A study of clinical profile and outcomes of paediatric patients with acute myeloid leukaemia
Suhani Barbhuiyan1, Munlima Hazarika1, Asif Iqbal1, Manasa Kakunje1, Helie P Raval1, Anupam Sarma2 and Roopam Deka3
1Department of Medical and Paediatric Oncology, Dr. B. Borooah Cancer Institute, Guwahati 781016, India
2Department of Oncopathology, Dr. B. Borooah Cancer Institute, Guwahati 781016, India
3Department of Pathology, All India Institute of Medical Sciences (AIIMS), Guwahati 781101, India
Abstract
Objective: To describe the clinical and socio-demographic profile, as well as the induction outcomes, of paediatric patients with acute myeloid leukaemia (AML).
Methods: A retrospective analysis was conducted of patients up to 18 years of age who presented between 1 January 2017 and 31 December 2021. Patients with a diagnosis of de novo AML confirmed by flow cytometry, or with extra-medullary soft tissue masses proven to be AML by immunohistochemistry, were included. All records were obtained from the Paediatric Oncology database and supplemented by treatment files.
Results: Among 56 patients analyzed, the male-to-female ratio was 1.15. The median age was 7 years (range: 0.2–18 years). The white blood cell (WBC) count at presentation ranged from 1,250/μL to 296,000/μL (median: 29,000/μL). Sixty percent of patients were malnourished, and the majority belonged to lower socioeconomic strata. Fever was the most common presenting symptom (93%), followed by bleeding (61%), easy fatigability (53%), bone pain (46%), hepatosplenomegaly (39%) and lymphadenopathy (14%). According to European LeukaemiaNet (ELN) 2017 risk stratification, 52% of patients were categorized as favourable risk, 18% as intermediate risk and 28% as high risk; one patient could not be risk-stratified. Remission was achieved in 47% of patients, 23% did not achieve remission, 16% died during induction and 14% abandoned treatment.
Conclusion: Demographic variables, nutritional status, WBC count and clinical presentations were not associated with induction outcomes, possibly due to the availability of extensive supportive care services such as accommodation near the hospital, free food and treatment and psychosocial support in the paediatric oncology unit. In contrast, ELN risk categories were strongly associated with outcomes.
Keywords: AML, clinical outcome
Correspondence to: Munlima Hazarika.
Email: munlima.hazarika@bbci.in
Published: 20/01/2026
Received: 16/06/2025
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Acute leukaemia is the most common paediatric cancer and the second leading cause of cancer-related deaths (after brain and nervous system tumours) among children and adolescents younger than 20 years [1]. In India, cancer is the ninth most common cause of death among children aged 5–14 years [2] and approximately 45,000 children are diagnosed with cancer annually [3]. A significant gap exists between the outcomes of acute lymphoblastic leukaemia (ALL) and acute myeloid leukaemia (AML) in India compared with those reported from high-income countries (HICs) [4, 5].
AML is characterized by clonal neoplastic proliferation of myeloid precursor cells in the bone marrow, with arrest of their maturation. Replacement of marrow by leukaemic blasts leads to anemia, thrombocytopenia and granulocytopenia, with or without leukocytosis. This accumulation of non-functional blast cells inhibits normal hematopoiesis, which, if untreated, results in bone marrow failure and death. Although the etiology of AML is unknown in most cases, several risk factors have been implicated, many of which cause DNA damage—consistent with the understanding that acute leukaemia arises from acquired genetic abnormalities in bone marrow cells. Reported risk factors include germline predisposition, radiation exposure, prior exposure to alkylating agents and electromagnetic radiation [6]. Epidemiological studies have suggested associations between childhood AML and factors such as maternal age, birth weight, previous fetal loss, birth order, maternal pesticide exposure and maternal alcohol use during pregnancy [7].
Three major reasons account for poor outcomes in children with acute leukaemias: treatment abandonment, relapse and treatment-related mortality [7, 8]. For AML, no clear biological differences have been demonstrated between patients in India and those in HICs, indicating that optimal treatment and supportive care are key to improving outcomes [9]. There is a paucity of literature from this geographic region [10], and given that AML is an acquired disease, region-specific factors may influence disease presentation, treatment response and toxicity profiles.
Justification for the study
Despite India’s high paediatric cancer burden, there is limited published data on paediatric AML from the North–East region. Regional factors—including genetic background, nutritional status, socioeconomic constraints and healthcare accessibility—may significantly impact presentation, tolerance to therapy and outcomes. Understanding these patterns is crucial to tailoring supportive care strategies, optimizing treatment protocols for resource-limited settings and guiding policy interventions aimed at reducing mortality in this underrepresented population.
