The impact of human immunodeficiency virus infection on cervical preinvasive and invasive neoplasia in South Africa
Louis-Jacques van Bogaert
National Health Laboratory Service, Polokwane/Mankweng Hospital Complex, University of Limpopo, Polokwane 0700, South Africa
Correspondence to: Louis-Jacques van Bogaert. Email: louis.vanBogaert@nhls.ac.za
Abstract
Objectives: Sub-Saharan Africa is at the epicentre of the human immunodeficiency virus (HIV) epidemic and has the highest incidence of invasive cervical cancer (ICC) in the world. Access to highly active antiretroviral treatment (HAART) in South Africa is still limited and provided only to nonpregnant women with a CD4 T-cell count <200 μg/L. We evaluated the relative distribution of cervical preinvasive and invasive neoplasia among HIV-infected (treated or not) and uninfected women in the Limpopo Province of South Africa.
Methods: We compared the consecutive biopsy-diagnosed cervical pathology of 1,023 HIV-infected and 1,023 uninfected women. We investigated the influence of the CD4 T-cell count and of HAART on the relative distribution of cervical pathology.
Results: There was a significantly higher proportion of cervical intraepithelial neoplasia (CIN)1 (P = 0.012) and 2 (P = 0.01) but a lower proportion of ICC (P = 0.015) among HIV-infected women. Patients on HAART had less CIN1 (P = 0.018), 2 (P = 0.18) and ICC (P = 0.019) that their untreated counterparts. The mean CD4 count was similar regardless of cervical lesions and HAART or no treatment.
Conclusion: Our data support the concept that HIV-infected women exhibit a higher rate of high-grade preinvasive lesions than uninfected controls. However, they have a significantly lower rate of ICC as compared with uninfected counterparts. The inclusion of ICC among acquired immune deficiency syndrome-defining illnesses is questionable.
Keywords: AIDS-defining illness, cervical neoplasia, HIV, South Africa.
Introduction
In 1987, the US Centers for Disease Control and Prevention created a list of clinical conditions called acquired immune deficiency syndrome (AIDS)-defining illnesses (ADI). Most of them were related to microbiological agents, such as viral, fungal, bacterial, and protozoan agents. In 1993, three more were added, such as Kaposi’s sarcoma, lymphoma, and invasive cervical cancer (ICC) [1]. Although the role of human papillomavirus (HPV) in cervical preinvasive and invasive neoplasia was already known, the vast body of investigation currently available was still relatively limited in those days [2]. This may explain why HPV was not listed among the microbiological agents involved in ADI until 2011 [3].
To qualify as an AIDS-defining cancer, the malignancy should be at an increased risk in the HIV-positive population, and the risk should be inversely proportional to the degree of immunosuppression as expressed by the CD4 T-cell count. In addition, partial immune reversal through highly active antiretroviral treatment (HAART) should decrease the incidence of AIDS-defining cancers [4]. As a whole, the incidence of HPV-induced diseases has increased since the introduction of HAART [5]. Cervical cancer incidence, however, among HIV-infected women in the US has been unchanged since the introduction of HAART [6, 7]. There is no convincing evidence that the HIV epidemic went parallel with an increase in ICC in sub-Saharan Africa [8].
In general, the rate of HPV-associated cancers is higher among people with AIDS; however, the relative risk of ICC linked to immunodepression is only 1.32 [95% confidence interval (CI) 0.96–1.80 and P = 0.077] [9, 10]. It has also been speculated that the increased longevity of patients on HAART would result in an increase in ICC among HIV-infected women. Perhaps it is still difficult to witness this within a timespan of ten years. However, the fact that the average age at diagnosis of ICC in HIV-infected women is at least ten years younger than that of HIV-naive women does not support this hypothesis [11].
It is well documented that HIV-infected women are at a risk of carrying one or more high-risk (HR) HPV and tend to have a low clearance rate [12]. Therefore, they are at HR of developing high-grade squamous intraepithelial lesions (HGSIL) or HR cervical intraepithelial neoplasia (CIN) 2 . Whether this places them at increased risk of ICC is controversial [4].
