Biweekly cetuximab in combination with capecitabine and oxaliplatin (XELOX) or irinotecan (XELIRI) in the first-line and second-line treatment of patients with RAS wild-type metastatic colorectal cancer
Jamal Zekri1,2, Mohammed Abbas Baghdadi3, Refaei Belal Ibrahim4, Abdelrazak Meliti5 and Turki M Sobahy3
1College of Medicine, Al-Faisal University, Riyadh 11533, Saudi Arabia
2King Faisal Specialist Hospital & Research Centre (Jeddah), Jeddah 21499, Saudi Arabia
3Research Centre, King Faisal Specialist Hospital & Research Centre (Jeddah), Jeddah 21499, Saudi Arabia
4Department of Clinical Oncology and Nuclear Medicine, Faculty of Medicine, Al Azhar University, Cairo 11884, Egypt
5Department of Pathology and Laboratory Medicine, King Faisal Specialist Hospital & Research Centre, Jeddah 21499, Saudi Arabia
Abstract
Background: Oral capecitabine in combination with intravenous oxaliplatin (XELOX) or irinotecan (XELIRI) are acceptable substitutions to fully intravenous regimens. Biweekly (as opposed to weekly) cetuximab is more convenient when combined with biweekly chemotherapy. Here, we report the tolerability and efficacy of biweekly cetuximab in combination with biweekly XELOX or XELIRI in patients with RAS wild-type metastatic colorectal cancer (RAS-WT mCRC).
Methods: Clinical data of consecutive patients with mCRC who received biweekly cetuximab (500 mg/m2) in combination with XELOX or XELIRI between January 2009 and May 2019 in the first- or second-line settings was extracted. Dosage of XEL (Capecitabine/XELODA) was 1,000 mg/m2 twice daily for 9 days, plus on day 1 oxaliplatin 85 mg/m2 or irinotecan 180 mg/m2. Treatment dose reduction and delay for ≥7 days was analysed as surrogates for toxicity. Extended RAS testing was performed in the context of this study for patients who received treatment based on limited KRAS-WT genotype.
Results: Sixty one patients with RAS-WT mCRC fulfilled the eligibility criteria. XELOX was administered to 26 (42.6%) and XELIRI to 35 (57.4%) of patients. For all patients in the first-line setting, the objective response rate (ORR), median progression free survival (PFS) and median overall survival (OS) were 54%, 8 months and 25 months, respectively. The corresponding outcomes for the subgroup of patients who received first-line XELOX were 68%, 10 months and not reached, respectively. For all patients in the second-line setting, the ORR, PFS and OS were 50%, 7 months and 20 months, respectively. Chemotherapy components dose reduction and delays were observed in 18 (29.5%) and 25 (41%) patients, respectively. The corresponding frequencies for cetuximab were 3 (5%) and 31 (50.8%).
Conclusion: Biweekly cetuximab in combination with XELOX or XELIRI is tolerable and effective. The addition of cetuximab to capecitabine and oxaliplatin is associated with favourable outcome.
Keywords: biweekly cetuximab, XELOX, XELIRI, colorectal cancer, anti-EGFR
Correspondence to: Jamal Zekri
Email: jmzekri@hotmail.com
Published: 15/12/2022
Received: 17/07/2022
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Recent reports based on Global Cancer Incidence, Mortality And Prevalence (GLOBOCAN) 2020 data estimate 19.3 million new cancer cases globally with colorectal cancer (CRC) representing 10% of these cases preceded only by lung (11.4%) and breast (11.7%) cancers [1].
Some countries are reporting stability or slight decline in the incidence of CRC, possibly due to screening. However, others are experiencing a significant rise in reported cases [2, 3].
Approximately a quarter to third of patients with CRC present with metastatic disease and up to 50% develop metastases after initial curative intent treatment [4, 5].
The outcome for patients with metastatic CRC (mCRC) is poor. However, there has been a measurable improvement in outcome over the recent two decades due to the adoption of the multidisciplinary team management and the successful development of newer and more effective chemotherapy and targeted agents [6, 7].
Infusional 5 Fluorouracil (5FU) is the most commonly used fluoropyrimidine backbone cytotoxic agent in landmark trials and in daily routine practice. The more convenient oral fluoropyrimidine, capecitabine is an attractive alternative to infusional 5FU as monotherapy and in combination with oxaliplatin and irinotecan in 3-weekly and biweekly regimens [8, 9].
Cetuximab is an IgG-1 monoclonal antibody that binds to and inhibits the epidermal growth factor receptor (EGFR). Randomised phase III trials investigated the addition of cetuximab to 5FU-based combination chemotherapy regimens (FOLFOX/FOLFIRI) in mCRC. Results of these studies confirmed the clinical activity of this approach in patients with RAS wild type (RAS-WT) tumours [10–12].
Currently, management guidelines endorse the use of anti-EGFR antibodies such as cetuximab in combination with chemotherapy in patients with RAS-WT mCRC in the first and subsequent lines of treatment.
The approved schedule of cetuximab consists of first a loading dose of 400 mg/m2 followed by 250 mg/m2 every week. Meanwhile, most chemotherapy regimens for the treatment of mCRC are administered biweekly. This creates frequent visits to the hospital and extra costs. Pharmacokinetic, pharmacogenomic and pharmacoproteomic studies show equivalence of weekly (250 mg/m2) and biweekly (500 mg/m2) administration [13].
Substituting weekly with biweekly cetuximab in combination with biweekly capecitabine based combination chemotherapy provides the prospect of convenience and cost effectiveness both for the patient and health care providers.
Encouraged by the above, we adopted biweekly regimens of cetuximab in combination with capecitabine and oxaliplatin (XELOX) and with irinotecan (XELIRI) in routine clinical practice. Here we report our experience with these regimens coupled with planned and structured evaluation of efficacy and tolerability.
Patients and methods
This was a single centre, non-randomised retrospective study. All consecutive patients with mCRC who received biweekly cetuximab (500 mg/m2) in combination with chemotherapy for mCRC were identified through the pharmacy records. Additional inclusion criteria were as follows: (a) the chemotherapy component of the regimen contains capecitabine combined with either oxaliplatin or irinotecan administered every 2 weeks and (b) treatment in the first- or second-line settings. Exclusion criteria were as follows: (a) chemotherapy component of the regimen containing infusional 5FU, (b) chemotherapy component regimen lacking fluoropyrimidine such as single-agent iriniotecan or cetuximab, (c) treatment in third or subsequent line settings and (d) receiving cetuximab in both first- and second-line settings. The above inclusion and exclusion criteria were designed to specifically select patients who received biweekly cetuximab combined with biweekly capecitabine and oxaliplatin (XELOX) or capecitabine and irinotecan (XELIRI). Dosage of capecitabine was 1,000 mg/m2 twice daily for 9 days, oxaliplatin 85 mg/m2 on day 1 and irinotecan 180 mg/m2 on day 1 administered every 2 weeks.
