Individual-level determinants of late-stage cervical cancer diagnosis and their implications for prevention and control
Adeniyi K Akiseku1,a, Taiwo O Adenuga2, Olusoji E Jagun1, Mutiu A Popoola2 and Adetola O Olatunji1
1Department of Obstetrics and Gynaecology, Olabisi Onabanjo University, Ago Iwoye, Ogun State 120107, Nigeria
2Department of Obstetrics and Gynaecology, Olabisi Onabanjo University Teaching Hospital, Ago Iwoye, Ogun State 121101, Nigeria
ahttps://orcid.org/0000-0003-4530-6479
Abstract
Background: Cervical cancer remains a significant public health issue, particularly in low-income countries. It is the fourth most common cancer among women globally, with an estimated 570,000 new cases and 311,000 deaths in 2018.
Objective: This study aimed to examine the stages of cervical cancer at diagnosis and identify factors contributing to late-stage presentation among women in a tertiary care hospital in Nigeria.
Methods: A retrospective study analysed data from women diagnosed with cervical cancer between 2017 and 2021. Demographic, reproductive and clinical data were extracted from medical records.
Results: Of the 102 women who presented during the study period, only 57 (55.9%) had complete staging, clinical and demographic data; these complete cases were included to ensure data integrity. From this population, 73.7% were aged 50 years or older and 56.1% presented with late-stage disease. Additionally, anaemia (packed cell volume <30%) was present in 75.4% of women. Postcoital bleeding was reported in 35.1% of cases. Women with no formal education had higher odds of late-stage diagnosis odds ratios (OR: 4.40, 95% CI: 1.08–17.82). Postmenopausal women also had higher odds of late-stage diagnosis (OR: 4.46, 95% CI: 1.27–15.70).
Conclusion: A late-stage cervical cancer diagnosis is prevalent among women in Nigeria, particularly among those with lower educational levels and postmenopausal women. Targeted awareness programmes, expanded screening (including integration into well-woman/postmenopausal care) and improved healthcare infrastructure, including consistent documentation of screening history and human papillomavirus vaccination, are essential for reducing the burden of cervical cancer in this context.
Keywords: cervical cancer, late-stage diagnosis, education, menopausal status, low-income countries, Nigeria
Correspondence to: Adeniyi K Akiseku
Email: niyikepler@yahoo.com
Published: 07/10/2025
Received: 01/04/2025
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cervical cancer remains one of the most pressing public health challenges globally, particularly in low-income countries (LICs) [1,2]. It is the fourth most common cancer among women worldwide, with an estimated 570,000 new cases and 311,000 deaths in 2018 [3–5]. Despite advancements in cancer prevention, diagnosis and treatment, cervical cancer continues to disproportionately affect LICs due to limited healthcare resources and infrastructure [5–7]. Research in sub-Saharan Africa has revealed alarming mortality rates, with cumulative deaths ranging from 65% to 68% over a 2–5-year period [8–10]. In Nigeria specifically, the 5-year cervical cancer mortality prevalence was estimated at 22.11 per 100,000 women [11], accounting for 14.8% of all cancer-related deaths among Nigerian women in 2018 [12].
The primary etiological factor for cervical cancer is persistent infection with high-risk human papillomavirus (HPV) types, particularly HPV-16 and HPV-18, which are responsible for approximately 70% of all cases [1, 5, 13]. In high-income countries, organised screening programs using Pap smears and HPV testing have led to substantial reductions in cervical cancer incidence and mortality [5, 14, 15]. However, in LICs, the implementation of cervical cancer prevention programs is hindered by inadequate healthcare infrastructure, insufficient healthcare personnel, limited public awareness, inadequate health funding and poor healthcare financing mechanisms, particularly the lack of comprehensive health insurance coverage [10, 14–16]. Consequently, cervical cancer is often diagnosed at advanced stages in these regions, leading to poorer prognoses and higher mortality rates [10, 14]. To combat this, the World Health Organisation (WHO) is developing a global plan of action to engage stakeholders and mobilise resources to make cervical cancer a rare disease globally [15, 17, 18]. Nigeria has also taken steps to introduce the HPV vaccine into its routine immunisation system, aiming to reach 7.7 million girls aged 9–14 years [13, 19].
