all patients, such as for those with a personal history of hormone-dependent cancer or of venous thromboembolism [5, 6].
In particular, for young women diagnosed with breast cancer, who experience premature ovarian failure due to cancer treatments, severe HFs are an unsolved problem that strongly impacts on quality of life [7]. Nowadays, for hormone-dependent tumours in high-risk patients, extended therapy with adjuvant antihormonal treatment up to 10 years is suggested and for premenopausal women, the combination of ovarian suppression plus aromatase inhibitors should be considered [8] with consequent negative influence on climacteric symptoms [9].
Moreover, many women with no contraindication to HRT refuse hormonal treatment. In particular, after the publication of the Women’s Health Initiative (WHI) randomised trial in 2003, a progressive reduction in HRT prescription happened worldwide because HRT emerged as a potential risk factor for breast cancer [10]. Physicians are often reluctant in prescribing HRT for this reason, and nowadays, two-thirds of healthy women who seek treatment for menopausal symptoms will not be treated with HRT [5].
As a consequence, a spread of non-hormonal therapies is observed: data from different surveys show that around 30%–80% of women with HFs employ non-hormonal treatments [11–13]. The prevalence of complementary and alternative medicine (CAM) therapies is increasing as showed by the Study of Women’s Health Across the Nation, being 48.5% in 2002 and 80% in 2008 [11]. Moreover, data from surveys show that women prefer CAM to conventional therapies because they consider CAM natural and safe, having a positive effect on maintaining good general health and having no or mild side effects. However, women often do not inform physicians about their decision to start using CAM since they feel that healthcare providers (HCPs) lack knowledge about it and prefer to receive information from different sources (media, friends and relatives) [12–14].
For these reasons, it is important that HCPs are to be well informed about alternative remedies for menopausal symptoms.
Moreover, when discussing non-oestrogenic alternatives for menopausal symptoms, we have to keep in mind the placebo effect. Throughout the literature, the placebo effect is reported in up to 59% of the different studies on menopausal treatment. For this reason, a result which is similar to that achieved by placebo must be a good one, even if it is not significant. Moreover, menopause is a complex period of life where many physical and psychological changes interact, determining a higher susceptibility to the relationship between the woman and HCPs [14].
In this narrative review, we provide an update on the available non-oestrogenic alternatives for HFs treatment in patients who do not wish to or cannot employ HRT.
We searched through PubMed articles in an interval period (2000–2017) using the following keywords: menopause, hot flushes, climacteric symptoms, vasomotor symptoms, non-hormonal treatment. Only the publications written in English were included.
As in a narrative review, we summarised evidence from randomised controlled trials (RCTs) about non-oestrogenic alternatives for menopause-related HFs in order to broaden HCPs’ knowledge on the topic. Since isoflavones have oestrogenic properties and it is not known if they can be safely consumed by women with previous hormone-dependent cancer, they were excluded from this review. Thus, the present review merely reflects the expert opinion of the authors on addressing the various non-oestrogenic strategies to manage vasomotor menopausal symptoms and their appropriate application on a patient-by-patient basis.
Management of vasomotor menopausal symptoms with non-hormonal strategies
Selective serotonin reuptake inhibitors and selective serotonin-norepinephrine reuptake inhibitors
Selective serotonin reuptake inhibitors (SSRIs) and selective serotonin-norepinephrine reuptake inhibitors (SNRIs) are effective non-hormonal alternatives for vasomotor symptoms [15], reducing HFs intensity and frequency in percentages ranging from 20% to 65% [15–18].
Since HFs are thought to occur due to the changes in thermoregulation induced by oestrogen deprivation, with a consequent decrease in serotonin levels [19], the block of serotonin and norepinephrine receptors induced by SSRIs and SNRIs may oppose this imbalance.
Even if many studies have assessed the efficacy of SSRIs and SNRIs in reducing HFs [15], paroxetine salt 7.5 mg/day is the only officially Food and Drug Administration (FDA)-approved product for the treatment of moderate-to-severe vasomotor symptoms in menopausal women [20].
Among SSRIs, paroxetine, sertraline, fluoxetine and escitalopram have been studied for HFs in menopausal women, with most of the studies reporting positive results (Table 1). As regards SNRIs, duloxetine, venlafaxine and its active metabolite O-desmethylvenlafaxine have also shown a benefit in HF reduction (Table 2).
Table 1. SSRIs for HFs.
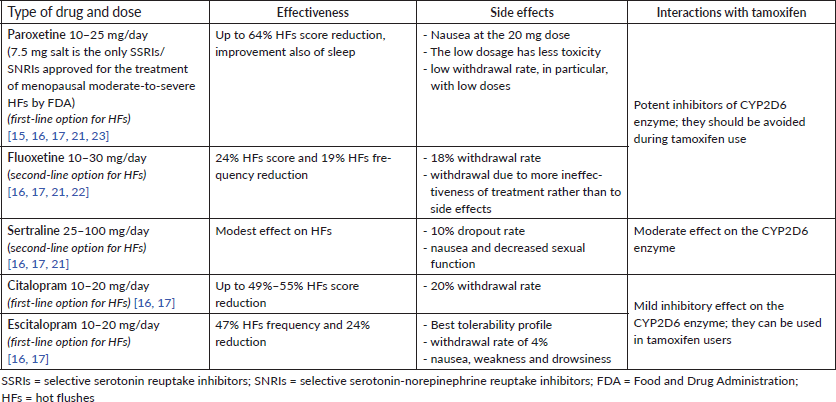
Table 2. SNRIs for HFs.
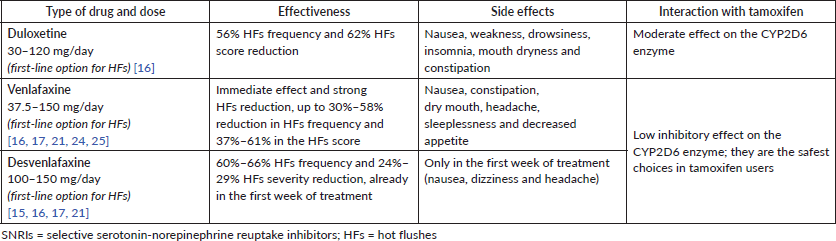
In breast cancer patients, SSRIs and SNRIs are proven to have a mild to moderate effect in reducing HFs, as assessed in a Cochrane Review on the efficacy of non-hormonal therapies for HFs in breast cancer survivors, including six RCTs on SSRIs/SNRIs (venlafaxine, paroxetine, fluoxetine and sertraline) conducted in 451 women [21].
