Immunophenotypic characterisation of non-Hodgkin lymphomas at a tertiary hospital in Ghana
Joseph Adomako1, Afua O D Abrahams2 and Yvonne A Dei-Adomakoh3
1G2 Medical Laboratory Services, 37, Accra, GA-007-6041, Ghana
2Department of Pathology, University of Ghana Medical School, University of Ghana, Accra, GA-221-9384, Ghana
3Department of Haematology, University of Ghana Medical School, University of Ghana, Accra, GA-221-9384, Ghana
Abstract
Background: Non-Hodgkin lymphomas (NHLs) are a heterogeneous group of clonal lymphoid tumours originating from lymphocytes. They constitute about 90% of an estimated 3%–4% worldwide distribution of malignant lymphomas among various cancers. Despite the continuous rise and associated deaths, research on NHLs, and in particular the area of immunophenotypic spectrum is limited in Ghana and sub-Saharan Africa.
Methods: A retrospective, descriptive study in which archived tissue blocks of histologically diagnosed NHLs at a tertiary hospital in Accra, Ghana, were used. Antigenic phenotypes were determined by immunohistochemistry.
Results: A total of 66 cases of NHLs, with a mean age of 50.2 ± 16.1 years, were selected for the study. Among the targeted markers, cluster of differentiation 20 (CD20) was the most commonly expressed in 89.4% (59) cases. Immunohistochemistry studies revealed a greater proportion of B cell lymphomas of 89.4%. Five subtypes were successfully identified, of which diffuse large B cell lymphoma constitutes the predominant group (40.9%). A significant association was observed between phenotypic cell types and outcomes of NHLs (p = 0.011).
Conclusion: Adult NHLs were mostly due to the malignant transformation of B cells with diffuse large B cell lymphoma being the commonest subtype. The present study therefore serves as preliminary data for further research towards the adoption of an improved treatment regimen and management of NHLs.
Keywords: non-Hodgkin lymphomas, immunohistochemistry, subtypes, diffuse large B cell lymphoma, Ghana
Correspondence to: Yvonne Dei-Adomakoh
Email: deiadom@yahoo.com
Published: 31/10/2022
Received: 27/04/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Non-Hodgkin lymphomas (NHLs) are a heterogeneous group of clonal lymphoid tumours originating from B cell, T cell or natural killer (NK) lymphocytes. NHLs significantly increase with age, with a peak incidence age of 55–60 years [8].
The global cancer statistics by the Global Cancer Observatory (GLOBOCAN) estimate 544,000 new cases of NHLs and 260,000 deaths due to NHLs in 2020 [16]. Haematological malignancies represent about 7% of all cancers worldwide [13]. Malignant lymphomas which comprise NHLs and Hodgkin Lymphomas (HLs) make up an estimated 3%–4% worldwide of the distribution of malignancies [9]. NHLs make up 80%–90%, and HLs account for the remaining of all malignant lymphomas [5]. They are the most common haematological malignancy and are currently the fifth most common cancer diagnosis among patients in some developed countries [8]. A national strategy for control of cancer document developed by the Ministry of Health, Ghana, showed that out of 830 adult haematological malignancies recorded over a decade at the Haematology/Oncology clinic, Korle Bu Teaching Hospital (KBTH), a tertiary hospital in Accra Ghana, NHLs recorded the highest frequency of 218 (26.3%) [12]. A retrospective review of already diagnosed NHLs of patients aged 13 and above at the same unit, in a period of 4 years and 10 months showed a dramatic figure of 279 [6].
The study sought to determine the immunophenotypic patterns of NHLs by immunohistochemistry and to characterise the NHLs according to pathological cell and subtypes. Association between cell types and outcomes of NHLs was also determined.
Diversity of NHLs requires the determination of a particular cell type and disease sub-type in order to maximise treatment through the selection of the most appropriate therapy. However, diagnosis and treatment are largely based on morphology due to limited resources and financial constraints. Dei-Adomakoh et al [6] reported 17 out of 279 (only 6.1%) NHL cases with phenotypic studies. Research on the NHLs and the immunophenotypic distribution is limited in Africa. As of now, not much systematic scientific assessment has been carried out on NHLs in the area of immunophenotypic spectrum in Ghana. There is therefore no clear-cut data on the distribution of phenotypic cells and sub-types necessary for policy directions on local treatment protocols.