Treatment with cytarabine and an anthracycline remains the backbone of AML therapy. At Dr. B. Borooah Cancer Institute (BBCI), all patients under 18 years are treated in the Paediatric Oncology department and receive a uniform induction regimen of 2–3 days of anthracycline combined with 5–7 days of infusional cytarabine. This uniformity allows observed outcomes to be more reflective of patient- and disease-related factors rather than treatment heterogeneity. This study was undertaken to generate region-specific data on paediatric AML and to serve as a reference for future clinical and epidemiological research.
Materials and methods
Primary objectives
- To describe the socio-demographic and clinical profile of paediatric patients with AML.
- To determine induction mortality, rates of complete remission and induction failure in these patients.
A retrospective analysis was conducted on patients presenting to the Paediatric Oncology Unit, Department of Medical and Paediatric Oncology, BBCI, Guwahati, between 1 January 2017 and 31 December 2021. Patients aged ≤18 years were included. Eligible patients had a diagnosis of de novo AML confirmed by flow cytometry of blood or bone marrow, or an extra-medullary soft tissue mass proven to be AML by immunohistochemistry.
Patients were excluded if they had:
- Acute leukaemia of ambiguous lineage or mixed phenotypic acute leukaemia.
- Secondary AML arising from a previous myeloproliferative disorder (e.g., juvenile myelomonocytic leukemia and chronic myeloid leukemia).
- Acute promyelocytic leukaemia.
- Incomplete treatment or follow-up details.
Demographic, clinical and follow-up data were extracted from the Paediatric Oncology database and supplemented with treatment files. Records with incomplete, ambiguous or missing information were excluded.
Socio-demographic and clinical variables studied included age, gender, ethnicity, state and district of residence, distance from home to BBCI, socioeconomic status (assessed using the Modified B.G. Prasad Scale, 2019) and nutritional status (assessed using World Health Organisation Z-score growth charts). Presenting symptoms and signs—including fever, bleeding, easy fatigability, bone pain, lymphadenopathy, hepatosplenomegaly and proptosis—were recorded.
Risk stratification was performed according to the European LeukaemiaNet (ELN) 2017 criteria.
Statistical analysis
Quantitative data were summarized using mean or median values, as appropriate; qualitative data were expressed as percentages. Paired t-test and chi-square test were used to assess associations between variables and outcomes. Data were analyzed using R version 4.2.0 software.
Definitions
- Induction mortality: Death due to any cause within 30 days of starting induction chemotherapy.
- Complete remission: Restoration of normal hematopoiesis, transfusion independence, absence of circulating blasts and bone marrow blasts/hematogones <5% of all nucleated cells.
- Failure of induction: Non-achievement of complete remission (CR) despite two induction chemotherapy cycles.
- Abandonment: Refusal to start induction or discontinuation of treatment before bone marrow evaluation for remission.
All definitions were based on morphological examination of the first pull of bone marrow aspirates. Minimal residual disease assessment by flow cytometry was not performed in this study.
Summary of study design
- Design: Retrospective descriptive study.
- Place of study: BBCI, Guwahati, Assam, India.
- Study period: January 2017–December 2021 (5 years).
- Study population: Paediatric patients (≤18 years) with AML.
- Inclusion criteria:
- Patients ≤18 years with de novo AML confirmed by flow cytometry.
- Patients ≤18 years with extra-medullary soft tissue mass proven to be AML by immunohistochemistry.
- Exclusion criteria:
- Patients >18 years.
- No documented evidence of AML surface antigen expression.
- Acute leukaemia of ambiguous lineage or mixed phenotypic acute leukaemia.
- Acute promyelocytic leukaemia.
Ethical clearance
This study was approved by the Institutional Ethics Committee of BBCI. The requirement for individual informed consent was waived due to the retrospective nature of the study.
Results
This retrospective audit included paediatric patients diagnosed with AML and treated in the Paediatric Oncology Unit of the Department of Medical and Paediatric Oncology at BBCI, Guwahati. Data from patients registered between 1 January 2017 and 31 December 2020 were analyzed. Only patients with confirmed diagnoses by surface antigen characterization and complete blood investigation and induction treatment details were included. A total of 56 patients were analyzed.
Tables 1–6 show the socio-demographic profile of the patients in the study
Table 1. Socio-demographic profile of patients.
