Changing paradigms in pediatric cancer care – the contemporary landscape and perspectives for India
Badira Cheriyalinkal Parambil1, Nirmalya Roy Moulik1, Venkata Rama Mohan Gollamudi1, Shyam Srinivasan1, Chetan Dhamne1, Akanksha Chichra1, Gaurav Narula1, Mukta Ramadwar2, Sumeet Gujral7, Tanuja Shet2, Epari Sridhar2, Poonam Panjwani2, Uma Sakhadeo2, Siddhartha Laskar3, Nehal Khanna3, Jifmi Jose Manjali3, Sajid Qureshi4, Vasundhara Patil5, Akshay Baheti5, Sneha Shah6, Kunal Gala5, Pappagudi Subramanian7 , Prashant Tembhare7, Nikhil Patkar7, Gaurav Chatterjee7, Sweta Rajpal7, Dhanlaxmi Shetty8, Maya Prasad1 and Girish Chinnaswamy1
1Division of Pediatric Oncology, Tata Memorial Hospital, Homi Bhabha National Institute (HBNI), Mumbai 400012, India
2Department of Pathology, Tata Memorial Hospital, Homi Bhabha National Institute (HBNI), Mumbai 400012, India
3Department of Radiation Oncology, Tata Memorial Hospital, Homi Bhabha National Institute (HBNI), Mumbai 400012, India
4Department of Surgical Oncology, Tata Memorial Hospital, Homi Bhabha National Institute (HBNI), Mumbai 400012, India
5Department of Radiodiagnosis, Tata Memorial Hospital, Homi Bhabha National Institute (HBNI), Mumbai 400012, India
6Department of Nuclear Medicine, Tata Memorial Hospital, Homi Bhabha National Institute (HBNI), Mumbai 400012, India
7Department of Hematopathology, Tata Memorial Hospital, Homi Bhabha National Institute (HBNI), Mumbai 400012, India
8Department of Cytogenetics, Tata Memorial Hospital, Homi Bhabha National Institute (HBNI), Mumbai 400012, India
Abstract
Advances in the diagnosis and management of childhood cancers have significantly improved survival, and 80% of those who have access to contemporary treatment are expected to survive into adulthood. Multimodality protocols incorporating high-intensity cytotoxic chemotherapy and radiotherapy may be associated with increased acute and delayed adverse effects, thereby compromising the quality of life. Furthermore, curative therapeutic options remain limited in the context of metastatic, relapsed or refractory disease as well as rare tumour entities. This has prompted a paradigm shift in pediatric oncology care in the contemporary era, encompassing multiple domains including cancer predisposition, immunotherapy, precision medicine and survivorship, aimed at optimising survival while minimising treatment-related toxicity and improving quality of life. While these advances are increasingly evident in high-income countries, several hurdles and challenges exist in the implementation of these strategies in low-income and middle-income countries (LMICs). Key barriers include restricted accessibility and affordability of newer and advanced diagnostic modalities and therapeutic agents, deficient infrastructure, non-availability of targeted agents and newer immunotherapy drugs, logistical and regulatory hurdles, limited access to clinical trials and inadequate long-term follow-up. Substantial changes are requisite to facilitate the translation of these changing paradigms into reality in India and LMICs.
Keywords: pediatric cancer, cancer predisposition, immunotherapy, precision medicine, survivorship, perspectives
Correspondence to: Badira Cheriyalinkal Parambil
Email: badiracp@yahoo.co.in
Published: 24/06/2025
Received: 13/01/2025
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Childhood cancer, although rare and constituting only around 3%–4% of all cancer diagnoses, has increased in incidence over the past several decades [1]. Dramatic advances in diagnosis and curative treatment strategies in pediatric malignancies have significantly improved the survival in this subset, and 80% who have access to contemporary treatment protocols are expected to survive into adulthood [2]. However, survival remains dismal with limited treatment options for various metastatic, relapse/refractory diseases and many rare tumours. Additionally, intense multimodality therapies incorporating cytotoxic chemotherapy have contributed to the high burden of late morbidity, impairing the quality of life [3]. This has led to a paradigm shift in pediatric oncology care, with risk-stratified, response-adapted treatment protocols replacing blanket treatment approaches. There has been increasing use of non-chemotherapy systemic treatments, which include immunotherapy and targeted therapy, genomics-focused pediatric precision oncology programs and establishment of varied models of survivorship programs, all of which focus on optimising cure with minimal morbidity and mortality.
The stark disparities in the incidence and patterns of childhood cancer between high-income countries (HICs) and low-income and middle-income countries (LMICs) extend to survival outcomes, with 80% of children with cancer cured of disease in HICs compared to 20% in LMICs [4, 5]. This article reviews the major shifts in pediatric oncology care and current perspectives, especially for India and applicable for other LMICs, in the fields of cancer predisposition syndromes, precision medicine, immunotherapy and survivorship.
Cancer predisposition syndromes
Rapidly evolving research in the field of genetic susceptibility has found several germline mutations in predisposing genes in childhood cancer. Knowledge of the specific germline mutations facilitates the understanding of tumourigenesis, and helps direct patient care and genetic counselling. Approximately 10% of children with cancer are affected by monogenic cancer predisposition syndromes (CPSs) [6, 7]. CPS working groups have been established worldwide to provide consensus recommendations for the purposes of clinical, diagnostic and surveillance programs for carriers [8, 9].
Cancer predisposition syndromes: current evidence
A landmark study by Zhang et al [7] utilised next generation sequencing (NGS) of 565 genes to identify germline mutations in 8.5% of 1,120 children and adolescents with diverse cancers [7]. Only 40% had a family history of cancer, of which only half had a cancer consistent with a known CPS [7]. Another study on the landscape of genomic alterations across childhood cancers showed that a pathogenic germline variant in one of the 162 genes tested was identified in 7.6% of the samples [6]. Some of the studies reporting on the genomic landscape in childhood cancer were restricted to certain tumour types and subtypes and may not be applicable to all childhood cancer cohorts [6, 7].
The varied approaches to diagnosis range from phenotype-driven, with candidate selection based on patient, tumour and physical characteristics, to a universal phenotype-agnostic comprehensive germline genetic testing or a combinatorial approach of both [10]. Each has its own advantages, with the phenotype-driven approach being clinically driven, thus creating an optimal balance of resource allocation, lower chance of ambiguous findings and identifying CPS in diseases associated with epigenetic changes or low-level mosaicism. Comprehensive genetic testing in all is best employed in research settings currently to understand the prevalence and spectrum of CPS [10].