Materials and methods
This was a retrospective, descriptive study in which archived formalin-fixed paraffin-embedded tissue blocks of morphologically diagnosed NHL cases were used. The cases of patients aged 15 and above were received at the Haematology Department, KBTH, Accra, Ghana, between 2015 and 2019.
A data abstraction form was used to retrieve personal, laboratory and clinical information from the folders of patients aged 15 and above. Archived tissue blocks and haematoxylin and eosin (H&E) stained slides of these patients were retrieved at the Pathology Department, KBTH.
Selected tissue blocks were sectioned at 4 microns and mounted on positively charged slides.
Immunohistochemistry studies were performed using BenchMark GX automated immunohistochemistry/in situ hybridization (IHC/ISH) staining instrument (Tucson, VENTANA-Roche, USA). Monoclonal antibodies, anti-CD20 (L26, VENTANA-Roche, Tucson, USA), anti-CD3 (2GV6, VENTANA-Roche, Tucson, USA), anti-CD5 (SP19, VENTANA-Roche, Tucson, USA) and anti-CD23 (SP23, VENTANA-Roche, Tucson, USA) were used with OptiView DAB IHC detection kit [2].
Examination of slides
Immunohistochemical stained slides were examined under transmitted light illumination. Two pathologists scored the slides independently based on the colour intensity and the percentage of involved tumour cells. Final scores were then determined by averaging independent scores. Discordant scores were re-examined by a third, independent individual and final score made from the two closest.
Data analysis
Data were entered into 2016 Microsoft excel and analysed using Statistical Package for Social Science version 22. A summary was presented using the descriptive statistics of mean, median, standard deviation and frequency of variables. Graphical displays such as pie chart, frequency distributions or scattergrams, were created where appropriate. Pearson’s chi-square test was used to determine associations between phenotypic cell types (based on immunological markers) and treatment outcome. In cases of sparse data, the Fisher’s exact test was used. All tests were two-sided and a p-value less than 0.05 was interpreted as significant.
Results
A total of 66 cases of NHL were successfully selected for immunohistochemistry studies. The youngest age recorded for the study was 16 years while the oldest was 78 years. The average age of participants in this study was 50.2 ± 16.1 years. Majority 45.5% (n = 30) of the participants fall within 41–60 year group. More than half 60.6% (n = 40) of the participants were males. These are shown in Table 1.
Antigen expression in various NHLs
Markers used in the study were CD3, CD5, CD20 and CD23. CD3 antigens were expressed in only 10.6% (n = 7) of cases. Also, 31.8% (n = 21) were positive for CD5 antigens. Majority 89.4% (n = 59) of the cases were positive for CD20. Additionally, 28.8% (n = 19) of the cases were positive for CD23 antibodies. These are shown in Table 2.
Characterisation of NHLs according to cell type
Figure 1 shows the distribution of NHLs according to pathological cell type. Majority 89.4% (n = 59) were due to malignant transformation of B cells.
Phenotypic distribution of NHLs
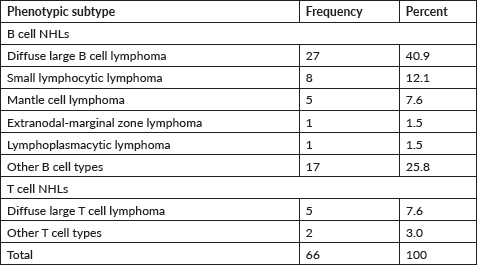
The commonest subtype 40.9% (n = 27) observed in the study was diffuse large B cell lymphoma. Small lymphocytic lymphoma was identified in 12.1% (n = 8) cases. Also, significant proportion 7.6% (n = 5) of cases were mantle cell lymphoma. T cell lymphomas were mostly diffuse large forms with 7.6% (n = 5). However, substantial percentage of B cell lymphomas 25.8% (n = 17) were not classified into specific subtypes. These are shown in Table 3.
Table 1. Demographic characteristics of participants.