Methods
Study settings and participants
The study was approved by the Research Ethics Committee of the Polokwane/Mankweng Hospital Complex and the Institutional Review Board of the University of Limpopo at Polokwane. The province has a population in excess of 5,000,000, which is mainly rural. The laboratory receives all surgical pathology specimens (in excess of 10,000 cases/annum) from the public health facilities serving at least 80% of the population. During the five-year study period (2008–2012), 5,500 specimens were cervical in origin and 1,023 (19.0%) were from HIV-infected women.
A consecutive series of cervical biopsies of 1,023 HIV-infected women was prospectively collected over a period of five years (2008–2012). Consecutive cervical biopsies from 1,023 HIV-uninfected women served as controls. The majority of specimens consisted of colposcopydirected large-loop excision of the transformation zone represented by an abnormal cytology, low grade (LG) or HGSIL. Punch biopsies were from symptomatic (contact bleeding and bloody vaginal discharge) women. Information was collected from the laboratory request form and included age, cytological diagnosis, HIV serostatus, CD4 T-cell count at the time of cervical pathology diagnosis (when available), and HAART (when applicable).
Laboratory tests
The surgical pathology specimens were routinely processed (10% buffered formalin fixation and paraffin embedding). Four micron sections were stained with haematoxylin and eosin. Preinvasive lesions were subdivided into CIN1, 2 and 3.
Statistical analysis
Data entries were carried out on site. Statistical analysis was done using GraphPad (Prism, San Diego, CA). We used column statistics, Student’s t test, chi-square (Χ2) analysis for contingency tables, and 95% CIs of proportions. The level of statistical significance was set at P < 0.05.
Results
Table 1 lists the relative distribution of preinvasive and invasive lesions according to HIV serostatus. HIV-positive women had significantly more CIN1 and 2 than HIV-uninfected women (P = 0.012 and 0.01). The proportion of CIN3 was similar (P = 0.07). HIV-negative patients had significantly more invasive cancers than the HIV positives (P = 0.015).
Table 2 lists the relative distribution of cervical neoplasia in HIV-infected women according to the treatment status. Untreated women had fewer preinvasive lesions but a higher rate of ICC than treated women (P = 0.019).
Table 1. Relative distribution of preinvasive and invasive lesions: HIV-negative versus HIV-positive women
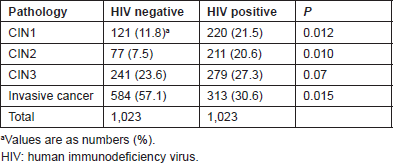
Table 2. Relative distribution of preinvasive and invasive lesions in HIV-positive women: not on HAART versus on HAART
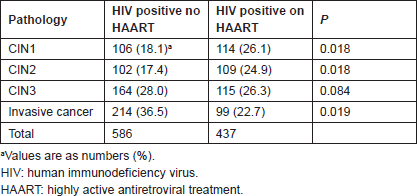
Table 3 lists the mean CD4 T-cell counts of uninfected and untreated infected women. No data were available for HIV-negative CIN1 and 3 cases. Among uninfected women, CD4 counts with CIN2 were significantly higher than with ICC (t = 2.2 and P = 0.032). Among infected untreated women, there was no difference in CD4 counts between CIN2 and ICC (t = 0.93 and P = 0.35).
Tables 4 and 5 list the average CD4 counts of HIV-infected women according to the cervical pathology and treatment. No significant difference was found between CD4 counts on HAART or not between CIN1–CIN3 and invasive cancer. CD4 counts were similar with increasing severity regardless of treatment or no treatment. Table 6 shows that the cervical pathology and treatment did not affect the proportion of cases with CD4 counts below 200/μL.
