Management of BRCA-associated breast cancer patients in low and middle-income countries: a review
Fen Saj1, Shona Nag2, Nita Nair3 and Bhawna Sirohi1
1Department of Medical Oncology, Balco Medical Centre-Vedanta Medical Research Foundation, Raipur 493661, India
2Department of Medical Oncology, Sahyadri Hospital, Pune 411004, India
3Department of Surgical Oncology, Apollo Hospitals, Mumbai 400614, India
Abstract
Breast cancer poses a significant global health challenge, with higher incidence rates in developed countries. However, low- and middle-income countries (LMICs) suffer from higher mortality rates due to various factors, including limited screening programs, delayed diagnosis and inadequate access to healthcare and advanced treatments. Approximately 5%–10% of breast cancer cases stem from germline mutations in BRCA-1/2 genes. A positive BRCA1/2 status obtained through genetic testing significantly influences surgical and medical treatment decisions. Therefore, genetic counseling, proper surveillance and customized interventions for BRCA1/2 carriers are essential to maximizing the benefits of monitoring, chemoprevention and risk-reducing surgeries for breast and ovarian cancers. Identification of BRCA mutations also impacts treatment strategies, leading to the integration of chemotherapeutic agents like platinum-based chemotherapy and PARP inhibitors. However, implementing these advanced treatment guidelines in LMICs with complex, fragmented and underfunded healthcare systems presents numerous challenges. In this review, we explore the current status and obstacles associated with managing BRCA1/2-associated breast cancer in LMICs.
Keywords: breast cancer, BRCA-associated, LMICs
Correspondence to: Bhawna Sirohi
Email: bhawna.sirohi13@gmail.com
Published: 22/08/2024
Received: 19/06/2024
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Breast cancer poses a significant health challenge, currently ranking as the most commonly diagnosed cancer and the primary cause of cancer-related deaths among women [1]. There are substantial global variations in breast cancer incidence, largely influenced by differences in hormonal, nutritional and reproductive factors, along with socioeconomic statuses across countries [2]. The most significant rise in breast cancer incidence is observed in low- and middle-income countries (LMICs) across South America, Africa and Asia, regions traditionally known for lower incidence rates compared to industrialized nations [3–5]. This trend is frequently linked to the ‘Westernization’ of these nations, characterised by the widespread adoption of Western dietary patterns and sedentary lifestyles [6, 7]. In LMICs, hormonal risk factors such as early onset of menstruation, delayed childbirth and reduced breastfeeding are increasingly prevalent [6]. Moreover, lifestyle factors such as smoking, alcohol use and obesity are becoming more prevalent in developing nations [8]. Significantly, breast cancer tends to impact younger women in LMICs more than it does in high-income countries [9].
Women living in LMICs experience a 17% higher mortality rate compared to those in developed nations [3]. Additionally, 70% of breast cancer deaths take place in the developing world [10]. The 5-year survival rates for breast cancer in LMICs are substantially lower, ranging from 12% to 52%, compared to rates above 80% in developed nations [11, 12]. In some regions of Middle, Eastern and West Africa, the mortality-to-incidence ratio can be as high as 0.55, whereas in North America, this ratio is 0.16 [13]. The lower survival rates in LMICs are attributed to several factors, such as the absence of screening programs, delayed diagnosis, restricted access to healthcare and inadequate application of contemporary treatment methods [14].
A notable 5%–10% of newly diagnosed breast cancer cases are due to hereditary factors, mainly resulting from germline autosomal dominant mutations in the Breast Cancer Susceptibility Genes, BRCA1 and BRCA2 [15]. These genes are crucial as dynamic regulators of genomic integrity, coordinating DNA repair processes and transcriptional regulation in response to DNA damage [16]. The cumulative risk of developing breast cancer by age 70 is approximately 55%–70% for individuals carrying BRCA1 mutations and 45%–70% for those carrying BRCA2 mutations [17]. However, the exact prevalence of hereditary breast cancers remains unclear in LMICs due to limited cancer registry data [18]. Although studies from India suggest a higher occurrence of germline BRCA1/2 mutations among breast cancer cases, it is uncertain whether this finding can be generalised to other LMICs [19, 20]. This review aims to explore the current status and challenges associated with managing BRCA1/2-associated hereditary breast cancer in LMICs.
Challenges in identifying and testing for BRCA1/2 mutations in populations at risk in LMICs
Early identification of individuals at risk for BRCA1/2 mutations is crucial for effective management and prevention of hereditary breast cancer. However, LMICs face numerous challenges in genetic counseling and treatment-focused genetic testing.
Many LMICs have a shortage of professional genetic counselors, national guidelines for genetic screening and insurance coverage for genetic counseling services [21]. Both patients and healthcare providers often lack awareness of the importance of genetic counseling. Despite specific guidelines for referring patients with suspected hereditary breast cancer, many eligible women are not referred for genetic counseling and screening, even in developed countries [22]. The utilisation of genetic counseling services in LMICs is notably limited, primarily due to intricate ethical, sociocultural and religious factors [23]. A study conducted in India highlighted a widespread lack of awareness regarding hereditary breast cancer and genetic counseling among breast cancer patients [24].
Access to cancer genetics testing services is constrained in LMICs mainly because of financial obstacles. Public healthcare systems in many LMICs are underfunded and disjointed, leaving patients responsible for the expenses associated with genetic counseling and testing. Insurance and public health funding agencies in LMICs typically do not cover the costs of genetic testing, and the health insurance coverage for the population is often insufficient [25]. Testing for germline BRCA mutation costs $200–400 in India, while the per-capita expenditure on health was nearly $60 [26, 27]. Besides privately run laboratories and hospitals driven by market forces and lacking standardised operating procedures, geographic and logistical challenges contribute to the subpar quality of genetic testing services in LMICs [23, 28]. Poor genetic literacy among healthcare personnel and the community exacerbates these challenges, leading to the underutilisation of genetic services [29, 30]. In LMICs, where social stigma and cultural conflicts are prevalent, a considerable number of women are uneducated and financially reliant on their families. A diagnosis of hereditary cancer can impose significant psychological burdens and negatively impact their quality of life [31, 32].
