Impact of exon 19 versus exon 21 EGFR-activating mutation on outcomes with upfront pemetrexed–carboplatin chemotherapy
Vanita Noronha1,*, Vijay Patil1,*, Amit Joshi1, Anuradha Chougule1, Atanu Bhattacharjee2, Rajiv Kumar3, Sucheta More1, Supriya Goud1, Ashay Karpe1, Anant Ramaswamy1, Nikhil Pande1, Arun Chandrasekharan1, Alok Goel1, Vikas Talreja1, Abhishek Mahajan4, Amit Janu4, Nilendu Purandare5 and Kumar Prabhash1
1Department of Medical Oncology, Tata Memorial Hospital, HBNI, Parel, Mumbai 400068, India
2Department of Biometrics, Chiltern International Limited, Residency Road, Bengaluru 560025, India
3Department of Pathology, Tata Memorial Hospital, HBNI, Parel, Mumbai 400068, India
4Department of Radiology, Tata Memorial Hospital, HBNI, Parel, Mumbai 400068, India
5Department of Nuclear Medicine, Tata Memorial Hospital, HBNI, Parel, Mumbai 400068, India
*These authors contributed equally to this work.
Correspondence to: Kumar Prabhash. E-mail: kumarprabhashtmh@gmail.com
Abstract
Background: EGFR mutation subtype is a recognised factor impacting outcomes of patients receiving oral tyrosine kinase inhibitors (TKIs) in non-small-cell lung cancer (NSCLC). Evidence for the effect of this factor on outcomes in patients receiving pemetrexed is limited.
Methods: We completed a study comparing pemetrexed–platinum combination versus oral TKI in EGFR mutation-positive patients in lung cancer. We analysed the impact of EGFR mutation subtype, specifically, exon 19 and 21 on the PFS and OS of patients treated with pemetrexed (500 mg/m2 on day 1) and carboplatin (AUC 5 on day 1) as first-line therapy. Patients underwent axial imaging for response assessment on D42, D84, D126 and subsequently every two months till progression. Patients post-progression were treated with gefitinib.
Results: Fifty-one patients (36%) had exon 21 mutation, while 92 patients (64%) had exon 19 mutation. Response rates in evaluable patients was 47.7% in exon 19 patients (41 patients, n = 86) and 42.9 % in exon 21 patients (18 patients, n = 42). There was a significant increase in median overall survival for patients with exon 19 mutations (24.5 months, 95% CI: 21.3–27.7 months ) over the exon 21-mutated patients (18.1 months, 95% Cl: 13.5–22.6 months, p = 0.002). This differential impact was due to second-line gefitinib having a differential outcome on these mutations.
Conclusion: Pemetrexed-based chemotherapy does not have a differential impact on exon 19- or exon 21-mutated patients. However, second-line treatment with gefitinib has a favourable response and outcome in exon 19-mutated patients.
Keywords: EGFR mutation, NSCLC, exon 19, pemetrexed, exon 21, gefitinib, prognosis
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Published: 24/10/2017; Received: 19/09/2017
Introduction
The heterogeneity in EGFR mutations in terms of biology and potential response to treatment has been a work in progress from the time of the discovery of these mutations in NSCLC [1]. Deletion in exon 19 and L858R substitution on exon 21 constitute a majority of these mutations and they predict for a high response rate to tyrosine kinase inhibitors. These mutations were termed as classic activating mutations [2, 3].
Multiple studies have established that upfront tyrosine kinase inhibitors (reversible or irreversible) are the treatment of choice in patients with classic activating mutations. However, these studies have clubbed exon 19 deletions and exon 21 L858R mutations together [4–6]. There is growing evidence to suggest that exon 19 deletion may have a better prognosis compared to exon 21 mutations [7]. A recent meta-analysis of over 2500 patients with classic activating mutations reported that patients with exon 19 deletions have better progression-free survival compared to exon 21 L858R mutations, when treated with irreversible TKIs [8]. Also, exon 19 deleted patients treated with irreversible TKI (afatinib) had an overall survival advantage according to the LUX-LUNG studies [9]. In the near future, it is likely that these mutations may need to be addressed separately when conducting studies.