In particular, as regards the use of SSRIs for treating HFs in breast cancer patients (Table 1), the double-blinded RCT by Loprinzi et al [22] showed that fluoxetine 20 mg/day was well tolerated and determined a significant but modest decrease in HFs’ score in 68 women, after 8 weeks of treatment.
Paroxetine, both the dose of 10 mg and 20 mg/day was evaluated in the RCT by Stearns et al [23] on 107 women with or without a history of breast cancer, of which more than 80% were breast cancer survivors mainly under tamoxifen. Both doses significantly decreased HFs, but the lower dose was less discontinued by women and even significantly improved sleep compared to placebo.
As regards SNRIs use for HFs in breast cancer survivors (Table 2), most studies focussed on venlafaxine [24, 25]. In the RCT by Loprinzi et al [24], 191 patients with or without previous breast cancer received placebo or venlafaxine at three different doses (37.5 mg, 75 mg and 150 mg/day). After week 4 of treatment, HFs severity decreased from baseline by 27%, 37%, 61% and 61%, respectively in the four groups, with significant results. Side effects (xerostomia, decreased appetite, nausea and constipation) were significantly more frequently reported in the venlafaxine 75 mg and 150 mg groups than in the placebo group. In the double-blind, placebo-controlled RCT by Boeckhout et al [25], venlafaxine 75 mg for 12 weeks significantly decreased by 41% HFs score in 38 breast cancer survivors (p < 0.001) compared to 17 patients who received placebo. The decrease in HFs score in the venlafaxine group was evident very soon, in 42% of cases as early as after 4 weeks of treatment (p < 0.01, compared to placebo). Interestingly, in the same study, a relevant placebo effect was seen (29% at 12 weeks, compared to baseline, p < 0.001). In the venlafaxine group, the more frequently reported side effects were nausea, constipation and severe appetite loss.
That being so, the American Cancer Society (ACS) and the American Society of Clinical Oncology (ASCO) recommend that primary care clinicians should offer SSRIs and SNRIs for HFs relief in breast cancer survivors [9].
SSRIs and SNRIs act rapidly, with a decrease in vasomotor symptoms as early as after 2 weeks of treatment [17]. Among SSRIs and SNRIs, paroxetine, citalopram and escitalopram carry the best safety profiles [16]. The more frequently reported side effects are nausea, asthenia, dizziness, xerostomia, constipation and sexual dysfunction [15]. SNRIs can increase blood pressure; therefore, this variable should be monitored in all patients [16].
Side effects, in combination with the fear of pharmacological interactions, may lead to early interruption of SSRIs/SNRIs treatment, with high dropout rates reported in the literature (up to 50% at 3 months) [26].
Due to the fast action in 2 weeks and to the safe tolerability profile, venlafaxine has been the more widely used antidepressant for HFs in clinical practice for many years. Nowadays, escitalopram is also prescribed as a first-line option for menopausal HFs. Indeed, due to its favourable tolerability profile, it is considered the antidepressant with the highest number of days of uninterrupted treatment, the best adherence to treatment and the lowest proportion of switching to other drugs [16, 26, 27].
For women with breast cancer, potential interference of antidepressants with tamoxifen has been reported since some SSRIs and SNRIs can inhibit CYP2D6 enzyme with a consequent decrease in the formation of the active metabolite from inactive tamoxifen. Among SSRIs, paroxetine and fluoxetine are the most potent inhibitors and they should be avoided during tamoxifen use; on the contrary, citalopram and escitalopram only have a limited inhibitory effect and can be used in tamoxifen users (Table 1). Among SNRIs, venlafaxine and desvenlafaxine are the safest choices while using tamoxifen [16] (Table 2).
Absolute contraindications to SSRIs and SNRIs use include previous neuroleptic and serotonin syndrome and the current use of monoamine oxidase inhibitors [17]. The North American Menopause Society (NAMS) suggests caution also in subjects affected by other conditions, such as bipolar disease, uncontrolled seizures, liver or kidney insufficiency, hypertension for SNRIs users or concurrent use of other SSRI or SNRI [16, 17].
All antidepressants need to be started at the lowest dose for 2 weeks and then the standard dose can be initiated. To stop the drug, in the same way, the lowest dose should be given for 2 weeks before ending the treatment. [16]
Gabapentin and pregabalin
Gabapentin and pregabalin are anticonvulsant drugs able to decrease the frequency of HFs by binding to calcium channels located in the hypothalamus and, consequently, better modulating thermoregulatory activity [7, 28].
A beneficial effect of gabapentin on HFs was seen both in healthy menopausal women [29, 30] and in breast cancer survivors [31, 32] (Table 3).
Results from clinical studies performed in healthy menopausal women demonstrated a reduction of frequency and severity of HFs by around 50% [29, 30].
Table 3. Main studies performed on gabapentin for HFs treatment.
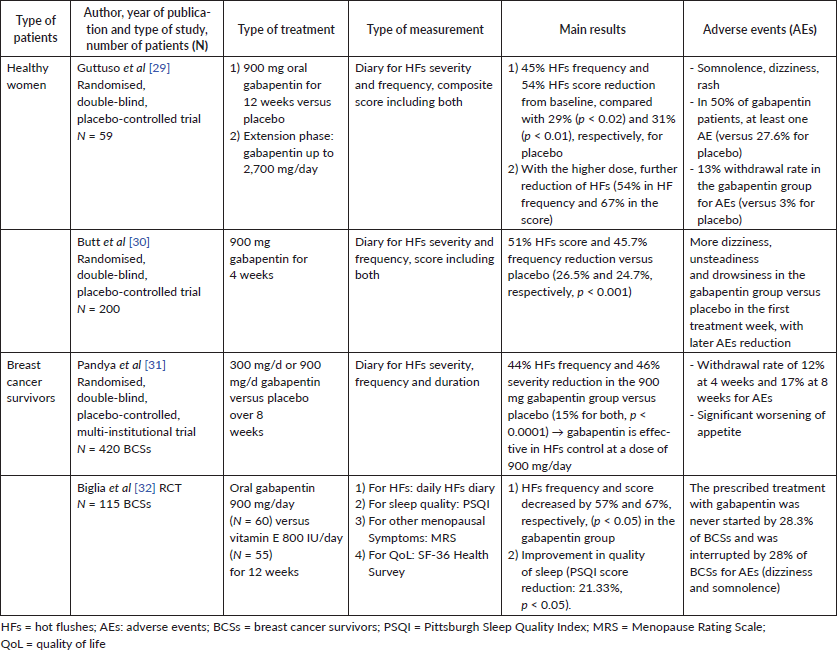
In breast cancer survivors, a 44%–57% decrease in HFs frequency and a 46%–67% decrease in HFs severity were reported with gabapentin in two studies on 420 and 115 breast cancer survivors, respectively [31, 32]. Furthermore, the quality of sleep improved with gabapentin 900 mg/day in these women [32].
