Characteristics and clinical outcomes of pulmonary sarcomatoid carcinoma: experience from Tata Memorial Centre
Suresh Kumar Bondili, Ravindra Nandhana, Aditya Dhanawat, Vanita Noronha, Amit Joshi, Vijay Maruti Patil, Nandini Menon, Rajiv Kumar Kaushal, Anuradha Choughule, Sabita S Jiwnani, Amit Janu and Kumar Prabhash
Tata Memorial Hospital, Mumbai, Maharashtra, 400 012, India
Abstract
Background: Pulmonary sarcomatoid carcinoma (PSC) constitutes a heterogeneous group of poorly differentiated non-small cell lung cancers. Since these are rare tumours, we sought to determine the characteristics and clinical outcomes of these patients treated at our centre.
Methods: We did a retrospective evaluation of all patients diagnosed with PSC between January 2013 and September 2020 at the Tata Memorial Hospital, Mumbai, India. Baseline demographic and treatment data and outcomes were obtained retrospectively from electronic medical records and survival was calculated by using the Kaplan–Meier method.
Results: Out of 151 patients diagnosed with PSC during this period, 129 were included in the final analysis. The clinical stage was stage I in 3 (2.03%), stage II in 4 (3.1%), stage III in 35 (27.1%) and stage IV in 87 (67.4%). The median follow-up duration was 32 months (range, 15.0–48.9). The median overall survival (OS) of patients who received curative surgery was 18 months (95% confidence interval (95% CI), 2.59–33.4); concurrent chemoradiation was 11 months (95% CI, 2.99–19); palliative chemotherapy was 8 months (95% CI, 5.24–10.75) and best supportive care was 1 month (95% CI, 0.43–1.57, p = 0.001). On multivariate analysis, the presence of brain metastasis (p = 0.018; hazard ratio (HR), 2.47; 95% CI, 1.34–4.49) and the administration of chemotherapy (p = 0.037; HR, 2.2; 95% CI, 1.04–4.94) were the only factors impacting the OS.
Conclusion: PSC usually presents in advanced stages and is associated with a poor prognosis.
Keywords: non small cell lung cancer, sarcomatoid carcinoma, retrospective analysis
Correspondence to: Kumar Prabhash
Email: kumarprabhashtmh@gmail.com
Published: 08/08/2022
Received: 02/02/2022
Publication costs for this article were supported by ecancer (UK Charity number 1176307).
Copyright: © the authors; licensee ecancermedicalscience. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Pulmonary sarcomatoid carcinoma (PSC) encompasses a heterogeneous group of non-small cell lung cancers (NSCLC) with poor differentiation. It is rare and comprises about 0.3%–3% of all lung cancer cases [1].
PSCs are epithelial tumours with a component of sarcomatous differentiation [2]. They include five different histological varieties as per the 2004 World Health Organization (WHO) classification of NSCLC, namely pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma, carcinosarcoma and pulmonary blastoma [1, 3].
PSC has a predilection for males and heavy smokers [4]. The usual age at the time of diagnosis is the sixth decade of life. However, pulmonary blastoma occurs with similar prevalence in both men and women and the age of presentation is lower than the other types of PSC and generally occurs in the fourth decade [5, 6]. There are no signs or symptoms which are specific to PSC; they resemble those of conventional NSCLC [7]. Patients with PSC are treated along the lines of NSCLC. Surgery is the mainstay of the management of the early-stage disease [8]. Many retrospective analyses and propensity score analyses have revealed that these patients tend to relapse early and have a worse survival in comparison with patients of similar stage NSCLC [9–12]. PSCs are aggressive, respond poorly to chemotherapy and are less sensitive to radiotherapy. Immune checkpoint inhibitors (ICIs) might have a critical role in the treatment of PSCs as they have high programmed death ligand-1 (PD-L1) scores and remarkable responses to ICIs have been described [13–19]. Moreover, the tumour mutational burden was also found to be high in about 40.6% of patients with PSC in a Chinese study [20].
Because of the complexity involved in the histological categorisation, a well-experienced onco-pathologist is required for an accurate diagnosis. Given the rarity of this disease, no randomised trials are available to guide appropriate clinical management, and the optimal treatment strategies for PSC are not known. Our study aims to evaluate the clinical characteristics, pathological features and outcomes of patients with PSC treated at our centre and may provide important clues for the optimal management of these patients.
