Borderline tumours of the ovary, current controversies regarding their diagnosis and treatment
Maria Guadalupe Patrono1, Lucas Minig1, Ivan Diaz-Padilla2, Nuria Romero2, Juan Francisco Rodriguez Moreno2 and Jesus Garcia-Donas2
1 Gynaecology Oncology Programme, Clara Campal Comprehensive Cancer Centre, HM Hospitals, Madrid 28050, Spain
2 Gynaecology Oncology Programme, Medical Oncology, Comprehensive Oncology Centre Clara Campal, HM Hospitals, Madrid 28050, Spain.
Correspondence to: Lucas Minig. Email: lucasminig@yahoo.com
Abstract
Borderline ovarian tumours generally affect women of reproductive age. The positive prognosis is related to the fact that over 80% of cases are diagnosed at an early stage of the disease. Although radical surgery is the standard of care for this disease, fertility-sparing surgery can be performed in selected cases. Since it was first described in 1929, the knowledge of the molecular and histologic characteristics has been significantly improved. In this review, advances in the clinical behaviour, pathologic characteristics, prognostics factors, and different strategies of treatment are discussed.
Keywords: borderline ovarian tumour, conservative treatment of fertility, gynaecological cancer, ovarian cancer surgery
Introduction
Epithelial tumours of the ovary can be either benign (cystadenomas) or malignant (cystadenocarcinomas). However, there is an intermediate state of epithelial tumours of the ovary called ‘borderline tumours’. Neither the oncological behaviour of this intermediate group of tumours nor the histological changes of the cells of the ovarian epithelium meet the specific criteria of benignity or malignancy. In 1973, the International Federation of Gynaecology and Obstetrics (FIGO) gave this group of ovarian tumours a ‘low malignant potential’ [1], and since then, the World Health Organization (WHO) has called them borderline ovarian tumours (BOTs) [2].
They are tumours that usually occur during the third to fourth decade of women’s lives and are diagnosed as being limited to the ovary in 80% of cases. Because of this, their biological–oncological behaviour is very good, with an overall survival rate of ten years for 90% of those in the initial stages [3] and 60–70% of those in the advanced stages [4, 5].
While the recommended treatment is a hysterectomy with double adnexectomy, this frequently raises the clinical dilemma of diagnosis in women who have not yet given birth. Here, the conservative treatment of fertility may be a safe possibility in selected cases. The effectiveness of the chemotherapy is limited due to the slow rate of growth of the altered cells, meaning its use is limited and not advised. This chapter will describe the main histological features and clinical behaviour, and the different therapeutic options at the time of both the diagnosis and the recurrences of BOTs.
Epidemiology and risk factors
BOTs comprise 15–20% of epithelial tumours [6]. Unlike invasive ovarian cancer, these tumours are diagnosed in the early stages in 80% of cases [7]. The average age at which such tumours are diagnosed is 40 years, but almost 30% of women with them are diagnosed before the age of 40 [8, 9].
Ritman et al [10] assessed the risk factors for BOTs in a case control study using the regional registry of tumours in Sweden. They randomly assigned 3899 control patients from the population register of all the residents in the country. The results showed that the women who had given birth more than once had a lower risk of developing borderline tumours compared with those women who had not given birth at all [odds ratio (OR): 0.44 (confidence interval (CI): 0.26–0.75) for serous tumours and 0.63 (CI: 0.34–1.19) for the mucinous tumours]. The authors found, in contrast, that breast-feeding served as a protective factor. This is similar to what has been observed for ovarian cancer. However, unlike the latter, the use of oral contraceptives was not a protective factor against the development of BOTs [OR: 1.4 (CI: 0.87–2.26)]. Thus, the authors suggested that this finding could support the concept that BOTs would represent a distinct disease to that of invasive ovarian cancer [10].
Classification and histological characteristics
BOT of the ovary is characterised histologically by the presence of epithelial cells with nuclear atypia and mitotic activity in 10% or more of the tumour but without ovarian stromal invasion [8, 11]. The histological subtypes of epithelial BOTs can be serous, mucinous, endometrioid, clear, and transition cells (Brenner). The first two variants include 95% of the total [9].
Serous BOTs
Serous tumours represent 65% of all the BOTs [9]; they are unilateral in 70% of cases, and 30% of them can occur with peritoneal implants. In turn, it is important to note that almost 30% of these implants have microscopic characteristics of stromal invasion [12] The implants with and without invasion are described in this way, reducing the ten-year survival from 95% to 60%, respectively [13].