Table 3. CD4 T-cell counts/μL: HIV-negative versus HIV-positive untreated women
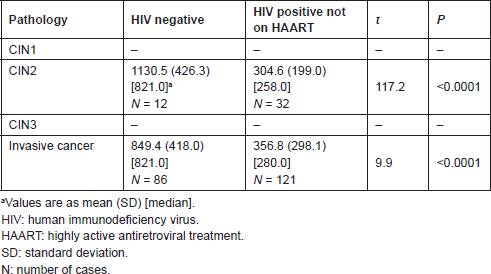
Table 4. CD4 T-cell counts/μL in HIV-positive women: not on HAART versus HAART
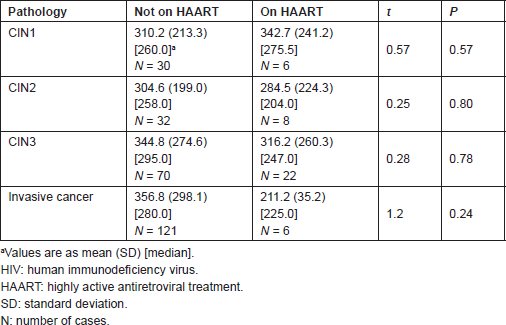
Table 5. Statistical significance of the difference in mean CD4 T-cell count/μL of preinvasive and invasive lesions on HAART and not on HAART
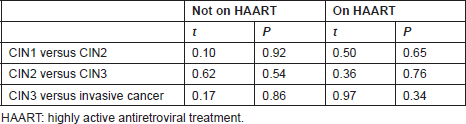
Table 6. Number of cases with CD4 T-cell count <200/μL

Discussion
Current South African cervical cancer statistics are lacking, since the latest National Cancer Registry report dates back to 1999 when ICC ranked first among female cancers [13]. The total prevalence of 19.0% of HIV seropositivity in the present series of preinvasive and invasive lesions was similar to the reported incidence of 20.0% HIV infection in the South African general female population between the ages of 15 and 45 [14].
The prevalence of HIV infection in women with ICC varies from 2.7 in Nigeria to 15%, 18% and 21% in Uganda, Kenya and Tanzania, respectively, [8, 15–17]. South African surveys of ICC with known HIV status found that 6.0% and 13.1%, respectively, were HIV positive, showing no excess risk of ICC [18, 19].
According to Bower et al, to qualify as ADI, the risk of an AIDS-related malignancy should increase [4]. The odds ratio of the association between HIV and ICC has been estimated to vary from 1.17 to 1.27, 1.32 and 1.60, showing only a small increased risk [6, 8, 10, 18]. Hence, the first requirement is not met as far as ICC is concerned.
Second, the risk of ADI should be inversely proportional to the degree of immunosuppression as expressed by the CD4 T-cell count [4]. This, however, is a controversial and hotly debated issue. It is a fact that HIV infection results in loss of CD4 T-cells, followed by an increase of certain but not all HPV-associated anogenital malignancies; cervical cancer, however, is an exception [5, 20]. The mean CD4 T-cell count in ICC has been shown to be 312/μL, showing that there is less immunosuppression in ICC than other cancers [21]. The fact that HPV is more likely to persist in HIV-infected persons is independent of their CD4 count [7]. It appears to result from an impaired anti-HIV IgA local response [22, 23]. Data suggest that immunosuppression may lead to higher levels of HPV replication with resulting higher HPV DNA levels and increased incidences of LGSIL and HGSIL [24]. However, there have been no similar findings in ICC; cellular genetic changes rather than immunosuppression may be the driving force of progression into ICC [24].
Immunosuppression is most strongly associated with preinvasive lesions; the progression to ICC is not solely associated with immunosuppression [5]. In HIV-infected women, the hazard ratio of developing any preinvasive lesion was 1.2 if the CD4 T-cell count was >500/μL, which is similar to HIV-uninfected controls [25]. The relative incidence of clearance has been said to decrease with a CD4 count <200 [26]. A Zimbabwean study, however, concluded that only HR HPV, and not HIV, was associated with CIN [27]. The rate of ICC in women with AIDS might be unrelated to immunosuppression and, therefore, not biologically associated with HIV [20].