The diagnostic and therapeutic implications of a diagnosis of CPS, though limited at present, could be significant in certain settings. In DICER1 syndrome, which is associated with multiple rare tumours, the use of a germline gene panel can facilitate early diagnosis. Studies have demonstrated the utility of immune checkpoint inhibitors (ICIs) in germline-mismatch repair deficiency (MMRD) hypermutated tumours [11]. Exposure to ionising radiation is to be avoided in patients with Li-Fraumeni or Gorlin syndrome and chromosomal breakage syndromes to prevent subsequent cancers. Conventional doses of myelosuppressive chemotherapy (specifically alkylating agents) with the attendant excess toxicity should be avoided in chromosomal breakage syndromes and those with defective DNA repair. Understanding of CPS helps in planning organ-sparing surgery (e.g., nephron-sparing surgery in Wilms tumour), risk-reducing surgery (e.g., prophylactic thyroidectomy in MEN2 syndrome) and in donor selection for allogenic stem cell transplantation. In rhabdomyosarcoma (RMS), significantly worse outcomes were demonstrated with germline TP53 or HRAS alterations, especially for fusion-negative RMS, suggesting a possible role of these for future risk stratification [12].
A study by Villani et al, [13] showed that a comprehensive surveillance protocol for individuals with pathogenic TP53 variants improved long-term survival, facilitating this approach in these patients [13]. Patients with constitutional MMRD may also benefit from cancer surveillance, especially for colorectal cancer and brain tumours, though surveillance may not guarantee detection at a curable stage [14]. Recommendations for surveillance and position statements in most key CPS have recently been published, based on expert opinion, though the impact of surveillance on survival is not established [15].
Cancer predisposition syndromes: Indian perspective
The expanding field of germline genetic testing is still in its nascent stage in LMICs, including India. Lack of awareness among patients and primary health-care providers, restricted access to germline genetic testing services, compounded by cost and limited availability of genetic counsellors, are hindrances to the implementation of these services. Moving ahead, the phenotypic spectra, frequency, genotype-phenotype correlations and geo-ethnic variations are hitherto unexplored. This necessitates a comprehensive germline genetic testing in consecutive patients in a research context to assess the prevalence and impact of a CPS diagnosis on treatment adaptation, survival outcomes, quality of life, economic resources and psychosocial outcomes. These baseline data could inform further testing policies and help develop an algorithm that could be integrated into routine clinical practice. Phenotype-driven, curated gene panels appear to be more cost-effective and practical in contemporary settings [10]. Incorporation of genetic services into the national cancer control programme would be a desirable step in the long term.
In India, the establishment of dedicated pediatric genetics clinics in tertiary care centers have been initiated [16]. The institution of a Cancer Genetics Subcommittee under the Indian Pediatric Hematology Oncology Group (INPHOG), a leading collaborative network in the country, is a welcome step toward streamlining the services [17]. Multi-center studies involving major institutes in the country are ongoing, and leverage the existing infrastructure, expertise and data-sharing frameworks. These studies are anticipated to further facilitate the development of nodal centers offering laboratory, online genetic counselling and other support in a hub and spoke model.
Immunotherapy
Immunotherapy has emerged as a ground-breaking approach in the treatment of various pediatric cancers, leveraging the body’s immune system to destroy cancer cells. The use of immunotherapy may potentially reduce long-term toxicity – a critical consideration in pediatric oncology. Additionally, immunotherapies are uniquely suited to achieving remission or positive responses in relapsed/refractory cancers that have proven resistant to traditional treatments like chemotherapy and radiation.
Immunotherapy: current evidence
Antibody-based immunotherapy
Unconjugated monoclonal antibodies attach directly to the target cell surface antigens, causing cell lysis. The prototype, rituximab, has now evolved as the standard of care in the management of high-risk mature B-NHL in children [18]. It is also widely used in salvage regimens in Hodgkin and other CD20-positive NHLs.
Antibody drug conjugates (ADCs) are coupled to cytotoxic drugs, targeting specific cell surface antigens and delivering the cytotoxic payload to the target cells. ADCs useful in pediatric cancers include Inotuzumab, Gemtuzumab (humanised anti-CD33-calicheamicin conjugate), Brentuximab [anti-CD30 monoclonal antibody-monomethyl auristatin E conjugate] for leukemia/lymphoma [19, 21]. Inotuzumab has shown remarkable efficacy in CD22-positive relapsed/refractory adult and pediatric acute lymphoblastic leukemia (ALL) and is being investigated in the frontline setting in childhood ALL. Gemtuzumab significantly reduced relapse risk in childhood acute myeloid leukemia in the Children’s Oncology Group (COG) AAML0531 study [20]. Similarly, Brentuximab has shown a remarkable response in CD30-expressing lymphomas (Hodgkin lymphoma, ALCL) and is currently projected as a standard treatment for children with high-risk Hodgkin lymphoma [21].
Bispecific antibodies are engineered antibodies recruiting immune cells to the close proximity of the cancer cells for effecting cytotoxicity. Blinatumomab (anti-CD19) is the most important molecule in this group and is now approved for the treatment of relapsed/refractory ALL in both children and adults [22]. Based on recent reports from the COG-AALL1731, children treated with blinatumomab in combination with chemotherapy had improved outcomes over chemotherapy alone in non-high-risk B-ALL in the frontline setting [23]. Blinatumomab has also shown exceptional efficacy in infant ALL and other subsets of very high-risk pre B-cell ALL.
The most prominent example of monoclonal antibodies developed for solid tumours is dinutuximab, targeting the GD2 antigen expressed on neuroblastoma cells. A reported 20% survival benefit over conventional therapy in high-risk neuroblastoma patients led to its approval in this setting [24]. A high response rate with chemoimmunotherapy is also demonstrated in the relapse/refractory setting [25]. Humanised or chimeric anti-GD2 are being explored in other GD2+ pediatric solid tumours, including osteosarcoma and soft tissue sarcomas.
Despite their efficacy, antibody-based therapies are not without limitations. Infusion reactions, cytokine release and neurotoxicity are common adverse effects [26]. Additionally, resistance can develop due to antigen loss or downregulation, necessitating combination therapies or sequential treatments [27].
Cell-based immunotherapy
Cell-based immunotherapy focuses on the use of modified immune cells to target and destroy cancer cells. Approval of tisagenlecleucel (Kymriah), an anti-CD19 chimeric antigen receptor (CAR) T-cell for pediatric and young adult patients with relapsed/refractory B-ALL, marked a significant milestone [28]. Clinical trials have reported remission rates exceeding 80%, even in heavily pre-treated populations [29]. CAR-Ts against other leukemia antigens such as CD-22 (B-cell ALL), CD7 (T-ALL/AML), CD5 (T-ALL) and CD123 (AML) are under various stages of development and clinical trials as is newer generations of CAR-T constructs aimed at increased specificity and efficacy with minimal toxicity.