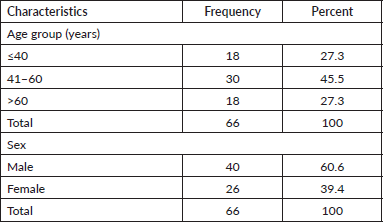
Table 2. Immunophenotypic pattern of NHLs.
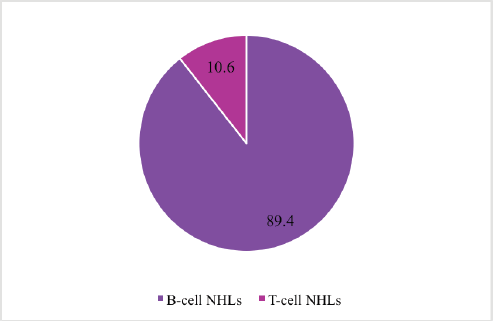
Figure 1. Distribution of NHLs by cell type.
Table 3. Distribution of subtypes of NHLs.
Histopathological distribution of NHLs
Table 4 shows that diffuse large forms were the majority of the histopathologically diagnosed NHLs constituting more than 33.3% (n = 22), and followed by small cell NHLs, 18.2% (n = 12).
Table 4. Diagnosis of NHLs by morphology.
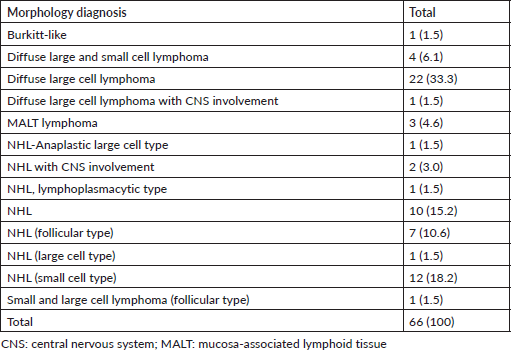
Selected images of slides of NHLs
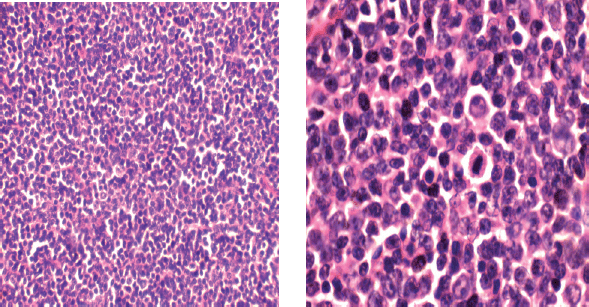
Figure 2. Photomicrograph of diffuse large cell lymphoma.
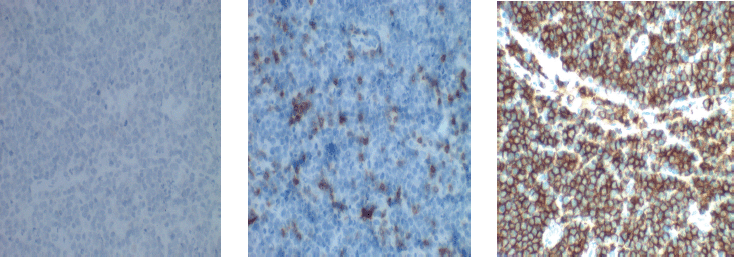
Figure 3. Photomicrograph of B-cell lymphoma.
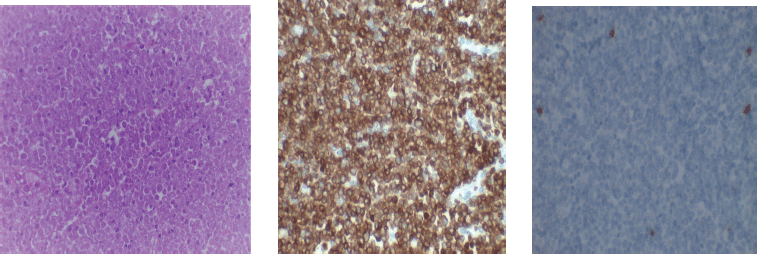
Figure 4. Photomicrograph of T-cell lymphoma.