Third, partial immunity reversal through HAART should decrease the incidence of AIDS-defining malignancies [4]. It has been expected that reversal of immunosuppression following HAART would have a dramatic effect on the natural history of cervical preinvasive lesions [26], but data on the effects of HAART in this regard are mixed [28, 29].
The increased risk of HIV-infected women to CIN2 may be due to different factors: a) high exposure to risk factors (coitarche, number of sex partners, and contraception), b) a direct effect of HIV, or c) a molecular interaction between HIV and HPV [30, 31].
It has been hypothesised that since more women with HIV survive due to access and use of HAART, the incidence of cervical cancer is likely to be increased by the HIV epidemic [32, 33]. However, so far the incidence of cervical cancer among HIV-infected women in the industrialised world has been unchanged since the introduction of HAART [7, 34]. The incidence of HPV-induced diseases has increased rather than decreased since the introduction of HAART; HAART restores the immune response to AIDS-defining opportunistic infections such as Cytomegalovirus and Kaposi’s sarcoma associated virus; 25% of the HIV-infected CIN1 subjects on HAART still progress to CIN2 , and HIV-infected patients with CIN2 often do not show regression when treated with HAART. Regression of CIN1 and CIN2 depends on the type of HPV (oncogenic or not) rather than on immunocompetence [5]. The longer survival of HIV-infected patients related to HAART may have a proportionally greater impact on the risk of HPV-related cancers than the partial reversal of immunosuppression that occurs with HAART [20].
The study of HPV-induced cervical neoplasia in HIV-infected women is complex and is riddled with contradictory reports. For instance, it has been reported, as already mentioned, that the incidence of cervical cancer has not decreased in the HAART era and that clinical research has not shown a clear benefit of HAART in decreasing HPV-related cervical disease in HIV-infected women [29, 35]. Other reports, however, claim the contrary [36, 37]. Still others claim the evolution to depend on the degree of immunosuppression [25]. However, contrary to Hawes et al [38], no impact in the development of SIL was evidenced for CD4 T-cell counts by Heard et al [39].
The strength of our investigation was that we carried out a large case-control study. The weakness was that we had to rely on the information provided by the request for biopsy diagnosis. Like others, we found a high prevalence of HGSIL. We found no effect of HAART on the relative distribution of preinvasive and invasive neoplasia and no effect of HAART on the degree of immunosuppression.
Conclusion
Many HIV-associated cancers, such as Kaposi’s sarcoma, lymphomas and HPV-associated head and neck, and lower anogenital squamous cancers, are known or suspected to be ADIs [40]. The numerous data from the literature show that this is not the case with cervical cancer. Our investigation shows that the prevalence of ICC is significantly lower in HIV-infected women than in uninfected women. HAART neither affects the distribution of preinvasive lesions nor the CD4 cell counts. This suggests that ICC should not be listed among ADIs.
Conflict of interest
The author declares that he has no conflict of interest.
References
1 Centers for Disease Control and Prevention (1993) Impact of the expanded AIDS surveillance case definition on AIDS case reporting—United States, first quarter, 1993 MMWR Morb Mortal Wkly Rep 42 308–10.
2 zur Hausen H (1976) Condylomata acuminata and human genital cancer Cancer Res 36 794. PMID: 175942
3 Hariri S et al Human papillomavirus In: Manual for the surveillance of vaccine preventable diseases. Available at: http://www.cdc.gov/vaccines/pubs/surv-manual/chpt05-hpv.html. Last accessed: 10/24/2012.