The International Federation of Gynaecology and Obstetrics (FIGO) staging system is the most widely used framework for classifying the extent of cervical cancer. It ranges from stage I, where the cancer is confined to the cervix, to stage IV, where it has spread to distant organs [5, 14, 20]. The treatment approach for cervical cancer depends on the stage at diagnosis, with early-stage cases typically managed with surgery or radiotherapy, while advanced stages often require a combination of radiotherapy and chemotherapy [5, 14, 20].
Studies have shown that in many LICs, a substantial proportion of women present with advanced-stage disease [1, 2, 21]. Studies in Uganda, Ghana and Nigeria showed that a significant proportion of cervical cancer patients presented at late stages, with 61% in Uganda, 67.2% in Ghana and 72.8% in Nigeria diagnosed at advanced stages [14, 21, 22]. This late presentation is being attributed to socioeconomic barriers, cultural beliefs and inadequate access to healthcare services [14].
This study examines the stages of cervical cancer in women when they first arrive at a tertiary care hospital. By analysing the stages at diagnosis and identifying factors that contribute to late-stage presentation, the study aims to explain strategies for early detection and reduce the burden of cervical cancer, particularly in vulnerable populations.
Methods
This retrospective study analysed data from women diagnosed with cervical cancer at a tertiary care hospital in Nigeria between January 2017 and December 2021. Inclusion criteria consisted of women aged 18 years or older with a confirmed histopathologic diagnosis of cervical cancer and complete staging information. Women with incomplete reproductive history or clinical staging data, as well as those with a history of prior cancer treatment, were excluded.
Data extraction involved reviewing medical records to collect demographic information (age, marital status), reproductive history variables (parity, age at menarche and age at coitarche) and clinical data (FIGO staging, presence of postcoital bleeding, menopause status and anaemia based on packed cell volume (PCV) levels). The primary outcome was the stage of cervical cancer at diagnosis, categorised as early-stage (stages I–IIA) or late-stage (stages IIB–IV) according to the FIGO 2018 classification [20].
Data analysis
Descriptive analysis: The distributions of reproductive variables, including parity, age at menarche and age at coitarche, were summarised using means, medians and standard deviations. FIGO cancer stages were categorised as early-stage (Stages One and Two) or late-stage (Stages Three and Four). Inferential statistical analyses were conducted to assess the factors influencing the likelihood of late-stage cervical cancer diagnosis. Specifically, odds ratios (ORs) and adjusted odds ratios (AORs) were calculated to determine the strength and direction of associations between key variables (such as parity, age at menarche and age at coitarche) and cancer stage at diagnosis.
Statistical software
All statistical analyses were conducted using SPSS version 25.0 (IBM Corp., Armonk, NY) and statistical significance was set at p-values less than 0.05.
Results
Of the 102 women who presented to the clinic during the study period, only 57 (55.9%) had complete demographic and clinical data records from the Obstetrics and Gynaecology Department of the Institution. We restricted analyses to these 57 cases to maintain data integrity and reliable statistical analysis. The participants' ages ranged from 27 to 85 years, with a mean age of 57.23 years and a median age of 55. Parity varied from 0 to 11 children, with a median of 5 children. The age at menarche ranged from 9 to 21 years, with a median of 15 years and a mean of 14.75 years, while the age of first sexual intercourse (coitarche) varied between 15 and 30 years, with a median of 19 years and a mean of 19.67 years.