However, not uncommonly, patients with EGFR mutations are treated with pemetrexed platinum chemotherapy. It is unknown whether the response, progression-free survival and overall survival of these patients are influenced by the type of EGFR mutation. A post hoc analysis of a phase-III trial was carried out to address this question.
Methods
Study
We completed a single-centre, phase-3, double arm, parallel-group, open-label exploratory randomised study comparing pemetrexed with carboplatin and oral TKI in EGFR mutation positive lung cancer patients (Clinical trial registry of India: CTRI/2015/08/006113). We have reported its results [10]. This was a post hoc analysis planned with a primary objective to compare the progression-free survival between exon 19 deleted and exon 21L858R-mutated cohort when treated with pemetrexed carboplatin. The secondary objective was to compare the response rate and overall survival.
Patient selection
The detailed eligibility criteria of the original study are published elsewhere [10] For this analysis, we selected patients subjected to the following selection criteria. We included adult (age > or = 18 years) patients with ECOG PS 0-2, with either exon 19 deletion or exon 21L858R mutation, with pathologically confirmed adenocarcinoma without uncontrolled comorbidities, with adequate organ function and receiving first-line treatment with pemetrexed–carboplatin chemotherapy. Patients who had received upfront gefitinib or who had exon 18 mutation were excluded from this analysis.
Intervention
Patients were treated with six cycles of pemetrexed (500 mg/m2) and carboplatin (AUC-5) with appropriate antiemetics and supportive care at three weekly intervals. Post-six cycles, patients who had non-progressive disease were offered pemetrexed maintenance. The chemotherapy was continued till development of progressive disease or intolerable side effects or any other protocol defined criteria.
Patients underwent response assessment scan after three cycles, six cycles and then at two monthly intervals. At progression, all patients were offered gefitinib and were followed up till death.
Statistical analysis
SPSS version 20 was used for analysis. Response rate at the end of third cycle was documented in accordance with RECIST version 1.1 and compared with Fisher’s exact test. Progression-free survival was defined as time in months from randomisation to objective PD (progressive disease), change in treatment or death from any cause. Patients who had not progressed at last follow up were censored on 14th July 2016. Overall survival was defined as time in months from randomisation to death from any cause. Patients who had not died at last follow-up were censored on 14th July 2016. Kaplan–Meier time to event analysis was carried out for the estimation of PFS and OS. Log rank test was used for the comparison of PFS and OS between exon 19 deleted and exon 21–mutated patients. The COX regression analysis was used to estimate the hazard ratio with its 95% confidence interval. A p value of 0.05 or below was considered as significant.
Results
Baseline details
A total of 143 patients received pemetrexed-based therapy as first-line treatment for stage-III/stage-IV NSCLC in the chemotherapy arm. 51 patients (36%) had exon 21 mutation, while 92 patients (64%) had exon 19 mutation. The median age was 54 years (27–74 years). There were 95 males (66.4%) and 48 females (33.6%). Twenty-eight patients (19.6%) had a history of previous smoking. The stage was stage IIIB in three patients and stage-IV disease in the remaining patients. The ECOG PS was 0–1 in 129 patients (90.2%) and two in 14 patients (9.8%). The distribution of baseline characteristics in accordance with the type of mutation is shown in Table 1. The ECOG PS was slightly imbalanced between the two cohorts with 98.0% having ECOG PS 0–1 status in exon 21 mutated cohort versus 93.5% in exon 19 cohort. Imbalance was also observed in incidence of brain metastasis at presentation; 9.8% in exon 21 cohort as against 19.6% in exon 19 deletion cohort.
Table 1. Baseline characteristics between the two cohorts.
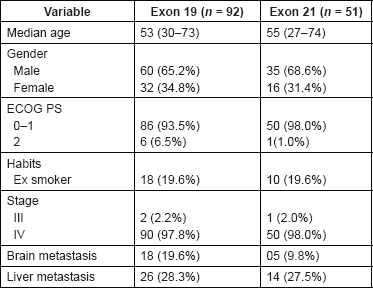
Table 2. Response to pemetrexed between the two cohorts.
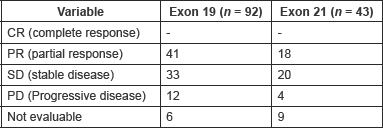
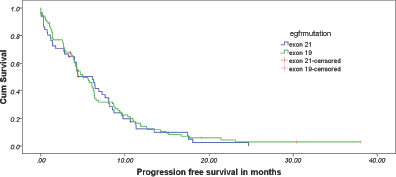
Figure 1. Estimated progression-free survival between the two cohorts.