The sample size is not based on statistical considerations and it represents the number of eligible patients who started treatment with the regimens of interest between January 2009 and May 2019. The study was approved by the Institutional Review Board.
Due to the historic nature of the study, some patients received cetuximab based on limited RAS results (only exon 2 KRAS-WT was confirmed). For the purpose of this study, additional KRAS and NRAS tumour mutational analysis of archival tissue was performed for these patients and only those with RAS-WT genotype were included in this report. Figure 1 illustrates the study flow diagram.
Data on chemotherapy and cetuximab components of treatment dose reduction and omission or treatment delay for ≥7 days due to toxicity were captured as surrogates for clinically significant intolerance. In this context, particular attention was paid to capture the grades of cetuximab specific toxicity, namely, skin rash, pruritus, fatigue and diarrhoea. Adverse events (AEs) severity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) V.4.03. Additionally, the rationale for cetuximab dose reduction/omission/delay was captured and categorised into five reasons (refer to the ‘Results’ section for more details).
Efficacy endpoints of interest were radiological best objective response rate (ORR), progression free survival (PFS) and overall survival (OS). Radiological response assessment CT scans were mostly performed every 6–10 weeks as per the routine practice of the treating oncologist unless an earlier assessment was clinically indicated. The ORR was interpreted and documented by the investigators as per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 criteria through carefully reading and interpreting the radiology reports. PFS was defined as the time from starting the index treatment (first or second line) to disease progression, death or loss to follow-up. OS was defined as the time from starting the index treatment to the date of death due to any cause, or to the date of censoring at the last time the subject was known to be alive. Statistical Package for the Social Sciences Version 20 (SPSS.20) software was used for statistical analysis including Kaplan–Meier and log-rank tests for survival outcome.
Results
Sixty-one patients with extended RAS-WT genotype fulfilled the inclusion criteria and are included in this analysis (Figure 1). Median age was 53 (27–75) years. XELOX was administered to 26 (42.6%) and XELIRI to 35 (57.4%) of patients in first line 33 (54.1%) or second line 28 (45.9%) settings. Table 1 depicts detailed patients’ characteristics. The median duration of follow-up for all the cohort was 39 months.
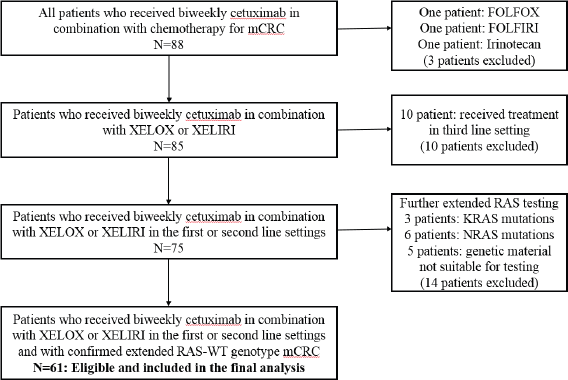
Figure 1. Study flow diagram.
The ORR was 54.5% (XELOX: 68.4% and XELIRI: 35.7%) and 50% (XELOX: 42.9% and XELIRI: 52.4%) in the first- and second-line settings, respectively.
At the time of last follow-up of each individual patient, 39 patients were dead and 22 were still alive. At the time of final data analysis in December 2020, 9/22 patient were lost to follow-up. The median PFS and OS were 8 and 25 months, respectively, for patients treated in the first-line setting and were 7 and 20 months, respectively, for patients treated in the second-line setting. Table 2 and Figures 2–4 illustrate the efficacy outcome results according to the line of treatment and the chemotherapy regimen.
Chemotherapy component dose reduction was observed in 18 (29.5%) and delay for ≥7 days in 25 (41%) patients. Cetuximab dose was reduced in only 3 (5%) patients, all due to skin rash. Frequencies of chemotherapy and cetuximab dose reduction, delay and discontinuation are depicted in Table 3.
There were no documented grade 4 cetuximab specific AEs. Grade 1&2 infusion reaction, skin rash, pruritus, diarrhoea and fatigue were reported in 0 (0%), 32 (52.5%), 27 (44.3%), 22 (36%) and 27 (44.3%) patients, respectively. The corresponding grade 3 AEs were reported in 1 (1.6%), 6 (9.8%), 0 (0%), 7 (11.5%) and 3 (4.9%), respectively (Table 4).
The median number of administered cycles of chemotherapy was 9 (1–60) and of cetuximab was 10 (1–70). Sixty-one percent and 65.9% of patients received ≥10 cycles of chemotherapy and cetuximab, respectively.
Cetuximab was discontinued permanently in one patient, due to an allergic infusion reaction. Additional 31 patients (50.8%) required dose delay for the following reasons: (a) In eight patients due to toxicities likely related to cetuximab such as rash (4), paronychia (2), fatigue (1) and diarrhoea (1). (b) In eight patients in conjunction with chemotherapy component dose delay due to toxicities related to the chemotherapy such as neutropenia (4), febrile neutropenia (2), thrombocytopenia (1) and hand-foot syndrome (1). (c) In seven patients due to toxicities of undetermined reason ‘could be chemotherapy or cetuximab’ such as diarrhoea (6) and conjunctivitis (1). (d) In five patients due to medical conditions that are unlikely to be related to any of the regimen components such as appendicitis (1), cholangitis (1), intestinal obstruction (1), chest infection (1) and vaginal infection (1). (e) Due to non-medical reasons in three patients who just wanted a short break from treatment.
Discussion
The substitution of infusional 5FU by oral capecitabine in oxaliplatin and irinotecan containing chemotherapy regimens allows patients to be managed more easily in an outpatient setting. In addition, it reduces the frequency of infusion visits and eliminates the need for central venous access and home infusion pumps, thereby offering a convenient treatment option for patients with mCRC. These regimens are usually administered triweekly [14, 15] with capecitabine administered for 14 days and followed by 7 days of break (2:1 ratio). However, biweekly administration with different dose intensities has been described in the literature and adopted in routine clinical practice albeit on a limited scale [16–19]. Combining these biweekly regimens with biweekly (instead of weekly) cetuximab is supported by pharmacokinetic, pharmacodynamic and some clinical data [13, 20, 21].