The relationship of serous BOT as a precursor of invasive carcinoma has been extensively studied in recent years [14], suggesting two morphology-pathogenic tracks of epithelial ovarian tumours, based on their molecular differences and on their clinical behavioural–biological differences [15]. In this way, the serous tumours of type 1 would have a slow growth, generally limited to the ovary at the time of diagnosis, and would be developed from well-established precursor lesions from the cystadenoma, serous BOT until the micropapillary carcinoma low-grade serous. Genetically, they are characterised by mutations in the track of the KRAS, BRAF, PTEN, and A-catenin [15].
Reinforcing this theory, a study published by Shvartsman et al [16] found that serous BOTs often coexist with low-grade serous ovarian carcinomas. The purpose of the study was to compare the oncological outcomes of patients with low-grade serous carcinoma stage II/IV (group 1), with patients with relapsing BOT as low-grade serous carcinoma (group 2). The time free of the disease was studied from its diagnosis in group 1 and from the first relapse in group 2. We identified 112 patients in group 1 and 41 in group 2. There were no statistically significant differences between the two groups in average age (42.7 versus 45.4 years, p = 0.37), progression-free survival (19.5 months versus 25, p = 0.92), nor in the overall survival (81.8 versus 82.8 months, p = 0.84) [16].
Ovarian tumours of type II, in contrast, are fast-growing without precursor lesions and with a high degree of aggressiveness [15]. They manifest themselves as high-grade ovarian carcinomas, with extensive peritoneal dissemination and extra-abdominal disease and include the high-grade serous carcinomas, malignant mixed mesodermal tumours (carcinosarcomas), and undifferentiated carcinomas [15]. This group of tumours has a high level of genetic instability and is characterised by the mutation of the P53 gene and the overexpression of the genes of HLA-G, HER2, and AKT [17]. Most of the malignant epithelial tumours of the ovary belong to this type II.
Borderline ovarian mucinous tumours
Mucinous BOTs represent 32% of all epithelial BOTs [7]. They are divided into two histologic subtypes: the intestinal (90%) and the Müllerian (endocervical type). In contrast to the serous BOT, the mucinous subtype is associated more rarely with peritoneal implants [9]. Like the serous tumours, the survival of women with stage I is 100%, while that in advanced stages is only 50% [9].
Following the type I morphology-pathogenic pathway previously described for the serous tumours, the mucinous BOT usually reaches a large size, tends to be unilateral, and can coexist with areas of mucinous cystadenoma or low-grade invasive carcinoma [14].
Diagnosis
Clinical differentiation between borderline ovarian cysts, benign or malignant, is difficult. The majority of patients show an asymptomatic adnexal mass, in the annual gynaecological exam as an incidental finding in a gynaecological ultrasound. Of all forms, the most frequent symptoms are those of any type of adnexal mass and include abdominal pain, changes in intestinal transit, pelvic pain, and dyspareunia among others. The ability to manifest itself with abdominal distension because of ascites is less likely than in cases of ovarian cancer.
Vine et al [18] evaluated the symptoms and their duration before the diagnosis of invasive cancer or BOT. The authors observed that patients with BOT were twice as likely to be asymptomatic at diagnosis. At the same time, women with BOT were two times more likely to be diagnosed during a routine examination. Among the women with symptoms, those with BOT had a greater duration of symptoms than women with ovarian cancer (six versus four months). These aspects of the clinical presentation of BOT are probably a reflection of the more indolent nature of these tumours.
The vaginal ultrasound is the first step in the evaluation of patients with an adnexal mass. Exacoustos et al [19] determined that the presence of papillae within the cyst was the most common finding in the BOT. However, neither the sonographic features nor the papillae are ultrasound markers of high sensitivity. A Japanese study [20] also showed the usefulness of employing magnetic resonance imaging (MRI) to distinguish between BOTs and other conditions that are invasive to the ovary.
The tumour marker CA-125 may be high in more than half of the patients with BOT [8]. Engelen et al [21] assessed the level of the CA-125 preoperatively, which was found to be high in 8 of 33 (24%) patients, levels of CEA in 3 of 32 (9%), and levels of CA 19-9 in 11 of 24 (46%) cases. In patients with mucinous BOT, CA 19-9 was most frequently high (8/14, 57%) when compared with the CA-125 (3/20, 15%) (p = 0.02) or the CEA (2/18, 11%) (p = 0.02). Ayhan et al [22] found that the positivity of the CA-125 in the BOT serous group was statistically greater than in the group of BOT mucinous, whereas positivity for CA 19-9 and CEA in the mucinous histology was significantly greater than those of the serous tumours after analysing 60 patients.