Patients and methods
Patients
This is a retrospective analysis of patients with PSC diagnosed and treated by the thoracic disease management group of the Department of Medical Oncology, Tata Memorial Hospital, a tertiary cancer centre in Mumbai, India. All patients with pathologically diagnosed PSC between January 2013 and September 2020 and aged > 15 years were identified from the pathology database and included in this study. The cut-off date for survival follow-up was 30 September 2020. The demographic details, comorbidities, family history, histopathological details, radiologic data, treatment administered, response to therapy, follow-up and survival data were obtained retrospectively from the electronic medical records. The study was conducted by the principles laid down by the Declaration of Helsinki and the International Council for Harmonization-Good Clinical Practice.
Diagnosis and management
The pathological diagnosis was made on the biopsy specimens or after surgical resection by an experienced onco-pathologist. The tumours were classified according to the revised 2004 WHO classification of lung tumours [3]. All tumours were staged as per the tumour (T), nodes (N) and metastasis (M) staging system, American Joint Committee on Cancer, seventh edition. Evaluation of driver mutation status was done by real-time polymerase chain reaction for epidermal growth factor receptor (EGFR), immunohistochemistry for Anaplastic lymphoma kinase (ALK), ROS proto-oncogene 1 (ROS1) and PD-L1. Next-generation sequencing was done in some patients. Patients were managed as per the decision taken in the multidisciplinary thoracic disease management group meeting. The choice of chemotherapy was decided by the treating physician. Patients who failed to follow-up were contacted by telephone to assess their status and survival. Patients for whom no medical records were available were excluded from the study.
Clinical outcomes
In patients with metastatic disease on palliative therapy, response assessment was done by imaging every 2–3 months or at the development of clinical features of progression and was classified according to the Response Evaluation Criteria in Solid Tumors version 1.1 [21]. The progression-free survival (PFS) was defined as the interval from the start of systemic therapy to the date of progression or death due to any cause before disease progression. The patients who were lost to follow-up were censored on the date of their last follow-up. The overall survival (OS) was defined as the interval from the date of diagnosis to the date of death. Patients who were alive at the end of the study were censored at the date of the last follow-up.
Statistical analysis
As this was a retrospective study of a rare tumour, the sample size was not calculated. We included all patients fulfilling the eligibility criteria during the study period. All statistical analyses were done using the SPSS software (Version 23, IBM Corp, Armonk, NY, USA), and the Kaplan–Meier curve was generated using R software (Vienna, Austria). The categorical variables were summarised by frequency and percentage and the continuous variables by median and range. Survival analysis was done using the Kaplan–Meier method [22] and the hazard ratio (HR) was calculated using the Cox proportional model [23, 24]. Univariate and multivariate analyses were performed to compare the OS to various factors using the log-rank test and Cox regression analysis, respectively. All p values were two-sided with a 95% confidence interval (95% CI) level. A p-value of less than 0.05 was considered statistically significant.
