Ovarian cancer in BRCA1 and BRCA2 gene mutation carriers: analysis of prognostic factors and survival
Nicoletta Biglia1, Paola Sgandurra2, Valentina Elisabetta Bounous1, Furio Maggiorotto2, Eleonora Piva3, Emanuele Pivetta4, Riccardo Ponzone2 and Barbara Pasini5
1Unit of Obstetrics and Gynaecology, Mauriziano Umberto I Hospital, Largo Turati, 62, 10128, Turin, Italy
2Gynecological Oncology Unit, Candiolo Cancer Institute FPO, IRCCS, Km 3,95, SP142, 10060 Candiolo (TO), Italy
3Obstetrics and Gynaecology I, Sant’Anna Hospital, AOU Città della Salute e della Scienza di Torino, Corso Spezia, 60, 10126, Turin, Italy
4AOU Città della Salute e della Scienza, Department of Medical Sciences, Corso Bramante, 88-10126, Turin, Italy
5AOU Città della Salute e della Scienza, SC Genetica Medica U, Via Santena, 19-10126, Turin, Italy
Correspondence to: Nicoletta Biglia. Email: nicoletta.biglia@unito.it
Abstract
Objectives: To compare clinical–pathological characteristics and outcome between sporadic ovarian cancer and ovarian cancer in patents with hereditary breast and ovarian cancer syndrome (HBOC).
Methods: Twenty-four patients with ovarian cancer treated between 2000 and 2009 who tested positive for BRCA1/2 mutation (BRCA ) and a control group of 64 age-matched patients with no family history of breast/ovarian cancer (controls) were enrolled. Clinical–pathological characteristics, surgical outcome, overall (OS), and progression-free survival (PFS) were compared between the two groups.
Results: The high-grade serous histotype was more represented in BRCA than in controls (70.8% versus 53.1%) (p > 0.05). BRCA cancers were more frequently diagnosed at stage II than controls (20.83% versus 4.69%) (p = 0.024). Radical primary surgery was performed in 70% of women in both groups, with no difference in debulking results. In patients undergoing surgery after neoadjuvant chemotherapy, in all BRCA patients, optimal cytoreduction was achieved (versus 70% of the controls). PFS was significantly longer for BRCA patients compared to controls (60 months versus 22 months; p = 0.039). No significant difference was observed in OS between BRCA patients and controls.
Conclusions: At a median follow-up time of 46 months, BRCA patients have a better prognosis than controls in terms of PFS. Higher chemosensitivity of BRCA tumours was observed.
Keywords: ovarian cancer, hereditary breast and ovarian cancer syndrome, BRCA1, BRCA2, gene mutations, prognosis
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Published: 03/05/2016; Received: 28/10/2015
Introduction
The estimated lifetime risk of ovarian cancer in BRCA1 mutation carriers is 40% to 50%, among BRCA2 mutation carriers the risk is lower, ranging from 20% to 30% [1], while the lifetime risk of ovarian cancer in the general population is 1.6%.
The mean age at diagnosis of ovarian cancer in patients with germ line BRCA1 mutations is younger than for patients with sporadic cancer [2, 3].
Most ovarian cancers associated with hereditary breast and ovarian cancer syndrome (HBOC) reported in the literature are high-grade and advanced-stage serous carcinomas, whereas borderline and mucinous tumours are uncommon [4].
Ovarian cancers in patients with HBOC are associated with unfavourable pathological characteristics but with a higher chemosensitivity, [2, 5, 6, 7, 8, 9] as described in the term ‘BRCAness’ phenotype [10, 11]. Retrospective studies [5, 10, 11, 12, 13, 14] describe also a superior outcome; however, more recent data show only a short term but not a long-term survival advantage [15].
In the present study, we compare clinical–pathological characteristics and outcome, in terms of overall survival (OS) and progression-free survival (PFS), of HBOC and sporadic ovarian cancer.
Materials and methods
Study population
The study was carried out on 24 ovarian cancer patients who tested positive for BRCA1/2 mutation (BRCA ) and 64 age-matched patients, without any family history of breast/ovarian cancer, not tested for BRCA mutation (controls), retrieved from the database of cases operated by the same surgical team between 2000 and 2009 at our institution. Somatic BRCA mutation in the tumours was not assessed.