For breast cancer patients, a Cochrane review includes gabapentin among the effective drugs in reducing with a mild to moderate effect HFs [20]. Furthermore, gabapentin is included among the suggested recommendations for HFs relief in breast cancer survivors by ACS and ASCO [9].
Pregabalin (150 to 300 mg/day) is effective in HF relief but it is less studied than gabapentin [7]. However, compared to SSRIs/SNRIs, gabapentin is as effective but has more side effects [6]. In a study on 115 breast cancer survivors using gabapentin 900 mg/day, 28.3% of the patients never started the treatment and a further 28% of them interrupted the treatment due to side effects [32].
The most common side effects on gabapentin are drowsiness, unsteadiness and dizziness [15, 30, 32], up to 50% in postmenopausal healthy women [29]. Consequently, withdrawal rates ranging from 12% to 17% are reported [29, 31]. NAMS alerts also for possible suicidal thoughts and behaviours with gabapentin and pregabalin [17].
Clonidine
Clonidine is an anti-hypertensive alpha-adrenergic agonist, which may inhibit flushing by reducing peripheral vascular reactivity [33]. However, the exact mechanism of action is still unclear. In a systematic review and meta-analysis on non-hormonal therapies for menopausal HFs [18], clonidine significantly reduced the frequency of HFs in four out of ten trials included.
For breast cancer patients, two placebo-controlled RCTs included in a Cochrane systematic review [21] showed a moderate reduction in the frequency and severity of HFs.
Furthermore, in the double-blind, placebo-controlled RCT by Boeckhout et al [25], clonidine 0.1 mg/day for 12 weeks significantly decreased by 26% HFs score in 28 breast cancer survivors (p < 0.045) compared to 17 patients who received placebo. In the same study, another group of 38 breast cancer patients employed venlafaxine and the decline in HFs score was faster with venlafaxine than with clonidine.
However, significant side effects (xerostomia, dizziness, constipation, hypotension and potential hypertension, if suddenly interrupted) have been often reported with clonidine [17, 18] and, due to safety problems, its clinical use is poor.
Purified pollen extract
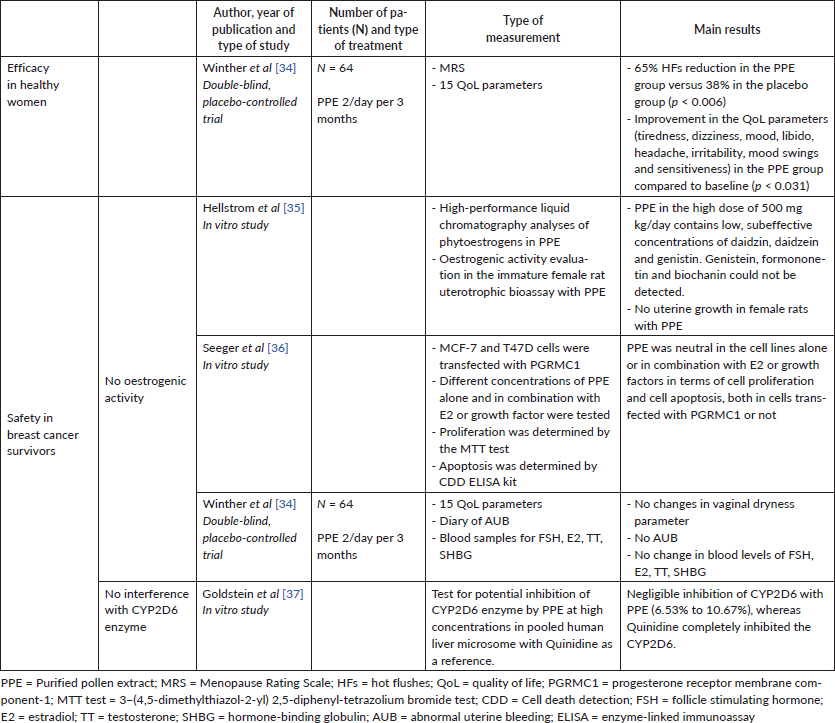
Purified pollen extract (PPE) is a supplement sourced from pure pollen extract green climacteric (GC Fem). It is a combination of pollen and pistil extracts (PI 82), from plants of the Poaceae family, and vitamin E. The pollen and pistil extracts have high antioxidant enzyme superoxide dismutase activity [34]. Each tablet contains 40 mg of GC Fem, 120 mg of PI 82 and 5 mg of vitamin E.
Beneficial effects of PPE on vasomotor symptoms may derive from the inhibition of serotonin uptake at the synaptosomal junction, with an SSRI ‘like’ mode of action [34]. PPE does not contain any of the common phytoestrogens and does not show uterotropic-oestrogenic effects [34, 35].
Furthermore, the production technology which allows the elimination of potential allergens from PPE ensures patient safety, without any contraindications of its use in patients with pollen allergy [34].
PPE has proven clinical efficacy in the treatment of menopausal symptoms like HFs and insomnia in healthy women. A double-blind, placebo-controlled RCT on 64 postmenopausal symptomatic women showed that PPE is effective in HF treatment [34]. In the PPE group, a 65% reduction in HFs was observed, compared to 38% in the placebo group (p < 0.006). PPE also showed positive effects on other quality of life-related symptoms, such as dizziness, mood swings and tiredness, which often accompany vasomotor symptoms.