Table 1 revealed that 73.7% of participants were 50 years or older, and a significant proportion (40.4%) had between 5 and 7 children. The most common age at first sexual intercourse was between 16 and 19 years, accounting for 43.9% of cases. Additionally, 59.6% of participants had their first menstruation between the ages of 11 and 15 years. The majority of women were traders (70.1%), while artisans, civil servants, clergy, unemployed individuals and teachers each represented less than 10% of the cohort. Marital status analysis showed that nearly 60% of participants were married at the time of diagnosis. Regarding education levels, 24.6% had no formal education, 21.0% completed primary education, 38.6% attained secondary education and 15.8% had tertiary education.
Clinical distribution can be seen in Table 2, which indicates that 56.2% of cases were diagnosed at an advanced stage (stages IIB and IV), Figure 1. Additionally, anaemia (PCV <30%) was present in 75.4% of women. Postcoital bleeding was reported in 35.1% of cases. Most participants (73.7%) were postmenopausal at the time of diagnosis.
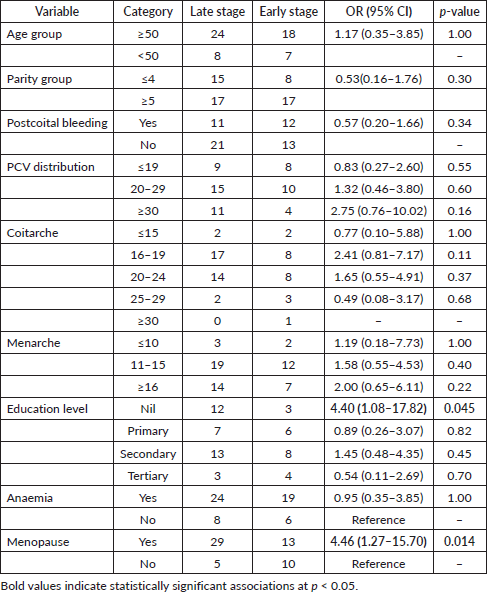
Analysis of associations revealed that women with no formal education had significantly higher odds of late-stage cervical cancer diagnosis compared to those with tertiary education (OR: 4.40, 95% CI: 1.08–17.82; AOR: 3.20, 95% CI: 1.02–10.05). Postmenopausal women were also significantly more likely to present with late-stage cervical cancer compared to premenopausal women (OR: 4.46, 95% CI: 1.27–15.70; AOR: 3.80, 95% CI: 1.25–11.55).
Several trends did not reach statistical significance but suggested potential associations. Women with coitarche at ages 16–19 had higher odds of late-stage diagnosis compared to those aged ≥30, though this was not statistically significant (OR: 2.41, 95% CI: 0.81–7.17; AOR: 1.90, 95% CI: 0.70–5.15). Similarly, women with a PCV of 30 or higher had increased odds of late-stage cancer (OR: 2.75, 95% CI: 0.76–10.02; AOR: 2.50, 95% CI: 0.70–9.50), though these findings were not significant (Tables 3 and 4).
Discussion
The present study provides a detailed analysis of demographic, clinical and associated risk factors for cervical cancer among women diagnosed at our institution. The findings are consistent with global trends in cervical cancer epidemiology, highlighting key associations between late-stage diagnosis and factors such as education level and menopausal status.
The mean age of the participants was 57.2 years, with 73.7% of cases occurring in women aged 50 years or older. This age distribution aligns with studies indicating that cervical cancer predominantly occurs in this age group in LIC [2, 21, 23–25]. However, a concerning trend has emerged globally, with an increasing incidence of cervical cancer among young women aged 15–49 years,
particularly in areas with high Socio-Demographic Index scores. This upward trend may be attributed to various factors, including increased exposure to HPV, lower participation rates in cervical cancer screening programs, earlier sexual debut and a history of multiple sexual partners [17, 26].
Table 1. Demographic distribution.