There is a lack of universally accepted guidelines for genetic counseling, testing and managing BRCA mutation carriers in LMICs. Genetic testing has become increasingly intricate, with commercial laboratories providing diverse testing options in various packages. These complexities present significant challenges for healthcare professionals in integrating genetic testing into clinical protocols [33].
Genetic counseling is recommended for individuals with BRCA1/2 mutations because of the well-documented advantages of surveillance, chemoprevention and risk-reducing surgeries in managing breast and ovarian cancer [34]. A positive BRCA1/2 genetic test outcome significantly influences the decisions regarding surgical and medical interventions [35]. The primary surgical decision involves choosing between breast-conserving surgery and bilateral risk-reducing mastectomy (RRM). Furthermore, BRCA carriers should consider risk-reducing bilateral salpingo-oophorectomy (RRBSO) to decrease ovarian cancer risk significantly [35, 36].
Challenges in screening BRCA-associated breast cancer in LMICs
Early detection and timely access to effective cancer therapy are critical measures in reducing breast cancer-related mortality. The prognosis of breast cancer improves when it is detected early, while the disease remains localised and has not spread to other parts of the body [37]. For women aged 40 years and older with an average risk of developing breast cancer, screening mammography continues to be the recommended approach [38]. Studies have demonstrated that it can decrease breast cancer mortality by 20%–35% among women aged 50–69 years and slightly less among those aged 40–49 years [39].
BRCA-related breast cancers are distinguished by their early onset. Research indicates that the median age of breast cancer onset is 42 years for BRCA1 carriers and 48 years for BRCA2 carriers [40]. Due to the higher breast tissue density typically observed in younger women, mammography may be less effective as a screening strategy for detecting BRCA1/2-associated cancers, which often occur at younger ages [41]. For BRCA1/2 carriers, magnetic resonance imaging (MRI) of the breasts emerges as a more sensitive albeit less specific screening tool than mammography [42]. In light of this, the American Cancer Society recommends a combination of annual mammography and breast MRI for breast cancer surveillance in BRCA mutation carriers [43]. This combined approach not only improves the sensitivity and specificity of breast cancer detection but also facilitates earlier detection of breast cancer in BRCA1/2 mutation carriers [44]. Before surgical planning, it is crucial to assess both the ipsilateral and unaffected contralateral breasts through imaging, as BRCA1/2 patients are at higher risk of multifocal or multicentric breast cancer [45].
In LMICs, the implementation of mammography screening is hindered by significant financial and technical challenges. It requires high-quality machines, well-trained radiologists and skilled technicians [46]. Studies demonstrate a marked disparity in the availability of mammography equipment across different income brackets: less than 16% in low-income countries, 23% in LMICs, 48% in upper-middle-income countries and 78% in high-income countries [47]. Moreover, the scarcity of MRI machines compounds the issue, exacerbated by a severe shortage of medical physicists, radiographers and radiologists. Low-income countries, for instance, have only 1.9 radiologists per million people, whereas high-income countries have 97.9 radiologists per million [48]. In facilities lacking mammography and MRI equipment, clinicians may resort to clinical breast examinations or ultrasound scans despite their suboptimal effectiveness as screening modalities [49].
Beyond the availability of screening facilities, ensuring high participation rates among eligible women is equally crucial. The World Health Organisation recommends a minimum 70% participation rate in screening programs to reduce mortality [50]. However, low compliance with screening tests poses a significant hurdle in LMICs. For instance, a screening trial involving clinical breast examination in the Philippines was terminated due to women's reluctance to participate in follow-up despite extensive counseling, transportation and home visitation efforts [51]. In another screening study in Egypt, over half of the women recalled for further evaluation were lost to follow-up [52]. Reasons for non-compliance in LMICs range from avoidance and denial to fatalism and financial constraints [53]. While early diagnosis through screening offers a meaningful survival benefit, the economic burden of biopsies and follow-up examinations could strain already fragmented healthcare systems in many LMICs [54].
Additionally, a lack of awareness among healthcare providers regarding which patients should undergo evaluation poses another barrier to effectively managing individuals with hereditary cancers. For example, BRCA-related cancers often present at a younger age and may be misdiagnosed by primary care physicians, who may not recommend a triple test, including biopsy [55]. Socioeconomic factors also contribute to delayed breast cancer diagnoses in LMICs, where women may lack awareness of cancer symptoms or encounter obstacles accessing timely investigations due to financial constraints, geographical remoteness from healthcare facilities and extended wait times in public hospitals [56]. Cultural and religious beliefs further complicate breast cancer control and prevention efforts, with fatalistic attitudes prevalent among women in LMICs, perceiving breast cancer as predestined or divine retribution [57]. In communities like Bangladesh, breast cancer may be viewed as a curse or punishment for sins [58], while in Pakistan, diagnosis may lead to social ostracism, psychological stress and family discord [59]. Stigmatisation also deters women from seeking medical help promptly, particularly for BRCA1/2-associated breast cancers affecting younger, financially dependent women in LMICs, exacerbating delays in diagnosis and impeding treatment outcomes [60].