At our institution and with the above in mind, we established triweekly and biweekly XELOX and XELIRI regimens for patients with mCRC. In the year 2009, we incorporated biweekly cetuximab (500 mg/m2) into the biweekly XELOX and XELIRI regimens to achieve matching and convenient treatment schedules for patients with RAS-WT mCRC. The dose of capecitabine (1,000 mg/m2 twice daily) was in line with that in other studies and clinical practice. However, in our biweekly regimen, the duration of capecitabine administration was reduced to 9 days to allow 5 days for recovery before the subsequent cycle is administered. Our intention was to maintain a capecitabine treatment duration ratio of 2:1 similar to that in the original 3-weekly regimen. There is no one standard regimen for the doses and schedules of oxaliplatin when combined with capecitabine and doses of 70 mg/m2 on days 1&8 and 130 mg/m2 on day 1 in 3-weekly regimens have been described [22, 23]. When combined with capecitabine, the doses of irinotecan are even more varied in weekly, biweekly and 3-weekly regimens. Doses between 70 mg and 300 mg have been described depending on the frequency of administration [15, 24–31].
Table 1. Patients’ characteristics.
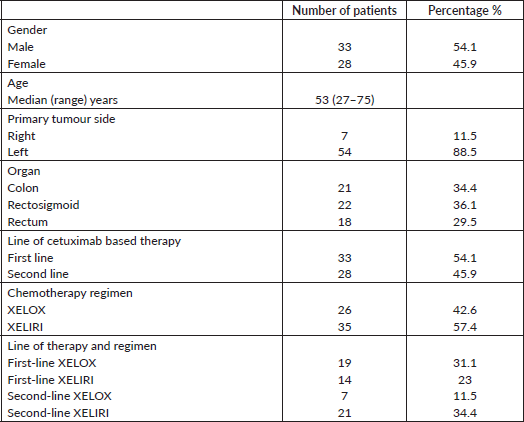
Table 2. Treatment outcome.
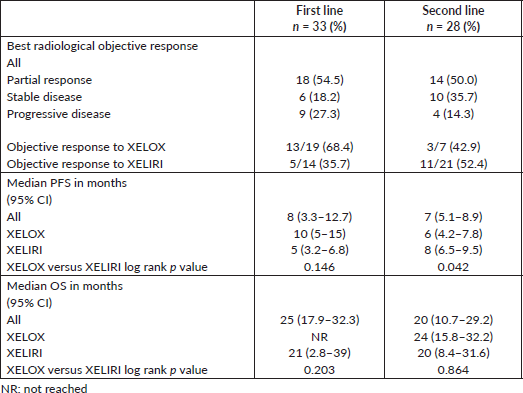
Table 3. Dose reduction, delay for ≥7 days and discontinuation of the chemotherapy and cetuximab components of the regimen.
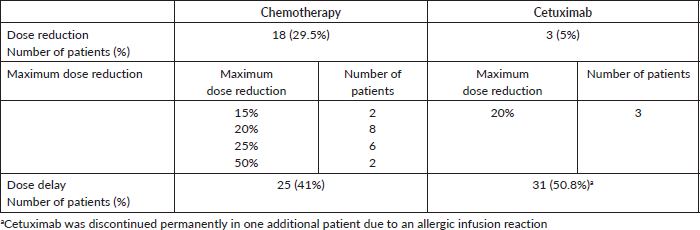
Table 4. Grades of documented cetuximab specific AEs.
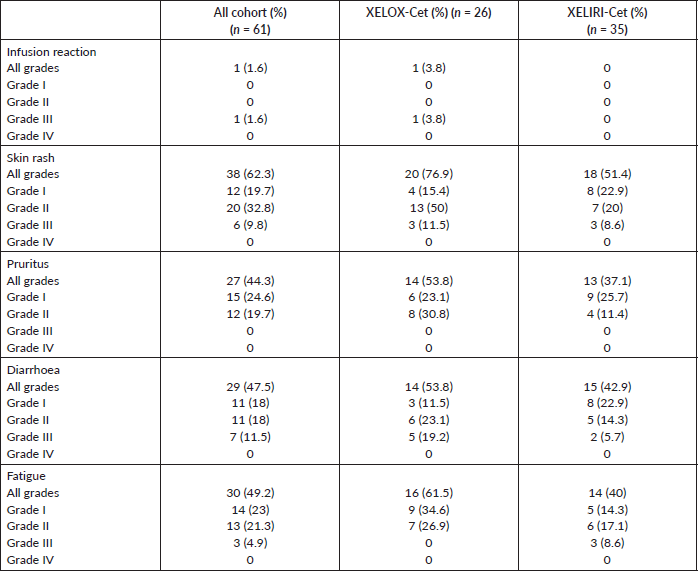
Figure 2. (a): PFS on first-line treatment regardless of the regimen. (b): PFS on second-line treatment regardless of the regimen. (c): OS on first-line treatment regardless of the regimen. (d): OS on second-line treatment regardless of the regimen.
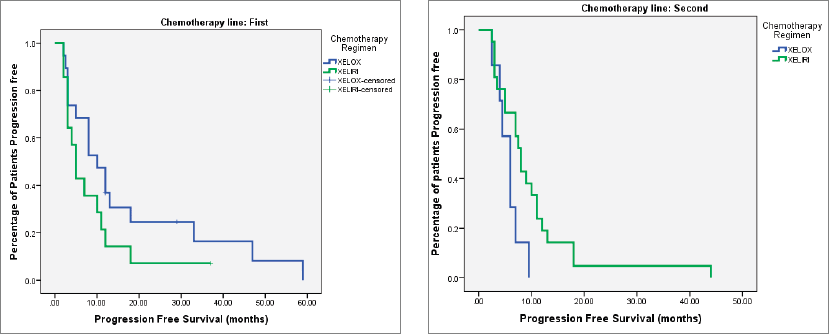
Figure 3. (a): PFS on first-line treatment according to chemotherapy regimen. (b): PFS on second-line treatment according to chemotherapy regimen.
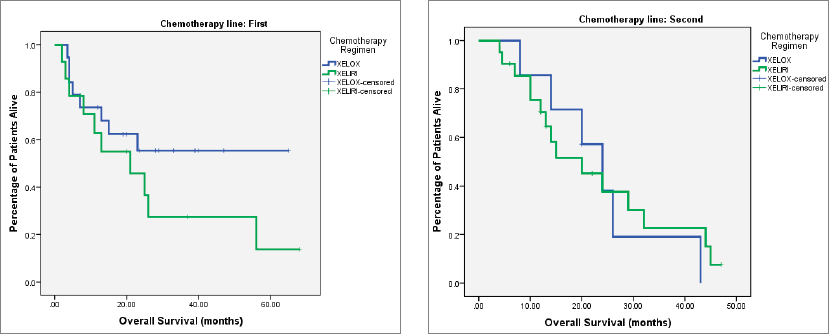
Figure 4. (a): OS on first-line treatment according to chemotherapy regimen. (b): OS on second-line treatment according to chemotherapy regimen.