No data are available for PPE in women with breast cancer, but the lack of oestrogenic effect demonstrated in a preclinical study by Hellstrom et al [35] suggests that PPE can be a suitable option. In this study, PPE was found to contain low, subeffective concentrations of daidzin, daidzein and genistin at high-performance liquid chromatography. Genistein, formononetin and biochanin could not be detected. Moreover, PPE was tested in the same study for oestrogenic activity in the immature female rat uterotrophic bioassay and no uterine growth was seen with PPE in the high dose of 500 mg kg/day.
Moreover, in a recent in vitro study, PPE was neutral in the cell lines alone or in combination with oestradiol or growth factors in terms of cell proliferation and cell apoptosis, both in cells transfected with the progesterone receptor membrane component-1 (PGRMC1) or not. This is important safety data since recent experimental data revealed that oestrogens could trigger a further proliferative effect on breast cancer cells via PGRMC1, in addition to the proliferative effect via intracellularly located receptors [36].
In the clinical study by Winther et al [34], which employed PPE 2/day per 3 months, the evaluation of vaginal dryness and menstrual bleeding showed no change during PPE treatment. Moreover, serum measurements of the follicle stimulating hormone (FSH), E2, testosterone and sex hormone-binding globulin before and after the study period did not suggest any hormone effect of PPE.
Furthermore, an in vitro study demonstrated that PPE does not inhibit the CYP2D6 enzyme and thus does not interfere with tamoxifen metabolism [37].
Available data on the safety and efficacy of PPE are shown in Table 4.
Black cohosh
Native American women have used the extract of black cohosh (Actae racemosa or Cimicifugae racemosae) for centuries as a phytotherapic cure for many different conditions. Nowadays, black cohosh is indicated only for the management of climacteric symptoms [38].
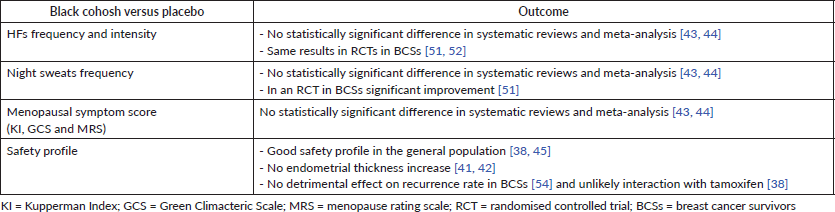
The mechanism underlying the bioactivity of black cohosh is still unclear. Selective modulation of oestrogen receptors (SERM), serotonin partial agonist mechanism, antioxidant and anti-inflammatory effects have been suggested [38, 39]. Initial studies using in vitro and in vivo assays suggested oestrogenic activity but these data have not been confirmed subsequently [38, 40].
Moreover, more recent different clinical trials showing no effect on the vaginal or endometrial thickness or on sexual hormones variation confirmed the lack of oestrogenic activity [38, 41, 42].
In the Herbal Alternatives for Menopause (HALT) double-blind, placebo-controlled RCT on 351 patients, black cohosh given up to 52 weeks determined no effects on vaginal epithelium, endometrium or sexual hormones [41].
In a large prospective open study on 400 postmenopausal women, the endometrial safety of black cohosh was assessed before and after 52 weeks of treatment, showing no increase in endometrial thickness on ultrasound and no case of endometrial hyperplasia or of serious adverse endometrial outcome [42].
RCTs showed HF reduction in healthy menopausal women and in breast cancer survivors [38]. However, a Cochrane systematic review of 16 RCTs, on 2,027 symptomatic menopausal women [43], did not show a significant difference between black cohosh and placebo in the frequency of HFs, concluding that there is insufficient evidence to support the use of black cohosh for controlling menopausal symptoms. The inconsistency of the evidence may be explained also by the heterogeneity of results among the studies due to the use of different parts of the plant or different kind of extract of black cohosh. In the review, at least five different oral preparations of black cohosh were included.
Another recent systematic review and meta-analysis, considering four RCTs on black cohosh, confirmed this data, showing that overall black cohosh was not associated with changes in the rate of HFs [44].
As regards side effects, suspected hepatotoxicity was previously reported, but a meta-analysis of five randomised, double-blind, controlled clinical trials on 1,020 women showed no evidence that black cohosh has any adverse effect on liver function [45].
The use of black cohosh in breast cancer patients is still controversial due to its SERM–like mechanism of action [46]. In vitro studies in MCF-7 cells during chronic use of black cohosh show that changes in the gene expression pattern are more similar to tamoxifen than to oestradiol [47]. In animal models, black cohosh given to rats for 40 weeks determines a dose-dependent reduction of breast cancer, suggesting a chemopreventive potential [48].
Table 4. PPE for HFs treatment.
Furthermore, since breast density is a biomarker for breast cancer risk, different studies showed no significant modification in mammary breast density while using black cohosh [42, 49, 50]. In the 52-weeks study by Raus et al [42] on 400 postmenopausal women, increase in breast density was observed only in one patient who developed an invasive breast cancer unrelated to black cohosh use [49, 50]. In a prospective study on 74 postmenopausal patients, Hirschberg et al [49] observed no increase in breast density, assessed by mammography, and of breast cancer proliferation by Ki 67 determination through agobiopsy, both performed at baseline and after 24 weeks of black cohosh use. In the comparative study of Lundström et al [50] on 65 postmenopausal patients using black cohosh and 154 under HRT (oestradiol 2 mg/norethisterone acetate or tibolone) or placebo, breast density assessed by mammography performed at baseline and after 24 weeks was significantly increased with HRT use but not with placebo or black cohosh.
Only few RCTs have been performed in order to understand the use and safety of black cohosh in breast cancer patients. In the RCT by Jacobson et al [51], 85 breast cancer survivors, most of whom were on tamoxifen, were assigned to black cohosh or placebo. No significant differences were seen in HFs frequency and intensity with both treatments; however, in the black cohosh group, a significant improvement in sweating was observed. Interestingly, changes in blood levels of FSH and luteinizing hormone (LH) did not differ between the placebo and the black cohosh group. The RCT by Pockaj et al [52] failed to provide any evidence that black cohosh 40 mg/day reduces HFs more than the placebo (mean decrease in HFs score: 20% in the black cohosh group versus 27% in the placebo group, p = 0.53). Results from the only available RCTs performed in breast cancer patients show that there is currently a lack of evidence to support the use of black cohosh for HFs relief in breast cancer survivors [38, 53]. An observational, retrospective, cohort study on 18,861 breast cancer survivors, 1,102 of which receiving black cohosh, analysing diseases-free survival after breast cancer, showed no detrimental effect on recurrence rate [54].