Challenges in diagnosis and treatment of BRCA-associated cancers in LMICs
In many LMICs, the absence of comprehensive population-based cancer registries hinders critical information regarding anatomic stage, receptor status, prognostic markers and genetic determinants [61]. Obtaining a prompt and thorough histopathological review poses a significant challenge in LMICs due to limited access to high-quality tissue processing facilities, prognostic marker evaluation and adequately trained pathology personnel [62]. At the time of breast cancer diagnosis, conducting a core-needle biopsy is recommended as it provides sufficient tissue for assessing invasive versus in-situ status and performing immunohistochemistry (IHC) for estrogen and progesterone hormone receptors (ER/PR) and human epidermal growth factor 2 (HER2/neu) testing [63]. It is noteworthy that triple-negative breast cancer, characterised by negative ER/PR and HER2/neu status, accounts for a significant proportion of BRCA1-positive and BRCA2-positive patients [64]. BRCA1-positive patients typically exhibit a high nuclear grade under microscopy, underscoring the importance of accurately determining the ER/PR/HER2 status and nuclear grade through access to high-quality histology and IHC facilities [65].
Ensuring adequate quality control in handling biopsy or excision specimens remains a considerable challenge in LMICs, where the pathology team often struggles with limited control over cold ischemia time, ideally less than 60 minutes, to prevent degradation of critical biomarker proteins and false-negative results in IHC [66, 67]. Additionally, longer turnaround times (TATs) for pathology results, ranging from weeks to months, as opposed to the recommended two-business-day TAT for biopsy specimens by the College of American Pathologists, have been reported in LMICs, potentially leading to inappropriate therapy choices or disease progression resulting in stage migration [68–70]. Timelier pathology reports are essential for intra-operative frozen section samples to identify patients who may benefit from more radical surgery, emphasizing the importance of an adequate pathology workforce in LMICs where availability varies widely across regions [71, 72].
With limited resources for early detection, most breast cancer patients in LMICs present with advanced-stage disease (stages III and IV), a considerably higher proportion compared to developed countries [8, 73, 74]. BRCA-associated cancers, known for their aggressive biology and significant tumour burden, often require a standard treatment protocol of neoadjuvant chemotherapy followed by surgery and adjuvant radiotherapy or targeted therapy tailored to individual tumour characteristics [75, 76]. Despite the potential benefits of breast-conserving surgeries, their utilisation remains low in LMICs due to advanced disease presentation and deficiencies in surgical expertise and radiotherapy facilities [77, 78].
Chemotherapy regimens for managing BRCA-mutated cancers are advancing quickly, with platinum agents and adjuvant capecitabine demonstrating potential for improving survival rates [79–81]. However, these options pose challenges in LMICs due to nutritional deficiencies and higher toxicity risks [82]. Therefore, in LMICs, careful patient selection is necessary for these newer treatment approaches. Additionally, advanced therapies such as PARP inhibitors and immunotherapy remain largely inaccessible due to their high costs, further widening the treatment gap in these regions [82, 83].
Addressing fertility and pregnancy-related concerns is especially important for BRCA-associated breast cancer patients, as many are within the reproductive age group. Research conducted among healthcare professionals and patients in LMICs has shown a lack of understanding, practices and attitudes regarding fertility preservation and post-treatment pregnancy in young women diagnosed with breast cancer [84, 85]. Research conducted among young women diagnosed with breast cancer in India revealed that only 8% of patients underwent fertility preservation, while 13% experienced significant postmenopausal symptoms following treatment [86]. Unfortunately, these vital concerns are frequently neglected in LMICs.
Challenges in risk-reducing strategies of BRCA-associated cancers in LMICs
Recommendations for lowering breast cancer risk in BRCA1/2 mutation carriers include regular surveillance, risk-reducing surgeries and chemoprevention [87]. These strategies should be personalised based on patients' preferences, quality of life concerns and life expectancy. While prophylactic RRM markedly decreases cancer risk, it does not eliminate the possibility of breast cancer. Multiple studies, both retrospective and prospective, suggest that bilateral mastectomy reduces the risk of breast cancer by approximately 90% in BRCA1/2 carriers [88]. RRBSO is an effective strategy for reducing the risk of ovarian and fallopian tube cancer in BRCA1/2 carriers [89]. However, its impact on reducing breast cancer risk in BRCA1 carriers remains a topic of debate [90, 91]. Furthermore, the uptake of RRM and RRBSO remains low in LMICs due to the absence of reconstructive surgery services in the public sector [92]. A study conducted in Pakistan found that many BRCA carriers declined RRM due to financial constraints, misunderstandings about their health status, familial or spousal pressures, worries about body image and societal perceptions and concerns about potential complications and their impact on quality of life [93].
Hormonal therapy for risk reduction, such as tamoxifen, is generally considered less effective compared to surgical interventions. Tamoxifen has shown effectiveness in BRCA2 carriers, who often have estrogen receptor-positive tumours, unlike BRCA1 tumours, which typically present as triple-negative [94]. Moreover, most studies examining preventive strategies originate from developed countries, with limited research available from LMICs. This scarcity of evidence undermines the confidence of healthcare providers in LMICs when it comes to making referrals for genetic counseling, testing and risk-reduction procedures [33].
Proposed strategies for managing BRCA-associated breast cancer in LMICs
The distinct challenges faced by LMICs demand a customized approach. Table 1 delineates these obstacles and proposes potential solutions. Initiatives such as implementing universal health coverage, developing national cancer care plans to guarantee access to essential diagnostic and treatment resources and establishing comprehensive population-based cancer registries are critical for strengthening healthcare systems in LMICs. These registries will provide valuable data on disease burden and facilitate targeted interventions tailored to specific populations, including individuals with BRCA1/2 germline mutations.