Considering the above variations, we designed the biweekly regimen of capecitabine (1,000 mg/m2 on days 1–9) with oxaliplatin at a dose of 85 mg/m2 on day 1 (XELOX) or with irinotecan 180 mg/m2 on day 1 (XELIRI) combined with biweekly cetuximab (500 mg/m2).
First-line XELOX-cetuximab
Our real-world experience in routine daily clinical practice has demonstrated the treatment efficacy and safety of biweekly cetuximab and capecitabine based combination regimens (XELOX and XELIRI). In the first-line setting, the ORR to XELOX-Cet was 68.4%. Several clinical trials have demonstrated that the ORR to FOLFOX plus weekly cetuximab varies between 57% and 72% in patients with KRAS-WT and extended RAS-WT mCRC [11, 32–35]. Thus, reassuringly, the ORR observed in our population falls within this reported range.
Our patients who received XELOX-Cet have a comparable outcome to those who received biweekly cetuximab in combination with traditional 5FU and oxaliplatin in relatively large phase II trials. The NORDIC-7.5 trial reported ORR, median PFS and median OS of 62%, 8 months and 23 months, respectively, in 152 patients with KRAS-WT mCRC treated with bolus oxaliplatin and bolus 5FU (FLOX-Cet) [36].
The OPTIMIX-ACROSS phase II trial reported ORR, median PFS and median OS of 60.6%, 10.1 months and 20.8 months, respectively, in 99 patient with KRAS-WT mCRC treated with FOLFOX-Cet [37].
A number of small to medium size studies investigated biweekly cetuximab and XELOX (at times referred to as CAPEOX). In a combined analysis of the FLEET and FLEET2 trials, the investigators identified 88 patients with extended RAS/BRAF-WT mCRC. The ORR was 61.5% in patients treated with XELOX-Cet (n = 52) and 66.7% in those treated with FOLFOX-Cet (n = 36). The authors concluded that CAPEOX-Cet should be considered as a treatment option for these patients [38]. Similarly, 68.4% of our patients achieved objective responses to XELOX-Cet albeit we did not exclude patients with BRAF mutations. Identification and exclusion of these patients would have likely improved the ORR even further. The multicentre two-arm phase II (SAKK) trial, confirmed the value of adding cetuximab to first-line 3-weekly XELOX. ORR, PFS and OS were 14%, 5.8 months and 16.5 months, respectively, in the XELOX arm and were and 41%, 7.2 months and 20.5 months in the XELOX-Cet arm [39].
In the COIN trial, patients were randomly assigned to oxaliplatin and fluoropyrimidine chemotherapy (arm A), the same combination plus cetuximab (arm B) or intermittent chemotherapy (arm C). The choice of fluoropyrimidine therapy (capecitabine or infused fluorouracil) was decided before randomisation. XELOX with or without cetuximab was administered every 3 weeks. ORR increased from 57% (n = 209) with chemotherapy alone to 64% (n = 232) with addition of cetuximab (p = 0·049) in patients with KRAS-WT tumours [40]. The outcome was further analysed according to the administered fluoropyrimidine. The ORRs were 51% and 57% with XELOX-Cet and FOLFOX-Cet, respectively, and the difference was not statistically different (Odds ratio: 1.26 (95% CI: 0.93, 1.70)) [33].
It is clear from the above data that the ORR of 68.4% to first-line XELOX-Cet observed in our patients compares favourably with that reported in large landmark and other smaller trials.
In the first-line setting, the median PFS was 10 months and OS was not reached in patients who received XELOX-Cet. These results compare favourably to those reported in the literature. In the FLEET2 trial, 14 patients with KRAS-WT mCRC received biweekly XELOX-Cet similar to our regimen except the duration of capecitabine treatment was limited to 7 days (instead of 9 days in our protocol). They reported response in 50% of patients while median PFS and OS were 6.5 and 24.3 months, respectively [20]. The FLEET trial randomised patients with previously untreated KRAS/BRAF-WT mCRC to FOLFOX-Cet or XELOX-Cet. The ORRs in the FOLFOX-Cet and XELOX-Cet were 64.9 % and 72% while the corresponding median PFS/OS was 13.1/38.1 and 13.4/47 months [41].
We appropriately excluded patients with additional novel KRAS and NRAS mutations and this could explain the longer PFS in our population than that reported the FLEET2 trial (10 versus 6.5 months). On the other hand, the exclusion of patients with BRAF mutations may explain the apparent longer PFS (13.1 months) observed in the FLEET trail.
First-line XELIRI-cetuximab
Combining irinotecan and capecitabine with weekly cetuximab (XELIRI-Cet and CAPIRI-Cet) is effective regardless of dosages and schedules. The AIO KRK-0104 phase II trial randomised 185 patients to 3-weekly CAPIRI-Cet or CAPOX-Cet. The ORR was 50% for CAPIRI-Cet in patients with KRAS-WT tumours and the median PFS and OS were of 6.2 and 21.1 months, respectively. The authors concluded that the addition of cetuximab to either CAPIRI or CAPOX is effective and safe in first-line treatment setting [42]. Similar results and conclusions were presented in an abstract form by another group of investigators [43]. In a dose finding phase I study, biweekly XELIRI was administered with irinotecan dose of 180 mg/m2 on day 1 and capecitabine was administered for 7 days at five different investigational dose levels. The identified maximum tolerated dose for capecitabine was 3,500 mg/m2/day [44].
As discussed above, different dosages and schedules exist with the XELIRI and CAPIRI regimens. We elected for a lower daily capecitabine dose but over a longer duration (9 days instead of 7 days). In our patients treated with XELIRI-Cet, the ORR was 35.7% and the median PFS was 5 months in the first-line setting. This outcome may be perceived as less than optimum. Possible explanations include (a) relatively small number (n = 14) of patients in this group, (b) less than optimum performance status of patients treated in routine daily practice, (c) difference in tumour biology which unfortunately cannot be confirmed as the majority of these tumours were not analysed for more modern biological markers such as BRAF, Microsatellite Instability (MSI) and Her2, and (d) differences in third and subsequent lines of therapy which we are unable to confirm as this information was not required when the study was designed. This outcome cannot be explained by tumour sidedness as only 2 (14.3%) of these patients had a right side tumour or by biological resistance to fluoropyrimidines as only 3 (21%) patients were pre-exposed to fluoropyrimidines in the adjuvant or neoadjuvant settings.