Kolwijck et al [23] found that preoperative serum levels of CA-125 were significantly higher in patients with advanced stages (median 181 IU/ml; reaching values of I413 U/ml) compared with patients with initial stage (median 28 IU/ml; reaching values of 1123 IU/ml). The median in patients with serous histology was 59 IU/ml compared with the mucinous histology, which was 25 IU/ml.
Other diagnostic methods such as MRI and positron emission tomography-computed tomography (PET-CT) are usually reserved for selected cases since the sonographic features of the adnexal mass, coupled with the value of the tumour marker CA-125, are usually sufficient to indicate diagnostic or therapeutic surgery. Computed tomography (CT) is useful in the case of an adnexal mass with suspected BOT or malignancy. The objective is to detect intra-abdominal presence of disease [24].
In any case, the definitive diagnosis of the adnexal mass is made by an intraoperative histological study. In this sense, many studies have evaluated the accuracy of the histological diagnosis of the adnexal mass [25, 26]. As a result, the diagnosis is not often easy, mainly in large and mucinous tumours [25, 26]. The intraoperative diagnosis has proven to be reliable in discriminating between a benign and a malignant mass in a BOT (overdiagnosis less than 10%). However, a subdiagnosis of 25–30 % has been shown in differentiating a BOT from a malignant tumour [27, 28].
The clinical consequence of this limit is the need to re-operate to surgically stage the cases diagnosed as ovarian cancer in the final histological analysis that had been BOT in the intraoperative diagnosis.
Treatment
Surgery is the initial treatment for BOTs. Its principle is the same as that in invasive cancer, to remove the whole of the disease that is macroscopically visible. The recommended surgical staging includes having a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, multiple biopsies, and peritoneal cytology. In the case of mucinous BOTs, an appendectomy should also be performed (Figure 1). The role of a lymphadenectomy has been extensively discussed in recent years [29–32]. However, according to the results of multiple studies, a pelvic and aortic lymphadenectomy does not improve the disease-free time or overall survival rate for women with BOT [29–33].
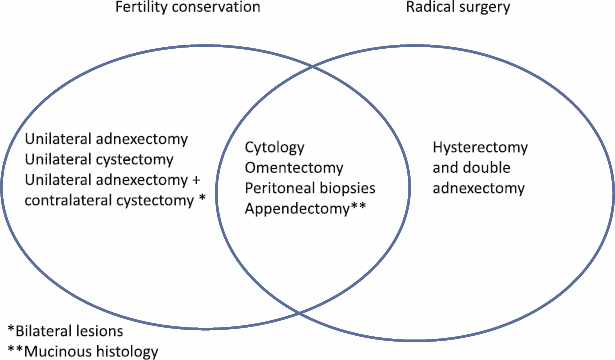
Figure 1: Surgical procedures in patients with borderline tumor with and without the desire to preserve fertility.
In contrast, the diagnosis may also be made at the time of the removal of a seemingly benign ovarian cyst. In that case, the dilemma is whether the patient should or should not be re-operated on, with the objective of completing the surgical staging and of making a careful inspection of the entire abdominal and pelvic cavity, to detect the presence of possible peritoneal implants. According to the data from the literature, this must be done mainly in the serous subtypes [33]. However, the majority of authors recommend this routinely, regardless of the histological subtype of the BOT [34, 35].
Staging of borderline tumours of the ovary
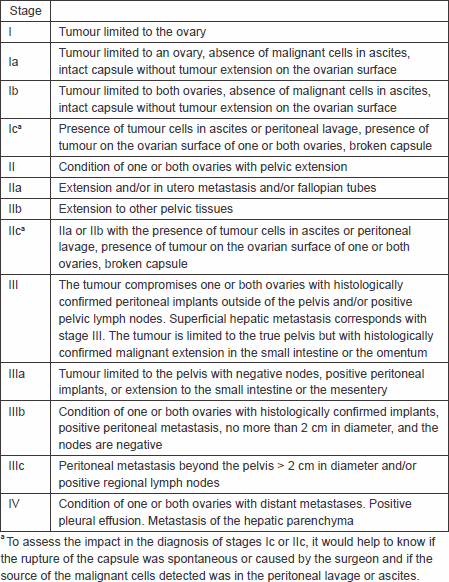
The FIGO and the American Joint Committee on Cancer (AJCC) have designated stages to define ovarian tumours of a low malignant potential. The FIGO system as seen below is the most frequently used (Table 1) [36, 37].
Table 1. Distribution of Recurrence Score categories by patient/tumour characteristics.
