Primary ovarian adenosarcoma with elevated Ca-125 levels and normal ascitic fluid cytology: a case report and review of literature
PN Shakuntala1, K Umadevi1, A Usha2, N Abhilasha1 and UD Bafna1
1 Department of Gynaecologic Oncology, Kidwai Memorial Institute of Oncology, Dr. M.H. Mari Gowda Road, Bengaluru 560029, Karnataka, India
2 Department of Pathology, Kidwai Memorial Institute of Oncology, Dr. M.H. Mari Gowda Road, Bengaluru 560029, Karnataka, India
Correspondence to: Shakuntala PN. Email: shakuntala_pn@yahoo.com
Abstract
Objective: Ovarian adenosarcoma is a very rare tumour for which treatment options vary. We will consider the option of optimal cytoreductive surgery followed by adjuvant chemotherapy consisting of ifosamide (mesna) and adriamycin to prevent systemic metastasis, and will observe the role of serial CA-125 levels as a follow-up marker.
Case report: We report a case of ovarian adenosarcoma in a 38-year-old woman presenting with abdominal pain, distention due to massive ascites. She had undergone total abdominal hysterectomy 8 months previously for abnormal uterine bleeding. She underwent paracentesis followed by optimal cytoreductive surgery. A post-operative histopathologic diagnosis of primary adenosarcoma was confirmed. She was assigned a stage III C cancer. She received five cycles of ifosamise (mesna) and adriamycin and is on follow-up with serial CA-125 levels. There is no evidence of recurrence clinically, biochemically, or radiologically for more than 12 months.
Conclusion: Multimodality treatment comprising optimal cytoreductive surgery followed by ifosamide (mesna) and adriamycin-based chemotherapy may be an option for treatment of these aggressive tumours. Follow-up with serial CA-125 values in advanced stage adenosarcoma of the ovary is a novel observation which needs to be researched.
Keywords: adenosarcoma ovary, CA-125, ascitic fluid cytology, chemotherapy
Introduction
Mullerian adenosarcoma is very rare. In 1974, Clement and Scully described uterine adenosarcomas for the first time [1], since then there have been 60 cases of ovarian adenosarcomas described in the literature [2–10], and only four cases of ovarian adenosarcoma with elevated levels of CA-125 have been reported [3–5, 8]. Most of the cases reported have associated endometriosis or an adenosarcoma arising from an endometriotic area, but the direct relation between this tumour and endometriosis has not been made clear in the literature [7, 10]. The present case was pure or homologous adenosarcoma of the ovary without associated endometriosis and an elevated CA-125 level. Adenosarcomas contain benign Müllerian type glands and generally low-grade sarcomatous stroma that resembles stromal sarcoma [9]. The recommended treatment of adenosarcoma is optimal cytoreduction followed by adjuvant therapy for high-grade sarcomatous component if present [4, 7]. Various treatment options ranging from oral progesterone [8], fertility sparing surgery to optimal cytoreductive surgery [4, 7], post-operative radiation therapy and adjuvant chemotherapy [2] have been described in the literature.
Case report
A 38-year-old multiparous woman presented with history of abdominal distension and pain for almost 3 weeks. She had undergone hysterectomy 8 months previously for abnormal uterine bleeding. Following abdominal paracentesis for massive ascites, a mobile mass was palpated measuring about 12 × 10 × 6 cm. Upper abdominal fullness was noted. Her haemogram, serum biochemistry, serum antibodies for HIV and HBsAg were negative with normal chest X-ray and cardiac evaluation. The serum CA-125 value was 142 U/ml. A CT scan of the abdomen and pelvis revealed multiple heterogeneously enhancing soft tissue masses within omentum, mesentry, perihepatic regions, and the pelvis. The ovaries were not seen separately. There was moderate ascites suggestive of malignant ovarian lesion with metastasis (Figures 1 and 2). Peritoneal fluid cytology was non-contributory.
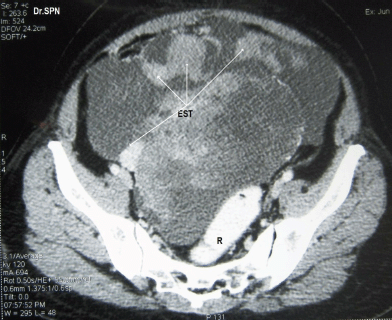
Figure 1: CT scan with oral and rectal contrast (R) showing a pelvic mass with multiple heterogeneously enhancing soft tissue masses with central necrotic areas within pelvis not separately seen from the ovaries (EST).