In Figure 2, H&E (×10, Left; ×40, right) showing a diffuse proliferation of large lymphoid cells. The cytoplasm is moderate to abundant, nuclei are irregular and nucleoli conspicuous. Mitotic figures are frequent.
Immuno-histochemical staining in Figure 3 shows strong membranous positivity for CD20. (×40, right). CD 3 staining (×10, left) is negative and CD 5 staining (×40, middle) highlights the reactive lymphocytes present.
In Figure 4, H&E (×40, Left) showing a diffuse proliferation of variably sized lymphocytes with irregular nuclei; the neoplastic cells show strong cell membrane positivity to CD 3 (×40, middle) and do not express CD 20 (×40, right)
Cell types and outcomes
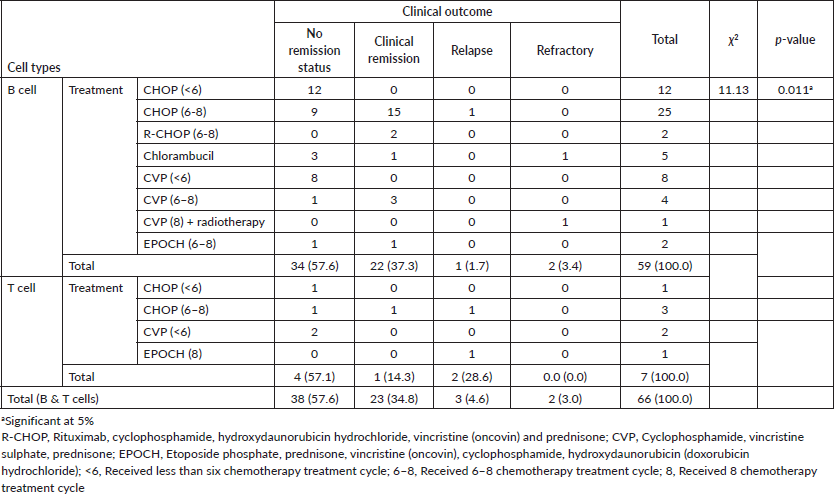
In this study, 37.3% (n = 22) of those with B cell lymphomas had clinical remission. A remarkable figure of 15 out of the 22 had completed at least a sixth treatment cycle for the cyclophosphamide, hydroxydaunorubicin hydrochloride, vincristine (oncovin) and prednisone (CHOP) regimen. A proportion of 14.7% (n = 1) of those with T cell lymphomas had clinical remission. Also, only 1.7% (n = 1) of those with B cell lymphoma had a relapse while 28.6% (n = 2) of those with T cell had a relapse. There was a significant association between cell types and clinical outcomes (p = 0.011). These are shown in Table 5.
Discussion
Immunohistochemistry studies throw more light and confirm already morphologically diagnosed NHLs. This study was able to determine the phenotype of abnormal forms revealed by morphology and further characterised these neoplastic populations according to specific lymphoid lineage.
Age and sex are known predisposing factors associated with NHLs according to Alyahya et al [3]. For the purpose of this study, the age of participants were put into adolescent and young adults (AYAs) (15–40) and older adults groups based on risk and guidance from National Cancer Institute, USA. Older adults were further put into two groups (41–60 and >60). The mean age was 50.2 ± 16.1 years and comparable to a previous report done in the same unit by Dei-Adomakoh et al [6]. Again, age distribution shows that a greater proportion (45.5%) of participants were within 41–60 older adult year group. Together, these indicate a relatively early onset of disease contrary to reports from Saudi Arabia and United states by Alyahya et al [3] and Teras et al [17], respectively, which follow the pattern that majority of NHLs occur after 60 years. The early occurrence of NHL may be attributed to younger age distribution among the population and personal risk factors. Majority of Ghana’s population is between 15 and 59 years according to the Ghana Statistical Service [11].
Table 5. Association between cell types of NHLs and clinical outcomes.
Meanwhile, the study observed majority (72.8%) of cases in the older adult group as a whole and therefore suggests that NHL generally increases with age. Additionally, this agrees with the data generated by the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program (SEER), USA, which shows a higher age-standardised rate of 10.5–116.4 per 100,000 in the older adult groups as compared to 1.8–7.2 per 100,000 for AYA group [15].