Prognostic factors
In patients with BOTs in advanced stages (II–IV), prognostic factors include age at the time of diagnosis, FIGO stage, residual disease following surgery, type of peritoneal implants (with or without invasion), presence of microinvasion in the ovarian tumour, micropapillary pattern, and the CA-125 value [38].
Approximately 30% of patients with serous BOTs have peritoneal implants at the time of diagnosis. Of these, 30% are invasive implants [39]. Morice et al [40] evaluated 80 patients with serous BOTs in FIGO stages II–III, 65 of whom had non-invasive implants, and 15 invasive implants. They found that the only prognostic factor for patients with advanced stage was the presence of invasive peritoneal implants. Reinforcing this idea, another study found that, although the overall survival rate of seven years for BOTs in advanced stages is 95% [41], in the presence of invasive implants, it can drop to 33% [42]. The extent of residual disease is another important prognostic factor. In a series of 168 patients with serous BOTs (FIGO stage II and III), the survival rate of five years was 75%, 76%, and 56% (p < 0.02) in patients without residual disease, with residual disease 1–20 mm or more than 20 mm, respectively [43]. In addition to the therapeutic potential, complete elimination of peritoneal implants allows for complete histological analysis of the disease [11].
Adjuvant chemotherapy
Stage I BOTs do not need adjuvant treatment. A retrospective study from the Gynaecologic Oncology Group (GOG) analysed 988 adequately staged patients with stage I BOTs who did not receive adjuvant treatment and observed a mortality rate of 0.7% at five years [44].
In contrast, the role of adjuvant therapy in women with advanced stage BOTs is debatable. A meta-analysis of from Cochrane on BOTs concluded that current evidence does not show any benefits in the use of adjuvant therapy, whether it is chemotherapy or radiotherapy, independently of the stage or tumour histology [45].
Trope et al [46], after a review of four randomised studies, concluded that adjuvant treatment in patients with stage I of the disease, significantly increases intestinal, neurological, and haematological toxicity, without therapeutic benefits. Nor did Sutton et al [47] find benefits in the use of chemotherapy in patients with stage III randomly assigned to receive treatment with cisplatin plus cyclophosphamide with or without doxorubicin in a prospective study from the GOG. Of the 32 patients selected after initial cytoreduction, 19 had residual disease less than 1 cm, and 13 remained without residual disease. Twenty (62.5%) received cisplatin plus cyclophosphamide and 12 cisplatin, cyclophosphamide, and doxorubicin as monotherapy; 75% of the patients received six or more cycles. A second exploration was done in 15 cases; only six were negative. However, with an average follow-up of 31.7 months (range 1–75), 31 patients remained alive without clinical evidence of disease.
Conservative fertility treatment
Conservative fertility treatment, which consists of removing the entire disease but preserving the uterus and at least a part of an ovary, is especially important in women with BOT since nearly 30% of women are diagnosed before 40 years old, and many of them have not even met their expectations for reproduction [8, 9]. The conservative treatment with BOT consists of doing peritoneal cytology, infracolic omentectomy, peritoneal biopsies, and appendectomy in the case of mucinous BOTs (Figure 1).
Conservative surgery has been extensively evaluated in recent years. After analysing more than 2000 published cases, conservative fertility surgery is associated with a major risk of recurrence of the disease but has no impact on the overall survival rate [48–50].
The radicalism of conservative surgery, either ovarian cystectomy or unilateral oophorectomy, must be based on the extension of the disease and the presence of factors associated with a bad prognostic advising against conservative treatment [11]. These include the presence of microinvasion, a micropapillary pattern, and invasive peritoneal implants [51]. In contrast, based on the data from the literature, there do not seem to be contraindications for the use of drugs for ovarian stimulation in case of getting future pregnancies following the diagnosis and treatment of the disease [52, 53].
Another controversial point is whether the patients who have received conservative treatment of fertility must later receive radical surgical treatment with removal of the uterus and remaining ovary after meeting their reproduction desires, once they reach menopause or waiting on a possible disease relapse, independently of the age of the patient.
Given that there are no uniform criteria in that regard, possibly the most important factor of which to take note is the stage of the tumour at the time of diagnosis. So one could contemplate completing the staging at the time of recurrence or after having met the reproduction desires in patients with early or advanced stages, respectively [40, 51].