Regarding tamoxifen users, a potential in vitro inhibition of CYP2D6 has been described, but clinical data suggest that the interaction of black cohosh with tamoxifen is unlikely [38].
Although black cohosh seems to have a good safety profile, more high-quality studies are needed to reach a definitive conclusion regarding its efficacy on HFs [43, 44, 46].
A summary of the evidence on the efficacy and safety of black cohosh on HFs relief is described in Table 5.
Oxybutinin
Studies suggest that oxybutynin, an anticholinergic generally employed for urinary incontinence due to overactive bladder, is an effective treatment for HFs, both in healthy women [55] and in breast cancer survivors [56].
Weight loss
Data from the WHI trial in healthy women show that weight loss determines a reduction in HFs [57]. Two RCTs confirm these findings, suggesting that weight loss is associated in overweight or obese healthy women with a reduction in HFs [58, 59]. In breast cancer survivors, prevention of weight gain after diagnosis can help in controlling HFs, whereas the role of intentional weight loss after diagnosis on vasomotor symptoms is still not defined [60].
Table 5. Black cohosh for HFs treatment.
Yoga and exercise
A Cochrane review failed to demonstrate a positive effect of exercise and yoga on HFs [61]. However, a recent study reported that exercise training may decrease the severity of HFs [62] and in an RCT by Cramer et al [63], yoga was effective in reducing vasomotor symptoms in breast cancer survivors.
In a systematic review and meta-analysis of 13 RCTs on 1,306 women, yoga compared with no treatment reduced total menopausal symptoms, HFs, psychological and urogenital symptoms without serious adverse events [64]
Cognitive behavioural therapy
Cognitive behavioural therapy (CBT) is, among mind-body techniques, an effective treatment for HFs both for healthy postmenopausal women and breast cancer survivors [17]. In the MENOS 2 trial, an RCT conducted on 140 healthy postmenopausal women, CBT significantly reduced vasomotor symptoms [65]. The MENOS 1 trial was an RCT specifically addressed to breast cancer patients in which CBT significantly reduced HFs after 9 weeks compared with usual care. The improvement was maintained at 26 weeks from randomisation and additional benefits to mood, sleep and quality of life were observed [66].
Relaxation therapy
For breast cancer patients, a Cochrane Review assessed that relaxation therapy has a mild to moderate effect in reducing HFs [21]. Nevertheless, in healthy perimenopausal and postmenopausal women, a more recent Cochrane review concluded that evidence is insufficient to prove the effectiveness of relaxation techniques [67].
Acupuncture
Acupuncture has been shown to reduce HFs in healthy women, compared with no treatment [68].
A meta-analysis including three systematic reviews and four RCTs assessed the effectiveness of acupuncture in reducing HF frequency and severity in peri or postmenopausal women, with improvement also in health-related quality of life items and without significant side effects [69].
However, in breast cancer patients, a Cochrane Review failed to confirm its effectiveness in reducing HFs [21]. Further data are needed to prove a positive effect of acupuncture and, eventually, to predict which subset of patients could benefit from it.
Cooling strategies
Moreover, since temperature is a trigger of flushing, cooling strategies have also been proposed in order to reduce HFs (dressing in layers, with light, cotton clothing and standing away from sources of warming); however, the efficacy of such strategies is not supported by scientific evidence [17].
Stellate ganglion block
Stellate ganglion block (SGB), consisting of a vertebral cervical block by local anaesthetic injection, has been proposed for HF treatment. It was suggested that SGB resets the temperature-regulating mechanisms by interrupting the connections between the central and sympathetic nervous system. Only one RCT on 40 postmenopausal women focussed on SGB for HFs [70], showing no significant difference in the overall HF frequency after SGB, while four open-label studies showed a 45%–90% reduction in HFs with SGB [7]. Larger RCTs are needed in order to evaluate its efficacy. Even if it is reported that SGB performed by skilled practitioners is safe [70], concerns related to the close proximity of critical structures may hinder the spread of this non-hormonal option for non-HF relief.
Conclusion
In menopausal women, vasomotor symptoms, and in particular, HFs, frequently remain underdiagnosed and undertreated, with a negative impact on the patient’s quality of life. Paying more attention to the needs and preferences of menopausal women may reduce the number of untreated cases of HFs. Psychoactive agents are commonly used to treat HFs in some countries, such as the USA but are less well accepted by physicians and patients in others. Whatever the perception toward these drugs is, they are mostly used as off-label drugs and for short periods. On the other hand, CAM approaches have provided interesting data in recent studies. Among them, PPE has a confirmed non-oestrogenic effect and has been shown to be effective in decreasing HFs, night sweats, irritability and improving the quality of sleep in menopausal women, with consequent improvement in the quality of life. Black cohosh is a well-known traditional medicine treatment, which is widely used in spite of the inconsistency of data collected so far requiring further research. Recent evidence supports a positive role of physical activity in the management of HFs.
In order to manage HFs in menopausal women effectively, clinicians should identify the patient’s health profile and personal preferences and then select an individualised and safe therapy.
Research agenda
Future research on the molecular mechanisms of flushing during menopause and on the effects of available and novel treatments will further improve the management of HFs in both healthy menopausal women and in women with contraindications to oestrogens.
Since limitations of the available evidence are in particular the imprecision or lack in the data and in the study method details, a great effort must be made to perform RCTs with large samples and with a strict methodology to allow comparison of the different strategies.
Acknowledgments
The authors thank Editamed srl, Tommaso Sacco M.D. for medical writing services and Raquel Carvalhosa, Ph.D. for editorial services.
Conflicts of interest
Anna Maria Paoletti, Francesco De Seta and Stefano Lello declare no conflict of interests. Nicoletta Biglia had a financial relationship (lecturer, member of advisory boards and/or consultant) with Gedeon Richter, Shionogi Limited and Italfarmaco. Rossella E Nappi has a financial relationship (lecturer, member of advisory boards and/or consultant) with Bayer HealthCare, Endoceutics, Gedeon Richter, HRA Pharma, MSD, Novo Nordisk, Pfizer, Shionogi and Teva. Valentina E Bounous had a financial relationship with Italfarmaco S.p.A. (medical writing) and has a financial relationship with Shionogi (medical writing, tables preparation).