Tailored breast cancer awareness initiatives that are culturally sensitive and focus on identifying risk factors and promoting early cancer detection are essential, with active involvement from advocacy groups. Effective implementation of breast cancer programs relies on a comprehensive assessment of local contexts, including disease prevalence, existing infrastructure, resource availability and sociocultural factors influencing women's participation [63]. Disseminating breast cancer awareness campaigns using culturally appropriate materials in local languages can help mitigate social stigma and cultural barriers related to breast cancer diagnosis and treatment. Embracing technology-driven solutions such as telemedicine, telepathology and affordable point-of-care molecular diagnostics can improve patient care in LMICs with complex and fragmented healthcare systems [95]. Mobile imaging technologies and screening camps offer cost-effective and accessible avenues for early cancer detection with quicker TAT [37]. Strengthening screening services involves ensuring adequate imaging equipment, trained personnel and pathology services. In regions with limited high-cost screening modalities, biennial clinical breast examinations conducted by trained primary health workers can be a feasible screening strategy [96].
Table 1. Critical challenges and suggested solutions.
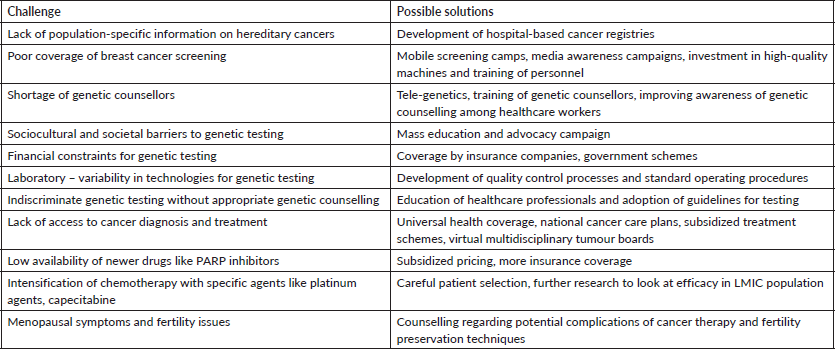
Promoting access to essential cancer treatments such as surgery, radiotherapy and chemotherapy at subsidized rates in public hospitals is highly recommended. Virtual multidisciplinary tumour boards can significantly improve treatment decisions and patient outcomes, especially in regions without specialized cancer centers [97]. Educating patients about clinical trial opportunities and addressing barriers to participation can promote clinical research and expand access to innovative treatment options.
International collaboration and cross-border medical research utilising open-access, de-identified genomic databases offer promise in advancing care for genetically predisposed breast cancer patients in LMICs [98]. Raising awareness about genetic counseling among healthcare providers and expanding the number of genetic counselors trained in risk reduction strategies are essential steps. Quality control measures and standard operating procedures in laboratories are crucial for ensuring the ethical and practical use of genetic testing with reliable reporting. LMICs must develop comprehensive guidelines for genetic testing, risk reduction strategies and follow-up protocols tailored to the local context. Advocacy efforts are vital to achieving equitable access to genetic testing and care, mobilizing support from caregivers, patients and families to address barriers posed by government facilities and insurance providers, including cost concerns, limited awareness and competing priorities.
In conclusion, addressing the multifaceted challenges of managing BRCA-associated breast cancer in LMICs requires a multifaceted approach encompassing tailored awareness initiatives, strengthened healthcare infrastructure, access to affordable treatments and robust international collaboration. By implementing these strategies, LMICs can significantly improve breast cancer outcomes and ensure equitable care for all patients, including those with BRCA1/2 germline mutations.
Acknowledgments
None.
Conflicts of interest/disclosure
There are no relevant conflicts of interest to declare.
Funding
No external sources of funding.
References
1. Giaquinto AN, Sung H, and Miller KD, et al (2022) Breast cancer statistics, 2022 CA Cancer J Clin 72(6) 524–541 https://doi.org/10.3322/caac.21754 PMID: 36190501
2. Parkin DM, Whelan SL, and Ferlay J, et al (1997) Cancer Incidence in Five Continents Vol. VII. IARC Scientific Publications No. 143 (Lyon: Wiley Online Library)
3. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
4. Bray F, McCarron P, and Parkin DM (2004) The changing global patterns of female breast cancer incidence and mortality Breast Cancer Res BCR 6(6) 229–239 https://doi.org/10.1186/bcr932 PMID: 15535852 PMCID: 1064079
5. Joko-Fru WY, Jedy-Agba E, and Korir A, et al (2020) The evolving epidemic of breast cancer in sub-Saharan Africa: results from the African cancer registry network Int J Cancer 147(8) 2131–2141 https://doi.org/10.1002/ijc.33014 PMID: 32306390
6. Porter P (2008) “Westernizing” women’s risks? Breast cancer in lower-income countries N Engl J Med 358(3) 213–216 https://doi.org/10.1056/NEJMp0708307 PMID: 18199859
7. Yoo KY, Kim Y, and Park SK, et al (2006) Lifestyle, genetic susceptibility and future trends of breast cancer in Korea Asian Pac J Cancer Prev APJCP 7(4) 679–682
8. Anyanwu SN (2008) Temporal trends in breast cancer presentation in the third world J Exp Clin Cancer Res CR 27(1) 17 https://doi.org/10.1186/1756-9966-27-17 PMID: 18620559 PMCID: 2486264
9. Gutnik LA, Matanje-Mwagomba B, and Msosa V, et al (2015) Breast cancer screening in low- and middle-income countries: a perspective from Malawi J Glob Oncol 2(1) 4–8 https://doi.org/10.1200/JGO.2015.000430 PMID: 28717676 PMCID: 5497737