Follow-up
Today there is no consensus with respect to the best way to follow-up with patients following the initial treatment for BOT for the early detection of recurrence. It seems reasonable that the sum of methods such as physical examination, CA-125 determination, and CT is a method of choice. Vaginal gynaecologic ultrasound is of vital importance in women who receive conservative treatment of fertility. It is important to know that the free time of disease for women with BOT who show a relapse is significantly major with respect to women with invasive ovarian carcinoma. Uzan et al [54] observed a time up until the recurrence of up to 31 months (range 4–242 months) in 162 patients with advanced stage serous BOT. Similarly, recurrences as far apart as 15 years following initial diagnosis have been described [55]. These data force the prolongation of the time of follow-up with said patients for many years following the diagnosis. In this way, the recommended follow-up must be every three months for the first two years, every six months from two to five years and must continue every year up to 15 years after the initial diagnosis [55].
Zanetta et al [56] analysed prospectively the follow-up of 164 women with stage I BOT who have undergone conservative surgery of fertility. The follow-up was done with a physical examination and vaginal ultrasound every three months for two years and every six months after that. The CA-125 determination was planned every six months in patients with a serous BOT. The authors concluded that the vaginal ultrasound is the most effective diagnosis technique in this group of patients. The clinical practice guide of the National Comprehensive Cancer Network (NCCN) [57] recommends follow-up with gynaecologic ultrasound; but in those patients with invasive implants the follow-up must be similar to that of ovarian cancer: CT of thorax-abdomen-pelvis and CA-125. Likewise, women who meet maternity expectations and were previously treated under conservative must receive the aforementioned standard surgical treatment [57].
With respect to hormonal contraception, the Centres for Disease Control and Prevention (CDC) classifies as category 1 (there is no restriction on the use of contraceptive methods) the use of combined contraceptive pills as well as progestins, patches, vaginal ring, copper intrauterine device and levonorgestrel, birth control methods for patients with ovarian cancer; hence the choice of contraceptive methods is not considered limited by the diagnosis of a BOT [58].
Something similar happened with the use of hormone replacement therapy (HRT): a Swiss study done by Mascarenhas et al [59] analysed the five-year survival rate in patients with BOT and invasives that received HRT. They did a prospective cohort study in which they included 799 women diagnosed with invasive cancer (n = 649) and BOT (n = 150) between the ages of 50 and 74 years in 1993–1995. Following five years of follow-up, 45% of patients with carcinoma and 93% of patients with BOT were alive. For women with BOT, there was no association between the use of HRT used before or after diagnosis and the survival rate. Nor was there any association for women with ovarian cancer.
Treatment of relapses
Surgical treatment with the objective of maximum cytoreduction is the goal of treatment for relapsed BOTs [7, 34, 60]. This concept is equally valid for women following a conservative fertility pretreatment who want to do it again but with adequate counsel, accepted the major risk of relapse and with the concrete possibility of a strict follow-up [34, 61].
Conclusion
BOTs constitute a group of epithelial tumours that can affect women of reproductive age who have excellent prognosis due to the low aggressiveness and the fact that they are largely diagnosed in initial stages. Conservative surgery of fertility is possible in select cases with disease limited to the ovary and when there is a strong desire to become a mother. Radical surgery of maximum cytoreduction is the goal of treatment in women with advanced or recurrent diseases. Residual disease following surgery and the presence of invasive peritoneal implants are the main prognostic factors. The benefits of adjuvant chemotherapy are limited by the fact that its use is still inadvisable. The follow-up of patients must be done up to 15 years following the initial diagnosis.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