Funding
Financial support for medical writing (table preparation) and editorial services (English supervision) was provided by Shionogi.
References
1. Blümel JE, Chedraui P, and Baron G, et al (2011) Collaborative group for research of the climacteric in Latin America (REDLINC), a large multinational study of vasomotor symptom prevalence, duration, and impact on quality of life in middle-aged women Menopause 18 778–785 https://doi.org/10.1097/gme.0b013e318207851d
2. Freeman EW and Sherif K (2007) Prevalence of hot flushes and night sweats around the world: a systematic review Climacteric 10 197–214 https://doi.org/10.1080/13697130601181486 PMID: 17487647
3. Avis NE, Crawford SL, and Greendale G, et al (2015) Study of women’s health across the nation (SWAN), study of women’s health across the nation, duration of menopausal vasomotor symptoms over the menopause transition JAMA Intern Med 175 531–539 https://doi.org/10.1001/jamainternmed.2014.8063 PMID: 25686030 PMCID: 4433164
4. Hunter M, Gentry-Maharaj A, and Ryan A, et al (2012) Prevalence, frequency and problem rating of hot flushes persist in older postmenopausal women: impact of age, body mass index, hysterectomy, hormone therapy use, lifestyle and mood in a cross-sectional cohort study of 10 418 British women aged 54–65: Prevalence, frequency and problem rating of hot flushes BJOG 119 40–50 https://doi.org/10.1111/j.1471-0528.2011.03166.x
5. Constantine GD, Graham S, and Clerinx C, et al (2016) Behaviours and attitudes influencing treatment decisions for menopausal symptoms in five European countries Post Reprod Health 22 112–122 https://doi.org/10.1177/2053369116632439 PMID: 26895640 PMCID: 5019289
6. Baber RJ, Panay N, and Fenton AT, et al, (2016) IMS Recommendations on women’s midlife health and menopause hormone therapy Climacteric 19(2) 109–150 https://doi.org/10.3109/13697137.2015.1129166 PMID: 26872610
7. Knobf MT (2006) The influence of endocrine effects of adjuvant therapy on quality of life outcomes in younger breast cancer survivors Oncologist 11 96–110 https://doi.org/10.1634/theoncologist.11-2-96 PMID: 16476831
8. Curigliano G, Burstein HJ, and Winer EP, et al (2017) De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen international expert consensus conference on the primary therapy of early breast cancer 2017 Ann Oncol 28 1700–1712 https://doi.org/10.1093/annonc/mdx308 PMID: 28838210 PMCID: 6246241
9. Runowicz CD, Leach CR, and Henry NL, et al (2016) American cancer society/American society of clinical oncology breast cancer survivorship care guideline J Clin Oncol 34(6) 611–635 https://doi.org/10.1200/JCO.2015.64.3809
10. Chlebowski RT, Hendrix SL, and Langer RD, et al (2003) WHI investigators, influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the women’s health initiative randomized trial JAMA 289(24) 3243–3253 https://doi.org/10.1001/jama.289.24.3243 PMID: 12824205
11. Bair YA, Gold EB, and Zhang G, et al (2008) Use of complementary and alternative medicine during the menopause transition: longitudinal results from the study of women’s health across the nation Menopause 15 32–43 https://doi.org/10.1097/gme.0b013e31813429d6
12. Posadzki P, Lee MS, and Moon TW, et al (2013) Prevalence of complementary and alternative medicine (CAM) use by menopausal women: a systematic review of surveys Maturitas 75 34–43 https://doi.org/10.1016/j.maturitas.2013.02.005 PMID: 23497959
13. Peng W, Adams J, and Sibbritt DW, et al (2013) Critical review of complementary and alternative medicine use in menopause: focus on prevalence, motivation, decision-making, and communication Menopause 21(5) 536–548 https://doi.org/10.1097/GME.0b013e3182a46a3e PMID: 24104604
14. Tonob D and Melby MK (2017) Broadening our perspectives on complementary and alternative medicine for menopause: a narrative review Maturitas 99 79–85 https://doi.org/10.1016/j.maturitas.2017.01.013 PMID: 28364873
15. American College of Obstetricians and Gynaecologists (2014) ACOG practice bulletin No. 141: management of menopausal symptoms Obstet Gynecol 123(1) 202–216 https://doi.org/10.1097/01.AOG.0000441353.20693.78 PMID: 24463691
16. Handley AP and Williams M (2015) The efficacy and tolerability of SSRI/SNRIs in the treatment of vasomotor symptoms in menopausal women: a systematic review J Am Assoc Nurse Pract 27 54–61
17. North American Menopause Society (NAMS) (2015) Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society Menopause 22 1155–1174 https://doi.org/10.1097/GME.0000000000000546 PMID: 26382310
18. Nelson D, Vesco KK, and Haney E, et al (2006) Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis JAMA 295 2057–2071 https://doi.org/10.1001/jama.295.17.2057 PMID: 16670414
19. Vilar-González S, Pérez-Rozos A, and Cabanillas-Farpón R, Mechanism of hot flashes Clin Transl Oncol 13(3) 143–147 PMID: 21421458
20. Orleans RJ, Li L, and Kim MJ, et al (2014) FDA approval of paroxetine for menopausal hot flushes N Engl J Med 370(19) 1777–1779 https://doi.org/10.1056/NEJMp1402080 PMID: 24806158
21. Rada G, Capurro D, and Pantoja T, et al (2010) Non-hormonal interventions for hot flushes in women with a history of breast cancer Cochrane Database Syst Rev (9) CD004923 PMID: 20824841
22. Loprinzi CL, Sloan JA, and Perez EA, et al (2002) Phase III evaluation of fluoxetine for treatment of hot flashes J Clin Oncol 20(6) 1578–1583 https://doi.org/10.1200/JCO.2002.20.6.1578 PMID: 11896107