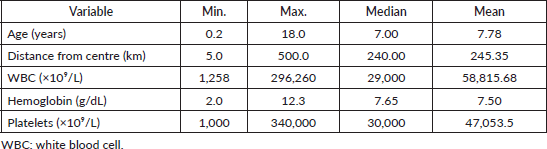
Table 2. State of origin.
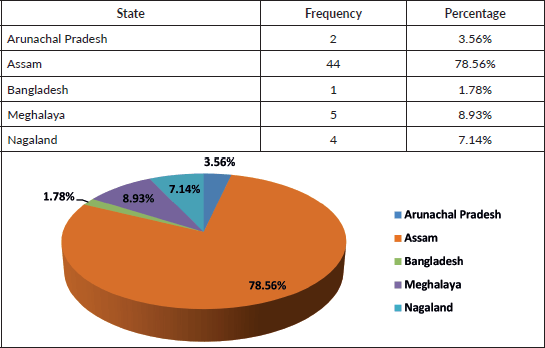
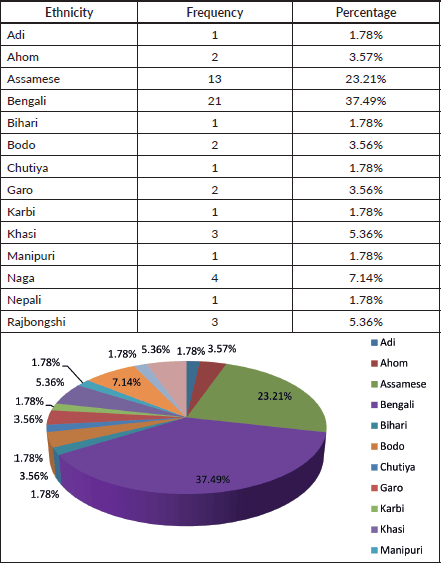
Table 3. Ethnicity.
Clinical parameters relevant to outcomes
The clinical parameters analyzed for their relevance to treatment outcomes included:
White blood cell (WBC) count at diagnosis
Clinical presentation
Biologic risk categories (ELN 2017)
The majority of patients presented with fever (92.8%), bleeding (60.7%) and easy fatigability (53.5%).
Other common findings included:
Bone pain: 46.4%
Hepatosplenomegaly: 39.2%
Lymphadenopathy: 28.5%
Proptosis: 25%.
Table 4. Age groups and gender.
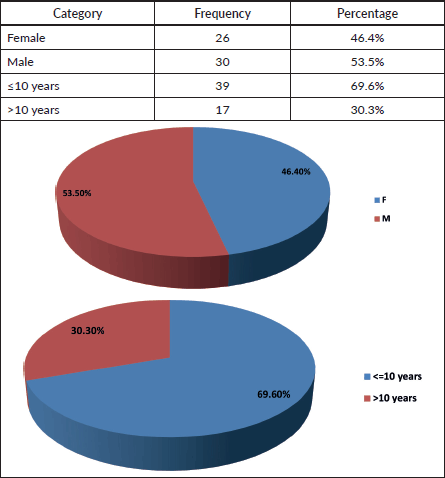
Table 5. Nutritional status.
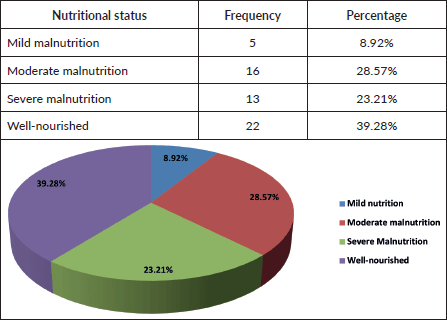
Table 6. Socio-economic status.
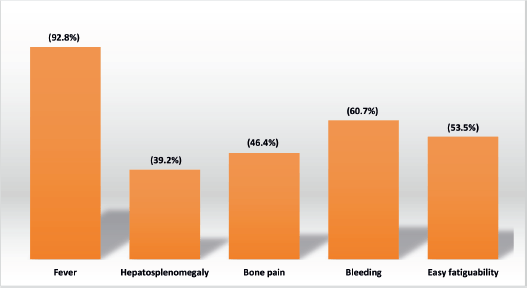
Figure 1. Clinical presentation at diagnosis.