CAR T-cell therapies are also being explored in solid tumours including relapsed/refractory neuroblastoma, though with less success [30]. The challenges include the heterogeneity of solid tumour antigens, the immunosuppressive tumour microenvironment and physical barriers preventing T-cell infiltration.
Immune checkpoint inhibitors
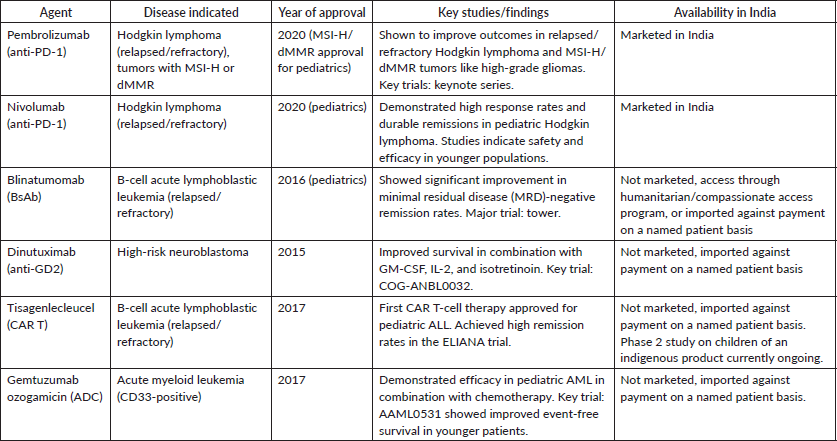
These block proteins are used by cancer cells to evade immune detection, thereby reactivating T-cells to attack the tumour. The most studied ICI target programmed cell death protein 1 (PD-1), its ligand PD-L1 and cytotoxic T-lymphocyte-associated protein 4. Their role in pediatric cancers is still emerging [31]. Pediatric tumours generally have a lower mutation burden, resulting in fewer neoantigens and reduced immunogenicity compared to adult tumours. However, certain pediatric cancers, particularly those with DNA repair deficiencies or microsatellite instability, may respond well to checkpoint blockade [11]. Among ICI, pembrolizumab and nivolumab have been extensively studied in pediatric cancers like relapsed/refractory Hodgkin lymphoma, high-grade gliomas with mismatch repair deficiency or high microsatellite instability 11,26 [11]. Despite their promise, ICI in children carries potential risks, including autoimmune toxicities like colitis, endocrinopathies and pneumonitis. Long-term follow-up is essential to monitor these effects and better understand the impact of immune modulation on developing organs. Table 1 lists the main immunotherapeutic agents approved for use in pediatric cancers.
Immunotherapy: Indian perspective
Significant challenges remain in the universal application of immunotherapy, ranging from toxicity management to the high cost of therapies [32, 33]. Furthermore, limited availability makes these therapies inaccessible to most children from the LMICs. Infrastructure deficiencies, including a lack of specialised facilities and trained personnel, hinder proper administration. Ongoing research and clinical trials are expected to expand the availability and effectiveness of these treatments [32]. Regulatory barriers and logistical issues, such as a lack of approvals/slow approvals and cold-chain requirements, further delay access. Financial constraints, including limited funding and prohibitive out-of-pocket costs, exacerbate disparities. Also, underrepresentation in clinical trials leads to a lack of region-specific data, compounded by ethical and logistical difficulties in conducting research in all ethnicities. Addressing these issues requires targeted investments, policy reforms, advent of generic formulations, humanitarian/compassionate access to these high-end therapies [33].
In India, the development of an indigenous CAR-T cell therapy is a promising development, with NexCAR19 approved for adolescents and young adults [34]. In children, this has successfully completed a phase 1 trial and is now progressing to phase 2 studies. Furthermore, blinatumomab is being offered to children at 7 hospitals in 4 LMICs through a humanitarian access program, organised by Amgen in collaboration with St. Jude Children’s Research Hospital in Memphis, USA [35]. Other therapeutic agents, such as Inotuzumab, are also marketed in India and are being used in clinical practice to treat children with ALL.
Table 1. Immunotherapeutic agents approved for use in pediatric cancers.
Precision medicine
Over the last decade, precision medicine has significantly advanced in oncology, offering tailored treatment approaches based on the molecular and genetic characteristics of tumours. While still in its incipient stage in pediatric oncology, early data underscores its potential, particularly for high-risk and rare cancers with poor outcomes [27, 28].
Precision medicine: current evidence
Studies such as the NCI-COG Pediatric MATCH trial have laid the foundation for the broader application of precision medicine in pediatric oncology [36]. This trial established a collaborative framework for efficiently collecting, processing and sequencing refractory pediatric cancers. Early results revealed actionable alterations in 31.5% of the first 1,000 tumours screened. Similarly, the European MoleculAr profiling for pediatric and young adult cancer treatment stratification (MAPPYACTS) trial identified potentially actionable targets in 69% of 624 patients screened [37]. Beyond identifying actionable mutations, these studies have unveiled other critical benefits. Germline mutations were detected in 7%–17% of enrolled patients, offering insights into hereditary cancer predisposition with significant implications for family members [38]. Molecular profiling has also occasionally led to changes in diagnosis, enhancing the precision of therapeutic strategies [39]. Once actionable targets are identified, patients can be enrolled in platform trials targeting specific survival pathways or have their cases reviewed in multidisciplinary tumour boards (MTBs). MTBs provide age-specific therapeutic recommendations, ensuring safety and efficacy for pediatric patients. Table 2 lists the Published pediatric precision medicine studies.
Table 2. Published pediatric precision medicine studies (38).