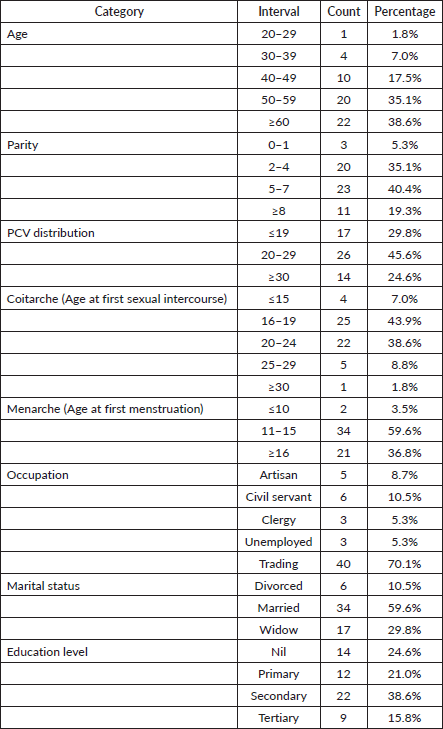
Table 2. Clinical distribution.
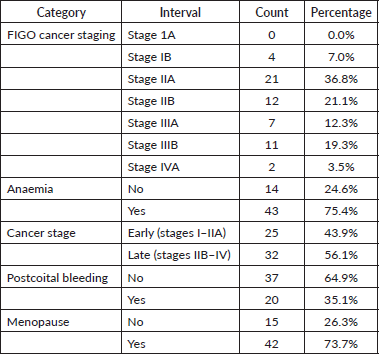
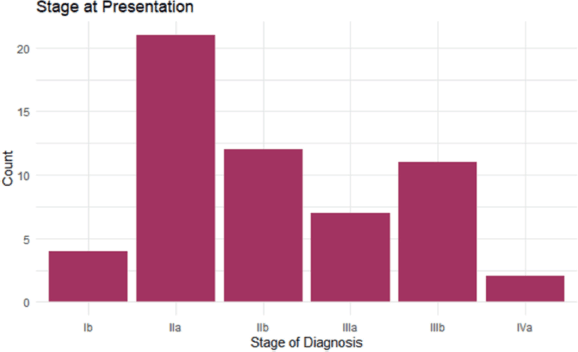
Figure 1. Bar chart showing stages of disease at presentation.
Table 3. Measure of association.
A substantial proportion of participants (40.4%) had between 5 and 7 children. High parity has been widely recognised as a risk factor for cervical cancer, likely due to prolonged exposure to HPV, cervical trauma and hormonal changes during pregnancy [4, 23, 27]. In contrast, the age at menarche and coitarche did not demonstrate statistically significant associations with late-stage diagnosis, despite previous studies suggesting an increased risk with earlier sexual debut [28, 29].
Late-stage cervical cancer (FIGO stages IIB–IV) was observed in 56.1% of cases. This is comparable to findings from other low-resource settings, where delayed diagnosis is frequently reported due to limited access to screening programs [2, 14, 21, 25]. A study conducted in Ethiopia similarly reported that over 60% of women presented with advanced-stage disease due to inadequate screening and late health-seeking behaviour [30]. Inadequacies in the healthcare system may also play a significant role in the late-stage diagnosis of cervical cancer [2, 26, 30]. Additionally, prolonged investigation times and facility-based delays can further hinder early diagnosis and timely initiation of appropriate care [21, 22].
Table 4. Measure of association (Crude and AOR).