23. Stearns V, Slack R, and Greep N, et al (2005) Paroxetine is an effective treatment for hot flashes: results from a prospective randomized clinical trial J Clin Oncol 23(28) 6919–6930 https://doi.org/10.1200/JCO.2005.10.081 PMID: 16192581
24. Loprinzi CL, Kugler JW, and Sloan JA, et al (2000) Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial Lancet 356(9247) 2059–2063 https://doi.org/10.1016/S0140-6736(00)03403-6
25. Boekhout AH, Vincent AD, and Dalesio OB, et al (2011) Management of hot flashes in patients who have breast cancer with venlafaxine and clonidine: a randomized, double-blind, placebo-controlled trial J Clin Oncol 29(29) 3862–3868 https://doi.org/10.1200/JCO.2010.33.1298 PMID: 21911720
26. Aguglia E, Ravasio R, and Simonetti M, et al (2012) Use and treatment modalities for SSRI and SNRI antidepressants in Italy during the period 2003–2009, Curr Med Res Opin 28(9) 1475–1484 https://doi.org/10.1185/03007995.2012.713341 PMID: 22809113
27. Wu EQ, Greenberg PE, and Yang E, et al (2009) Treatment persistence, healthcare utilisation and costs in adult patients with major depressive disorder: a comparison between escitalopram and other SSRI/SNRIs J Med Econom 12 124–135 https://doi.org/10.3111/13696990903093537
28. Hayes LP, Carroll DG, and Kelley KW (2011) Use of gabapentin for the management of natural or surgical menopausal hot flashes Ann Pharmacother 45 388–394 https://doi.org/10.1345/aph.1P366 PMID: 21343402
29. Guttuso TJ Jr, Kurlan R, and McDermott MP, et al (2003) Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial Obstet Gynecol 101 337–345 PMID: 12576259
30. Butt DA, Lock M, and Lewis JE, et al (2008) Gabapentin for the treatment of menopausal hot flashes: a randomized controlled trial Menopause 15 310–318 https://doi.org/10.1097/gme.0b013e3180dca175
31. Pandya KJ, Morrow GR, and Roscoe JA, et al (2005) Gabapentin for hot flashes in 420 women with breast cancer: a randomised double-blind placebo-controlled trial Lancet 366 818–824 https://doi.org/10.1016/S0140-6736(05)67215-7 PMID: 16139656 PMCID: 1627210
32. Biglia N, Sgandurra P, and Peano E, et al (2009) Non-hormonal treatment of hot flushes in breast cancer survivors: gabapentin versus vitamin E Climacteric J Int Menopause Soc 12 310–318 https://doi.org/10.1080/13697130902736921
33. Sassarini J, Fox H, and Ferrell W, et al (2012) Hot flushes, vascular reactivity and the role of the α-adrenergic system Climacteric 15 332–338 https://doi.org/10.3109/13697137.2011.636847 PMID: 22208784
34. Winther K, Rein E, and Hedman C (2005) Femal, a herbal remedy made from pollen extracts, reduces hot flushes and improves quality of life in menopausal women: a randomized, placebo-controlled, parallel study Climacteric 8 162–170 https://doi.org/10.1080/13697130500117987 PMID: 16096172
35. Hellström AC and Muntzing J (2012) The pollen extract femal-a nonestrogenic alternative to hormone therapy in women with menopausal symptoms Menopause 19 825–829 https://doi.org/10.1097/gme.0b013e31824017bc
36. Seeger H, Ruan X, and Neubauer H, et al (2017) Membrane-initiated effects of serelys® on proliferation and apoptosis of human breast cancer cells Gynecol Endocrinol 34(4) 353–356 https://doi.org/10.1080/09513590.2017.1407751
37. Goldstein SR, Espié M, and Druckmann R (2015) Does purified Swedish pollen extract, a nonhormonal treatment for vasomotor symptoms, inhibit the CYP2D6 enzyme system? Menopause 22 1212–1214 https://doi.org/10.1097/GME.0000000000000535 PMID: 26325084
38. Drewe J, Bucher KA, and Zahner C (2015) A systematic review of non-hormonal treatments of vasomotor symptoms in climacteric and cancer patients SpringerPlus 4 65 https://doi.org/10.1186/s40064-015-0808-y PMID: 25713759 PMCID: 4331402
39. Ruhlen RL, Sun GY, and Sauter ER (2008) Black cohosh: insights into its mechanism(s) of action Integr Med Insights 3 21–32 https://doi.org/10.4137/117863370800300002 PMID: 21614156 PMCID: 3046019
40. Jarry H, Metten M, and Spengler B, et al (2003) In vitro effects of the Cimicifuga racemosa extract BNO 1055 Maturitas 44(1) S31–S38 https://doi.org/10.1016/S0378-5122(02)00346-8 PMID: 12609557
41. Reed SD, Newton KM, and LaCroix AZ, et al (2008) Vaginal, endometrial, and reproductive hormone findings: randomized, placebo-controlled trial of black cohosh, multibotanical herbs, and dietary soy for vasomotor symptoms: the herbal alternatives for menopause (HALT) study Menopause 15(1) 51–58 PMID: 18257142
42. Raus K, Brucker C, and Gorkow C, et al (2006) First-time proof of endometrial safety of the special black cohosh extract (Actaea or Cimicifuga racemosa extract) CR BNO 1055 Menopause 13 678–691 https://doi.org/10.1097/01.gme.0000196813.34247.e2 PMID: 16837890
43. Leach MJ and Moore V (2012) Black cohosh (Cimicifuga spp.) for menopausal symptoms Cochrane Database Syst Rev 9 CD007244
44. Franco OH, Chowdhury R, and Troup J, et al (2016) Use of plant-based therapies and menopausal symptoms: a systematic review and meta-analysis JAMA 315 2554–2563 https://doi.org/10.1001/jama.2016.8012 PMID: 27327802
45. Naser B, Schnitker J, and Minkin MJ, et al (2011) Suspected black cohosh hepatotoxicity: no evidence by meta-analysis of randomized controlled clinical trials for isopropanolic black cohosh extract Menopause 18(4) 366–375 https://doi.org/10.1097/gme.0b013e3181fcb2a6 PMID: 21228727
46. Fritz H, Seely D, and McGowan J, et al (2014) Black cohosh and breast cancer: a systematic review Integr Cancer Ther 13 12–29 https://doi.org/10.1177/1534735413477191