1. Kottmeier HL, Kolstad P and McGarrity KA (1973) Annual Report on the Results of Treatment in Gynaecologic Cancer vol 17, Statements of results obtained in 1969–1972, inclusive (Stockholm, Sweden, Editorial office, Radiumhemmet: FIGO)
2. Serov SF, Scully RE and Sobin LH (1973) International histological classification of tumors, no 9 Histoloigcal Typing of Ovarian Tumours (Geneva: World Health Organization)
3. Nikrui N (1981) Survey of clinical behavior of patients with borderline tumors of the ovary. Gynecol Oncol 12 107–19 DOI: 10.1016/0090-8258(81)90102-5 PMID: 6268484
4. Bostwick DG, Tazelaar HD and Ballon SC (1983) Ovarian epithelial tumors of borderline malignancy: a clinical and pathologic study of 109 cases Cancer 58 2052–64 PMID: 3756820
5. Leake JF, Currie JL and Rosenshein NB (1992) Long-term follow-up of serous ovarian tumors of low malignant potential Gynecol Oncol 47 150–8 DOI: 10.1016/0090-8258(92)90099-5 PMID: 1468692
6. Lenhard MS, Mitterer S and Kümper C (2009) Long-term follow-up after ovarian borderline tumor: relapse and survival in a large patient cohort Eur J Obstet Gynecol Reprod Biol 145 189–94 DOI: 10.1016/j.ejogrb.2009.04.031 PMID: 19477060
7. Trillsch F, Mahner S and Ruetzel J (2010) Clinical management of borderline ovarian tumors Expert Rev Anticancer Ther 10 1115–24 DOI: 10.1586/era.10.90 PMID: 20645700
8. Jones MB (2006) Borderline ovarian tumors: current concepts for prognostic factors and clinical management Clin Obstet Gynecol 49(3) 517–25 DOI: 10.1097/00003081-200609000-00011 PMID: 16885658
9. Lalwani N, Shanbhogue AK, Vikram R (2010) Current update on borderline ovarian neoplasms AJR Am J Roentgenol 194(2) 330–6 DOI: 10.2214/AJR.09.3936 PMID: 20093592
10. Riman T, Dickman PW and Nilsson S (2001) Risk factors for epithelial borderline ovarian tumors: results of a Swedish case– control study Gynecol Oncol 83 575–85 DOI: 10.1006/gyno.2001.6451 PMID: 11733975
11. Morice P, Uzan C and Fauvet R (2012) Borderline ovarian tumour: pathological diagnostic dilemma and risk factors for invasive or lethal recurrence Lancet Oncol 13(3) e103–15 DOI: 10.1016/S1470-2045(11)70288-1 PMID: 22381933
12. Seidman JD, Horkayne-Szakaly I and Haiba M (2004) The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin Int J Gynecol Pathol 23 41–44 DOI: 10.1097/01.pgp.0000101080.35393.16
13. Sherman ME, Mink PJ and Curtis R (2004) Survival among women with borderline ovarian tumors and ovarian carcinoma: a population-based analysis Cancer 100 1045–52 DOI: 10.1002/cncr.20080 PMID: 14983501
14. Kurman RJ and Shih IeM (2008) Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications Int J Gynecol Pathol 27(2)151–60 PMID: 18317228 PMCID: 2794425
15. Shih I and Kurman R (2004) Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis Am J Pathol 164 1511–8 DOI: 10.1016/S0002-9440(10)63708-X PMID: 15111296 PMCID: 1615664
16. Shvartsman HS, Sun CC and Bodurka DC (2007) Comparison of the clinical behavior of newly diagnosed stages II-IV low-grade serous carcinoma of the ovary with that of serous ovarian tumors of low malignant potential that recur as low-grade serous carcinoma Gynecol Oncol 105(3) 625–9 DOI: 10.1016/j.ygyno.2007.01.030 PMID: 17320156
17. Landen CN Jr, Birrer MJ and Sood AK (2008) Early events in the pathogenesis of epithelial ovarian cancer J Clin Oncol 26(6) 995–1005 DOI: 10.1200/JCO.2006.07.9970 PMID: 18195328
18. Vine MF, Ness RB and Calingaert B (2001) Types and duration of symptoms prior to diagnosis of invasive or borderline ovarian tumor Gynecol Oncol 83(3) 466–71 DOI: 10.1006/gyno.2001.6411 PMID: 11733956
19. Exacoustos C, Romanini ME and Rinaldo D (2005) Preoperative sonographic features of borderline ovarian tumors Ultrasound Obstet Gynecol 25(1) 50–9 DOI: 10.1002/uog.1823
20. Takemori M, Nishimura R and Hasegawa K (2002) Clinical evaluation of MRI in the diagnosis of borderline ovarian tumors Acta Obstet Gynecol Scand 81(2) 157–61 DOI: 10.1034/j.1600-0412.2002.810212.x PMID: 11942907