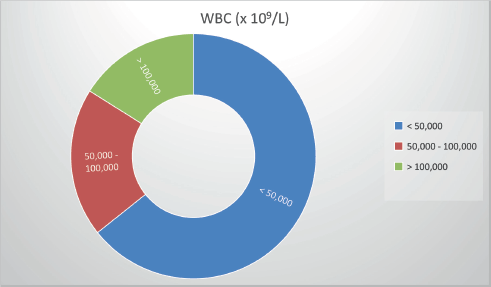
Figure 2. WBC counts at presentation.
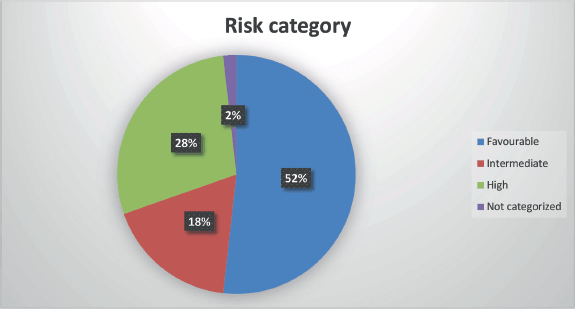
Figure 3. Risk stratification.
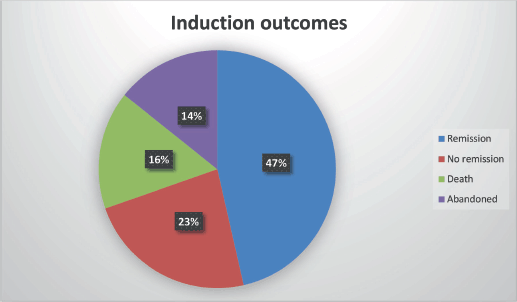
Figure 4. Induction outcomes.
The WBC count at presentation ranged from 1,250 × 10⁹/L to 296,000 × 10⁹/L (median: 29,000 × 10⁹/L; mean: 58,000 × 10⁹/L). For analysis, patients were grouped as follows:
≤50,000 × 10⁹/L: 64.2%
50,000–100,000 × 10⁹/L: 20%
100,000 × 10⁹/L: 16%.
Risk stratification (ELN 2017)
Favourable: 29 patients (52.2%)
High risk: 16 patients (28.5%)
Intermediate risk: 10 patients (17.8%)
Not categorized: 1 patient (1.8%)
Induction outcomes
Of the 56 patients analyzed:
CR: 26 (46.4%)
No remission: 13 (23.2%)
Death during induction: 9 (16.0%)
Treatment abandonment: 8 (14.2%)
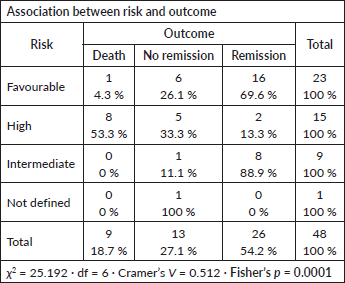
Associations between risk factors and outcomes
Risk category versus outcome
A strong statistical association was observed between ELN risk category and induction outcome (Fisher’s p = 0.0001; Cramer’s V = 0.512).
High-risk patients were more likely to experience non-remission or death.
Favourable-risk patients were more likely to achieve remission.
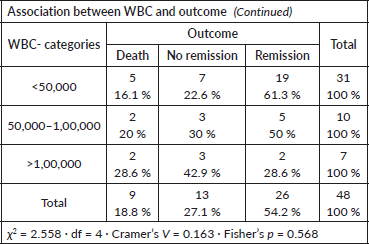
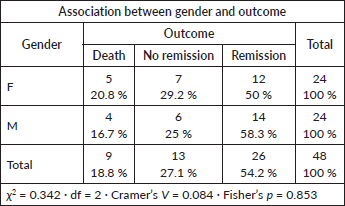
WBC count at presentation versus outcome
No statistically significant association was found (Fisher’s p = 0.568; Cramer’s V = 0.163).
- Higher WBC counts (>100,000 × 10⁹/L) showed a trend toward poorer outcomes, but without statistical significance.
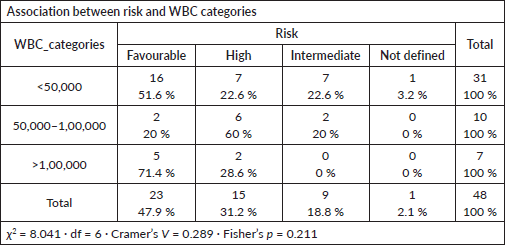
WBC count versus risk category
No significant association observed (Fisher’s p = 0.211; Cramer’s V = 0.289).