Second-line XELOX-cetuximab and XELIRI-cetuximab
In our study, 28 patients received XELOX-Cet or XELIRI-Cet in the second-line setting. ORR was 50% while the PFS and OS were 7 and 20 months, respectively. This compares favourably to the results of FOLFOX/FOLFIRI combined with weekly cetuximab reported in the literature. In an extension of the ITACa trial, patients were randomised to second-line chemotherapy (FOLFOX or FOLFIRI) with or without cetuximab. ORRs were not reported. In patients with RAS-WT (regardless of BRAF status), the median PFS was 7.0 months and 3.3 months for chemotherapy-Cet and chemotherapy alone, respectively, with an adjusted hazard ratio (HR) of 0.56 (95% confidence interval (95% CI): 0.29–1.09, p = 0.088). The corresponding results for OS were 11.1 and 7.8 months, respectively, with an adjusted HR of 1.27 (95% CI: 0.64–2.50, p = 0.489) [45]. The phase II second-line FLIER trial reported ORR in 31.7% of patients with median PFS and OS of 7.4 and 18.2 months, respectively, with weekly second-line cetuximab and FOLFIRI [46]. The ORR was 35% while the median PFS and OS were 7.1 and 14.3 months, respectively, in retrospective analysis of 40 patients who received second-line FOLFOX and weekly cetuximab [47]. In a retrospective analysis of ten patients treated with second-line FOLFIRI and two patients with irinotecan combined with biweekly cetuximab, relative risk (RR) was 33.3% and the median PFS was 4.6 months [48].
Weekly and biweekly cetuximab
The results of a recent health record-derived database study comparing the outcome of weekly with biweekly cetuximab combined with FOLFIRI/FOLFOX/irinotecan or cetuximab monotherapy in first-, second- or third-line therapy for patients with stage IV or recurrent KRAS-WT mCRC were presented at the American Society of Clinical Oncology Gastrointestinal Cancers Symposium (ASCO-GI 2021). OS in performance status-matched weekly versus biweekly cohorts was not significantly different. The median OS was 23.36 and 29.14 months (log-rank p = 0.347) in weekly and biweekly cetuximab groups (each group; 121 patients), respectively, in the first-line setting. The corresponding median OS was 12.93 and 15.39 months (log-rank p = 0.504) (each group; 159 patients) in the second-line setting. The investigators concluded that weekly and biweekly cetuximab had comparable effectiveness in real-world environment [49]. The median OS (25 months) in our patients treated in the first-line setting is comparable to these results while the OS (20 months) of patients treated in second-line seems to compare favourably.
The interest in biweekly cetuximab became more apparent with the recent publications (year 2020) of the results of a meta-analysis and a pooled analysis of weekly versus biweekly cetuximab. Both analyses confirmed the non-inferiority of the biweekly regimen and support its use instead of weekly cetuximab [50, 51]. On 6 April 2021, the Food and Drug Administration approved the biweekly regimen of cetuximab at a dose of 500 mg/m2 for patients with mCRC and squamous cell carcinoma of the head and neck [52].
Toxicity and tolerance of XELOX-cetuximab and XELIRI-cetuximab
The combinations XELOX-Cet and XELIRI-Cet were generally well tolerated. The identified cetuximab specific AEs of any grade were rash (62%), diarrhoea (48%) and pruritus (44%) (Table 4). The OPUS trial reported any grade AEs were skin and subcutaneous tissue disorders (90%) and GI disorders (78%) in the FOLFOX-Cet group [32]. The CRYSTAL trial reported grades 3/4 diarrhoea in 15.7% of patients in the FOLFIRI-Cet arm. Grade 3 skin reactions (19.7%), acne-like rash (16.2%) and none of them were grade 4 [53]. The FLEET trial reported any grade acneiform rash in 60% and 70% in the biweekly XELOX-Cet and FOLFOX-Cet groups, respectively [41]. The FLEET2 trial reported any grade rash (80%), diarrhoea (12.5%) while infusion reaction occurred in 3 (7.5%) patients (grade 2: 2 & grade 3: 1) in patients receiving biweekly XELOX-Cet [20].
In a meta-analysis of 47 studies, the rate of infusion reactions in patients treated with cetuximab was 6% compared to 1.6% with the human monoclonal antibody anti-EGFR panitumumab [54]. We found only one patient (1.6%) with documented infusion reaction (grade 3). However, under-documentation of mild grades of infusion reactions could explain our observation.
Fatigue is common in patients with cancer and can be due the disease itself, anaemia, systemic treatment, electrolyte disturbances, radiotherapy, emotional burden and excessive travel for medical care. It can be difficult to identify one cause for fatigue as it is usually multifactorial. A systematic review and meta‑analysis of randomised controlled trials found that anti-EGFR antibodies (cetuximab and panitumumab) increased the risk of all grades fatigue (RR: 1.10, 95% CI: 1.05–1.14) and high-grade fatigue (RR: 1.31, 95% CI: 1.19–1.45). For the cetuximab trials, the frequency of high-grade fatigue was 12.3% in patients receiving cetuximab regimens and 9% in patients receiving corresponding regimens without cetuximab (RR: 1.33, 95% CI: 1.20–1.47) [55]. We report fatigue in 30 (49%) of patients (including grade 3: 4.9% and grade 4: none). The CRYSTAL trial reported a similar rate (5.3%) of grade 3/4 fatigue.
There was no grade IV diarrhoea. All grades & grade III diarrhoea were recorded in 53.8% & 19.2% and 42.9% & 5.7% in the XELOX and XELIRI groups, respectively. There is a general perception that severe diarrhoea is common with XELIRI (or CAPIRI). However, clinical trials did not always show consistent results in this context. For example, The AIO KRK-0104 randomised phase II trial reported grades III/IV diarrhoea in 19.3% and 15.7% of patients on 3-weekly CAPIRI-Cet and CAPOX-Cet, respectively [42]. The available evidence (albeit limited) suggests that biweekly XELIRI is associated with even lower rates of diarrhoea. In a single- arm study, 46 patients received irinotecan (175 mg/m2 on day 1), capecitabine (1,000 mg/m2 twice daily on days 2–8) and bevacizumab every 2 weeks. Grade III/IV diarrhoea was reported in 7% of the patients [56]. Similarly, only 2% of patients reported grade III diarrhoea and none reported grade IV after treatment with capecitabine at 1,000 mg/m2 twice on days 1–8, irinotecan at 150 mg/m2 on day 1 and bevacizumab in a Japanese second-line phase II study [57]. It is reassuring that severe diarrhoea was infrequent (5.7%) with XELIRI-Cet regimen in our study. This finding supports the available data that biweekly XELIRI regimens are associated with lower rate of severe diarrhoea and therefore are safe in routine clinical practice.