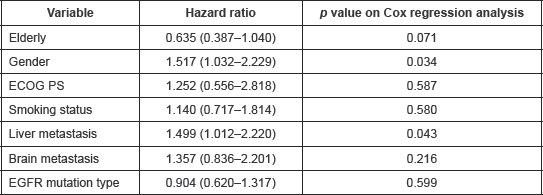
Table 3. Details of multivariate analysis for progression-free survival.
Response rate
Out of 143 patients, 15 patients were ineligible for response assessment. The overall response rate among evaluable patients was 46.1% without any complete responses (Table 2). Response rates in evaluable patients was 47.7% in exon 19 patients (41 patients, n = 86) and 42.9 % in exon 21 patients (18 patients, n = 42) (p = 0.706, Fisher’s exact two-sided p value).
PFS
At the data cut-off, 90.6% of the patients had progressed. The overall median PFS was 5.033 months (95% CI: 3.596–6.471). The median PFS in exon 19 and 21 cohorts were 5.033 months (95% CI: 3.428–6.638) and 6.133 months (95% CI: 3.815–8.452), respectively (Figure 1). There was no differential impact of EGFR mutation on PFS (p = 0.599, HR = 0.904, 95% CI: 0.620–1.317). Table 3 provides details of cox regression analysis results.
Table 4. Details of multivariate analysis for overall survival.
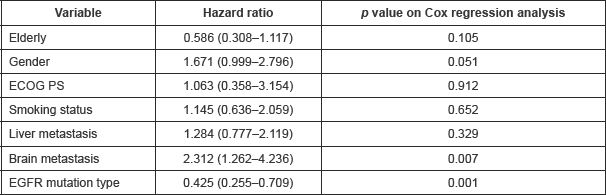

Figure 2. Estimated overall survival between the two cohorts.
OS
At the data cut-off, 65.2% of the patients had died. The overall median survival was 21.533 months (95%CI: 17.558–25.508 months). The median overall survival in exon 19 patients was 24.5 months (95% CI: 21.3–27.7 months ) which was significantly better than that seen in exon 21 mutated patients (18.1 months, 95% Cl: 13.5–22.6 months, p = 0.002) (Table 4 and Figure 2).
Impact of second-line gefitinib
Out of 128 patients who had progressed, 16 patients had not received gefitinib. The second-line treatment with gefitinib was received in 112 patients, of which 76 patients were in the exon 19 cohort and 36 in the exon 21 cohort. Ninety-eight patients were evaluable for response and the response rate was 69.7% in the exon 19 cohort as opposed to 43.8% in the exon 21 cohort. The median progression-free survival was 8.067 months (95% CI: 6.556–9.577 months) in exon 19 cohort versus 5.767 months (95%CI: 3.814–7.719 months) in exon 21 cohort (p = 0.141). The median overall survival was 17.867 months (95%CI: 13.079–22.655 months) in the exon 19 cohort as opposed to 9.633 months (95% CI: 5.959–13.308 months) in the exon 21 cohort (p = 0.001). The patients who had not received gefitinib had poor survival and the median OS was only 9.4 months versus 22.6 months in patients receiving it (p = 0.012).
Discussion
Biomarkers are frequently used in oncology and certain biomarkers like activating EGFR mutations have both prognostic and predictive implications [2]. Both exon 19 deletions and exon 21 L858R substitutions have been considered together when landmark trials were done and reported in first line setting [4–6]. Recent data suggest that both these mutations have different biological behaviours and have different prognostic significance when treated with reversible and irreversible tyrosine kinase inhibitors like gefitinib, erlotinib or afatinib [7–9]. However, it is not known how tumours carrying these mutations would behave if they were exposed to chemotherapy. The current analysis was done to answer this question.