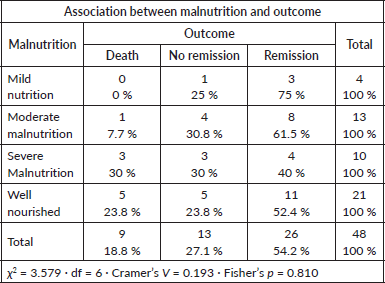
Nutritional status versu outcome
No significant relationship was noted (Fisher’s p = 0.810; Cramer’s V = 0.193).
- Severe malnutrition was present in 23.2% of patients, but this did not significantly influence induction outcome.
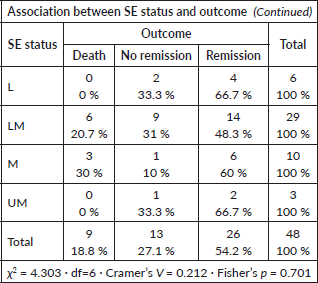
Socio-economic status versus outcome
No significant association observed (Fisher’s p = 0.701; Cramer’s V = 0.212).
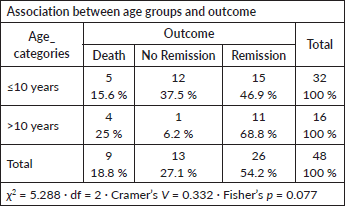
Age group versus outcome
No significant association found (Fisher’s p = 0.077; Cramer’s V = 0.332).
- However, a trend toward higher remission rates was observed in patients >10 years.
Gender versus outcome
No statistically significant relationship was found (Fisher’s p = 0.853; Cramer’s V = 0.084).
Summary of associations
- Only ELN risk category showed a statistically significant association with induction outcomes.
- Other demographic and clinical factors (WBC count, nutritional status, socio-economic status, age and gender) did not significantly influence induction remission, non-remission or mortality rates in this cohort.
Discussion
AML in children is a biologically heterogeneous malignancy associated with substantial risk of mortality [1, 5, 20]. Standard therapy consists of intensive induction chemotherapy followed by consolidation or allogeneic hematopoietic stem cell transplantation, while targeted and hypomethylating agents remain largely investigational in paediatric AML [5, 6]. In our cohort, all patients received the standard ‘7 + 3’ regimen—3 days of daunorubicin with 7 days of cytarabine—or acceptable protocol modifications based on clinical status.
Our analysis included 56 paediatric AML patients treated from 2017 to 2021. The male-to-female ratio (1.15:1) and median age of 7 years align with other Indian and international studies [11–19]. Most patients (78%) were from Assam, with the remainder from other Northeast Indian states and Bangladesh, reflecting the center’s role as a regional referral facility. Fourteen ethnic groups were represented, the largest being Bengali (37%) and Assamese (21%), highlighting the genetic diversity of the catchment population.
Leukocyte count at diagnosis is a recognized prognostic marker in AML [20–23]. In our study, median WBC was 29,000/µL, with 64.2% presenting below 50,000/µL. Although hyperleukocytosis has been linked to worse outcomes in other series [22, 23], we did not observe a statistically significant association, similar to the findings of Morais et al [24].
Nutritional status is another potential determinant of outcome. The CCG 2961 trial reported inferior survival in underweight and overweight paediatric AML patients due to higher treatment-related mortality [25]. None of our patients were overweight; however, 60% were malnourished (23% severely). Nutritional status did not significantly influence induction outcomes, possibly reflecting the impact of robust supportive measures at our center, including free treatment, nutritional supplementation, social work support and NGO partnerships.
Host factors such as age, sex, ethnicity and socio-economic status have variably been linked to prognosis [20, 26–28]. In our study, none showed a statistically significant association with induction response, consistent with Gupta et al [15], but differing from studies that demonstrated age- or sex-related effects[26–28]. This may again be attributable to comprehensive institutional supportive care reducing disparities in treatment tolerance.
The clinical presentation pattern—fever (93%), bleeding (61%), easy fatigability (53%), bone pain (46%), hepatosplenomegaly (39%), lymphadenopathy (14%) and proptosis (25%)—was broadly in line with other paediatric AML cohorts [16, 17, 29, 30]. ELN 2017 risk categorization revealed a predominance of favourable-risk patients (52%), followed by high (28%) and intermediate (18%) risk. This contrasts with previous Indian and international series where intermediate or high-risk categories dominate [14, 29, 30], likely due to selection bias, as very high-risk patients may be referred directly to transplant-capable centers.