10. Rivera-Franco MM and Leon-Rodriguez E (2018) Delays in breast cancer detection and treatment in developing countries Breast Cancer Basic Clin Res 12 1178223417752677 https://doi.org/10.1177/1178223417752677
11. Coleman MP, Quaresma M, and Berrino F, et al (2008) Cancer survival in five continents: a worldwide population-based study (CONCORD) Lancet Oncol 9(8) 730–756 https://doi.org/10.1016/S1470-2045(08)70179-7 PMID: 18639491
12. Jedy-Agba E, McCormack V, and Adebamowo C, et al (2016) Stage at diagnosis of breast cancer in sub-Saharan Africa: a systematic review and meta-analysis Lancet Glob Health 4(12) e923–e935 https://doi.org/10.1016/S2214-109X(16)30259-5 PMID: 27855871 PMCID: 5708541
13. Martei YM, Pace LE, and Brock JE, et al (2018) Breast cancer in low- and middle-income countries Clin Lab Med 38(1) 161–173 https://doi.org/10.1016/j.cll.2017.10.013 PMID: 29412880 PMCID: 6277976
14. Sankaranarayanan R, Swaminathan R, and Brenner H, et al (2010) Cancer survival in Africa, Asia, and Central America: a population-based study Lancet Oncol 11(2) 165–173 https://doi.org/10.1016/S1470-2045(09)70335-3
15. Hill AD, Doyle JM, and McDermott EW, et al (1997) Hereditary breast cancer Br J Surg 84(10) 1334–1339 PMID: 9361585
16. Paul A and Paul S (2014) The breast cancer susceptibility genes (BRCA) in breast and ovarian cancers Front Biosci Landmark Ed 19(4) 605–618 https://doi.org/10.2741/4230 PMID: 24389207 PMCID: 4307936
17. Kuchenbaecker KB, Hopper JL, and Barnes DR, et al (2017) Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers JAMA 317(23) 2402–2416 https://doi.org/10.1001/jama.2017.7112 PMID: 28632866
18. Pramesh CS, Badwe RA, and Bhoo-Pathy N, et al (2022) Priorities for cancer research in low- and middle-income countries: a global perspective Nat Med 28(4) 649–657 https://doi.org/10.1038/s41591-022-01738-x PMID: 35440716 PMCID: 9108683
19. Mittal A, Deo SVS, and Gogia A, et al (2022) Profile of pathogenic mutations and evaluation of germline genetic testing criteria in consecutive breast cancer patients treated at a North Indian Tertiary Care Center Ann Surg Oncol 29(2) 1423–1432 https://doi.org/10.1245/s10434-021-10870-w
20. Kulkarni SS, Nag S, and Patra A, et al (2023) Prevalence of germline mutations in women with breast and/or ovarian cancer in a tertiary care center in Pune, India Int J Mol Immuno Oncol 8(2) 65–71 https://doi.org/10.25259/IJMIO_5_2023
21. Moukadem HA, Al Masry A, and Atwani RW, et al (2021) Genetic counseling, screening and risk-reducing surgery in patients with primary breast cancer and germline BRCA mutations: unmet needs in low- and middle-income countries Eur J Breast Health 18(1) 16–20 https://doi.org/10.4274/ejbh.galenos.2021.2021-5-1
22. Wood ME, Kadlubek P, and Pham TH, et al (2014) Quality of cancer family history and referral for genetic counseling and testing among oncology practices: a pilot test of quality measures as part of the American society of clinical oncology quality oncology practice initiative J Clin Oncol 32(8) 824–829 https://doi.org/10.1200/JCO.2013.51.4661 PMID: 24493722 PMCID: 4876350
23. Zhong A, Darren B, and Loiseau B, et al (2021) Ethical, social, and cultural issues related to clinical genetic testing and counseling in low- and middle-income countries: a systematic review Genet Med 23(12) 2270–2280 https://doi.org/10.1038/s41436-018-0090-9
24. Pramanik R, Vats S, and Mitra S, et al (2024) Assessment of knowledge and attitude of breast and ovarian cancer patients regarding hereditary breast-ovarian cancer syndrome at a Tertiary Cancer Institute: a cross-sectional observational study Indian J Med Paediatr Oncol 45(01) 028–034 https://doi.org/10.1055/s-0043-1768178
25. Hooley B, Afriyie DO, and Fink G, et al (2022) Health insurance coverage in low-income and middle-income countries: progress made to date and related changes in private and public health expenditure BMJ Glob Health 7(5) https://doi.org/10.1136/bmjgh-2022-008722 PMID: 35537761 PMCID: 9092126
26. World Bank Open Data (2023) World Bank Open Data [https://data.worldbank.org]
27. Pillai N (2023) BRCA Testing Cost in India – BRCA1 & BRCA2 Test Costs & Best Hospitals (Mumbai: Impact Guru) [https://www.impactguru.com/info/brca-testing-cost-in-india/] Date accessed: 17/12/23
28. Nippert I (2013) “CAPABILITY” and “genetic testing in emerging economies” (GenTEE) J Community Genet 4(3) 293–296 https://doi.org/10.1007/s12687-013-0158-9 PMID: 23934260 PMCID: 3739846
29. Murff HJ, Byrne D, and Syngal S (2004) Cancer risk assessment: quality and impact of the family history interview Am J Prev Med 27(3) 239–245 PMID: 15450637
30. Mohanty D and Das K (2011) Genetic counselling in tribals in India Indian J Med Res 134(4) 561–571 PMID: 22089621 PMCID: 3237257
31. Hafeez Bhatti AB (2015) Discussing genetic testing with patients with breast cancer in developing countries: should we be judicious? J Clin Oncol 33(35) 4232–4233 https://doi.org/10.1200/JCO.2015.63.3974 PMID: 26371136