In the study, males outnumbered females with 1.54:1 male to female ratio. This observation is in agreement with other authors (Onwubuya et al [14]; Yakubu et al [18]; Alyahya et al [3]. Also, male predominance was evident in both B and T cell types, although B cell lymphomas especially subtypes such as diffuse large B-cell lymphoma (DLBCL) and Burkitts lymphoma occur more in males according to SEER [7].
The greater occurrence of NHLs in males may be attributed to socioeconomic factors such as occupation. Thus, the issue of sex-segregation continues to linger in labour especially in the traditional (informal) occupations in Ghana according to Abukari & Odai [1] such that certain male dominated jobs including pesticide applicators, grain millers, wood and forestry workers and farmers may be associated with higher NHLs in men in the study. Moreover, Horesh and Horowitz [7] linked increasing number of pregnancies and live births as well as sex hormones such as oestrogen to the reduction in the risk of developing NHLs in females.
This work mainly utilised the pattern of expression of markers of differentiation. A panel of differentiation markers CD20, CD23 and CD5 were used to confirm neoplasms of B cells while T cell neoplasms were identified with CD3 and CD5. CD20, a pan-B cell maker, was observed in 59 (89.4%) of tumours while 27 of these co-expressed CD23. There was simultaneous co-expression of CD5 with some CD3 and largely with some CD20 proteins. CD3, a pan T cell marker, was least expressed (10.6%). This is similar to findings in a previous study where at least CD20, CD5, CD23 and CD3 markers were used. CD20 was the commonest expressed marker (81.3%) followed by CD5 (50%). However, CD23 was the least expressed in that study [4].
The immunophenotypic findings show that 89.4% of the NHLs were due to malignant transformation of B cells, whereas relatively few cases (10.6%) were derived from T cells. Research work done by Onwubuya et al [14], Perry et al [19], Sharma et al [20], Jairajpuri et al [21] and Laurini et al [22] in Nigeria, South Africa, India, Iran and Central and South America, respectively, indicates overall greater proportions of B cell lymphomas and hence, consistent with the findings of this study. Documented B cell lymphomas for these studies ranged from 66% to 98.8%. Despite this similarity, differences are observed in the incidence rates of B and T cell lymphomas. T cell lymphomas reported by Jairajpuri et al [21] were about three times higher (34%) than that reported in my study while Onwubuya et al [14] had only 1.2%. The disparities may be attributed to different geographical locations with varied but related underlying risk factors. For example, some infectious agents [such as H. pylori, Human T-lymphotropic virus type 1 (HTLV-1) and HIV/AIDS] are known to be strongly associated with particular NHLs such that higher prevalence of those infections in various locations may correspond to the development of greater proportion of specific cell and sub-types of NHLs. Karin [10] associates much higher adult T cell lymphoma in Japan and Caribbean to the endemic nature of HTLV-1 in these locations. Karin [10] also reports frequent occurrence of some rare T-cell neoplasms in Asia than other continents.
Moreover, in this study, NHLs were put into broad subtypes based on immunophenotype and knowledge on the relevant clinical features and morphology. CD20 positive diffuse large forms were broadly categorised as diffuse large B cell lymphomas without specific division into germinal center B-cell (GCB) and activated B cell (ABC)-like types. CD20 positive small B cell neoplasms with simultaneous co-expression of CD5 and CD23 were identified as small lymphocytic lymphoma. Mantle cell lymphomas were confirmed by the expression of CD20 and CD5 without CD23. About a quarter of B cell neoplasms (25.8%) with morphology and features other than the ones explained above could not be further categorised beyond the cell types. Also, CD3 positive diffuse large cell types and others identified as T cell lymphomas in the study could not be classified into specific subtypes. The vast majority of subtypes recorded were diffuse large B cell lymphoma (40.9%). Although other subtypes, small lymphocytic lymphoma (12.1%), mantle B cell lymphoma (7.6%), extra-nodal B cell marginal zone lymphoma (1.5%) and lymphoplasmacytic lymphoma (1.5%) were observed in the study, ranking of rate of occurrences cannot be appropriately done as significant proportion (25.8%) remain unclassified. The findings of the study are consistent with previous work in Nigeria by Onwubuya et al [14] and in India by Atri et al [4]. Onwubuya et al [14] reported 47% for DLBCL while Atri et al [4] had 66.66% for DLBCL and 3.7% for marginal B cell lymphoma. Conversely, more mantle cell lymphoma (7.4%) than small lymphocytic lymphoma (3.7%) was obtained by Atri et al [4] and therefore indicates geographical locations with increased possible triggers of particular lymphoma types.