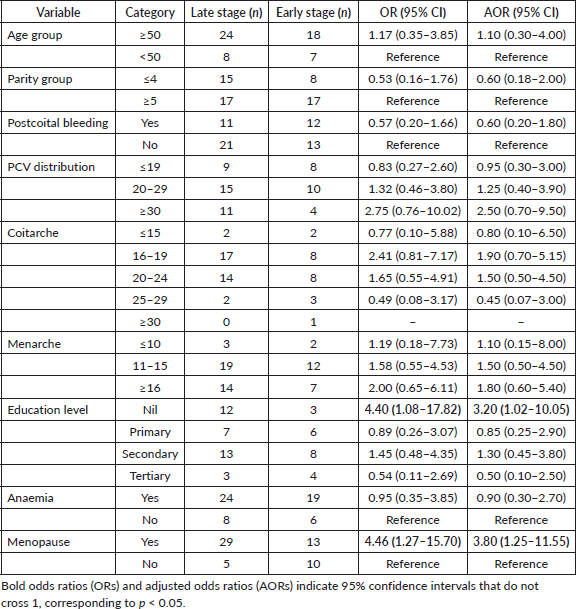
Anaemia was present in 75.4% of cases, highlighting the significant nutritional and systemic health challenges faced by cervical cancer patients in resource-limited settings. Reports indicate that 40%–64% of cervical cancer patients presenting for treatment are anaemic, with some studies showing rates exceeding 80% at diagnosis [2, 31]. Notably, anaemia is approximately twice as prevalent in patients with advanced-stage cervical cancer compared to those diagnosed at an earlier stage [31]. This, however, differs from what was seen in this study, which showed marginal differences. This high prevalence of anaemia in cervical cancer patients can be attributed to multiple factors, including tumour-related bleeding, bone marrow invasion, malnutrition, disrupted iron metabolism, renal impairment and compromised bone marrow function [2, 31, 32].
Women with no formal education were significantly more likely to be diagnosed at an advanced stage (OR = 4.4, p = 0.045). After adjusting for confounders, the odds remained high (AOR = 3.20, 95% CI: 1.02–10.05). This finding supports previous studies linking lower educational attainment to delayed diagnosis, as education influences health literacy, awareness of cervical cancer symptoms and healthcare-seeking behaviour [21, 33]. Notably, in a systematic review, women with low or no formal education are more likely to present with late-stage cervical cancer [34]. The findings highlight the urgent need for targeted awareness programs to improve cervical cancer screening uptake among women with lower educational backgrounds. Furthermore, the lack of education is often linked to early marriage and high parity, which are independent risk factors for cervical cancer [35]. Therefore, improving women's literacy levels can be a crucial strategy in controlling cervical cancer, emphasising the importance of education in cancer prevention.
From this study, postmenopausal women had 4.5 times higher odds of late-stage diagnosis (p = 0.014), which remained significant after adjustment (AOR = 3.80, 95% CI: 1.25–11.55). The significantly higher odds of late-stage diagnosis among postmenopausal women may be attributed to reduced engagement with gynaecological care after menopause. Younger women, due to their reproductive health needs, tend to have more frequent interactions with healthcare providers, facilitating earlier detection [21]. A Vietnamese study further asserted that the postmenopausal state increases the susceptibility to late-stage presentation as a result of hormonal and immunological changes [27]. The establishment of well-woman clinics can significantly improve early detection rates, ultimately leading to a lower prevalence of late-stage cervical cancer.
Eliminating cervical cancer is possible with high-coverage screening and vaccination, yet progress will likely be slowest in low-income regions, particularly in sub-Saharan Africa, due to existing healthcare gaps [36]. To combat this, LICs must strengthen health systems to support widespread screening and vaccination efforts. Achieving the WHO's global strategy targets requires significant investment in healthcare infrastructure, capacity building and public engagement [36–38].
Implications and recommendations
Targeted awareness campaigns: Develop literacy-focused educational interventions (for example, radio programmes and community workshops) aimed at women with low or no formal education to increase screening uptake.
Integration of screening into postmenopausal care: Incorporate Pap smears and HPV testing into routine care for postmenopausal women (well-woman clinics) to minimise delays in diagnosis.
Enhance health infrastructure: Ensure a reliable supply of screening equipment, consistent documentation of screening history and HPV vaccination and develop capacity for healthcare workers in early detection protocols.
Future research directions: Conduct multicentre, mixed-methods studies to explore cultural, socioeconomic and health system barriers in greater depth.