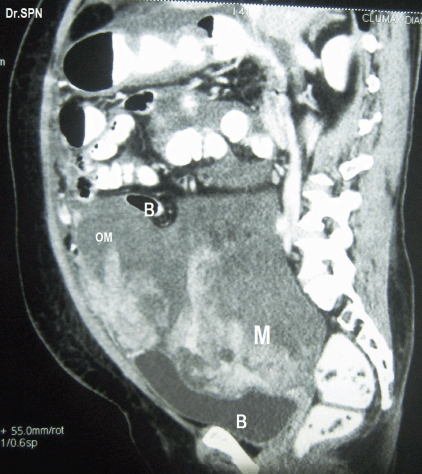
Figure 2: Sagittal section showing heterogeneously enhancing soft tissue lesions within omentum (OM), M- pelvic mass seen indenting bladder base (B), Bowel (B) loops are displaced upwards.
During laparotomy, 1 L of ascites was drained, a huge, vascular omental cake with multiple nodular deposits were seen (Figure 3). The right ovary measured 16 × 15 × 11 cm and was nodular and irregular with capsular breach (Figure 4). Metastatic deposits were seen on the mesentry, peritoneum, descending colon and bladder (Figure 5). On the table, frozen section of omentum showed short spindle cells with scanty hyalinized stroma with possibility of malignant stromal tumour of uterine or ovarian origin with metastasis to omentum was opined. Therefore, a complete tumour debulking, total omentectomy, left salphingo-oophorectomy, bilateral pelvic node dissection, peritonectomy, appendicectomy, excision of deposits on the bladder, bowel mesentry were performed to achieve optimal tumour load reduction. The post-operative period was uneventful. Histopathologic examination of the ovarian neoplasm and the peritoneal deposists revealed adenosarcoma of ovary (Figure 6).
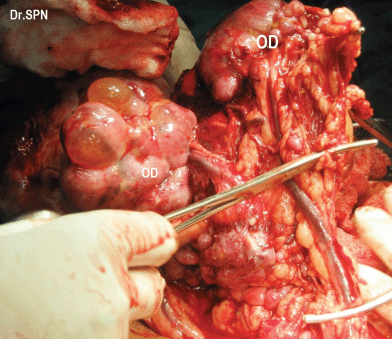
Figure 3: Intraoperative omental nodular metastatic deposits (OD).
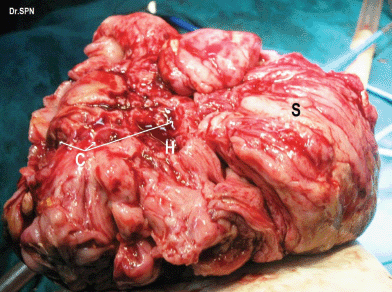
Figure 4: Right ovarian mass which appears nodular, breach in capsule, areas of haemorrhage (H), cystic spaces (C) and solid appearing areas (S).
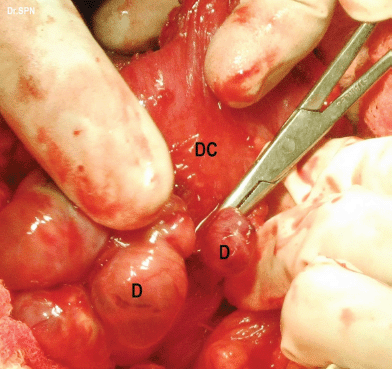
Figure 5: metastatic deposits (D) on the descending colon (DC).
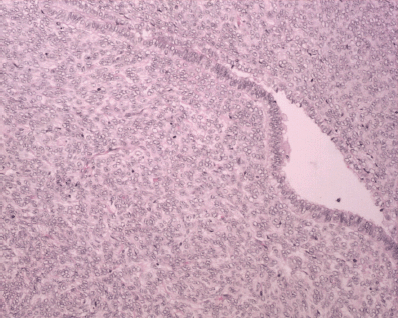
Figure 6: H&E x 20: biphasic neoplasm showing both benign epithelial component and sarcomatous mesenchymal component.