Results
Baseline patient characteristics
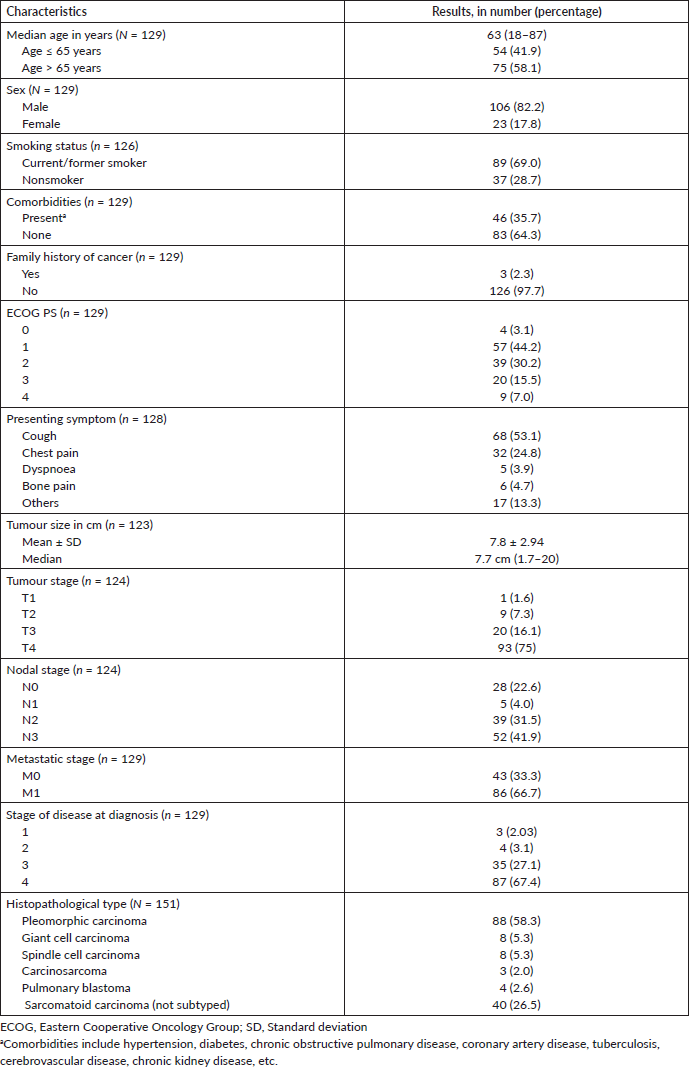
A total of 151 patients were diagnosed with PSC during the period between January 2013 and September 2020 (Figure 1). Follow-up data were available for 129 patients and were included in the analysis. The baseline demographic, clinicopathological and imaging characteristics are summarised in Table 1. The median age was 63 years (range, 18–87). Almost half the patients presented with poor performance status (PS) of 2–4.
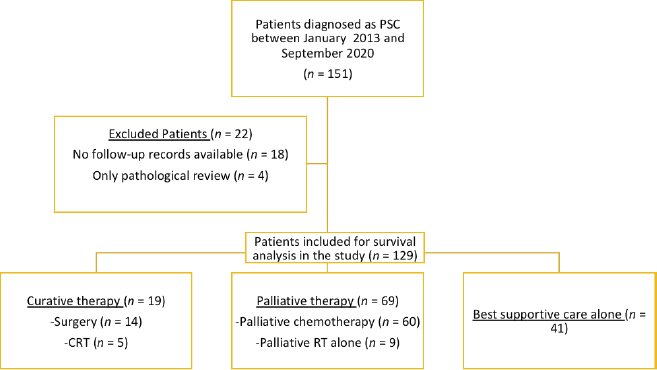
Figure 1. Patient flow diagram, showing the details of included and excluded patients. PSC, Pulmonary sarcomatoid carcinoma; CRT, Concurrent chemotherapy and radiotherapy.
Table 1. Demographic and tumour characteristics.
Disease-related characteristics
Eighty-six patients (66.7%) had metastatic cancer at the time of diagnosis. A majority of patients had multiple sites of metastasis including lung in 45 (34.9%), pleura in 35 (27.1%), bone in 30 (23.3%), adrenal in 29 (22.5%), brain metastases in 27 (20.9%) and non-regional lymph node in 24 (18.6%). Baseline PET-CT scan data was available in 71 (47%) patients, which revealed a high maximum standardized uptake value (SUVmax) in the majority of the patients. The median SUVmax was 20.2 (range, 5–45.7). Pleomorphic carcinoma was the predominant subtype of PSC, seen in about 88 (58%) patients.
Molecular characteristics
The reports of EGFR mutation testing were available for 57 patients, out of which 4 (7%) patients had an EGFR mutation (two patients had exon 19 deletions, one had exon 21 L858R mutation and one had both exon 19 deletion and exon 21 L858R mutation). ALK reports were available in 40 cases [either Immunohistochemisty (IHC) or fluorescence in situ hybridisation (FISH)] and of these, two patients (5%) had ALK rearrangement. ROS1 rearrangement was not detected in any of the 40 patients who were tested. FISH for mesenchymal epithelial transition factor (MET) amplification was negative in all the six tested patients. Next-generation sequencing was performed in four patients which revealed TP53 mutation with EGFR amplification in one patient, Kirsten ras oncogene homolog (KRAS) exon 2 missense mutation (p.G12D) with PIK3CA (Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha) exon 1 mutation in one, BRAF (B-Raf Proto-Oncogene, Serine/Threonine Kinase) V600E and KRAS exon 2 mutation (p.G12V) in one patient each.
Treatment received
Surgery
Curative surgery was performed in 14 (10.9%) of the 129 evaluable patients. One patient had an R+ resection. One patient died in the immediate postoperative period due to aspiration pneumonia. The various treatment modalities are summarised in Table 2.