The patients in both groups were treated according to the same protocol (primary cytoreductive surgery followed by chemotherapy). Patients unsuitable for upfront surgery received neoadjuvant chemotherapy, including carboplatin and paclitaxel, and secondary interval surgery when feasible. Surgical stage and histological grade and cell type were categorised according to the International Federation of Gynaecology and Obstetrics (FIGO) and World Health Organisation (WHO) standards. The relevant information was obtained from pathology reports and operative notes in the medical record.
Cytoreduction was categorised as no residual disease (TR = 0), residual disease ≤1 cm (TR ≤ 1) and residual disease >1 cm (TR > 1).
Data regarding familial and personal history, BRCA status, type of surgery performed for ovarian cancer, residual disease, pathological report and follow-up status (date of the last follow-up visit, patient condition, relapse) were recorded in the database.
Clinical data, tumour characteristics, surgical treatment, PFS, and OS were compared, evaluating differences in prognosis and tumour biology between sporadic and HBOC ovarian cancer.
The median follow-up time was 46 months (interquartile range 37.5).
Patients underwent clinical follow-up examination (clinical examination and CA 125) every three months in the first two years, every six months afterwards until the fifth year after diagnosis and every year subsequently. Abdomino–pelvic computerised tomography (CT) scan was prescribed in case of symptoms, clinical findings, or CA 125 elevation.
All patients had signed a written informed consent allowing their blinded clinical data and biological material to be used for research purposes.
Statistical analysis
Statistical analyses were performed using the SPSS 15.0 software for Windows and STATA 13.1 SE (Stata Corporation). The date of primary treatment was used as the date of diagnosis. OS was calculated according to date of death or most recent follow-up if still alive. PFS was estimated as the time from the date of primary treatment to the date of recurrence.
Two-sided p-value <0.05 was considered statistically significant. Normality of the variables distribution was tested by the Kolmogorov–Smirnov test. Categorical variables were compared using the Pearson’s chi square test, or Fisher’s exact test, and the analysis of variance (ANOVA), as count variables were compared using independent-samples t-test. Survival curves were estimated using the Kaplan–Meier method and compared with the log-rank test.
Results
Patients
Among BRCA patients, 19 women (79.2%) carried a BRCA1 gene mutation and five carried a mutation of the BRCA2 gene.
Median age at diagnosis was 54 years old, both for controls and for the BRCA group.
Characteristics of ovarian tumours in patients with and without HBOC
The distribution of tumour characteristics in patients with and without HBOC ovarian cancer is reported in Table 1.
Table 1. Histological characteristics and treatment of ovarian tumours in BRCA patients and in controls.
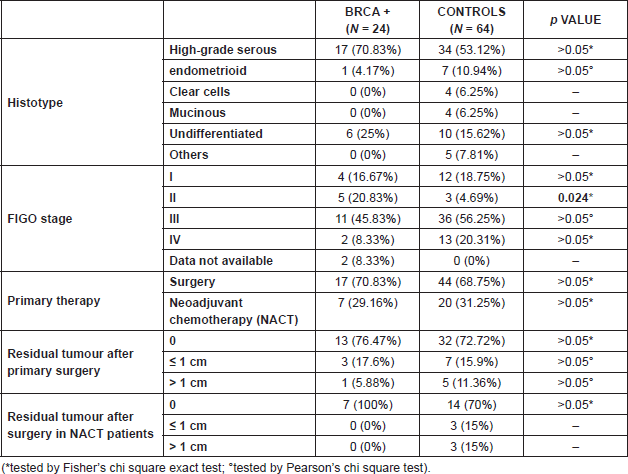
In the BRCA group, 70.8% of the tumours had high-grade serous histotype. In the control group, only 53.1% of tumours were high-grade serous (p > 0.05). Moreover, among BRCA patients, 25% of tumours were of undifferentiated histotype. In the control group, seven endometrioid, 10 undifferentiated, four clear cells, and four mucinous tumours were found.
Sporadic ovarian tumours were diagnosed at a more advanced stage (56.2% were stage III and 20.3% stage IV at diagnosis) as compared to BRCA cancers (45.8% stage III and 8.3% stage IV) (p > 0.05 for both stages). BRCA patients were significantly more frequently diagnosed at stage II than controls (20.83% versus 4.69%, p = 0.024).