21. Engelen MJ, de Bruijn HW and Hollema H (2000) Serum CA 125, carcinoembryonic antigen, and CA 19-9 as tumor markers in borderline ovarian tumors Gynecol Oncol. 78(1) 16–20 DOI: 10.1006/gyno.2000.5811 PMID: 10873403
22. Ayhan A, Guven S and Guven ES (2007) Is there a correlation between tumor marker panel and tumor size and histopathology in well staged patients with borderline ovarian tumors? Acta Obstet Gynecol Scand 86(4) 484–90 DOI: 10.1080/00016340701226138 PMID: 17486473
23. Kolwijck E, Thomas CM and Bulten J (2009) Preoperative CA-125 levels in 123 patients with borderline ovarian tumors: a retrospective analysis and review of the literature Int J Gynecol Cancer 19(8) 1335–8 DOI: 10.1111/IGC.0b013e3181a83e04 PMID: 20009886
24. Liu JH and Zanotti KM (2011) Management of the adnexal mass Obstet Gynecol 117(6) 1413–28 DOI: 10.1097/AOG.0b013e31821c62b6 PMID: 21606754
25. Tangjitgamol S, Jesadapatrakul S and Manusirivithaya S (2004) Accuracy of frozen section in diagnosis of ovarian mass Int J Gynecol Cancer 14(2) 212–9 DOI: 10.1111/j.1048-891X.2004.014202.x PMID: 15086718
26. Kim JH, Kim TJ and Park YG (2009) Clinical analysis of intra-operative frozen section proven borderline tumors of the ovary J Gynecol Oncol 20(3) 176–80 DOI: 10.3802/jgo.2009.20.3.176 PMID: 19809552 PMCID: 2757563
27. Tropé CG, Kristensen G and Makar A (2000) Surgery for borderline tumor of the ovary Semin Surg Oncol 19 69–75 PMID: 10883027
28. Tempfer CB, Polterauer S and Bentz EK (2007) Accuracy of intraoperative frozen section analysis in borderline tumors of the ovary: a retrospective analysis of 96 cases and review of the literature Gynecol Oncol 107 248–252 DOI: 10.1016/j.ygyno.2007.06.008 PMID: 17631951
29. Winter WE 3rd, Kucera PR and Rodgers W (2002) Surgical staging in patients with ovarian tumors of low malignant potential Obstet Gynecol 100(4) 671–6 DOI: 10.1016/S0029-7844(02)02171-3 PMID: 12383532
30. Rao GG, Skinner E and Gehrig PA (2004) Surgical staging of ovarian low malignant potential tumors Obstet Gynecol 104(2) 261–6 DOI: 10.1097/01.AOG.0000133484.92629.88 PMID: 15291997
31. Kaern J, Tropé CG and Abeler VM (1993) A retrospective study of 370 borderline tumors of the ovary treated at the Norwegian Radium Hospital from 1970 to 1982 Cancer 71(5) 1810–20 PMID: 8383580
32. Camatte S, Morice P and Thoury A (2004) Impact of surgical staging in patients with macroscopic ‘stage I’ ovarian borderline tumours: analysis of a continuous series of 101 cases Eur J Cancer 40 1842–9 DOI: 10.1016/j.ejca.2004.04.017 PMID: 15288285
33. Snider DD, Stuart GC and Nation JG (1991) Evaluation of surgical staging in stage I low malignant potential ovarian tumors Gynecol Oncol 40 129–32 DOI: 10.1016/0090-8258(91)90103-C PMID: 2010103
34. Cadron I, Leunen K and Van Gorp T (2007) Management of borderline ovarian neoplasms J Clin Oncol 25 2928–37 PMID: 17617524
35. Trimble EL and Trimble LC (1993) Epithelial ovarian tumors of low malignant potential Cancer of the Ovary, ed M Markman and W Hoskins (New York: Raven Press) pp 415–29
36. FIGO Committee on Gynecologic Oncology (2009) Current FIGO staging for cancer of the vagina, fallopian tube, ovary, and gestational trophoblastic neoplasia Int J Gynaecol Obstet 105(1) 3–4 PMID: 19322933
37. American Joint Committee on Cancer (2010) Ovary and primary peritoneal carcinoma AJCC Cancer Staging Manual 7th edn, ed SB Edge, DR Byrd, CC Compton, et al (New York: Springer) pp 419–28
38. Gherhenson D (2002) Clinical management potential tumours of low malignancy Best Pract Res Clin Obstet Gynaecol 6(4) 513–27 DOI: 10.1053/beog.2002.0308
39. Gershenson DM, Silva EG and Tortolero-Luna G (1998) Serous borderline tumors of the ovary with noninvasive peritoneal implants Cancer 83(10) 2157–63 PMID: 9827720
40. Morice P, Camatte S and Rey A (2003) Prognostic factors for patients with advanced stage serous borderline tumours of the ovary Ann Oncol 14(4) 592–8 DOI: 10.1093/annonc/mdg173 PMID: 12649107
41. Seidman JD and Kurman RJ (2000) Ovarian serous borderline tumors: a critical review of the literature with emphasis on prognostic indicators Hum Pathol 31 539–57 DOI: 10.1053/hp.2000.8048 PMID: 10836293