32. Wevers MR, Hahn DEE, and Verhoef S, et al (2012) Breast cancer genetic counseling after diagnosis but before treatment: a pilot study on treatment consequences and psychological impact Patient Educ Couns 89(1) 89–95 https://doi.org/10.1016/j.pec.2012.03.019 PMID: 22543000
33. Yip CH, Evans DG, and Agarwal G, et al (2019) Global disparities in breast cancer genetics testing, counselling and management World J Surg 43(5) 1264–1270 https://doi.org/10.1007/s00268-018-04897-6 PMID: 30610270
34. Pal T, Radford C, and Vadaparampil S, et al (2013) Practical considerations in the delivery of genetic counseling and testing services for inherited cancer predisposition Community Oncol 10(5) 147–153 https://doi.org/10.12788/j.cmonc.0010
35. Trainer AH, Lewis CR, and Tucker K, et al (2010) The role of BRCA mutation testing in determining breast cancer therapy Nat Rev Clin Oncol 7(12) 708–717 https://doi.org/10.1038/nrclinonc.2010.175 PMID: 21060331
36. Metcalfe K, Gershman S, and Ghadirian P, et al (2014) Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis BMJ 348 g226 https://doi.org/10.1136/bmj.g226 PMID: 24519767 PMCID: 3921438
37. Manson EN and Achel DG (2023) Fighting breast cancer in low-and-middle-income countries – what must we do to get every woman screened on regular basis? Sci Afr 21 e01848
38. Elmore JG, Armstrong K, and Lehman CD, et al (2005) Screening for breast cancer JAMA 293(10) 1245–1256 https://doi.org/10.1001/jama.293.10.1245 PMID: 15755947 PMCID: 3149836
39. Grimm LJ, Avery CS, and Hendrick E, et al (2022) Benefits and risks of mammography screening in women ages 40 to 49 years J Prim Care Community Health 13 21501327211058322 https://doi.org/10.1177/21501327211058322
40. Litton JK, Ready K, and Chen H, et al (2012) Earlier age of onset of BRCA mutation-related cancers in subsequent generations Cancer 118(2) 321–325 https://doi.org/10.1002/cncr.26284
41. Foxcroft LM, Evans EB, and Porter AJ (2004) The diagnosis of breast cancer in women younger than 40 Breast Edinb Scotl 13(4) 297–306 https://doi.org/10.1016/j.breast.2004.02.012
42. Le-Petross HT, Whitman GJ, and Atchley DP, et al (2011) Effectiveness of alternating mammography and magnetic resonance imaging for screening women with deleterious BRCA mutations at high risk of breast cancer Cancer 117(17) 3900–3907 https://doi.org/10.1002/cncr.25971 PMID: 21365619
43. Saslow D, Boetes C, and Burke W, et al (2007) American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography CA Cancer J Clin 57(2) 75–89 https://doi.org/10.3322/canjclin.57.2.75 PMID: 17392385
44. Warner E, Hill K, and Causer P, et al (2011) Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging J Clin Oncol Off J Am Soc Clin Oncol 29(13) 1664–1669 https://doi.org/10.1200/JCO.2009.27.0835
45. McCrorie AD, Ashfield S, and Begley A, et al (2020) Multifocal breast cancers are more prevalent in BRCA2 versus BRCA1 mutation carriers J Pathol Clin Res 6(2) 146–153 https://doi.org/10.1002/cjp2.155 PMID: 32022473 PMCID: 7164372
46. Black E and Richmond R (2019) Improving early detection of breast cancer in sub-Saharan Africa: why mammography may not be the way forward Glob Health 15(1) 3 https://doi.org/10.1186/s12992-018-0446-6
47. Francies FZ, Hull R, and Khanyile R, et al (2020) Breast cancer in low-middle income countries: abnormality in splicing and lack of targeted treatment options Am J Cancer Res 10(5) 1568–1591 PMID: 32509398 PMCID: 7269781
48. Hilabi BS, Alghamdi SA, and Almanaa M (2023) Impact of magnetic resonance imaging on healthcare in low- and middle-income countries Cureus 15(4) e37698 PMID: 37081900 PMCID: 10112545
49. Frija G, Blažić I, and Frush DP, et al (2021) How to improve access to medical imaging in low- and middle-income countries ? EClinicalMedicine 38 101034 https://doi.org/10.1016/j.eclinm.2021.101034
50. Cancer Control: Knowledge into Action: WHO Guide for Effective Programmes: Module 3: Early Detection (2007) (Geneva: World Health Organization) [http://www.ncbi.nlm.nih.gov/books/NBK195408/] Date accessed: 13/12/23
51. Pisani P, Parkin DM, and Ngelangel C, et al (2006) Outcome of screening by clinical examination of the breast in a trial in the Philippines Int J Cancer 118(1) 149–154 https://doi.org/10.1002/ijc.21343
52. Salem DS, Kamal RM, and Helal MH, et al (2008) Women health outreach program; a new experience for all Egyptian women J Egypt Natl Cancer Inst 20(4) 313–322
53. Weinmann S, Taplin SH, and Gilbert J, et al (2005) Characteristics of women refusing follow-up for tests or symptoms suggestive of breast cancer J Natl Cancer Inst Monogr 35 33–38 https://doi.org/10.1093/jncimonographs/lgi035
54. Harford JB (2011) Breast-cancer early detection in low-income and middle-income countries: do what you can versus one size fits all Lancet Oncol 12(3) 306–312 https://doi.org/10.1016/S1470-2045(10)70273-4 PMID: 21376292
55. Heena H, Durrani S, and Riaz M, et al (2019) Knowledge, attitudes, and practices related to breast cancer screening among female health care professionals: a cross sectional study BMC Womens Health 19 122 https://doi.org/10.1186/s12905-019-0819-x PMID: 31640681 PMCID: 6806575