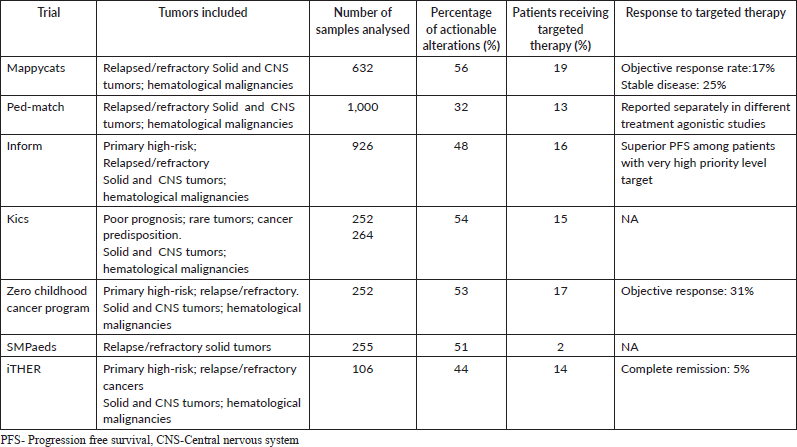
Despite progress, challenges persist. While actionable mutations are identified in a significant proportion of cases, only a fraction of patients receive matched targeted therapies. For instance, in the MAPPYACTS trial, only 30% of patients with actionable mutations accessed targeted treatments [37]. Barriers include the unavailability of drugs within clinical trials and uncertainties surrounding efficacy and benefit-risk balance. Another recurring concern is the outcomes of targeted therapies resulting from a precision medicine-based approach. Preliminary results from the NCI-MATCH study showed limited responses for agents such as palbociclib (CDK4/6 inhibitor), ulixertinib (ERK1/2 inhibitor) and samotolisib (PI3K/mTOR inhibitor) [40–42]. However, encouraging results have been seen in specific high-risk or relapsed cancers with key driver mutations such as NTRK fusions, ALK mutations and BRAF mutations, as shown in the MAPPYACTS and INFORM registry [37]. Based on these results, agents are now being explored in frontline settings. However, a notable gap in existing studies is the lack of comprehensive survival outcome data, with most focusing on objective response rates. To unlock the full potential of precision medicine, long-term outcomes must be prioritised in future research.
Precision medicine: Indian perspective
Implementing precision medicine in LMICs presents unique challenges. Access to advanced diagnostic tools like whole-genome sequencing, whole-exome sequencing and methylation studies is limited, necessitating adaptive and alternative strategies (for example, use of clinicoradiological and histological characteristics to make a molecular diagnosis in medulloblastoma) [43]. Another concern in LMIC is the availability and affordability of corresponding targeted therapies for actionable mutations. Unlike conventional chemotherapy, most targeted therapies are non-myelosuppressive, which significantly improves the quality of life for patients and their caregivers, due to the decreased incident morbidities. Continuous efforts need to be made to enrich and update the WHO essential medicine list for children to increase access to more targeted therapies. Standardised reporting of treatment responses and survival outcomes is crucial to assess the true impact of precision medicine in LMICs. National MTBs can play a critical role in LMICs by providing a platform to discuss molecular profiling results and therapeutic options for complex cases. Based on current evidence, even in LMICs, precision-based approaches should initially be restricted to clinical trials focusing on high-risk pediatric cancers with survival rates below 30%. Establishing funded clinical trials with centralised testing and collaborative frameworks is essential, which can ensure equitable access to precision medicine, even in resource-constrained settings, while generating robust data to guide treatment strategies. There is a need for strategic investments in research infrastructure, international collaborations and clinical trial frameworks.
In India, initiatives such as virtual tumour board organised by the National Cancer Grid, have enabled clinicians to incorporate reports of molecular testing in the risk stratification and treatment algorithms, and to promote the judicious resource-adapted use of NGS and molecular testing, providing a framework for future molecular tumour boards [44]. Government initiatives, like the Department of Health Research’s collaboration with the Indian Council of Medical Research under the DIAMOnDS project, have established guidelines for advanced molecular oncology diagnostics, ensuring wider implementation of these technologies [45]. The main objective of the project is to strengthen the diagnostic research and geographical spread of services and provide free-of-cost diagnostic services and evidence-based health care for patients with cancer. Enhancing access to targeted therapies by implementing patient assistance and compassionate-use programs, along with promoting the availability of cost-effective generic formulations, will significantly advance equitable and affordable care.
Survivorship
The flipside of improved long-term survival is the increased risk of a wide spectrum of life-altering and life-threatening late effects in a proportion of childhood cancer survivors (CCSs) contributing to morbidity and mortality [3]. A report from the childhood cancer survivor study (CCSS) cohort identified that two out of three survivors will develop a chronic health condition, and more than one third will develop a condition that is severe or life-threatening [46]. Acknowledging the need for evidence-based, lifelong surveillance of health-related outcomes, the COG and subsequently, the International Late Effects of Childhood Cancer Guideline Harmonisation Group (IGHG) have developed risk-based exposure-related guidelines for follow-up care of CCSs [47, 48].
Survivorship: current evidence
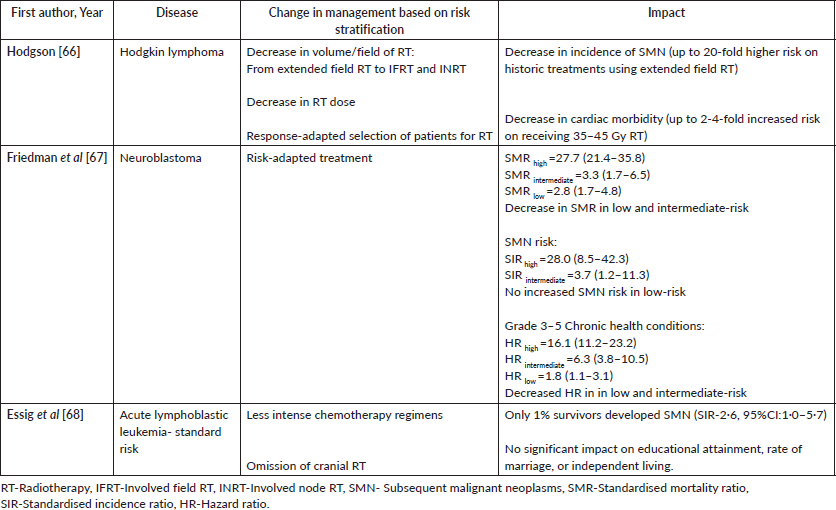
As childhood cancer remains a rare disease, recognition of low prevalence, yet clinically relevant late effects, poses a challenge due to the low event rate. Multiple institutions and cooperative groups have built and followed up cohorts of CCSs [49–51]. Combining data from various cohorts could make way for early identification of late effects, both known and novel, and updating of surveillance recommendations in long-term follow-up clinical practice guidelines. A recent pooled data analysis of more than 25,000 survivors from the CCSS, the Dutch Children’s Oncology Group’s later study and the St Jude lifetime study demonstrated the dose equivalence among anthracyclines with regards to cardiomyopathy, thereby impacting the screening recommendations for recipients of anthracycline with/without chest radiotherapy [52, 53]. Moving forward, similar collaborations facilitated in predicting the late effect risks of specific therapeutic exposures such as development and validation of a risk-prediction model for heart failure, ischemic heart disease and stroke, prediction of risk for acute ovarian failure and calculation of polygenic risk scoring in assessing the risk for developing subsequent thyroid cancer in CCSs [54–56]. Recognition of specific exposure-related late effects initiated risk-adapted treatment approaches, thereby modifying the side-effect profile. Details in Table 3. The contributions of genetic factors/pharmacogenomics in the genesis of late effects in CCSs, especially in health conditions like SMN, cardiovascular disease, obesity and hearing loss remains elusive till date. Multinational collaborative efforts with clinical, laboratory