The IHC revealed neoplastic spindle cells which were positive for CD10 and negative for inhibin, C-kit, Calretiniun, SMA, S100, and Mic2, and the epithelium was positive for CK7 and EMA (Figures 7 and 8). She was allotted Stage IIIC.
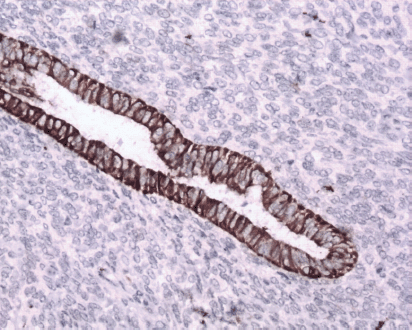
Figure 7: Immunostain CK7x20—epithelium is positive for CK7 (brown).
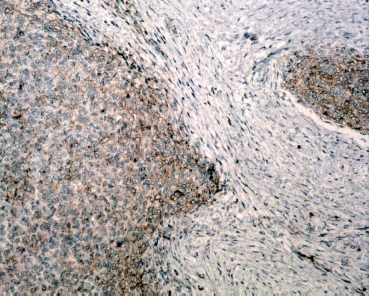
Figure 8: Immunostain, CD10x- Mesenchymal component is positivity for CD-10 (brown).
She received five cycles of ifosamide with mesna and adriamycin every third week. She is on follow-up for more than 12 months, and there is no clinical, radiological, or biochemical evidence of recurrence.
Discussion
Extrauterine Müllerian adenosarcomas are rare tumours. They can arise from the ovary, fallopian tubes, round ligament, pouch of Douglas, vagina, bladder, colon, and even peritoneum [2, 7, 9, 11].
The World Health Organization defines adenosarcoma as a biphasic tumour characterized by the proliferation of Müllerian epithelium of benign appearance or occasionally labelled atypical when absorbed in or covering a predominant sarcomatous stroma [12].
The present case has many clinical, therapeutic, and prognostic implications.
First, the adenosarcoma was arising from a previously normal ovary, which was salvaged for hormonal functions just 8 months earlier. These extrauterine Müllerian adenosarcomas occur at a younger age than their uterine counterparts and have more aggressive clinical behaviour because of invasion to adjacent pelvic organs at the time of diagnosis. Similar observations have been confirmed by many authors [4, 7, 9].
Second, homologous (pure) adenosarcomas arising from the ovary without associated endometriosis in either of the ovaries is very rare. Majority of the case reported have been associated with past, present, or concurrent evidence of endometriosis. The exact association is not clear in the literature as shared by many authors [4, 6, 8, 10, 11].
Third, ascitic fluid cytology may not be contributory, a frozen section may reveal the presence of malignant stromal tumour which needs further categorization and hence the importance of histopathology to delineate the benign and the malignant component of the tumour and immunohistochemistry to categorise the sarcomatous stromal component around which revolves the adjuvant therapy, prognosis and survival of the patients [10, 11]. To date, there is only one report of ascitic fluid cytology which could speculate neoplasm in an ovarian adenosarcoma [5].
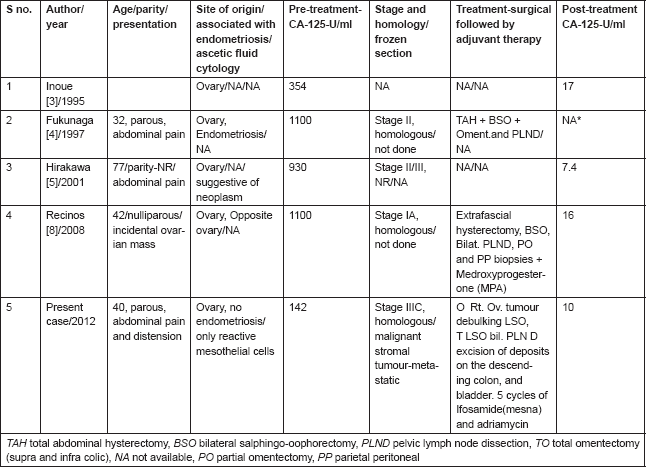
Fourth, a raised CA-125 level being uncommon in adenosarcoma has been observed by authors as depicted in Table 1. The clinical implication of elevated CA-125 was first reported by Recinos et al [8] in an early stage disease. The present case was an advanced stage disease associated with elevated CA125 levels. She had optimal cytoreduction followed by 5 cycles of ifosamide with mesna and adriamycin every three weeks. Post-operative serum CA-125 level was 10 U/l. Serial serum CA-125 was used as a biochemical marker for follow-up. Associated fallacies of ascitic fluid cytology, frozen section and absence of endometriosis are reported for the first time in English medical literature (Table 1). She is on follow-up and there is no clinical, biochemical, or radiological evidence of recurrence for more than 12 months.