47. Gaube F, Wolfl S, and Pusch L, et al (2007) Gene expression profiling reveals effects of cimicifuga racemosa (L.) NUTT. (black cohosh) on the estrogen receptor positive human breast cancer cell line MCF-7 BMC Pharmacol 7 11 https://doi.org/10.1186/1471-2210-7-11 PMID: 17880733 PMCID: 2194763
48. Einbond LS, Soffritti M, and Degli Esposti D, et al (2012) Chemopreventive potential of black cohosh on breast cancer in Sprague–Dawley rats Anticancer Res 32(1) 21–30 PMID: 22213284
49. Hirschberg AL, Edlund M, Svane G, et al (2007) An isopropanolic extract of black cohosh does not increase mammographic breast density or breast cell proliferation in postmenopausal women Menopause 14(1) 89–96 https://doi.org/10.1097/01.gme.0000230346.20992.34
50. Lundström E, Hirschberg AL, and Söderqvist G (2011) Digitized assessment of mammographic breast density–effects of continuous combined hormone therapy, tibolone and black cohosh compared to placebo Maturitas 70(4) 361–364 https://doi.org/10.1016/j.maturitas.2011.08.009 PMID: 21958943
51. Jacobson JS, Troxel AB, and Evans J, et al (2001) Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer J Clin Oncol 19(10) 2739–2745 https://doi.org/10.1200/JCO.2001.19.10.2739 PMID: 11352967
52. Pockaj BA, Gallagher JG, and Loprinzi CL, et al (2006) Phase III double-blind, randomized, placebo-controlled crossover trial of black cohosh in the management of hot flashes: NCCTG Trial N01CC1 J Clin Oncol 24(18) 2836–2841 https://doi.org/10.1200/JCO.2005.05.4296 PMID: 16782922
53. National Institute for Care and Health Excellence (NICE) (2017) Early and locally advanced breast cancer: diagnosis and treatment Clinical guideline [nice.org.uk/guidance/cg80] Date accessed: 24/2/2019
54. Henneicke-von Zepelin HH, Meden H, and Kostev K, et al (2007) Isopropanolic black cohosh extract and recurrence-free survival after breast cancer Int J Clin Pharmacol Ther 45(3) 143–154 https://doi.org/10.5414/CPP45143 PMID: 17416109
55. Simon JA, Gaines T, and LaGuardia KD (2016) Extended-release oxybutynin therapy for vasomotor symptoms in women: a randomized clinical trial Menopause 23(11) 1214–1221 https://doi.org/10.1097/GME.0000000000000773 PMID: 27760081
56. San Antonio Breast Cancer Symposium (SABCS) (2018) ‘It’s Going to Be a Useful Agent’: Oxybutynin for Hot Flashes Medscape 10 Dec 10 2018 (Abstract GS6-02, presented 7 December 2018)
57. Kroenke CH, Caan BJ, and Stefanick ML, et al (2012) Effects of a dietary intervention and weight change on VMS in the women’s health initiative Menopause 19 980–988 https://doi.org/10.1097/gme.0b013e31824f606e PMID: 22781782 PMCID: 3428489
58. Thurston RC, Ewing LJ, and Low CA, et al (2015) Behavioral weight loss for the management of menopausal hot flashes: a pilot study Menopause 22 59–65 https://doi.org/10.1097/GME.0000000000000274
59. Huang AJ, Subak LL, and Wing R, et al (2010) An intensive behavioral weight loss intervention and hot flushes inwomen Arch Intern Med 170 1161–1167 https://doi.org/10.1001/archinternmed.2010.162 PMID: 20625026 PMCID: 3030922
60. Caan BJ, Emond JA, and Su HI, et al (2012) Effect of postdiagnosis weight change on hot flash status among early-stage breast cancer survivors J Clin Oncol 30 1492–1497 https://doi.org/10.1200/JCO.2011.36.8597 PMID: 22430275 PMCID: 4874147
61. Daley A, Stokes-Lampard H, and Thomas A, et al (2014) Exercise for vasomotor menopausal symptoms Cochrane Database Syst Rev (11) CD006108 PMID: 25431132
62. Bailey TG, Cable NT, and Aziz N, et al (2016) Exercise training reduces the acute physiological severity of post-menopausal hot flushes J Physiol 594 657–667 https://doi.org/10.1113/JP271456
63. Cramer H, Rabsilber S, and Lauche R, et al (2015) Yoga and meditation for menopausal symptoms in breast cancer survivors-A randomized controlled trial Cancer 13 2175–2184 https://doi.org/10.1002/cncr.29330
64. Cramer H, Peng W, and Lauche R (2018) Yoga for menopausal symptoms-A systematic review and meta-analysis Maturitas 109 13–25 https://doi.org/10.1016/j.maturitas.2017.12.005 PMID: 29452777
65. Ayers B, Smith M, and Hellier J, et al (2012) Effectiveness of group and self-help cognitive behavior therapy in reducing problematic menopausal hot flushes and night sweats (MENOS 2): a randomized controlled trial Menopause 19 749–759 https://doi.org/10.1097/gme.0b013e31823fe835 PMID: 22336748
66. Mann E, Smith MJ, and Hellier J, et al (2012) Cognitive behavioural treatment for women who have menopausal symptoms after breast cancer treatment (MENOS 1): a randomised controlled trial Lancet Oncol 13 309–318 https://doi.org/10.1016/S1470-2045(11)70364-3 PMID: 22340966 PMCID: 3314999
67. Saensak S, Vutyavanich T, and Somboonporn W, et al (2014) Relaxation for perimenopausal and postmenopausal symptoms Cochrane Database Syst Rev 7 CD008582
68. Dodin S, Blanchet C, and Marc I, et al (2013) Acupuncture for menopausal hot flushes ed The Cochrane Collaboration, Cochrane Database Systematic Reviews (Chichester, UK: John Wiley & Sons Ltd) [http://doi.wiley.com/10.1002/14651858.CD007410.pub2]
69. Befus D, Coeytaux RR, and Goldstein KM, et al (2018) Management of menopause symptoms with acupuncture: an umbrella systematic review and meta-analysis J Altern Complement Med 4 314–323 https://doi.org/10.1089/acm.2016.0408
70. Walega DR, Rubvvbounoussin LH, and Banuvar S, et al (2014) Effects of stellate ganglion block on vasomotor symptoms: findings from a randomized controlled clinical trial in postmenopausal women Menopause 21(8) 807–814 https://doi.org/10.1097/GME.0000000000000194 PMID: 24496086 PMCID: 4110158