In conclusion, this study's findings highlight the persistent challenges in cervical cancer diagnosis and treatment in low-income settings. The results show that late-stage diagnosis is prevalent. Significant associations were found between late-stage diagnosis and factors such as lower educational attainment and postmenopausal status. The study's findings emphasise the need for targeted interventions, including improved access to screening and education, particularly for high-risk groups. By addressing these disparities and strengthening healthcare systems, progress can be made towards eliminating cervical cancer in low-income settings.
List of abbreviations
AOR, Adjusted odds ratios; FIGO, The International Federation of Gynaecology and Obstetrics; HPV, Human papillomavirus; LICs, Low-income countries; OR, Odds ratios; WHO, World Health Organisation.
Acknowledgments
The authors express their appreciation to the staff of the Department of Obstetrics and Gynaecology, as well as the Medical Records Unit, for their support and contributions during this study.
Conflicts of interest
The authors declare that they have no competing interests.
Funding
This study was not funded.
Consent for publication
Not applicable.
References
1. Sowemimo O, Ojo O, and Fasubaa O (2017) Cervical cancer screening and practice in low resource countries: Nigeria as a case study Trop J Obstet Gynaecol 34(3)170–176 https://doi.org/10.4103/TJOG.TJOG_66_17
2. Ola IO, Okunowo AA, and Habeebu MY, et al (2023) Clinical and non-clinical determinants of cervical cancer mortality: a retrospective cohort study in Lagos, Nigeria Front Oncol 13 1105649 https://doi.org/10.3389/fonc.2023.1105649 PMID: 36874121 PMCID: 9978796
3. Kong Y, Zong L, and Yang J, et al (2019) Cervical cancer in women aged 25 years or younger: a retrospective study Cancer Manag Res 11 2051-2058 https://doi.org/10.2147/CMAR.S195098 PMID: 30881129 PMCID: 6411317
4. Tekalegn Y, Sahiledengle B, and Woldeyohannes D, et al (2022) High parity is associated with increased risk of cervical cancer: systematic review and meta-analysis of case-control studies Womens Health (Lond) 18 17455065221075904 https://doi.org/10.1177/17455065221075904 PMID: 35114865 PMCID: 8819811
5. Bhatla N, Aoki D, and Sharma DN, et al (2021) Cancer of the cervix uteri: 2021 update Int J Gynaecol Obstet 155(1) 28–44 https://doi.org/10.1002/ijgo.13865 PMID: 34669203 PMCID: 9298213
6. Singh D, Vignat J, and Lorenzoni V, et al (2023) Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative Lancet Glob Health 11(2) e197–e206 https://doi.org/10.1016/S2214-109X(22)00501-0 PMCID: 9848409
7. Cetina-Pérez L, Luvián-Morales J, and Delgadillo-González M, et al (2024) Sociodemographic characteristics and their association with survival in women with cervical cancer BMC Cancer 24 161 https://doi.org/10.1186/s12885-024-11909-3 PMID: 38302893 PMCID: 10832171
8. Seifu B, Fikru C, and Yilma D, et al (2022) Predictors of time to death among cervical cancer patients at Tikur Anbesa specialized hospital from 2014 to 2019: a survival analysis PLoS One 17(2) e0264369 https://doi.org/10.1371/journal.pone.0264369 PMID: 35202442 PMCID: 8870501
9. Wassie M, Fentie B, and Asefa T (2021) Determinants of mortality among cervical cancer patients attending in Tikur Anbessa Specialized Hospital, Ethiopia: institutional-based retrospective study J Oncol 9916050 https://doi.org/10.1155/2021/9916050 PMID: 34239565 PMCID: 8233077