4 Bower M, Mazhar D and Stebbing J (2006) Should cervical cancer be an acquired immunodeficiency syndrome-defining cancer? J Clin Oncol 24(16) 2417–19. DOI: 10.1200/JCO.2005.05.4908 PMID: 16735700
5 Van den Burg SH and Palefsky JM (2009) Human immunodeficiency virus and human papilloma virus—why HPV-induced lesions do not spontaneously resolve and why therapeutic vaccination can be successful J Transl Med 7 108–16. DOI: 10.1186/14795876-7-108
6 Palefsky J (2006) HPV infection and HPV-associated neoplasia in immunocompromised women Int J Gynecol Obstet 94(Suppl 1) 556–64. DOI: 10.1016/S0020-7292(07)60011-3
7 Stier E (2008) Human papillomavirus related diseases in HIV-infected individuals Curr Opin Oncol 20 541–46. DOI: 10.1097/CCO.0b013e3283094ed8 PMID: 19106657
8 Sekirime WK and Gray R (2007) HIV infection among Ugandan women with cervical cancer: a retrospective study Gynecol Obstet Invest 63 222–28. DOI: 10.1159/000098197
9 Serraino D et al (2002) Invasive cervical cancer as an AIDS-defining illness in Europe AIDS 16(5) 781–86. DOI: 10.1097/00002030-200203290-00014 PMID: 11964535
10 Chaturvedi AK et al (2009) Risk of human papillomavirus-associated cancers among persons with AIDS J Natl Cancer Inst 101(16) 1120–30. DOI: 10.1093/jnci/djp205 PMID: 19648510 PMCID: 2728745
11 Van Bogaert LJ (2011) Age at diagnosis of preinvasive and invasive cervical neoplasia in South Africa. HIV-positive versus HIV-negative women Int J Gynecol Cancer 21 363–66. DOI: 10.1097/IGC.0b013e3182094d78 PMID: 21270617
12 Dim CC et al (2011) Prevalence of cervical squamous intraepithelial lesions among HIV-positive women in Enugu, South-eastern Nigeria J Obstet Gynaecol 31(8) 759–62. DOI: 10.3109/01443615.2011.598967 PMID: 22085071
13 Mqoqi N et al (eds) (2004) Cancer in South Africa, 1998–1999 (Johannesburg: National Cancer Registry, National Health Laboratory Service).
14 www.stats.sa.gov.sa Mid-year population estimates 2010 (Last accessed 20/01/2013).
15 Kahesa C et al (2008) Association between invasive cancer of the cervix and HIV-1 infection in Tanzania: the need for dual screening BMC Public Health 8 262. DOI: 10.1186/1471-2458-8-262 PMID: 18664298 PMCID: 2527006
16 Gichangi PB et al (2003) Impact of HIV-infection on invasive cervical cancer in Kenyan women AIDS 17 1963–68. DOI: 10.1097/00002030-200309050-00015 PMID: 12960829
17 Abdus-Salam AA, Ogunnorin OB and Abdus-Salam RA (2008) HIV seroprevalence in patients with carcinoma of the cervix in Ibadan, Nigeria Ghana Med J 42(4) 141–3. PMCID: PMC2673837
18 Moodley JR et al (2006) HIV and pre-neoplastic and neoplastic lesions of the cervix in South Africa: a case-control study BMC Cancer 6 135. DOI: 10.1186/1471-2407-6-135 PMID: 16719902 PMCID: 1481580