inputs in these domains would expedite research across a diverse patient profile and foster the development of preventive as well as frontline treatment strategies [57]. Longitudinal studies in the survivors of recipients of novel treatments with survival benefit and varied late-effect profiles like targeted therapy, immunotherapy, cellular therapy and proton beam radiotherapy are the need of the hour. A recent study reported on the long-term toxicity profile of CAR-T cell therapy [58]. One of the evolving areas in cancer survivorship research is the focus on chronic health conditions in young adult survivors, leading to frailty, which can lead to early mortality. With the increase in the proportion of adult survivors in various cooperative groups, the focus is on identifying risk factors for frailty and determining the effectiveness of pharmacologic and lifestyle interventions in preventing or delaying the premature aging process [59]. The complexity of survivorship care, with the continued emergence of new knowledge, poses a challenge in its implementation. One important way used in bridging the gap between the knowledge and practice is developing and timely updating the survivorship guidelines. The IGHG is developing a living guidelines information and communication technology (ICT) tool in collaboration with a European project (PanCareFollowUp). This ICT tool, ‘the living guideline’, will be able to regularly search the literature and provide the guideline groups with new evidence. It will also support the guideline groups to grade new evidence and, if needed, adjust current recommendations. This will be instrumental in translating new knowledge into guidelines in an efficient way [60].
Survivorship: Indian perspective
Accessibility, diversity in care models and barriers to childhood cancer survivorship care is a well-known, unique factors determining the delivery of quality care to the survivors [61]. In HICs, most CCSs have access to long-term follow-up (LTFU) care by their primary oncology team or a dedicated LTFU clinic. Research suggests that survivors who attend LTFU care programs have better long-term physical and psychosocial health outcomes than non-attenders [62]. Replicating similar models in LMICs is fraught with challenges [63]. Implementation barriers can relate to survivor, health-care provider and health-care domains. Survivors’ issues include knowledge deficits, lack of endorsement of need for LTFU care by the treating physician, perception that LTFU was not necessary because they felt well and were not experiencing problems, and competing demands related to school, work or family [64]. Health-care provider issues consist of knowledge deficits, lack of experience with survivorship care, especially with management of survivors with multisystem morbidity, lack of resources, lower priority for survivorship versus acute oncology care and suboptimal communication among providers [61, 65]. To optimise the delivery of CCS care, concerted efforts are required to enhance knowledge in patient and family about the need and potential benefits of LTFU care, improve provider education and training, and bring in policy change to ensure that childhood cancer survivors have access to essential services and resources to improve their quality of life. Importantly, there needs to be a focus on pre-empting the development of late toxicities by using up-to-date risk-adapted protocols, monitoring for toxicities on treatment and improving capacity for procedures such as fertility preservation [64].
Table 3. Impact of risk-adapted treatment in pediatric oncology on late effects profile in CCSs (66–68).
India’s first dedicated multidisciplinary survivorship clinic was established at Tata Memorial Hospital, Mumbai, which has registered more than 5,500 CCS till date. A report published by this group identified the spectrum of late effects prevalent in their cohort of survivors, reflective of the issues more commonly described in LMICs [63]. There is a promising future with the implementation of innovative steps to improve survivorship care in India with a focus on decentralisation of care, shared-care and remote follow-up models for low-risk survivors, increased participation from non-profit organisations, incorporation of technology in generating individualised survivorship-care plans, telesurvivorship and collaborative research within the Late Effects subcommittee of INPHOG and Indian childhood cancer initiative [64, 65].
Summary
In the contemporary era, a paradigm shift in pediatric oncology care is evident across multiple domains, including cancer predisposition, immunotherapy, precision medicine and survivorship, designed to harmonise survival with quality of life, ensuring the best possible outcomes. Though the above changes are increasingly evident in HICs, several hurdles and challenges exist in the implementation of these strategies in LMICs. Substantial changes are requisite to facilitate the translation of these changing paradigms into reality in LMICs.
List of abbreviations
LMIC, Low and middle-income countries; HIC, High-income countries; CPS, Cancer predisposition syndrome; NGS, Next generation sequencing; MMRD, Mis match repair deficiency; ALL, Acute lymphoblastic leukemia; AML, Acute myeloid leukemia; ADC, Antibody drug conjugate; COG, Children’s oncology group; CAR T, Chimeric antigen receptor T; MTB, Multidisciplinary tumour boards; CCS, Childhood cancer survivor; LTFU, Long term follow up; IGHG, International Late Effects of Childhood Cancer Guideline Harmonisation Group; ICI, Immune checkpoint inhibitors; INPHOG, Indian Pediatric Hematology Oncology Group.
Conflicts of interest
The authors declare that there is no conflicts of interest.
Funding
Nil funding received.
Author contributions
Dr Badira Cheriyalinkal Parambil conceptualised and designed the article, drafted the initial manuscript and reviewed and revised the manuscript. Drs Nirmalya Roy Moulik, Venkata Rama Mohan Gollamudi, Shyam Srinivasan, drafted the initial manuscript and reviewed and revised the manuscript. Drs Maya Prasad and Girish Chinnaswamy conceptualised and designed the article, and reviewed and revised the manuscript. Drs Gaurav Narula, Chetan Dhamne, Akanksha Chichra, Mukta Ramadwar, Poonam Panjwani, Siddhartha Laskar, Nehal Khanna, Jifmi Jose Manjali, Sajid Qureshi, Vasundhara Patil, Akshay Baheti, Sneha Shah, Kunal Gala, P G Subramanian, Prashant Tembhare, Nikhil Patkar, Gaurav Chatterjee, Swetha Rajpal, Dhanlaxmi Shetty, critically reviewed the manuscript for important intellectual content and revised the same. All authors have contributed in significant ways, have reviewed and agreed upon the manuscript content.