We acknowledge that retrospective analysis of data collected for a purpose other than the specific study intent has methodological limitations. The presence or extent of symptoms may not be completely recorded [58]. Consequently, accurate reporting of frequency and grades of AEs remains one of the limitations of retrospective studies. Therefore, we decided to use chemotherapy and cetuximab dose reduction/omission and treatment delay as surrogate markers for clinically significant intolerance. Cetuximab was omitted in one patient due to grade 3 infusion reaction and was reduced by 20% due to skin rash in only 3 (5%) patients. Although dose delay was observed in 31 (50.8%) patients, it was needed due to cetuximab specific toxicity in only 8 (13.3%) patients indicating that majority of patients can tolerate this dose and schedule of cetuximab.
Conclusion
In patients with extended RAS-WT mCRC, biweekly cetuximab in combination with biweekly XELOX or XELIRI is tolerable and has a manageable toxicity profile and associated with favourable outcome. The efficacy of these schedules is comparable to other established schedules considering the heterogeneous population treated in routine clinical practice. Our results add to the already accumulating clinical evidence and support the administration of the biweekly cetuximab regimen. Our findings should reassure oncologists to consider combining this regimen with the convenient biweekly oral fluoropyrimidine based chemotherapy combinations (XELOX and XELIRI).
Ethics approval
The study has been approved by the Research Institutional Review Board (Research Ethics Committee) at King Faisal Specialist Hospital & Research Centre (Jeddah). Approval number is 2018-46.
Consent to participate
The consent to participate has been waived by the Institutional Review Board (as it has been deemed that consent would be impossible or impracticable to obtain).
Authors’ contributions
All listed authors made a substantial contribution to the concept or design of the work; or acquisition, analysis or interpretation of data, drafted the article or revised it critically for intellectual content and approved the version to be published. All listed authors have participated sufficiently in the work and take public responsibility for the content of the manuscript.
Declaration of funding
The study was financially supported by Merck Serono, Middle East FZ-Ltd, Dubai, UAE, an affiliate of Merck KGaA Darmstadt, Germany (Cross Ref Funder ID: 10.13039/100009945).
Conflicts of interest
JZ declares acting in advisory and speaker roles for Merck Serono, Saudi Arabia. Other authors declared no potential conflicts of interest.
Acknowledgments
The final manuscript was reviewed by the healthcare business of Merck KGaA, Darmstadt for medical and language accuracy before submission. Merck KGaA had no access to the original data and did not have any input or intervention in the data analysis, results or interpretations. The contents, views and opinion described in this manuscript reflect solely those of the authors.
References
1. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
2. Arnold M, Abnet CC, and Neale RE, et al (2020) Global burden of 5 major types of gastrointestinal cancer Gastroenterology 159 335–349 https://doi.org/10.1053/j.gastro.2020.02.068 PMID: 32247694 PMCID: 8630546
3. Arnold M, Sierra MS, and Laversanne M, et al (2017) Global patterns and trends in colorectal cancer incidence and mortality Gut 66 683–691 https://doi.org/10.1136/gutjnl-2015-310912
4. Maringe C, Walters S, and Rachet B, et al (2013) Stage at diagnosis and colorectal cancer survival in six high-income countries: a population-based study of patients diagnosed during 2000-2007 Acta Oncol (Madr) 52 919–932 https://doi.org/10.3109/0284186X.2013.764008
5. Joachim C, Macni J, and Drame M, et al (2019) Overall survival of colorectal cancer by stage at diagnosis: data from the Martinique cancer registry Medicine (Baltimore) 98 e16941 https://doi.org/10.1097/MD.0000000000016941 PMID: 31464932 PMCID: 6736397
6. Mitry E, Rollot F, and Jooste V, et al (2013) Improvement in survival of metastatic colorectal cancer: are the benefits of clinical trials reproduced in population-based studies? Eur J Cancer 49 2919–2925 https://doi.org/10.1016/j.ejca.2013.04.001 PMID: 23642328
7. Brouwer NPM, Bos ACRK, and Lemmens VEPP, et al (2018) An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients Int J Cancer 143 2758–2766 https://doi.org/10.1002/ijc.31785 PMID: 30095162 PMCID: 6282554
8. Field KM, Kosmider S, and Jefford M, et al (2008) Chemotherapy treatments for metastatic colorectal cancer: is evidence-based medicine in practice? J Oncol Pract 4 271–276 https://doi.org/10.1200/JOP.0852002 PMID: 20856756 PMCID: 2793914
9. Aguado C, García-Paredes B, and Sotelo MJ, et al (2014) Should capecitabine replace 5-fluorouracil in the first-line treatment of metastatic colorectal cancer? World J Gastroenterol 20 6092–6101 https://doi.org/10.3748/wjg.v20.i20.6092 PMID: 24876731 PMCID: 4033448
10. Van Cutsem E, Lenz HJ, and Köhne CH, et al (2015) Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer J Clin Oncol 33 692–700 https://doi.org/10.1200/JCO.2014.59.4812 PMID: 25605843
11. Bokemeyer C, Köhne CH, and Ciardiello F, et al (2015) FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer Eur J Cancer 51 1243–1252 https://doi.org/10.1016/j.ejca.2015.04.007 PMID: 25937522 PMCID: 7508202
12. Sobrero A, Lenz HJ, and Eng C, et al (2021) Extended RAS analysis of the phase III EPIC trial: irinotecan + cetuximab versus irinotecan as second-line treatment for patients with metastatic colorectal cancer Oncologist 26 e261–e269 https://doi.org/10.1002/onco.13591
13. Tabernero J, Ciardiello F, and Rivera F, et al (2009) Cetuximab administered once every second week to patients with metastatic colorectal cancer: a two-part pharmacokinetic/pharmacodynamic phase I dose-escalation study Ann Oncol 21 1537–1545 https://doi.org/10.1093/annonc/mdp549 PMID: 19940007