Surgery
Radical primary surgery was performed in 70% of women in both groups; in 30% of patients, with no difference between the two groups, a carboplatin–paclitaxel neoadjuvant chemotherapy was administered.
Optimal cytoreduction was achieved in 76% of patients with HBOC ovarian cancer and in 72% of controls (p > 0.05). After neoadjuvant chemotherapy, optimal cytoreduction was obtained in all BRCA patients, versus 70% in controls (p > 0.05).
Data are shown in Table 1.
Survival
Median PFS was significantly longer in the BRCA group (60 months) as compared to controls (22 months) (p = 0.039) (Figure 1). In the control group, all recurrences occurred in the first three years of follow-up. In the BRCA group, recurrences have been observed up to nine years of follow-up.
No significant difference was observed in terms of OS between BRCA patients and controls (p = 0.379).
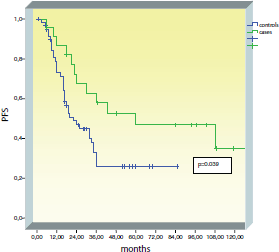
Figure 1. Progression-free survival (PFS) in the BRCA group compared to controls.
Discussion
Ovarian cancer in BRCA mutation carriers seems to have peculiar features compared to sporadic ovarian cancer, generally showing a better outcome.
In the present study, we compared clinical–pathological characteristics and outcome of sporadic and HBOC ovarian cancer, treated at a single institution by the same team of surgeons
Ovarian cancer in HBOC is often diagnosed at a younger age compared with sporadic ovarian cancer, in particular for patients with BRCA1 mutation [1, 2, 3, 5]. Mean age at diagnosis among BRCA mutation carriers is reported to range from 45 to 48 years old in the literature [14, 16]. In our series, median age at diagnosis was 54 years old both for sporadic and for HBOC ovarian cancer.
HBOC ovarian tumours are generally diagnosed at a higher stage and grade as compared to sporadic cancers [1, 4]. On the contrary, in our study, a significant higher proportion of patients in the BRCA group was diagnosed at stage II when compared to the sporadic group.
Furthermore, HBOC ovarian tumours are typically of serous histology [1, 2, 16] Generally, among HBOC ovarian cancers, no borderline or mucinous cancers are reported [4]. Consistent with the literature, in the present series, most of the HBOC ovarian tumours (70%) were high-grade serous and 25% were undifferentiated tumours. On the contrary, among the sporadic tumours, only around 50% of tumours were high-grade serous and endometrioid; clear cells, and mucinous tumours were observed as well.
In our series, radical primary surgery was performed in more than 70% of patients in both groups. In one-third of the patients, both in BRCA and in control groups, a neoadjuvant chemotherapy was administered. In our series, in patients who underwent surgery after neoadjuvant chemotherapy, optimal cytoreduction was achieved in all BRCA and in 70% of controls. According to many studies, patients carrying BRCA mutation have a higher response rate to platinum-based chemotherapy [2, 5, 6, 7, 9, 10, 17]. In the study of Cass et al., a significantly greater response to first-line platinum-based chemotherapy and a longer disease-free interval was observed among patients with BRCA ovarian cancer [5]. Also, in the study of Tan et al., in BRCA patients, a significantly higher response rate to first-, second-, and third-line platinum-based chemotherapy as compared to controls was observed [10]. In the study of Gorodnova et al., complete clinical response was documented in 34% of BRCA mutation carriers versus 4% of non-carriers (p < 0.05) treated by platinum-based neoadjuvant therapy [7]. It has been suggested that the more favourable response to chemotherapy of HBOC ovarian tumours is related to their increased sensitivity to DNA-damaging agents, such as platinum-containing regimen since in the absence of BRCA1/2 function, there is a deficiency in the homologous-recombination repair, resulting in an impaired ability of tumour cells to repair platinum-induced double-strand breaks [2, 6].
Whether the superior response to platinum-based chemotherapy translates into a better outcome is unclear. Tan et al. described the ‘BRCAness’ phenotype, with superior outcomes following platinum-based therapy in patients with BRCA mutations [10, 18], with survival advantage demonstrated by other retrospective studies [3, 5, 8, 12, 13, 19].