10. Musa J, Nankat J, and Achenbach CJ, et al (2016) Cervical cancer survival in a resource-limited setting-North Central Nigeria Infect Agent Cancer 11 15 https://doi.org/10.1186/s13027-016-0062-0 PMID: 27014366 PMCID: 4806480
11. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
12. World Health Organization (2020) Cancer Country Profile: Nigeria (Geneva: WHO) [https://cdn.who.int/media/docs/default-source/country-profiles/cancer/nga-2020.pdf?sfvrsn=16c2215b_2&download=true] Date accessed: 20/02/25
13. Okunade KS, Adejimi AA, and Adekanye TV, et al (2024) Impact of mobile health technologies on human papillomavirus vaccination uptake among mothers of unvaccinated girls aged 9-14 years in Lagos, Nigeria (mHealth-HPVac): study protocol of a randomised controlled trial BMC Cancer 24(1) 751 https://doi.org/10.1186/s12885-024-12538-6 PMID: 38902718 PMCID: 11191157
14. Appiah-Kubi A, Konney TO, and Amo-Antwi K, et al (2022) Factors associated with late-stage presentation of cervical cancer in Ghana Ghana Med J 56(2) 86–94 https://doi.org/10.4314/gmj.v56i2.5 PMID: 37449260 PMCID: 10336471
15. Babu M, Thakur A, and Sravyasri M, et al (2024) Empowering women’s health: examining the impact of human Papillomavirus (HPV) vaccination on cervical cancer treatment and beyond Cureus 16(8) e67287 https://doi.org/10.7759/cureus.67287 PMID: 39310467 PMCID: 11413974
16. Biddell CB, Spees LP, and Smith JS, et al (2021) Perceived financial barriers to cervical cancer screening and associated cost burden among low-income, under-screened women J Womens Health (Larchmt) 30(9) 1243–1252 https://doi.org/10.1089/jwh.2020.8807 PMID: 33851854 PMCID: 8558088
17. Arbyn M, Weiderpass E, and Bruni L, et al (2020) Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis Lancet Glob Health 8(2) e191–e203 https://doi.org/10.1016/S2214-109X(19)30482-6 PMCID: 7025157
18. Petersen Z, Jaca A, and Ginindza TG, et al (2022) Barriers to uptake of cervical cancer screening services in low-and-middle-income countries: a systematic review BMC Womens Health 22(1) 486 https://doi.org/10.1186/s12905-022-02043-y PMID: 36461001 PMCID: 9716693
19. Agha S, Bernard D, and Francis S, et al (2024) Determinants of human Papillomavirus vaccine acceptance among caregivers in Nigeria: a fogg behavior model-based approach Vaccines (Basel) 12(1) 84 https://doi.org/10.3390/vaccines12010084 PMID: 38250897 PMCID: 10820200
20. Bhatla N, Berek JS, and Cuello Fredes M, et al (2019) Revised FIGO staging for carcinoma of the cervix uteri Int J Gynaecol Obstet 147(2) 279–280 https://doi.org/10.1002/ijgo.12969
21. Deogratius Mwaka A, Garimoi CO, and Maloba E, et al (2016) Social, demographic and healthcare factors associated with stage at diagnosis of cervical cancer: cross-sectional study in a tertiary hospital in Northern Uganda BMJ Open 6(1) e007690 https://doi.org/10.1136/bmjopen-2015-007690
22. Awofeso O, Roberts AA, and Salako O, et al (2018) Prevalence and pattern of late-stage presentation in women with breast and cervical cancers in Lagos University teaching Hospital, Nigeria Niger Med J 59(6) 74–79 https://doi.org/10.4103/nmj.NMJ_112_17
23. Thulaseedharan JV, Malila N, and Hakama M, et al (2012) Socio demographic and reproductive risk factors for cervical cancer - a large prospective cohort study from rural India Asian Pac J Cancer Prev 13(6) 2991–2995 https://doi.org/10.7314/APJCP.2012.13.6.2991 PMID: 22938495
24. Adefuye PO, Adefuye BO, and Oluwole AA (2014) Female genital tract cancers in Sagamu, Southwest, Nigeria East Afr Med J 91(11) 398–406 PMID: 26866088