References
1. Sultan I, Alfaar AS, and Sultan Y, et al (2025) Trends in childhood cancer: incidence and survival analysis over 45 years of SEER data PLoS One 20(1) e0314592 https://doi.org/10.1371/journal.pone.0314592 PMID: 39752445 PMCID: 11698462
2. National Childhood Cancer Registry Explorer (NCCR*Explorer) [Internet]. [https://nccrexplorer.ccdi.cancer.gov/] Date accessed: 07/12/24
3. Armstrong GT, Kawashima T, and Leisenring W, et al (2014) Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study J Clin Oncol Off J Am Soc Clin Oncol 32(12) 1218–1227 https://doi.org/10.1200/JCO.2013.51.1055
4. Lubega J, Kimutai RL, and Chintagumpala MM (2021) Global health disparities in childhood cancers Curr Opin Pediatr 33(1) 33–39 https://doi.org/10.1097/MOP.0000000000000984
5. Steliarova-Foucher E, Colombet M, and Ries LAG, et al (2017) International incidence of childhood cancer, 2001–10: a population-based registry study Lancet Oncol 18(6) 719–731 https://doi.org/10.1016/S1470-2045(17)30186-9 PMID: 28410997 PMCID: 5461370
6. Gröbner SN, Worst BC, and Weischenfeldt J, et al (2018) The landscape of genomic alterations across childhood cancers Nature 555(7696) 321–327 https://doi.org/10.1038/nature25480 PMID: 29489754
7. Zhang J, Walsh MF, and Wu G, et al (2015) Germline mutations in predisposition genes in pediatric cancer N Engl J Med 373(24) 2336–2346 https://doi.org/10.1056/NEJMoa1508054 PMID: 26580448 PMCID: 4734119
8. Ripperger T, Bielack SS, and Borkhardt A, et al (2017) Childhood cancer predisposition syndromes-a concise review and recommendations by the Cancer Predisposition Working Group of the Society for Pediatric Oncology and Hematology Am J Med Genet A 173(4) 1017–1037 https://doi.org/10.1002/ajmg.a.38142 PMID: 28168833
9. Brodeur GM, Nichols KE, and Plon SE, et al (2017) Pediatric cancer predisposition and surveillance: an overview, and a tribute to Alfred G. Knudson Jr Clin Cancer Res Off J Am Assoc Cancer Res23(11) e1–e5 https://doi.org/10.1158/1078-0432.CCR-17-0702
10. Bakhuizen JJ, Bourdeaut F, and Wadt KAW, et al (2024) Genetic testing for childhood cancer predisposition syndromes: controversies and recommendations from the SIOPE Host Genome Working Group meeting 2022 EJC Paediatr Oncol 4 100176 https://doi.org/10.1016/j.ejcped.2024.100176
11. Das A, Tabori U, and Sambira Nahum LC, et al (2023) Efficacy of nivolumab in pediatric cancers with high mutation burden and mismatch repair deficiency Clin Cancer Res 29(23) 4770–4783 https://doi.org/10.1158/1078-0432.CCR-23-0411 PMID: 37126021 PMCID: 10690097
12. Germline Genetic Testing and Survival Outcomes Among Children With Rhabdomyosarcoma: A Report From the Children’s Oncology Group | Pediatrics | JAMA Network Open | JAMA Network [Internet]. [https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2816828] Date accessed: 10/12/24
13. Villani A, Tabori U, and Schiffman J, et al (2011) Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: a prospective observational study Lancet Oncol 12(6) 559–567 https://doi.org/10.1016/S1470-2045(11)70119-X PMID: 21601526
14. Westdorp H, Kolders S, and Hoogerbrugge N, et al (2017) Immunotherapy holds the key to cancer treatment and prevention in constitutional mismatch repair deficiency (CMMRD) syndrome Cancer Lett 403 159–164 https://doi.org/10.1016/j.canlet.2017.06.018 PMID: 28645564
15. Kuhlen M, Wieczorek D, and Siebert R, et al (2019) How I approach hereditary cancer predisposition in a child with cancer Pediatr Blood Cancer 66(11) e27916 https://doi.org/10.1002/pbc.27916 PMID: 31342632
16. Parambil B, Prasad M, and Sarin R, et al (2023) Impact of dedicated out-patient pediatric cancer genetics services on the detection of cancer predisposition syndromes in childhood cancers abstracts Pediatr Blood Cancer 70(S8) e30748 https://doi.org/10.1002/pbc.30748
17. Vol 10, Issue 3.pdf [Internet] [https://www.inphog.org/Newsletter/Vol%2010,%20Issue%203.pdf] Date accessed: 05/01/25
18. Minard-Colin V, Aupérin A, and Pillon M, et al (2020) Rituximab for high-risk, mature B-cell non-Hodgkin’s lymphoma in children N Engl J Med 382(23) 2207–2219 https://doi.org/10.1056/NEJMoa1915315 PMID: 32492302 PMCID: 7720281
19. Agrusa JE, Egress ER, and Lowe EJ (2023) Brentuximab vedotin use in pediatric anaplastic large cell lymphoma Front Immunol 14 1203471 https://doi.org/10.3389/fimmu.2023.1203471 PMID: 37275877 PMCID: 10232850
20. Gamis AS, Alonzo TA, and Meshinchi S, et al (2014) Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531 J Clin Oncol Off J Am Soc Clin Oncol32(27) 3021–3032 https://doi.org/10.1200/JCO.2014.55.3628
21. Castellino SM, Pei Q, and Parsons SK, et al (2022) Brentuximab vedotin with chemotherapy in pediatric high-risk Hodgkin’s lymphoma N Engl J Med 387(18) 1649–1660 https://doi.org/10.1056/NEJMoa2206660 PMID: 36322844 PMCID: 9945772
22. Locatelli F, Zugmaier G, and Rizzari C, et al (2021) Effect of blinatumomab vs chemotherapy on event-free survival among children with high-risk first-relapse B-cell acute lymphoblastic leukemia: a randomized clinical trial JAMA 325(9) 843–854 https://doi.org/10.1001/jama.2021.0987 PMID: 33651091 PMCID: 7926287
23. Gupta S, Rau RE, and Kairalla JA, et al (2024) Blinatumomab in standard-risk B-cell acute lymphoblastic leukemia in children N Engl J Med [Internet] 392(9) 875–891 Date accessed: 11/12/24 https://doi.org/10.1056/NEJMoa2411680
24. Yu AL, Gilman AL, and Ozkaynak MF, et al (2010) Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma N Engl J Med 363(14) 1324 https://doi.org/10.1056/NEJMoa0911123