14. Díaz-Rubio E, Tabernero J, and Gómez-España A, et al (2007) Phase III study of capecitabine plus oxaliplatin compared with continuous-infusion fluorouracil plus oxaliplatin as first-line therapy in metastatic colorectal cancer: final report of the Spanish cooperative group for the treatment of digestive tumors trial J Clin Oncol 25 4224–4230 https://doi.org/10.1200/JCO.2006.09.8467
15. Koopman M, Antonini NF, and Douma J, et al (2007) Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial Lancet 370 135–142 https://doi.org/10.1016/S0140-6736(07)61086-1 PMID: 17630036
16. HS H (2005) Capecitabine plus oxaliplatin vs infusional 5-fluorouracil plus oxaliplatin in the treatment of colorectal cancer. con: pumpin’ FU (or, avoiding that oral fixation) - PubMed Clin Adv Hematol Oncol 3(5) 405–406 [https://pubmed.ncbi.nlm.nih.gov/16167014/] Date accessed 19/07/21
17. Fukui T, Suzuki K, and Ichida K, et al (2017) Sequential administration of XELOX and XELIRI is effective, feasible and well tolerated by patients with metastatic colorectal cancer Oncol Lett 13 4947–4952 https://doi.org/10.3892/ol.2017.6100 PMID: 28599498 PMCID: 5452954
18. Sakar B, Gumus M, and Basaran M, et al (2008) XELOX followed by XELIRI or the reverse sequence in advanced colorectal cancer Oncology 73 298–304 https://doi.org/10.1159/000132395 PMID: 18477855
19. Cui C, Shu C, and Yang Y, et al (2014) XELIRI compared with FOLFIRI as a second-line treatment in patients with metastatic colorectal cancer Oncol Lett 8 1864–1872 https://doi.org/10.3892/ol.2014.2335 PMID: 25202427 PMCID: 4156196
20. Hazama S, Maeda H, and Iwamoto S, et al (2016) A phase II study of XELOX and cetuximab as first-line therapy in patients with KRAS wild type metastatic colorectal cancer (FLEET2 Study) Clin Colorectal Cancer 15 329–336 https://doi.org/10.1016/j.clcc.2016.07.003 PMID: 27507128
21. Moosmann N and Heinemann V (2008) Cetuximab plus XELIRI or XELOX for first-line therapy of metastatic colorectal cancer Clin Colorectal Cancer 7 110–117 https://doi.org/10.3816/CCC.2008.n.015 PMID: 18501070
22. Porschen R, Arkenau HT, and Kubicka S, et al (2007) Phase III study of capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: a final report of the AIO colorectal study group J Clin Oncol 25 4217–4223 https://doi.org/10.1200/JCO.2006.09.2684 PMID: 17548840
23. Cassidy J, Clarke S, and Díaz-Rubio E, et al (2008) Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer J Clin Oncol 26 2006–2012 https://doi.org/10.1200/JCO.2007.14.9898 PMID: 18421053
24. Bajetta E, Di Bartolomeo M, and Mariani L, et al (2004) Randomized multicenter phase II trial of two different schedules of irinotecan combined with capecitabine as first-line treatment in metastatic colorectal carcinoma Cancer 100 279–287 https://doi.org/10.1002/cncr.11910 PMID: 14716761
25. Borner MM, Bernhard J, and Dietrich D, et al (2005) A randomized phase II trial of capecitabine and two different schedules of irinotecan in first-line treatment of metastatic colorectal cancer: efficacy, quality-of-life and toxicity Ann Oncol 16 282–288 https://doi.org/10.1093/annonc/mdi047 PMID: 15668285
26. Rea DW, Nortier JWR, and Ten Bokkel Huinink WW, et al (2005) A phase I/II and pharmacokinetic study of irinotecan in combination with capecitabine as first-line therapy for advanced colorectal cancer Ann Oncol 16 1123–1132 https://doi.org/10.1093/annonc/mdi227 PMID: 15939714
27. Cartwright T, Lopez T, and Vukelja SJ, et al (2005) Results of a phase II open-label study of capecitabine in combination with irinotecan as first-line treatment for metastatic colorectal cancer Clin Colorectal Cancer 5 50–56 https://doi.org/10.3816/CCC.2005.n.016 PMID: 15929806
28. Jordan K, Kellner O, and Kegel T, et al (2004) Phase II trial of capecitabine/irinotecan and capecitabine/oxaliplatin in advanced gastrointestinal cancers Clin Colorectal Cancer 4 46–50 https://doi.org/10.3816/CCC.2004.n.009 PMID: 15207020
29. Köhne CH, De greve J, and Hartmann JT, et al (2008) Irinotecan combined with infusional 5-fluorouracil/folinic acid or capecitabine plus celecoxib or placebo in the first-line treatment of patients with metastatic colorectal cancer EORTC study 40015 Ann Oncol 19 920–926 https://doi.org/10.1093/annonc/mdm544
30. Koopman M, Antonini NF, and Douma J, et al (2006) Randomised study of sequential versus combination chemotherapy with capecitabine, irinotecan and oxaliplatin in advanced colorectal cancer, an interim safety analysis. A Dutch colorectal cancer group (DCCG) phase III study Ann Oncol 17 1523–1528 https://doi.org/10.1093/annonc/mdl179 PMID: 16873425
31. Fuchs CS, Marshall J, and Mitchell E, et al (2007) Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C study J Clin Oncol 25 4779–4786 https://doi.org/10.1200/JCO.2007.11.3357 PMID: 17947725
32. Bokemeyer C, Bondarenko I, and Makhson A, et al (2009) Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer J Clin Oncol 27 663–671 https://doi.org/10.1200/JCO.2008.20.8397
33. Madi A, Fisher D, and Wilson RH, et al (2012) Oxaliplatin/capecitabine vs oxaliplatin/infusional 5-FU in advanced colorectal cancer: the MRC COIN trial Br J Cancer 107 1037–1043 https://doi.org/10.1038/bjc.2012.384 PMID: 22935584 PMCID: 3461171
34. Douillard JY, Zemelka T, and Fountzilas G, et al (2014) FOLFOX4 with cetuximab vs. UFOX with cetuximab as first-line therapy in metastatic colorectal cancer: the randomized phase II FUTURE study Clin Colorectal Cancer 13 https://doi.org/10.1016/j.clcc.2013.11.009
35. Tabernero J, Van Cutsem E, and Díaz-Rubio E, et al (2007) Phase II trial of cetuximab in combination with fluorouracil, leucovorin, and oxaliplatin in the first-line treatment of metastatic colorectal cancer J Clin Oncol 25 5225–5232 https://doi.org/10.1200/JCO.2007.13.2183 PMID: 18024868
36. Pfeiffer P, Sorbye H, and Qvortrup C, et al (2015) Maintenance therapy with cetuximab every second week in the first-line treatment of metastatic colorectal cancer: the NORDIC-7.5 study by the nordic colorectal cancer biomodulation group Clin Colorectal Cancer 14 170–176 https://doi.org/10.1016/j.clcc.2015.03.002 PMID: 25956187