25. Mwaliko E, Itsura P, and Keter A, et al (2023) Survival of cervical cancer patients at Moi teaching and Referral Hospital, Eldoret in western Kenya BMC Cancer 23(1) 1104 https://doi.org/10.1186/s12885-023-11506-w PMID: 37957644 PMCID: 10644535
26. Yang M, Du J, and Lu H, et al (2022) Global trends and age-specific incidence and mortality of cervical cancer from 1990 to 2019: an international comparative study based on the Global Burden of Disease BMJ Open 12(7) e055470 https://doi.org/10.1136/bmjopen-2021-055470 PMID: 35868828 PMCID: 9316042
27. Van Pham H, Phan VM, and Van Bui C, et al (2024) Significant associations between age, menstrual status, and histopathological types of cervical carcinoma in vietnamese patients: insights from a retrospective and prospective analysis Biomed Res Therapy 11(6) 6511–6519 https://doi.org/10.15419/bmrat.v11i6.897
28. Louie KS, de Sanjose S, and Diaz M, et al (2009) Early age at first sexual intercourse and early pregnancy are risk factors for cervical cancer in developing countries Br J Cancer 100(7) 1191–1197 https://doi.org/10.1038/sj.bjc.6604974 PMID: 19277042 PMCID: 2670004
29. Sulistyawati D, Faizah Z, and Kurniawati EM (2020) An association study of cervical cancer correlated with the age of Coitarche in Dr. Soetomo Hospital Surabaya Indones J Cancer 14(1) 3–7 https://doi.org/10.33371/ijoc.v14i1.639
30. Zewdie A, Shitu S, and Kebede N, et al (2023) Determinants of late-stage cervical cancer presentation in Ethiopia: a systematic review and meta-analysis BMC Cancer 23(1) 1228 https://doi.org/10.1186/s12885-023-11728-y PMID: 38097989 PMCID: 10720221
31. Wassie M, Aemro A, and Fentie B (2021) Prevalence and associated factors of baseline anemia among cervical cancer patients in Tikur Anbesa Specialized Hospital, Ethiopia BMC Women’s Health 21 36 https://doi.org/10.1186/s12905-021-01185-9
32. Shin NR, Lee YY, and Kim SH, et al (2014) Prognostic value of pretreatment hemoglobin level in patients with early cervical cancer Obstet Gynecol Sci 57(1) 28–36 https://doi.org/10.5468/ogs.2014.57.1.28 PMID: 24596815 PMCID: 3924748
33. Dunyo P, Effah K, and Udofia EA (2018) Factors associated with late presentation of cervical cancer cases at a district hospital: a retrospective study BMC Public Health 18(1) 1156 https://doi.org/10.1186/s12889-018-6065-6 PMID: 30285699 PMCID: 6171232
34. Tekalign T and Teshome M (2022) Prevalence and determinants of late-stage presentation among cervical cancer patients, a systematic review and meta-analysis PLoS One 17(4) e0267571 https://doi.org/10.1371/journal.pone.0267571 PMID: 35476851 PMCID: 9045598
35. Alegbeleye JO, Nyeche S, and Jaja MA (2023) Socio-demographic and clinico-pathological analysis of cervical cancer patients at a tertiary care centre in South-south Nigeria Int J Reprod Contracept Obstet Gynecol 12(6) 1538–1545 https://doi.org/10.18203/2320-1770.ijrcog20231517
36. Jedy-Agba E, Joko WY, and Liu B, et al (2020) Trends in cervical cancer incidence in Sub-Saharan Africa Br J Cancer 123(1) 148–154 https://doi.org/10.1038/s41416-020-0831-9 PMID: 32336751 PMCID: 7341858
37. World Health Organization (2020) Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem (Geneva: WHO) [https://www.who.int/publications/i/item/9789240014107] Date accessed: 27/02/25
38. Zou Z, Fairley CK, and Ong JJ, et al (2024) Impact of achieving WHO’s 90-70-90 targets on cervical cancer elimination and potential benefits in preventing other HPV-related cancers in China: a modelling study EClinicalMedicine 77 102878 https://doi.org/10.1016/j.eclinm.2024.102878