Induction outcomes comprised complete remission in 47%, no remission in 23%, induction death in 16% and treatment abandonment in 14%. Literature from both developed and developing countries shows remission rates of 56%–83% and induction mortality between 3% and 25% [16, 17, 29–42]. When patients who abandoned therapy were excluded, only ELN risk category retained a statistically significant association with outcome: high-risk patients were more likely to fail induction or die, while favourable-risk patients achieved higher remission rates. This association has been consistently reported in prior studies [30–33].
In summary, our findings reinforce the prognostic importance of cytogenetic/molecular risk stratification in paediatric AML, whereas demographic and nutritional parameters were not predictive of induction outcomes in this cohort. The presence of strong supportive care infrastructure may attenuate the influence of socio-demographic risk factors. Multicentre studies in varied resource settings are warranted to determine the generalizability of these results.
Acknowledgments
General: Patients and their families.
Funding
None.
Ethical approval
Approved by institutional ethics committee- Dr.B.Borooah Cancer Institute.
Ethical declaration
None.
Author contributions
Dr.Suhani Barbhuiyan- Concept, Design, Data Collection, Maintaining master file, Drafting final report, Assigning duties to the study team, Communication with IEC.
Dr.Munlima Hazarika-Design, Interpretation of data, Drafting final report, Publication.
Dr.Asif Iqbal- Concept, Interpretation of data, Statistical analysis.
Dr.Manasa Kakunje- Data Collection, Maintaining master file.
Dr. Helie P.Raval- Data Collection, Maintaining master file.
Data availability
In EMR and Hard copy at room 15, OPD block, BBCI.
Study registration
None.
References
1. Puumala SE, Ross JA, and Aplenc R, et al (2013) Epidemiology of childhood acute myeloid leukemia Pediatr Blood Cancer 60(5) 728–733 https://doi.org/10.1002/pbc.24464 PMID: 23303597 PMCID: 3664189
2. Summary- Report on causes of Death: 2001–03 in India [http://censusindia.gov.in/ Vital_Statistics/ Summary_Report_Death_01_03.pdf]
3. Arora R, Eden T, and Kapoor G (2009) Epidemiology of childhood cancer in India Indian J Cancer 46 264–273 https://doi.org/10.4103/0019-509X.55546 PMID: 19749456
4. Pui CH, Yang JJ, and Hunger SP, et al (2013) Childhood Acute Lymphoblastic Leukemia Pediatr Blood Cancer 60(5) 728–733 https://doi.org/10.1002/pbc.24464
5. Zwaan CM, Kolb EA, and Reinhardt D, et al (2015) Collaborative efforts driving progress in pediatric acute myeloid leukemia J Clin Oncol 33 2949–2962 https://doi.org/10.1200/JCO.2015.62.8289 PMID: 26304895 PMCID: 4567700
6. ICMR Consensus Document on Management of Acute Myeloid Leukemia- Prepared as an outcome of ICMR subcommittee on Acute Myeloid Leukemia (AML). Division of non communicable diseases Indian Council of Medical Research 2019
7. Kulkarni KP, Arora RS, and Marwaha RK (2011) Survival outcome of childhood acute lymphoblastic leukemia in India: a resource-limited perspective of more than 40 years J Pediatr Hematol Oncol 33 475–479 https://doi.org/10.1097/MPH.0b013e31820e7361 PMID: 21792045
8. Abboud MR, Ghanem K, and Muwakkit S (2014) Acute lymphoblastic leukemia in low and middle-income countries: disease characteristics and treatment results Curr Opin Oncol 26 650–655 https://doi.org/10.1097/CCO.0000000000000125 PMID: 25202926
9. Arora RS and Arora B (2016) Acute leukemia in children: a review of the current Indian data South Asian J Cancer 5(3) 155–160 https://doi.org/10.4103/2278-330X.187591 PMID: 27606304 PMCID: 4991139
10. Bhattacharyya J, Nath S, and Saikia KK, et al (2018) Prevalence and clinical significance of FLT3 and NPM1 mutations in acute myeloid leukaemia patients of Assam, India Indian J Hematol Blood Transfus 34 32–42 https://doi.org/10.1007/s12288-017-0821-0 PMID: 29398797 PMCID: 5786617