Post-operative staging as per the histopathological report according to the TNM classification was stage I in 3 (21.4%), stage II in 3 (21.4%) and stage III in 8 (57.1%) patients.
A total of six patients with locally advanced disease (three had an unresectable primary tumour and three had single station N2 on endobronchial ultrasonography) received neoadjuvant chemotherapy. Of these six patients, three subsequently underwent surgery; the remaining three patients developed progressive metastatic disease following neoadjuvant chemotherapy. Platinum-based doublet combination was used in five patients; one patient who had pulmonary blastoma received an ifosfamide and doxorubicin combination. The response rate to neoadjuvant chemotherapy is summarised in Table 3. One patient received neoadjuvant chemo-radiotherapy and had a partial response.
Adjuvant therapy
Nine patients received adjuvant chemotherapy. Three received adjuvant postoperative radiotherapy (two due to N2 disease, one due to a positive margin). Platinum-based doublet combination was the most common regimen used in the adjuvant setting.
Radical radiotherapy
Five patients received radical intent radiotherapy with concomitant chemotherapy – indication being N2/N3 disease in three patients and unfit for surgery in two patients. Weekly paclitaxel 80 mg/m2 and carboplatin area under curve 2 mg/mL/minute were used as the concurrent chemotherapy regimen in all patients. One patient received stereotactic body radiation therapy to the lung as the primary treatment modality because of poor lung function.
Palliative therapy
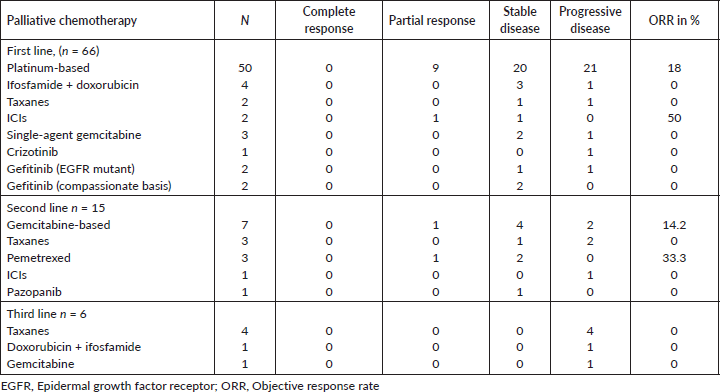
Of the 129 evaluable patients, palliative intent systemic therapy was given to 66 patients (51.2%), of which six patients received therapy post-progression on curative intent therapy. The systemic therapy regimens used were platinum-based chemotherapy in 50 (75.8%), non-platinum based in 9 (13.6), tyrosine kinase inhibitors in 5 (7.6%) and ICIs in 2 (3.0%). Four patients had an EGFR mutation, of which two patients received targeted treatment with gefitinib; one patient died due to sepsis and another patient defaulted before any targeted therapy could be given. The PFS for the two patients who received EGFR tyrosine kinase inhibitors (TKI) was 5 and 11 months. One patient had ALK rearrangement and received crizotinib in the first line; this patient progressed on crizotinib within 2 months. Two patients who received erlotinib on a compassionate basis in the first-line setting due to poor PS went on to receive second-line chemotherapy. One patient who had metastatic disease with BRAF V600E mutation was treated with only palliative chemotherapy as BRAF inhibitor was not feasible. Second-line chemotherapy could be administered in 15 (22.7%) patients. Gemcitabine was the most frequently used second-line agent. Only about 6 (9.1%) patients could receive third-line therapy. The response rates to the different lines of palliative chemotherapy are summarised in Table 4. The objective response rate (ORR) for first, second and third-line chemotherapy was 15.2%, 13.3% and 0%, respectively. Two patients received ICIs in the first line with pembrolizumab and the PFS were 4 and 8 months. One patient who received ICIs in the second-line setting with nivolumab had a PFS of 3 months. Forty-one (31.8%) patients were unfit for any form of therapy due to poor PS. These patients have been advised the best supportive care (BSC) alone.