Table 1. Ovarian adenosarcoma. Characteristics of women, associated with elevated CA-125 (Normal-35 U/ml) levels, ascitic fluid cytology, and frozen section.
Conclusions
Acknowledgements
We thank our patient and her family members for their cooperation. Our sincere gratitude to all the members of Department of Pathology at Kidwai who left no leaf unturned to arrive at a diagnosis. We thank Dr. VR Pallavi, Dr. K Shobha, Dr. SR Praveen, Dr. RN Patil, Dr. Rajshekar for their input into patient care. We thank our OT, ICU, and Shanthidhama ward nursing staff whose dedication helped the patient to recover.
References
1. Clement PB and Scully RE (1974) Müllerian adenosarcoma of the uterus: a clinicopathologic analysis of ten cases of a distinctive type of Müllerian mixed tumor Cancer 34 1138–49 DOI: 10.1002/1097-0142(197410)34:4<;1138::AID-CNCR2820340425>;3.0.CO;2-9 PMID: 4371193
2. Valdez VA, Planas AT, Lopez VF, Goldberg M and Herrera NE (1979) Adenosarcoma of the uterus and ovary: a clinicopathologic study of two cases Cancer 43 1439–47 DOI: 10.1002/1097-0142(197904)43:4<;1439::AID-CNCR2820430434>;3.0.CO;2-A PMID: 221090
3. Inoue M, Fukuda H and Tanizawa O (1995) Adenosarcoma originating from sites other than uterine endometrium Int J Gynecol Obstet 48 299–306 DOI: 10.1016/0020-7292(94)02267-3
4. Fukunaga M, Nomura K, Endo Y, Ushigome S and Aizawa S (1997) Ovarian adenosarcoma Histopathology 30 283–7 DOI: 10.1046/ j.1365-2559.1997.d01-596.x PMID: 9088962
5. Hirakawa E, Kobayashi S, Miki H et al (2001) Ascitic fluid cytology in adenosarcoma of the ovary: a case report Diagn Cytol 24 343–6 DOI: 10.1002/dc.1074
6. Sykiotis C, Kouvaris J, Karvouni H, Vitoratos N, Loghis C, Salamalekis E and Creatsas G (2001) J Gynecol Surg 17(2) 57–60 DOI: 10.1089/10424060152474677
7. Eichhorn JH, Young RH, Clement PB and Scully RE (2002) Mesodermal (Müllerian) adenosarcoma of the ovary: a clinicopathologic analysis of 40 cases and a review of the literature Am J Surg Pathol 26 1243–58 DOI: 10.1097/00000478-200210000-00001 PMID: 12360039
8. Recinos-Money E, Escobar-Alfaro G, Contreras J, Zepeda-Castilla E, Parra-Torres C, Di Castro P (2008) Ovarian adenosarcoma with elevated CA125 antigen. Case report and literature review Cirugia y Cirujanos 76(1) 71–5 PMID: 18492424
9. McCluggage WG (2010) Mullerian adenosarcoma of the female genital tract Adv Anat Pathol 17(2) 122–9 DOI: 10.1097/PAP.0b013e3181cfe732 PMID: 20179434
10. Shintaku M and Mise Y (2012) Müllerian adenosarcoma with a neuroectodermal component associated with an endometriotic cyst of the ovary: a case report Pathol Int 62(4) 271–5 DOI: 10.1111/j.1440-1827.2011.02782.x PMID: 22449231
11. Can B, Küçükali T, Ayhan A, Özkaya Ö and Durukan T (2008) Primary extrauterine adenosarcoma: report of 2 cases Turkish J Cancer 38(1) 26
12. Tavassoli FA and Devilee P (ed) (2003) World Health Organization classification of tumours. In: Pathology and Genetics of Tumours of the Breast and Female Genital Organs (Lyon: IARC Press) pp 114–5