25. Mody R, Yu AL, and Naranjo A, et al (2020) Irinotecan, temozolomide, and dinutuximab with GM-CSF in children with refractory or relapsed neuroblastoma: a report from the children’s oncology group J Clin Oncol Off J Am Soc Clin Oncol 38(19) 2160–2169 https://doi.org/10.1200/JCO.20.00203
26. Vedi A and Ziegler DS (2014) Antibody therapy for pediatric leukemia Front Oncol 4 82 https://doi.org/10.3389/fonc.2014.00082 PMID: 24795859 PMCID: 4000992
27. Bosse KR, Majzner RG, and Mackall CL, et al (2020) Immune-based approaches for the treatment of pediatric malignancies Annu Rev Cancer Biol 4 353–370 https://doi.org/10.1146/annurev-cancerbio-030419-033436 PMID: 34113750 PMCID: 8189419
28. Awasthi R, Maier HJ, and Zhang J, et al (2023) Kymriah® (tisagenlecleucel)—an overview of the clinical development journey of the first approved CAR-T therapy Hum Vaccines Immunother 19(1) 2210046 https://doi.org/10.1080/21645515.2023.2210046
29. Maude SL, Laetsch TW, and Buechner J, et al (2024) Tisagenlecleucel in children and young adults with B-Cell lymphoblastic leukemia N Engl J Med [Internet] 378(5) 439–448 Date accessed: 11/12/24 https://doi.org/10.1056/NEJMoa1709866
30. Del Bufalo F, De Angelis B, and Caruana I, et al (2023) GD2-CART01 for relapsed or refractory high-risk neuroblastoma N Engl J Med 388(14) 1284–1295 https://doi.org/10.1056/NEJMoa2210859 PMID: 37018492
31. Casey DL and Cheung NKV (2020) Immunotherapy of pediatric solid tumors: treatments at a crossroads, with an emphasis on antibodies Cancer Immunol Res 8(2) 161 https://doi.org/10.1158/2326-6066.CIR-19-0692 PMID: 32015013 PMCID: 7058412
32. Gupta S, Howard SC, and Hunger SP, et al (2015) Treating childhood cancer in low- and middle-income countries Cancer: Disease Control Priorities Third Edition vol 3 [Internet], eds H Gelband, P Jha, R Sankaranarayanan, S Horton (Washington (DC): The International Bank for Reconstruction and Development / The World Bank) [http://www.ncbi.nlm.nih.gov/books/NBK343626/] Date accessed: 23/12/24
33. Morin S, Segafredo G, and Piccolis M, et al (2023) Expanding access to biotherapeutics in low-income and middle-income countries through public health non-exclusive voluntary intellectual property licensing: considerations, requirements, and opportunities Lancet Glob Health 11(1) e145–e154 https://doi.org/10.1016/S2214-109X(22)00460-0
34. India’s First Homegrown CAR T-Cell Therapy—NCI [Internet] 2024 [https://www.cancer.gov/news-events/cancer-currents-blog/2024/nexcar19-car-t-cell-therapy-india-nci-collaboration] Date accessed: 5/1/25
35. Duffy C, Santana V, and Inaba H, et al (2022) Evaluating blinatumomab implementation in low- and middle-income countries: a study protocol Implement Sci Commun 3(1) 62 https://doi.org/10.1186/s43058-022-00310-5 PMID: 35690878 PMCID: 9187890
36. Parsons DW, Janeway KA, and Patton DR, et al (2022) Actionable tumor alterations and treatment protocol enrollment of pediatric and young adult patients with refractory cancers in the National Cancer Institute-Children’s Oncology Group pediatric MATCH trial J Clin Oncol Off J Am Soc Clin Oncol 40(20) 2224–2234 https://doi.org/10.1200/JCO.21.02838
37. Berlanga P, Pierron G, and Lacroix L, et al (2022) The european MAPPYACTS trial: precision medicine program in pediatric and adolescent patients with recurrent malignancies Cancer Discov 12(5) 1266–1281 https://doi.org/10.1158/2159-8290.CD-21-1136 PMID: 35292802 PMCID: 9394403
38. McCabe MG, Geoerger B, and Chesler L, et al (2024) Precision medicine for childhood cancer: current limitations and future perspectives JCO Precis Oncol 8 e2300117 https://doi.org/10.1200/PO.23.00117 PMID: 38207228
39. Wong M, Mayoh C, and Lau LM, et al (2020) Whole genome, transcriptome and methylome profiling enhances actionable target discovery in high-risk pediatric cancer Nat Med [Internet] 26(11) 1742–1753 Date accessed: 11/12/24 https://doi.org/10.1038/s41591-020-1072-4
40. Laetsch TW, Ludwig K, and Williams PM, et al (2024) Phase II study of samotolisib in children and young adults with tumors harboring phosphoinositide 3-kinase/mammalian target of rapamycin pathway alterations: pediatric MATCH APEC1621D JCO Precis Oncol 8 e2400258 https://doi.org/10.1200/PO.24.00258 PMID: 39298693 PMCID: 11581706
41. Vo KT, Sabnis AJ, and Williams PM, et al (2024) Phase II study of ulixertinib in children and young adults with tumors harboring activating mitogen-activated protein kinase pathway alterations: APEC1621J of the National Cancer Institute-Children’s Oncology Group pediatric MATCH trial JCO Precis Oncol 8 e2400103 https://doi.org/10.1200/PO.24.00103
42. Macy ME, Mody R, and Reid JM, et al (2024) Palbociclib in solid tumor patients with genomic alterations in the cyclinD-cdk4/6-INK4a-Rb pathway: results from National Cancer Institute-Children’s Oncology Group pediatric molecular analysis for therapy choice trial arm I (APEC1621I) JCO Precis Oncol 8 e2400418 https://doi.org/10.1200/PO-24-00418
43. Bailey S, Davidson A, and Parkes J, et al (2022) How can genomic innovations in pediatric brain tumors transform outcomes in low- and middle-income countries? JCO Glob Oncol [Internet] 8 e2200156 [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9812475/] Date accessed: 21/12/2024 https://doi.org/10.1200/GO.22.00156
44. The Virtual Tumor Board (VTB) [Internet] https://www.ncgindia.org/key-initiatives/virtual-tumor-board] Date accessed: 7/1/25
45. DHR-ICMR Advanced Molecular Oncology Diagnostic Services (DIAMOnDS) Guidelines | Department of Health Research | MoHFW | Government of India [Internet] [https://dhr.gov.in/dhrguidelines/dhr-icmr-advanced-molecular-oncology-diagnostic-services-diamonds-guidelines] Date accessed: 7/1/25