56. Yip CH and Taib NA (2012) Challenges in the management of breast cancer in low- and middle-income countries Future Oncol Lond Engl 8(12) 1575–1583 https://doi.org/10.2217/fon.12.141
57. George TO, Allo TA, and Amoo EO, et al (2019) Knowledge and attitudes about breast cancer among women: a wake-up call in Nigeria Open Access Maced J Med Sci 7(10) 1700–1705 https://doi.org/10.3889/oamjms.2019.221 PMID: 31210826 PMCID: 6560304
58. Hossain MS, Ferdous S, and Karim-Kos HE (2014) Breast cancer in South Asia: a Bangladeshi perspective Cancer Epidemiol 38(5) 465–470 https://doi.org/10.1016/j.canep.2014.08.004 PMID: 25182670
59. Banning M, Hafeez H, and Faisal S, et al (2009) The impact of culture and sociological and psychological issues on Muslim patients with breast cancer in Pakistan Cancer Nurs 32(4) 317–324 https://doi.org/10.1097/NCC.0b013e31819b240f PMID: 19444089
60. Nnaji CA, Ezenwankwo EF, and Kuodi P, et al (2022) Timeliness of diagnosis of breast and cervical cancers and associated factors in low-income and middle-income countries: a scoping review BMJ Open 12(2) e057685 https://doi.org/10.1136/bmjopen-2021-057685 PMID: 35121607 PMCID: 8819798
61. Bray F, Znaor A, and Cueva P, et al (2014) Planning and Developing Population-Based Cancer Registration in Low- or Middle-Income Settings (Lyon: International Agency for Research on Cancer) [http://www.ncbi.nlm.nih.gov/books/NBK566957/]
62. Sayed S, Lukande R, and Fleming KA (2015) Providing pathology support in low-income countries J Glob Oncol 1(1) 3–6 https://doi.org/10.1200/JGO.2015.000943 PMID: 28804765 PMCID: 5551652
63. Apantaku LM (2000) Breast cancer diagnosis and screening Am Fam Physician 62(3) 596–602, 605–606 PMID: 10950215
64. Atchley DP, Albarracin CT, and Lopez A, et al (2008) Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer J Clin Oncol 26(26) 4282–4288 https://doi.org/10.1200/JCO.2008.16.6231 PMID: 18779615 PMCID: 6366335
65. Chen H, Wu J, and Zhang Z, et al (2018) Association between BRCA status and triple-negative breast cancer: a meta-analysis Front Pharmacol 9 909 https://doi.org/10.3389/fphar.2018.00909 PMID: 30186165 PMCID: 6111442
66. Pekmezci M, Szpaderska A, and Osipo C, et al (2012) The effect of cold ischemia time and/or formalin fixation on estrogen receptor, progesterone receptor, and human epidermal growth factor receptor-2 results in breast carcinoma Pathol Res Int 2012 947041 https://doi.org/10.1155/2012/947041
67. Yildiz-Aktas IZ, Dabbs DJ, and Bhargava R (2012) The effect of cold ischemic time on the immunohistochemical evaluation of estrogen receptor, progesterone receptor, and HER2 expression in invasive breast carcinoma Mod Pathol Off J U S Can Acad Pathol Inc 25(8) 1098–1105
68. Zarbo RJ, Gephardt GN, and Howanitz PJ (1996) Intralaboratory timeliness of surgical pathology reports. Results of two College of American Pathologists Q-Probes studies of biopsies and complex specimens Arch Pathol Lab Med 120(3) 234–244 PMID: 8629897
69. Mpunga T, Tapela N, and Hedt-Gauthier BL, et al (2014) Diagnosis of cancer in rural Rwanda: early outcomes of a phased approach to implement anatomic pathology services in resource-limited settings Am J Clin Pathol 142(4) 541–545 https://doi.org/10.1309/AJCPYPDES6Z8ELEY PMID: 25239422
70. Masamba LPL, Mtonga PE, and Kalilani Phiri L, et al (2017) Cancer pathology turnaround time at Queen Elizabeth Central Hospital, the Largest Referral Center in Malawi for Oncology Patients J Glob Oncol 3(6) 734–739 https://doi.org/10.1200/JGO.2015.000257 PMID: 29244984 PMCID: 5735957
71. Rana MK, Rana APS, and Sharma U, et al (2022) Evolution of frozen section in carcinoma breast: systematic review Int J Breast Cancer 2022 4958580 https://doi.org/10.1155/2022/4958580 PMID: 35655582 PMCID: 9152418
72. Nelson AM, Milner DA, and Rebbeck TR, et al (2016) Oncologic care and pathology resources in Africa: survey and recommendations J Clin Oncol Off J Am Soc Clin Oncol 34(1) 20–26 https://doi.org/10.1200/JCO.2015.61.9767
73. Aziz Z, Iqbal J, and Akram M, et al (2008) Effect of social class disparities on disease stage, quality of treatment and survival outcomes in breast cancer patients from developing countries Breast J 14(4) 372–375 https://doi.org/10.1111/j.1524-4741.2008.00601.x PMID: 18540953
74. El Saghir NS, Khalil MK, and Eid T, et al (2007) Trends in epidemiology and management of breast cancer in developing Arab countries: a literature and registry analysis Int J Surg Lond Engl 5(4) 225–233 https://doi.org/10.1016/j.ijsu.2006.06.015
75. Asaoka M, Gandhi S, and Ishikawa T, et al (2020) Neoadjuvant chemotherapy for breast cancer: past, present, and future Breast Cancer Basic Clin Res 14 1178223420980377 https://doi.org/10.1177/1178223420980377
76. Narod SA and Salmena L (2011) BRCA1 and BRCA2 mutations and breast cancer Discov Med 12(66) 445–453 PMID: 22127115
77. Freitas-Junior R, Ferreira-Filho DL, and Soares LR, et al (2019) Oncoplastic breast-conserving surgery in low- and middle-income countries: training surgeons and bridging the gap Curr Breast Cancer Rep 11(3) 136–142 https://doi.org/10.1007/s12609-019-00317-3