A better OS in BRCA1 and 2 patients was observed by Bolton et al. in an analysis on 21 studies evaluating the five years overall mortality [12]. Also, the data from the Cancer Genome Atlas (TCGA) project reported a better OS in the group of BRCA mutation carriers, but only for BRCA2 carriers [13]. In the study by Gallagher et al. on stage III and IV ovarian cancer, a better OS but not a longer disease-free survival was observed in the BRCA group compared to the sporadic cancers [19]. Furthermore, Synowiec et al. observed a better OS in patients with BRCA1 mutations in comparison with patients with sporadic ovarian cancer, seeming BRCA1 mutation an independent prognostic factor for ovarian cancer [3]. Moreover, two recent meta-analyses show that BRCA1 and 2 carriers have a survival advantage compared to non-carriers [20, 21].
More recently, Mclaughlin et al. [22] Candido Dos Reis et al. [23] observed a short-term survival advantage for BRCA patients but not a long-term survival benefit. It seems that after a diagnosis of ovarian cancer, BRCA mutation confers a transient mortality benefit that diminishes with time [15]. In the recent study by Kotsopoulous et al., at 10 years of follow-up, 43% of non-carriers, 57% of BRCA1 mutation carriers, and 69% of BRCA2 mutation carriers died from ovarian cancer, being no residual disease at resection the strongest predictor of long-term survival [15]. According to Kotsopoulous et al., the initial survival advantage among women with BRCA mutations may reflect a higher initial sensitivity of BRCA carriers to chemotherapy, but this response does not predict long-term survival [15].
Previous studies found a worst outcome for BRCA patients, instead [24, 25]. However, their reliability has been questioned because the analysis was performed without stratifying for tumour stage.
In our study, no significant difference was observed in OS between BRCA patients and controls. A longer PFS was observed among BRCA patients as compared to patients of the sporadic group. In the control group, all recurrences occurred in the first three years of follow-up. In the BRCA group, recurrences occurred up to nine years after diagnosis.
Conclusion
In our experience, BRCA patients have more chemosensitive tumours and longer PFS, but no OS benefit when compared to sporadic ovarian tumours.
The major limit of our study is the small sample size, while its strength is the uniformity of treatment, since all patients were treated according to the same protocol and by the same surgical team. Further multicentre studies are needed in order to obtain a larger population of carriers.
Acknowledgments
All authors disclaim any financial and personal relationships with other people or organisations that could inappropriately influence their work.
The study did not have any sponsorship.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
References
1. Boyd J (2003) Specific keynote: hereditary ovarian cancer: what we know Gynecol Oncol 88 S8–10 DOI: 10.1006/gyno.2002.6674 PMID: 12586076
2. Boyd J, Sonoda Y and Federici MG et al (2000) Clinicopathologic features of BRCA-linked and sporadic ovarian cancer JAMA 283 2260–5 DOI: 10.1001/jama.283.17.2260 PMID: 10807385
3. Synowiec A, Wcisło G and Bodnar L et al (2016) Hereditary Cancer in Clinical Practice 14 1
4. Werness BA, Ramus SJ and Whittemore AS et al (2000) Histopathology of familial ovarian tumors in women from families with and without germline BRCAI mutations Hum Pathol 31 1420–4 DOI: 10.1016/S0046-8177(00)80014-3 PMID: 11112219
5. Cass I, Baldwin RL and Varkey T et al (2003) Improved survival in women with BRCA-associated ovarian carcinoma Cancer 97 2127–9 DOI: 10.1002/cncr.11310
6. Tan DS, Kaye SB (2015) Chemotherapy for patients with BRCA1 and BRCA2-mutated ovarian cancer: same or different? Am Soc Clin Oncol Educ Book 114–21 DOI: 10.14694/EdBook_AM.2015.35.114