Treatment outcomes were categorised as clinical remission, refractory and relapse. Remission status for greater proportion of both B cell (57.6%) and T cell (57.1%) lymphomas could not be determined as patients were lost to follow-up before completion of a required treatment cycle. A distribution of 37.3% and 14.3% for B and T cell NHLs, respectively, was recorded for clinical remission. This is consistent with the second year outcome of a 3-year follow-up on the outcomes of NHLs by Dei-Adomakoh et al [6], which recorded an impressive outcome (78.9% for clinical remission) at the first year with a sharp decline in clinical remission (31.5% for second year and 11.8% for third year) in the following years due to loss to follow-up. Furthermore, a significant association between cell types and clinical outcome was observed for the study (p = 0.011), which indicates a varied treatment response and behaviour of NHLs as a result of heterogeneous cell types.
Additional immunological markers including CD10, B cell lymphoma (BCL) 2, BCL6, multiple myeloma oncogene (MUM) 1, CD79a, CD2, CD30 and TdT of B or T/NK cell lineage would have been useful to determine the distribution according to specific subtypes. Clinical outcome of some selected cases could not be determined due to failure of clinical attendance and treatment discontinuation. Co-morbidities such as HIV which would have had an impact on outcomes were not included in the data analysis as it was not routinely done at our department until 5 years ago. In spite of the limitations, the reliability and validity of the study was not compromised.
Conclusion
NHLs were mostly due to the malignant transformation of B cell lineage with phenotypic distribution of 89.4% for B cells and 10.6% for T cells. The majority of B and T cell lymphomas occurred in males. The predominant subtype observed in the study was diffuse large B cell lymphoma. A significant association was observed between phenotypic cell types and outcomes of NHLs. The present study therefore serves as preliminary data for further research towards the adoption of an improved treatment regimen and management of NHLs in Ghana.
Acknowledgments and funding
The authors wish to express their profound appreciation to Roche Pharmaceuticals for the grant support that made it possible for them to conduct this study. The authors are also grateful to lecturers and staff of Department of Haematology and Pathology University of Ghana Medical School for their support.
Conflicts of interest
The authors declare no competing financial interests.
References
1. Abukari R and Odai RO (2018) Gender and the labour market in Ghana: the relationship in terms of the family, the market and the State Adv Appl Sociol 8 285–294 https://doi.org/10.4236/aasoci.2018.84015
2. Adomako J (2020) Immunophenotypic Characterization of Non-Hodgkin Lymphomas (University of Ghana) [http://ugspace.ug.edu.gh/bitstream/handle/123456789/35918/Immunophenotypic Characterization of Non-Hodgkin Lymphomas.pdf?sequence=1&isAllowed=y]
3. Alyahya N, Adiga B, and Alwadei A, et al (2019) The clinico-pathological profile of non‑Hodgkin’ s lymphoma in Aseer region of Saudi Arabia BMC Res Notes 12(418) 1–7 https://doi.org/10.1186/s13104-019-4447-1
4. Atri SK, Singhal A, and Kataria SP, et al (2020) Correlation between clinical features and immunohistochemistry profile of lymphoma patients Int J Biomed Adv Res 11(01) 1–6