78. Afaya A, Ramazanu S, and Bolarinwa OA, et al (2022) Health system barriers influencing timely breast cancer diagnosis and treatment among women in low and middle-income Asian countries: evidence from a mixed-methods systematic review BMC Health Serv Res 22(1) 1601 https://doi.org/10.1186/s12913-022-08927-x
79. Chen X, Qian X, and Xiao M, et al (2023) Survival outcomes and efficacy of platinum in early breast cancer patients with germline BRCA1 or BRCA2 mutation: a multicenter retrospective cohort study Breast Cancer Dove Med Press 15 671–682 PMID: 37692097 PMCID: 10487706
80. Holánek M, Bílek O, and Nenutil R, et al (2019) Effectiveness of neoadjuvant therapy with platinum-based agents for patients with BRCA1 and BRCA2 germline mutations – a retrospective analysis of breast cancer patients treated at MMCI Brno Klin Onkol Cas Ceske Slov Onkol Spolecnosti 32(Supplementum2) 31–35
81. de Boo LW, Jóźwiak K, and Joensuu H, et al (2022) Adjuvant capecitabine-containing chemotherapy benefit and homologous recombination deficiency in early-stage triple-negative breast cancer patients Br J Cancer 126(10) 1401–1409 https://doi.org/10.1038/s41416-022-01711-y PMID: 35124703 PMCID: 9090783
82. Bajpai J, Kashyap L, and Vallathol DH, et al (2022) Outcomes of non-metastatic triple negative breast cancers: real-world data from a large Indian cohort Breast Off J Eur Soc Mastol 63 77–84
83. Liang MI, Chen L, and Hershman DL, et al (2021) Total and out-of-pocket costs for PARP inhibitors among insured ovarian cancer patients Gynecol Oncol 160(3) 793–799 https://doi.org/10.1016/j.ygyno.2020.12.015 PMCID: 7902421
84. Khan SZ, Arecco L, and Villarreal-Garza C, et al (2022) Knowledge, practice, and attitudes of physicians in low- and middle-income countries on fertility and pregnancy-related issues in young women with breast cancer JCO Glob Oncol 8 e2100153 https://doi.org/10.1200/GO.21.00153 PMID: 35025688 PMCID: 8769103
85. Nair NS, Ali BA, and Siddique S, et al (2023) Patient-related awareness of impact of cancer-directed therapy on fertility in young women diagnosed of breast cancer South Asian J Cancer https://doi.org/10.1055/s-0043-1771385 ISSN 2278-330X
86. Bajpai J, Ventrapati P, and Joshi S, et al (2021) Unique challenges and outcomes of young women with breast cancers from a tertiary care cancer centre in India Breast Edinb Scotl 60 177–184 https://doi.org/10.1016/j.breast.2021.09.008
87. Collins JM and Isaacs C (2020) Management of breast cancer risk in BRCA1/2 mutation carriers who are unaffected with cancer Breast J 26(8) 1520–1527 https://doi.org/10.1111/tbj.13970 PMID: 32652823
88. Ludwig KK, Neuner J, and Butler A, et al (2016) Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review Am J Surg 212(4) 660–669 https://doi.org/10.1016/j.amjsurg.2016.06.010 PMID: 27649974
89. Bertozzi S, Londero AP, and Xholli A, et al (2023) Risk-reducing breast and gynecological surgery for BRCA mutation carriers: a narrative review J Clin Med 12(4) https://doi.org/10.3390/jcm12041422
90. Choi YH, Terry MB, and Daly MB, et al (2021) Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants JAMA Oncol 7(4) 585–592 https://doi.org/10.1001/jamaoncol.2021.2040 PMID: 33630024 PMCID: 7907985
91. Conduit C, Milne RL, and Friedlander ML, et al (2021) Bilateral salpingo-oophorectomy and breast cancer risk for BRCA1 and BRCA2 mutation carriers: assessing the evidence Cancer Prev Res Phila PA 14(11) 983–994 https://doi.org/10.1158/1940-6207.CAPR-21-0141
92. Grimes CE, Bowman KG, and Dodgion CM, et al (2011) Systematic review of barriers to surgical care in low-income and middle-income countries World J Surg 35(5) 941–950 https://doi.org/10.1007/s00268-011-1010-1 PMID: 21360305
93. Mooghal M, Vohra LM, and Khan W, et al (2023) Prophylactic risk-reducing mastectomy (PRRM): a set practice or catch-22 situation in LMIC. A single-centre prospective cohort study World J Surg 47(9) 2154–2160 https://doi.org/10.1007/s00268-023-07033-1 PMID: 37145137
94. King MC, Wieand S, and Hale K, et al (2001) Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) breast cancer prevention trial JAMA 286(18) 2251–2256 https://doi.org/10.1001/jama.286.18.2251 PMID: 11710890
95. Barrios CH (2022) Global challenges in breast cancer detection and treatment Breast Off J Eur Soc Mastol 62(Suppl 1) S3–S6
96. Mittra I, Mishra GA, and Dikshit RP, et al (2021) Effect of screening by clinical breast examination on breast cancer incidence and mortality after 20 years: prospective, cluster randomised controlled trial in Mumbai BMJ 372 n256 https://doi.org/10.1136/bmj.n256 PMID: 33627312 PMCID: 7903383
97. Pangarsa EA, Rizky D, and Tandarto K, et al (2023) The effect of multidisciplinary team on survival rates of women with breast cancer: a systematic review and meta-analysis Ann Med Surg 85(6) 2940–2948 https://doi.org/10.1097/MS9.0000000000000914
98. Abou-Alfa GK and Norton L (2023) Global oncology medical diplomacy working group inaugural meeting: defining worldwide barriers to germline genomics in cancer prevention and management Ann Glob Health 89(1) 16 https://doi.org/10.5334/aogh.3967 PMID: 36843667 PMCID: 9951627