11. Swaminathan R, Rama R, and Shanta V (2008) Childhood cancers in Chennai, India, 1990-2001: incidence and survival Int J Cancer 122 2607–2611 https://doi.org/10.1002/ijc.23428
12. Kavcic M, Fisher BT, and Li Y, et al (2013) Induction mortality and resource utilization in children treated for acute myeloid leukemia at free-standing pediatric hospitals in the United States Cancer 119 1916–1923 https://doi.org/10.1002/cncr.27957
13. Gupta S, Bonilla M, and Fuentes SL, et al (2009) Incidence and predictors of treatment-related mortality in paediatric acute leukemia in El Salvador Br J Cancer 100(7) 1026–1031 https://doi.org/10.1038/sj.bjc.6604895 PMID: 19293804 PMCID: 2669993
14. Housou B, Cherkaoui S, and Lamchahab M, et al (2018) Outcome of acute myeloid leukemia in children adolescents and young adults treated with an uniform protocol in Casablanca, Morocco Indian J Hematol Blood Transfus 35(2) 255–259 https://doi.org/10.1007/s12288-018-1013-2
15. Kersten E, Scanlan P, and Dubois SG, et al (2013) Current treatment and outcome for childhood acute leukemia in Tanzania Pediatr Blood Cancer 60(12) 2047–2053 https://doi.org/10.1002/pbc.24576
16. Seth R, Pathak N, and Singh A, et al (2017) Pediatric acute myeloid leukemia: improved survival rates in India Indian J Pediatr 84(02) 166–167 https://doi.org/10.1007/s12098-016-2234-8
17. Peyam S, Trehan A, and Jain R, et al (2018) PO-205, acute myeloid leukemia: incremental improvement in outcome in a developing country Pediatr Blood Cancer 2(2) S186
18. Uppuluri R, Swaminathan VV, and Ravichandran N, et al (2020) Chemotherapy for childhood acute myeloid leukemia and associated infections over two decades in India: timeline and impact on outcome Indian J Med Paediatr Oncol 41(06) 869–873 https://doi.org/10.4103/ijmpo.ijmpo_211_20
19. Saşmaz I, Tanyeli A, and Bayram I, et al (2004) The results of treatment with idarubicin in childhood acute nonlymphoblastic leukemia Turk J Pediatr 46(1) 32–37
20. Arceci RJ and Meshichi S Acute myeloid leukemia and myelodysplastic syndromes Principles and Practice of Pediatric Oncology by Pizzo and Poplack 7th
21. Woods WG, Kobrinsky N, and Buckley JD, et al (1996) Timed sequential induction therapy improves post remission outcome in acute myeloid leukemia: report from Children’s Cancer Group Blood 87(12) 4979–4989 https://doi.org/10.1182/blood.V87.12.4979.bloodjournal87124979 PMID: 8652810
22. Ghafoor T, Sharif I, and Bashir F, et al (2020) Mortality in paediatric acute myeloid leukaemia J Pak Med Assoc 70(12) 2316–2322
23. Zeller B, Glosli H, and Forestier E, et al (2017) Hyperleucocytosis in paediatric acute myeloid leukaemia - the challenge of white blood cell counts above 200×10(9)/l. The NOPHO experience 1984-2014 Br J Haematol 178(9) 448–456 https://doi.org/10.1111/bjh.14692 PMID: 28542715
24. Morais RV, De Souza RV, and de Souza Silva KA, et al (2021) Epidemiological evaluation of children with acute myeloid leukemia J De Pediatris 97(2) 204–210 https://doi.org/10.1016/j.jped.2020.02.003
25. Lange BJ (2005) Mortality in overweight and underweight children with acute myeloid leukemia JAMA 293(2) 203–211 https://doi.org/10.1001/jama.293.2.203 PMID: 15644547
26. Gamis AS, Alonzo TA, and Meshinchi S, et al (2014) Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomised phase III Children’s Oncology Group trial AAMLO531 J Clin Oncol 32 2021–2032 https://doi.org/10.1200/JCO.2014.55.3628
27. Aplenc R, Alonzo TA, and Gerbing RB, et al (2006) Ethnicity and survival in childhood acute myeloid leukemia: a report from the Children’s Oncology Group Blood 108(1) 74–80 https://doi.org/10.1182/blood-2005-10-4004 PMID: 16537811 PMCID: 1895824
28. Riley LC, Hann IM, and Wheatley K, et al (1999) Treatment-related deaths during induction and first remission of acute myeloid leukemia in children treated on the Tenth medical Research Council Acute Myeloid Leukaemia Trial (MRC AML10) Br J Haematol 106 436–444 https://doi.org/10.1046/j.1365-2141.1999.01550.x PMID: 10460604