37. Fernandez-Plana J, Pericay C, and Quintero G, et al (2014) Biweekly cetuximab in combination with FOLFOX-4 in the first-line treatment of wild-type KRAS metastatic colorectal cancer: final results of a phase II, open-label, clinical trial (OPTIMIX-ACROSS Study) BMC Cancer 14 https://doi.org/10.1186/1471-2407-14-865 PMID: 25417182 PMCID: 4251687
38. Iwamoto S, Maeda H, and Hazama S, et al (2018) Efficacy of CapeOX plus cetuximab treatment as a first-line therapy for patients with extended RAS/BRAF/PIK3CA Wild-type advanced or metastatic colorectal cancer J Cancer 9 4092–4098 https://doi.org/10.7150/jca.26840 PMID: 30519308 PMCID: 6277612
39. Borner M, Koeberle D, and Von moos R, et al (2008) Adding cetuximab to capecitabine plus oxaliplatin (XELOX) in first-line treatment of metastatic colorectal cancer: a randomized phase II trial of the swiss group for clinical cancer research SAKK Ann Oncol 19 1288–1292 https://doi.org/10.1093/annonc/mdn058 PMID: 18349029
40. Maughan TS, Adams RA, and Smith CG, et al (2011) Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial Lancet 377 2103–2114 https://doi.org/10.1016/S0140-6736(11)60613-2 PMID: 21641636 PMCID: 3159415
41. Soda H, Maeda H, and Hasegawa J, et al (2015) Multicenter phase II study of FOLFOX or biweekly XELOX and Erbitux (cetuximab) as first-line therapy in patients with wild-type KRAS/BRAF metastatic colorectal cancer: the FLEET study BMC Cancer 15 https://doi.org/10.1186/s12885-015-1685-z PMID: 26467662 PMCID: 4607014
42. Moosmann N, Von Weikersthal LF, and Vehling-Kaiser U, et al (2011) Cetuximab plus capecitabine and irinotecan compared with cetuximab plus capecitabine and oxaliplatin as first-line treatment for patients with metastatic colorectal cancer: AIO KRK-0104 - a randomized trial of the German AIO CRC study group J Clin Oncol 29 1050–1058 https://doi.org/10.1200/JCO.2010.31.1936 PMID: 21300933
43. Ocvirk J, Boc M, and Rebersek M, et al (2011) Efficacy and safety of XELIRI plus cetuximab versus XELOX plus cetuximab in first-line treatment of metastatic colorectal cancer (mCRC) J Clin Oncol 29 e14122–e14122 https://doi.org/10.1200/jco.2011.29.15_suppl.e14122
44. Mazard T, Ychou M, and Thezenas S, et al (2012) Feasibility of biweekly combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in patients with metastatic solid tumors: results of a two-step phase I trial: XELIRI and XELIRINOX Cancer Chemother Pharmacol 69 807–814 https://doi.org/10.1007/s00280-011-1764-z
45. Passardi A, Scarpi E, and Gelsomino F, et al (2017) Impact of second-line cetuximab-containing therapy in patients with KRAS wild-type metastatic colorectal cancer: results from the ITACa randomized clinical trial Sci Rep 7 https://doi.org/10.1038/s41598-017-11048-9 PMID: 28874797 PMCID: 5585399
46. Iwamoto S, Hazama S, and Kato T, et al (2014) Multicenter phase II study of second-line cetuximab plus folinic acid/5-fluorouracil/irinotecan (FOLFIRI) in KRAS wild-type metastatic colorectal cancer: the FLIER study Anticancer Res 34 1967–1974 PMID: 24692733
47. Ozaslan E, Topaloglu US, and Inanc M, et al (2017) Efficacy and safety of cetuximab plus FOLFOX in second-line and third-line therapy in metastatic colorectal cancer J B U ON 22 863–868
48. Chen Y, Cao D, and Bi F, et al (2014) Biweekly cetuximab plus FOLFIRI/irinotecan as first/second-line chemotherapy for patients with KRAS wild-type metastatic colorectal cancer: a retrospective analysis in Southwest Chinese population Med Oncol 31 https://doi.org/10.1007/s12032-014-0935-2
49. Agg H, Han Y, and Cui ZL (2021) Real-world data on overall survival associated with biweekly versus weekly cetuximab among metastatic colorectal cancer (mCRC) patients in the United States J Clin Oncol 39 33–33 https://doi.org/10.1200/JCO.2021.39.3_suppl.33
50. Matsuda A, Yamada T, and Jamjittrong S, et al (2020) Comparison between biweekly and weekly cetuximab in patients with metastatic colorectal cancer: a meta-analysis Anticancer Res 40 3469–3476 https://doi.org/10.21873/anticanres.14333 PMID: 32487646
51. Kasper S, Foch C, and Messinger D, et al (2021) Noninferiority of cetuximab every-2-weeks versus standard once-weekly administration schedule for the first-line treatment of RAS wild-type metastatic colorectal cancer Eur J Cancer 144 291–301 https://doi.org/10.1016/j.ejca.2020.11.013 PMID: 33383349
52. FDA (2020) FDA Approves New Dosing Regimen for Pembrolizumab (FDA) pp 2020–2021
53. Van Cutsem E, Köhne C-H, and Hitre E, et al (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer N Engl J Med 360 1408–1417 https://doi.org/10.1056/NEJMoa0805019 PMID: 19339720
54. Bylsma LC, Dean R, and Lowe K, et al (2019) The incidence of infusion reactions associated with monoclonal antibody drugs targeting the epidermal growth factor receptor in metastatic colorectal cancer patients: a systematic literature review and meta-analysis of patient and study characteristics Cancer Med 8 5800–5809 https://doi.org/10.1002/cam4.2413 PMID: 31376243 PMCID: 6745824
55. Zhu J, Zhao W, and Liang D, et al (2018) Risk of fatigue in cancer patients receiving anti-EGFR monoclonal antibodies: results from a systematic review and meta-analysis of randomized controlled trial Int J Clin Oncol 23 389–399 https://doi.org/10.1007/s10147-017-1218-7
56. García-Alfonso P, Mũoz-Martin AJ, and Alvarez-Suarez S, et al (2010) Bevacizumab in combination with biweekly capecitabine and irinotecan, as first-line treatment for patients with metastatic colorectal cancer Br J Cancer 103 1524–1528 https://doi.org/10.1038/sj.bjc.6605907 PMID: 20978503 PMCID: 2990572
57. Suzuki N, Hazama S, and Nagasaka T, et al (2019) Multicenter phase II study of biweekly CAPIRI plus bevacizumab as second-line therapy in patients with metastatic colorectal cancer (JSWOG-C3 study) Int J Clin Oncol 24 1223–1230 https://doi.org/10.1007/s10147-019-01473-3 PMID: 31144145 PMCID: 6736909
58. Hoffman RS (2007) Understanding the limitations of retrospective analyses of poison center data Clin Toxicol 45 943–945 https://doi.org/10.1080/15563650701233370