We failed to identify any major difference in baseline characteristics or tumour metastasis pattern between the cohorts carrying the two mutations. The response rate to chemotherapy and median PFS was similar between the two groups indicating that pemetrexed carboplatin therapy with pemetrexed maintenance had a similar impact on both groups irrespective of the type of EGFR mutation. Similar findings were reported in an ASCO 2015 annual conference abstract by Kogure et al. [11]. In their study of 32 patients, they reported response rates of 33.3% with exon 21 L858R substitution versus 22.2 % with exon 19 deletion, a median PFS of 5.13 months with exon 21 L858R substitution versus 5.40 with exon 19 deletion and a similar OS.
In our study, the median OS was different between the two groups favouring the exon 19 cohort with a hazard ratio of 0.425. The improvement in OS without any improvement in PFS indicated influence of therapy provided post progression on first-line treatment. Hence, the post hoc analysis of the influence of type of EGFR mutation on response to second-line therapy, second-line PFS and second-line OS was performed. The outcomes were similar to those reported in the literature for first-line setting. Exon 19 deleted patients had a better response rate, a better median PFS and a better median OS in comparison to exon 21-mutated cohort. This implies that the differential behaviour of exon 19 and 21 mutations is seen in the presence of tyrosine kinase inhibitors and not with chemotherapy irrespective whether the exposure to TKI was in the first-line setting or second-line setting.
The current analysis has its own limitations. It was a single-centre study, post hoc analysis with a small sample size and the cohorts were not balanced for important prognostic factors like the presence or absence of brain metastasis and ECOG PS.
Conclusions
Pemetrexed-based chemotherapy does not have a differential impact on exon 19 or exon 21-mutated patients. However, second-line treatment with gefitinib has a favourable response and outcome in exon 19-mutated patients.
References
1. Shigematsu H, Lin L, and Takahashi T, et al (2005) Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers J Natl Cancer Inst 97(5) 339–346 https://doi.org/10.1093/jnci/dji055 PMID: 15741570
2. Sharma SV, Bell DW, and Settleman J, et al (2007) Epidermal growth factor receptor mutations in lung cancer Nat Rev Cancer 7(3) 169–181 https://doi.org/10.1038/nrc2088 PMID: 17318210
3. Mok TS, Wu YL, and Thongprasert S, et al (2009) Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma N Engl J Med 361(10) 947–957 https://doi.org/10.1056/NEJMoa0810699 PMID: 19692680
4. Rosell R, Carcereny E, and Gervais R, et al (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial Lancet Oncol 13(3) 239–246 https://doi.org/10.1016/S1470-2045(11)70393-X PMID: 22285168
5. Maemondo M, Inoue A, and Kobayashi K, et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR N Engl J Med 362(25) 2380–2388 https://doi.org/10.1056/NEJMoa0909530 PMID: 20573926
6. Zhou C, Wu YL, and Chen G, et al (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study Lancet Oncol 12(8) 735–742 https://doi.org/10.1016/S1470-2045(11)70184-X PMID: 21783417
7. Li M, Zhang Q, and Liu L, et al (2011) The different clinical significance of EGFR mutations in exon 19 and 21 in non-small cell lung cancer patients of China Neoplasma 58(1) 74–81 https://doi.org/10.4149/neo_2011_01_74
8. Kuan F-C, Kuo LT, and Chen MC, et al (2015) Overall survival benefits of first-line EGFR tyrosine kinase inhibitors in EGFR-mutated non-small-cell lung cancers: a systematic review and meta-analysis Br J Cancer 113(10) 1519–1528 https://doi.org/10.1038/bjc.2015.356 PMID: 26461059 PMCID: 4815883
9. Yang J, Wu YL, and Schuler M, et al (2015) Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from Lancet Oncol [Internet] Available from: http://www.sciencedirect.com/science/article/pii/S1470204514711738 https://doi.org/10.1016/S1470-2045(14)71173-8
10. Patil VM, Noronha V, and Joshi A, et al (2017) Phase III study of gefitinib or pemetrexed with carboplatin in EGFR-mutated advanced lung adenocarcinoma ESMO Open 2(1) e000168 https://doi.org/10.1136/esmoopen-2017-000168 PMID: 28761735 PMCID: 5519810
11. Kogure Y, Saka H, and Oki M, et al (2015) Efficacy of pemetrexed for EGFR mutated lung carcinoma between L858R and Exon 19 deletion Available from: http://ascopubs.org/doi/abs/10.1200/jco.2015.33.15_suppl.e19073