29. Philip C, George B, and Ganapule A, et al (2015) Acute myeloid leukaemia: challenges and real world data from India Br J Haematol 170(01) 110–117 https://doi.org/10.1111/bjh.13406 PMID: 25858293 PMCID: 4864448
30. Radhakrishnan V, Thampy C, and Ganesan P, et al (2016) Acute myeloid leukemia in children: experience from tertiary cancer centre in India Indian J Hematol Blood Transfus 32(03) 257–261 https://doi.org/10.1007/s12288-015-0591-5 PMID: 27429516 PMCID: 4930761
31. Gupta N, Seth T, and Mishra P, et al (2011) Treatment of acute myeloid leukemia in children: experience from a tertiary care hematology centre in India Indian J Pediatr 78(10) 1211–1215 https://doi.org/10.1007/s12098-010-0300-1 PMID: 21553210
32. Yadav SP, Ramzan M, and Lall M, et al (2011) Pediatric acute myeloid leukemia: final frontier for pediatric oncologists in developing world Pediatr Hematol Oncol 28(08) 647–648 https://doi.org/10.3109/08880018.2011.601435 PMID: 21875321
33. Mohammed R, Yadav SP, and Meena L, et al (2013) PUB-0194, cytogenetic findings in acute myeloid leukemia: a developing country experience In Abstracts of the 45th Congress of the International Society of Paediatric Oncology (SIOP) 2013 (Hong Kong: ) 60(3) 228 p
34. Kota R, Linga VG, and Gullipalli M, et al (2013) PUB-0228, outcome of childhood acute myeloid leukaemia-Institutional experience from Indian subcontinent. Abstracts of the 45th Congress of the International Society of Paediatric Oncology (SIOP) 2013. Hong Kong, China. September 25-28 Pediatr Blood Cancer 60(3) 236
35. Sharawat SK, Bakhshi R, and Vishnubhatla S, et al (2014) FLT3-ITD mutation in relation to FLT3 expression in pediatric AML: a prospective study from India Pediatr Hematol Oncol 31(02) 131–137 https://doi.org/10.3109/08880018.2013.870624 PMID: 24498869
36. Jain K, Udgire S, and Mudaliar S, et al (2014) EP-316, maintenance therapy in acute myeloid leukemia: experience from a developing country In SIOP Abstracts (Toronto: ) 61(2) 324 p
37. Jayabose S, Kasi VT, and Vignesh SR, et al (2014) EP-317, Use of codified MRC-10 protocol for acute myeloblastic leukemia in Indian children In 46th Congress of The International Society of Paediatric Oncology (SIOP) 2014 Toronto, Canada, 22nd -25th October, 2014 SIOP Abstracts (Toronto: Pediatr Blood Cancer) 61(2) 324 p
38. Siddaiahgari S, Jillella B, and Manikyam A (2014) Improving survival rates of acute myeloid leukemia in developing countries using AML_15 protocol In 46th Congress of The International Society of Paediatric Oncology (SIOP) 2014 Toronto, Canada, 22nd -25th October, 2014 SIOP Abstracts (Toronto: Pediatr Blood Cancer) 61 326 p
39. Ramamoorthy J, Trehan A, and Bansal D, et al (2015) PD-053, acute myeloid leukemia: treatment related mortality is abate in a developing country In 47th Congress of the International Society of Paediatric Oncology (SIOP) Cape Town, South Africa (Cape Town: ) 62(4) 223 p
40. Narula G, Prasad M, and Jatia S, et al (2017) Clinicoepidemiological profiles, clinical practices, and the impact of holistic care interventions on outcomes of pediatric hematolymphoid malignancies - a 7-year audit of the pediatric hematolymphoid disease management group at Tata Memorial Hospital Indian J Cancer 54(04) 609–615 https://doi.org/10.4103/ijc.IJC_487_17
41. Naseer M, Ankit P, and Purva K, et al (2017) PO-012, acute myeloid leukemia: correlation of cytogenetics and outcome in a tertiary care pediatric centre. Abstracts From the 49th Congress of the International Society of Paediatric Oncology (SIOP) Washington, DC, USA October 12–15, 2017 Pediatr Blood Cancer 64(3) S442
42. Kapoor R and Yadav SP (2018) Genetics-based risk stratification of pediatric acute myeloid leukemia in India Indian Pediatr 55(11) 1006–1007 PMID: 30587658