42. Seidman JD and Kurman RJ (1996) Subclassi!cation of serous borderline tumors of the ovary into benign and malignant types. A clinicopathologic study of 65 advanced stage cases Am J Surg Pathol 20 1331–45 DOI: 10.1097/00000478-199611000-00004 PMID: 8898837
43. Uzan C, Kane A, Rey A and Gouy S (2010) Outcomes after conservative treatment of advanced-stage serous borderline tumors of the ovary Ann Oncol 21(1) 55–60 DOI: 10.1093/annonc/mdp267
44. Barnhill DR, Kurman RJ and Brady MF (1995) Preliminary analysis of the behavior of stage I ovarian serous tumors of low malignant potential: a Gynecologic Oncology Group study J Clin Oncol 13(11) 2752–6 PMID: 7595734
45. Faluyi O, Mackean M and Gourley C (2010) Interventions for the treatment of borderline ovarian tumours Cochrane Database Syst Rev 9 CD007696 PMID: 20824864
46. Tropé C, Kaern J and Vergote IB (1993) Are borderline tumors of the ovary overtreated both surgically and systemically? A review of four prospective randomized trials including 253 patients with borderline tumors Gynecol Oncol 51 236–43 DOI: 10.1006/gyno.1993.1279 PMID: 8276300
47. Sutton GP, Bundy BN and Omura GA (1991) Stage III ovarian tumors of low malignant potential treated with cisplatin combination therapy (a Gynecologic Oncology Group study) Gynecol Oncol 41 230–33 DOI: 10.1016/0090-8258(91)90314-U PMID: 1869100
48. Kane A, Uzan C and Rey A (2009) Prognostic factors in patients with ovarian serous low malignant potential (borderline) tumors with peritoneal implants Oncologist 14 591–600 DOI: 10.1634/theoncologist.2008-0263 PMID: 19487334
49. Zanetta G, Rota S and Chiari S (2001) Behavior of borderline tumors with particular interest to persistence, recurrence, and progression to invasive carcinoma: a prospective study J Clin Oncol 19 2656–64
50. Uzan C, Kane A and Rey A (2010) Outcomes after conservative treatment of advanced-stage serous borderline tumors of the ovary Ann Oncol 21 55–60 DOI: 10.1093/annonc/mdp267
51. Nam JH (2010) Borderline ovarian tumors and fertility Curr Opin Obstet Gynecol 22(3) 227–34 DOI: 10.1097/GCO.0b013e3283384928 PMID: 20386444
52. Beiner ME, Gotlieb WH and Davidson B (2001) Infertility treatment after conservative management of borderline ovarian tumors Cancer 92(2) 320–5 PMID: 11466685
53. Fortin A, Morice P and Thoury A (2001) Impact of infertility drugs after treatment of borderline ovarian tumors: results of a retrospective multicenter study Fertil Steril 87(3) 591–6 DOI: 10.1016/j.fertnstert.2006.07.1503
54. Uzan C, Kane A and Rey A (2011) How to follow up advanced-stage borderline tumours? Mode of diagnosis of recurrence in a large series stage II-III serous borderline tumours of the ovary Ann Oncol 22(3) 631–5 DOI: 10.1093/annonc/mdq414
55. Tropé CG, Kaern J and Davidson B (2012) Borderline ovarian tumours Best Pract Res Clin Obstet Gynaecol 26(3) 325–36 DOI: 10.1016/j.bpobgyn.2011.12.006 PMID: 22321906
56. Zanetta G, Rota S and Lissoni A (2001) Ultrasound, physical examination, and CA 125 measurement for the detection of recurrence after conservative surgery for early borderline ovarian tumors Gynecol Oncol 81(1) 63–6 DOI: 10.1006/gyno.2000.6099 PMID: 11277651
57. NCCN Guidelines Version 1.2013 Epithelial ovarian cancer/fallopian tube cancer/primary peritoneal cancer
58. Centers for Disease Control and Prevention (CDC) (2010) US Medical Eligibility Criteria for contraceptive use MMWR Recomm Rep 59(RR-4) 1–86
59. Mascarenhas C, Lambe M and Bellocco R (2006) Use of hormone replacement therapy before and after ovarian cancer diagnosis and ovarian cancer survival Int J Cancer 119(12) 2907–15 DOI: 10.1002/ijc.22218 PMID: 16998830
60. Silva EG, Gershenson DM and Malpica A (2006) The recurrence and the overall survival rates of ovarian serous borderline neoplasms with noninvasive implants is time dependent Am J Surg Pathol 30 1367–71 DOI: 10.1097/01.pas.0000213294.81154.95 PMID: 17063075
61. Cadron I, Amant F and Van Gorp T (2006) The management of borderline tumours of the ovary Curr Opin Oncol 18 488–93 DOI: 10.1097/01.cco.0000239889.98289.ce PMID: 16894298