5. Batra R, Kaur H, and Jindal S (2014) Extranodal large B-cell type aggressive non-Hodgkin’s lymphoma Indian J Dent 5(4) 225–228 https://doi.org/10.4103/0975-962X.144742 PMCID: 4260391
6. Dei-Adomakoh YA, Asare EV, and Amedonu ESA, et al (2017) Sub-types and treatment outcomes of adolescent and adult non-Hodgkin lymphomas in a resource poor setting J Hematol Oncol Res 2(3) 15–23 https://doi.org/10.14302/issn.2372-6601.jhor-17-1423
7. Horesh N and Horowitz NA (2014) Does gender matter in non-Hodgkin lymphoma? Differences in epidemiology, clinical behavior, and therapy Rambam Maimonides Med J 5(4) 1–8 https://doi.org/10.5041/RMMJ.10172
8. Howard RM and Hamilton JP (2013) Haematology: An Illustrated Colour Text 4th edn, (Edinburgh: Churchill Livingstone Elsevier)
9. Huh J (2012) Epidemiologic overview of malignant lymphoma Korean J Hematol 47 92–104 https://doi.org/10.5045/kjh.2012.47.2.92 PMID: 22783355 PMCID: 3389073
10. Karin E (2006) Epidemiology and etiology of non-Hodgkin lymphoma – a review Acta Oncol 45(3) 258–271 https://doi.org/10.1080/02841860500531682
11. Kpessa-Whyte M (2018) Aging and demographic transition in Ghana: state of the elderly and emerging issues Gerontologist 58(3) 403–408 https://doi.org/10.1093/geront/gnx205 PMID: 29381779
12. Ministry of Health-Ghana (2011) National Strategy on Cancer Control in Ghana (2012-2016) [https://www.iccp-portal.org/sites/default/files/plans/Cancer Plan Ghana 2012-2016.pdf] Date accessed 25/09/19
13. Miranda‑Filho A, Piñeros M, and Znaor A, et al (2019) Global patterns and trends in the incidence of non-Hodgkin lymphoma Cancer Causes Control 30 489–499 https://doi.org/10.1007/s10552-019-01155-5
14. Onwubuya IM, Adelusola KA, and Durosinmi MA, et al (2015) Lymphomas in Ile-Ife, Nigeria: immunohistochemical characterization and detection of Epstein-Barr virus encoded RNA J Clin Diagn Res 9(6) 14–19
15. Sandlund JT and Martin MG (2016) Non-Hodgkin lymphoma across the pediatric and adolescent and young adult age spectrum Hematol Am Soc Hematol Educ Program 2016(1) 589–597 https://doi.org/10.1182/asheducation-2016.1.589
16. Sung H, Ferlay J, and Siegel RL, et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 71(3) 209–249 https://doi.org/10.3322/caac.21660 PMID: 33538338
17. Teras LR, Desantis CE, and Cerhan JR, et al (2016) 2016 US lymphoid malignancy statistics by World Health Organization subtypes CA Cancer J Clin 66(6) 443–459 https://doi.org/10.3322/caac.21357 PMID: 27618563
18. Yakubu M, Ahmadu BU, and Yerima TS, et al (2015) Prevalence and clinical manifestation of lymphomas in North Eastern Nigeria Indian J Cancer 52(4) 551–555 https://doi.org/10.4103/0019-509X.178435
19. Perry AM, Perner Y, and Diebold J, et al (2016) Non-Hodgkin lymphoma in Southern Africa: review of 487 cases from The International Non-Hodgkin Lymphoma Classification Project British J Haematol 172 716–723 https://doi.org/10.1111/bjh.13885
20. Sharma M, Mannan R, and Madhukar M, et al (2014) Immunohistochemical (IHC) Analysis of Non-Hodgkin’s Lymphoma (NHL) Spectrum According to WHO/REAL Classification: A Single Centre Experience from Punjab, India J Clin Diagn Res 8(1) 46–49 https://doi.org/10.7860/JCDR/2014/8173.3988
21. Jairajpuri ZS, Ghai R, and Saluja S, et al (2017) Expression of apoptosis related and proliferative proteins in malignant lympho-proliferative disorders Iran J Pathol 12(3) 231–240
22. Laurini JA, Perry AM, and Boilesen E, et al (2012) Classification of non-Hodgkin lymphoma in Central and South America: a review of 1028 cases Blood 120(24) 4795–4802 https://doi.org/10.1182/blood-2012-07-440073