46. Oeffinger KC, Mertens AC, and Sklar CA, et al (2006) Chronic health conditions in adult survivors of childhood cancer N Engl J Med 355(15) 1572–1582 https://doi.org/10.1056/NEJMsa060185 PMID: 17035650
47. Kremer LCM, Mulder RL, and Oeffinger KC, et al A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group Pediatr Blood Cancer 60(4) 543–549
48. Landier W, Bhatia S, and Eshelman DA, et al Development of risk-based guidelines for pediatric cancer survivors: the Children’s Oncology Group long-term follow-up guidelines from the children’s Oncology Group Late Effects Committee and Nursing Discipline J Clin Oncol Off J Am Soc Clin Oncol 22(24) 4979–4990
49. Winther JF, Kenborg L, and Byrne J, et al (2015) Childhood cancer survivor cohorts in Europe Acta Oncol Stockh Swed 54(5) 655–668 https://doi.org/10.3109/0284186X.2015.1008648
50. Robison LL, Armstrong GT, and Boice JD, et al (2009) The childhood cancer survivor study: a National Cancer Institute-supported resource for outcome and intervention research J Clin Oncol Off J Am Soc Clin Oncol 27(14) 2308–2318 https://doi.org/10.1200/JCO.2009.22.3339
51. Hudson MM, Ness KK, and Nolan VG, et al (2011) Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study Pediatr Blood Cancer 56(5) 825–836 https://doi.org/10.1002/pbc.22875 PMID: 21370418 PMCID: 3088729
52. Feijen EAM, Leisenring WM, and Stratton KL, et al (2019) Derivation of anthracycline and anthraquinone equivalence ratios to doxorubicin for late-onset cardiotoxicity JAMA Oncol 5(6) 864–871 https://doi.org/10.1001/jamaoncol.2018.6634 PMID: 30703192 PMCID: 6490232
53. Feijen EAM, Leisenring WM, and Stratton KL, et al (2015) Equivalence ratio for daunorubicin to doxorubicin in relation to late heart failure in survivors of childhood cancer J Clin Oncol Off J Am Soc Clin Oncol 33(32) 3774–3780 https://doi.org/10.1200/JCO.2015.61.5187
54. Chow EJ, Chen Y, and Hudson MM, et al (2018) Prediction of ischemic heart disease and stroke in survivors of childhood cancer J Clin Oncol Off J Am Soc Clin Oncol 36(1) 44–52 https://doi.org/10.1200/JCO.2017.74.8673
55. Chen Y, Chow EJ, and Oeffinger KC, et al (2020) Traditional cardiovascular risk factors and individual prediction of cardiovascular events in childhood cancer survivors J Natl Cancer Inst 112(3) 256–265 https://doi.org/10.1093/jnci/djz108
56. Im C, Sharafeldin N, and Yuan Y, et al (2023) Polygenic risk and chemotherapy-related subsequent malignancies in childhood cancer survivors: a Childhood Cancer Survivor Study and St Jude Lifetime Cohort Study report J Clin Oncol Off J Am Soc Clin Oncol 41(27) 4381–4393 https://doi.org/10.1200/JCO.23.00428
57. Gramatges MM and Bhatia S (2018) Evidence for genetic risk contributing to long-term adverse treatment effects in childhood cancer survivors Annu Rev Med 69 247–262 https://doi.org/10.1146/annurev-med-041916-124328
58. Cappell KM and Kochenderfer JN (2023) Long-term outcomes following CAR T cell therapy: what we know so far Nat Rev Clin Oncol 20(6) 359–371 https://doi.org/10.1038/s41571-023-00754-1 PMID: 37055515 PMCID: 10100620
59. Fried LP, Tangen CM, and Walston J, et al (2001) Frailty in older adults: evidence for a phenotype J Gerontol A Biol Sci Med Sci 56(3) M146-M156 https://doi.org/10.1093/gerona/56.3.M146
60. Dixon SB, Chow EJ, and Hjorth L, et al (2020) The future of childhood cancer survivorship: challenges and opportunities for continued progress Pediatr Clin North Am 67(6) 1237–1251 https://doi.org/10.1016/j.pcl.2020.07.013 PMID: 33131544 PMCID: 7773506
61. Cai J, Cheung YT, and Hudson MM (2024) Care models and barriers to long-term follow-up care among childhood cancer survivors and health care providers in Asia: a literature review JCO Glob Oncol 10 e2300331 https://doi.org/10.1200/GO.23.00331 PMCID: 10939639
62. Signorelli C, Wakefield CE, and Fardell JE, et al (2017) The impact of long-term follow-up care for childhood cancer survivors: a systematic review Crit Rev Oncol Hematol 114 131–138 https://doi.org/10.1016/j.critrevonc.2017.04.007 PMID: 28477741
63. Prasad M, Goswami S, and Chinnaswamy G, et al (2022) Long-term outcomes in survivors of childhood cancer: a 30-year experience from India JCO Glob Oncol 8 e2200044 https://doi.org/10.1200/GO.22.00044 PMID: 36332172 PMCID: 9668554
64. Prasad M, Goswami S, and Kurkure PA (2024) The care of childhood cancer survivors in India: challenges and solutions Indian J Med Paediatr Oncol 45(2) 167–172 https://doi.org/10.1055/s-0043-1761262
65. Arora RS, Arora PR, and Seth R, et al (2020) Childhood cancer survivorship and late effects: the landscape in India in 2020 Pediatr Blood Cancer 67(9) e28556 https://doi.org/10.1002/pbc.28556 PMID: 32649000
66. Hodgson DC (2011) Late effects in the era of modern therapy for Hodgkin lymphoma Hematol Am Soc Hematol Educ Program 2011 323–329 PMID: 22160053
67. Friedman DN, Goodman PJ, and Leisenring WM, et al (2024) Impact of risk-based therapy on late morbidity and mortality in neuroblastoma survivors: a report from the Childhood Cancer Survivor Study J Natl Cancer Inst 116(6) 885–894 https://doi.org/10.1093/jnci/djae062 PMID: 38460547 PMCID: 11160496
68. Essig S, Li Q, and Chen Y, et al (2014) Risk of late effects of treatment in children newly diagnosed with standard-risk acute lymphoblastic leukaemia: a report from the Childhood Cancer Survivor Study cohort Lancet Oncol 15(8) 841–851 https://doi.org/10.1016/S1470-2045(14)70265-7 PMID: 24954778 PMCID: 4142216