7. Gorodnova TV, Sokolenko AP and Ivantsov AO et al (2015) High response rates to neoadjuvant platinum-based therapy in ovarian cancer patients carrying germ-line BRCA mutation Cancer Lett 369 363–7 DOI: 10.1016/j.canlet.2015.08.028 PMID: 26342406
8. Lorusso D, Cirillo F and Mancini M et al (2013) The different impact of BRCA mutations on the survival of epithelial ovarian cancer patients: a retrospective single-center experience Oncology 85 122–7 DOI: 10.1159/000353786 PMID: 23941904
9. Vencken PM, Kriege M and Hoogwerf D et al (2011) Chemosensitivity and outcome of BRCA1- and BRCA2- associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients Ann Oncol 22 1346–52 DOI: 10.1093/annonc/mdq628 PMID: 21228333
10. Tan DS, Rothermundt C and Thomas K et al (2008) “BRCAness” syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations J Clin Oncol 26 5530–6 DOI: 10.1200/JCO.2008.16.1703 PMID: 18955455
11. Pal T, Permuth-Wey J and Kapoor R et al (2007) Improved survival in BRCA2 carriers with ovarian cancer Fam Cancer 6 113–9 DOI: 10.1007/s10689-006-9112-x
12. Bolton KL, Chenevix-Trench G and Goh C et al (2012) Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer JAMA 307 382–90 DOI: 10.1001/jama.2012.20 PMID: 22274685 PMCID: 3727895
13. Yang D, Khan S and Sun Y et al (2011) Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer JAMA 306 1557–65 DOI: 10.1001/jama.2011.1456 PMID: 21990299 PMCID: 4159096
14. Chen S, Parmigiani G (2007) Meta-analysis of BRCA1 and BRCA2 penetrance J Clin Oncol 25 1329–33 DOI: 10.1200/JCO.2006.09.1066 PMID: 17416853 PMCID: 2267287
15. Kotsopoulos J, Rosen B and Fan I et al (2016) Ten-year survival after epithelial ovarian cancer is not associated with BRCA mutation status Gynecol Oncol 140 42–7 DOI: 10.1016/j.ygyno.2015.11.009
16. Rubin SC, Benjamin I and Behbakht K et al (1996) Clinical and pathological features of ovarian cancer in women with germ-line mutations of BRCA1 N Engl J Med 335 1413–6 DOI: 10.1056/NEJM199611073351901 PMID: 8875917
17. Dann RB, DeLoia JA and Timms KM et al (2012) BRCA1/2 mutations and expression: response to platinum chemotherapy in patients with advanced stage epithelial ovarian cancer Gynecol Oncol 125 677–82 DOI: 10.1016/j.ygyno.2012.03.006 PMID: 22406760
18. Muggia F, Safra T (2014) ‘BRCAness’ and its implications for platinum action in gynecologic cancer Anticancer Res 34 551–6 PMID: 24510983 PMCID: 4682661
19. Gallagher DJ, Konner JA and Bell-McGuinn KM et al (2011) Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity Ann Oncol 22 1127–32 DOI: 10.1093/annonc/mdq577
20. Zhong Q, Peng HL and Zhao X et al (2015) Effects of BRCA1- and BRCA2- related mutations on ovarian and breast cancer survival: a meta-analysis Clin Cancer Res 21 211–20 DOI: 10.1158/1078-0432.CCR-14-1816 PMCID: 4286460
21. Sun C, Li N and Ding D et al (2014) The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis PLoS One 9 e95285 DOI: 10.1371/journal.pone.0095285 PMID: 24788697 PMCID: 4006804
22. Mclaughlin JR, Rosen B and Moody J (2013) Long-term ovarian cancer survival associated with mutation in BRCA1 or BRCA2 J Natl Cancer Inst 105 141–8 DOI: 10.1093/jnci/djs494 PMCID: 3611851
23. Candido-dos-Reis FJ, Song H and Goode EL et al (2015) Germline mutation in BRCA1 or BRCA2 and ten-year survival for women diagnosed with epithelial ovarian cancer Clin Cancer Res 21 652–7 DOI: 10.1158/1078-0432.CCR-14-2497 PMCID: 4338615
24. Jo´annsson OT, Ranstam J and Borg A et al (1998) Survival of BRCA1 breast and ovarian cancer patients: a population-based study from southern Sweden J Clin Oncol 16 397–404
25. Pharoah PD, Easton DF and Stockton DL et al (1999) Survival in familial, BRCA1-associated, and BRCA2- associated epithelial ovarian cancer United Kingdom coordinating committee for cancer res (UKCCCR) familial ovarian cancer study group Cancer Res 59 868–71 PMID: 10029077