19 Nel CPG et al (2006) The prevalence of HIV amongst women with cervix cancer SA Fam Pract 48 17.
20 Strickler HD (2009) Does HIV/AIDS have a biological impact on the risk of human papillomavirus-related cancers? J Natl Cancer Inst 101 1103–05. DOI: 10.1093/jnci/djp236 PMID: 19648509 PMCID: 2734113
21 Maiman M et al (1993) Human immunodeficiency virus infection and invasive cervical carcinoma Cancer 71 402–06. DOI: 10.1002/1097-0142(19930115)71:2<402::AID-CNCR2820710222>3.0.CO;2-Y PMID: 8093678
22 Marais DJ et al (2000) The impact of human immunodeficiency virus type 1 status on human papillomavirus (HPV) prevalence and HPV antibodies in serum and cervical secretions J Infect Dis 182 1239–42. DOI: 10.1086/315815 PMID: 10979925
23 Agaba PA et al (2009) Cervical dysplasia in Nigerian women infected with HIV Int J Gynecol Obstet 107(2) 99–102. DOI: 10.1016/j.ijgo.2009.06.006
24 Palefsky JM and Holly EA (2003) Immunosuppression and co-infection with HIV J Natl Cancer Inst Monogr 2003(31) 41–6. DOI: 10.1093/oxfordjournals.jncimonographs.a003481
25 Harris TG et al (2005) Incidence of cervical squamous intraepithelial lesions associated with HIV serostatus, CD4 cell counts, and human papillomavirus test results JAMA 293(12) 1471–76. DOI: 10.1001/jama.293.12.1471 PMID: 15784870
26 Ahdieh L et al (2000) Cervical neoplasia and repeated positivity of human papillomavirus infection in human immunodeficiency virus seropositive and seronegative women Am J Epidemiol 151 1148–57. DOI: 10.1093/oxfordjournals.aje.a010165 PMID: 10905527
27 Baay MF et al (2004) Human papillomavirus in a rural community in Zimbabwe: the impact of HIV co-infection on HPV genotype distribution J Med Virol 73 481–85. DOI: 10.1002/jmv.20115 PMID: 15170646
28 Palefsky JM (2003) Cervical human papillomavirus infection and cervical intraepithelial neoplasia in women positive for human immunodeficiency virus in the era of highly active antiretroviral therapy Curr Opin Oncol 15 382–88. DOI: 10.1097/00001622-200309000-00007 PMID: 12960521
29 De Vuyst H et al (2008) HIV, human papillomavirus, and cervical neoplasia and cancer in the era of highly active antiretroviral therapy Eur J Cancer Prev 17 545–54. DOI: 10.1097/CEJ.0b013e3282f75ea1 PMID: 18941376
30 Mandelblatt JS et al (1999) Is HIV infection a cofactor for cervical squamous neoplasia? Cancer Epidemiol Biomarkers Prev 8 97–106. PMID: 9950246
31 Branca M et al (2004) Assessment of risk factors and human papillomavirus (HPV) related pathogenetic mechanisms of CIN in HIV-positive and HIV-negative women. study design and baseline data of the HPV-pathogen ISS study Eur J Gynaecol Oncol 25 689–98.
32 Denny L (2010) Cervical cancer in South Africa: an overview of current status and prevention strategies SA Cont Med Educ 28 70–3.
33 Vasconcelos de Andrade AC et al (2011) Factors associated with colposcopy–histopathology confirmed cervical intraepithelial neoplasia among HIV-infected women from Rio de Janeiro, Brazil PLos One 6 e18297. DOI: 10.1371/journal.pone.0018297
34 Launay O and Guillevin L (2003) Epidemiology of HIV-associated malignancies Bull Cancer 90 387–92 (French). PMID: 12850760
35 Adler DH (2010) The impact of HAART on HPV-related cervical disease Curr HIV Res 1(87) 493–97. DOI: 10.2174/157016210793499240
36 Sirera G et al (2007) Evolution of cervical cytology changes among HIV-infected women with normal cytology in the HAART era AIDS Res Hum Retrov 23(8) 965–71. DOI: 10.1089/aid.2006.0293
37 Soncini E et al (2007) Reduction of the risk of cervical intraepithelial neoplasia in HIV-infected women treated with highly active antiretroviral therapy Acta Biomed 78 36–40. PMID: 17687815
38 Hawes SE et al (2006) Incident high-grade squamous intraepithelial lesions in Senegalese women with and without human immunodeficiency virus type 1 (HIV 1) and HIV 2 J Natl Cancer Inst 98(2) 100–9. DOI: 10.1093/jnci/djj010 PMID: 16418512
39 Heard I, Potard V and Costagliola D (2006) Limited impact of immunosuppression and HAART on the incidence of cervical squamous intraepithelial lesions in HIV-positive women Antivir Ther 11 1091–96. PMID: 17302379
40 Urban MI et al (2009) The spectrum of HIV-associated cancers in a South African black population: results from a case-control study, 1995–2004 Infect Agents Cancer. DOI: 10.1186/1750-9378-4-S2-P44