Survival outcomes
One hundred and twenty-nine patients were included in the survival analysis (Figure 1). The median follow-up duration for the whole cohort was 32 months (range, 15.0–48.9). The median OS for the entire patient cohort was 5 months (95% CI, 3.4–6.5) (Figure 2). The median OS of patients who received curative-intent treatment (surgery or concurrent chemo-radiotherapy (CTRT)) was 14 months (95% CI: 6.5–21.4) and the corresponding 1- and 5-year survivals were 56% (Standard error (SE): 11.1) and 23% (SE: 9.9), respectively. The median OS for patients treated with palliative intent was 4 months (95% CI: 2.6–5.3, p = 0.00052) (Figure 2), and the corresponding 1- and 5-year survival rates were 20% (SE: 4.5) and 6% (SE: 3.2), respectively. The median OS according to the various treatment modalities is summarised in Table 5. For patients who received palliative treatment for locally advanced or metastatic disease, the median PFS for first-line chemotherapy was 4 months (95% CI: 2.95–5.04), second-line 4 months (95% CI: 0.33–7.67) and third-line 1 month (95% CI 0.21–1.78)
Factors affecting survival
The median OS for patients according to the stage of the disease was 28 months (95% CI: 0–71.20 months) for stage I, 9 months (95% CI: 0–18.80) for stage II, 6 months (95% CI: 2.85–9.14) for stage III and 4 months (95% CI: 2.83–5.16) for stage IV (HR: 1.52, 95% CI: 1.12–2.06), p = 0.007. The corresponding 2-year survival rates were 66.7% (SE: 27.2) for patients with stage I, 25% (SE: 21.7) for II, 17% (SE: 7.0) for stage III and 9% (SE: 4.1) for stage IV disease.
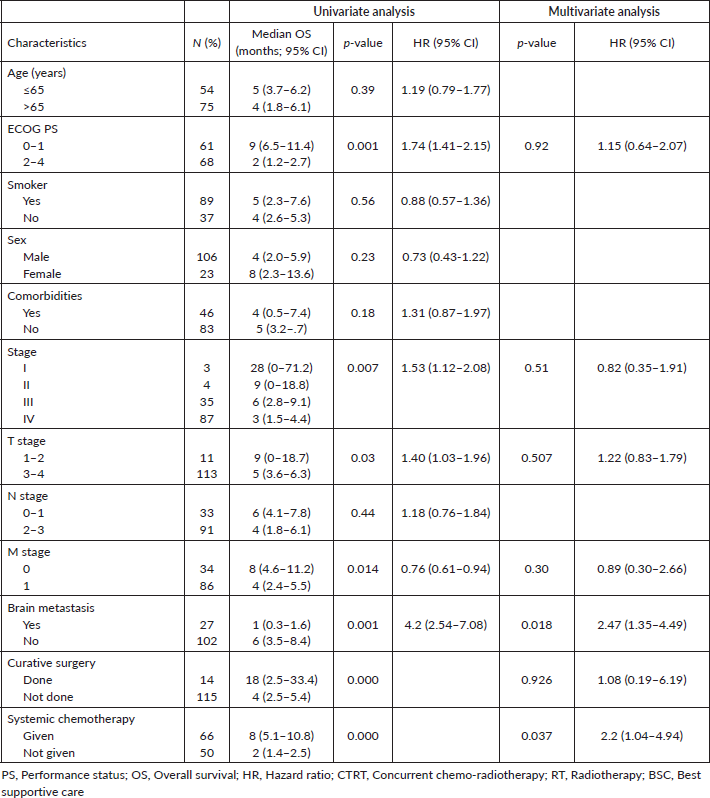
The factors significantly affecting the survival outcomes in the univariate analysis were Eastern Cooperative Oncology Group PS (ECOG PS), the intent of treatment, stage of the disease, T stage, M stage, presence of brain metastasis and the type of initial treatment received. On multivariate analysis, only brain metastasis and administration of palliative chemotherapy were found to be significant. The univariate and multivariate analyses are summarised in Supplementary Table 1.
Discussion
Our study represents one of the largest single-institution case series of patients with PSC.
In our study, the majority of the patients were smokers (70.6%), which is similar to that reported in other studies [25]. However, in an earlier study from our group, we found that 52% of patients with NSCLC were non-smokers; thus, smoking appears to be more common in patients with PSC than NSCLC in our patients [26]. However, we found that smoking did not significantly influence the survival of our patients with PSC. PSC was also seen more frequently in males than females (81.5% in males) [2, 11, 27]. The majority of patients who had metastatic disease had poor PS and the proportion of patients who received first-line or subsequent lines of therapy was low, which reflects the aggressive disease biology.
Table 2. Treatment modalities.
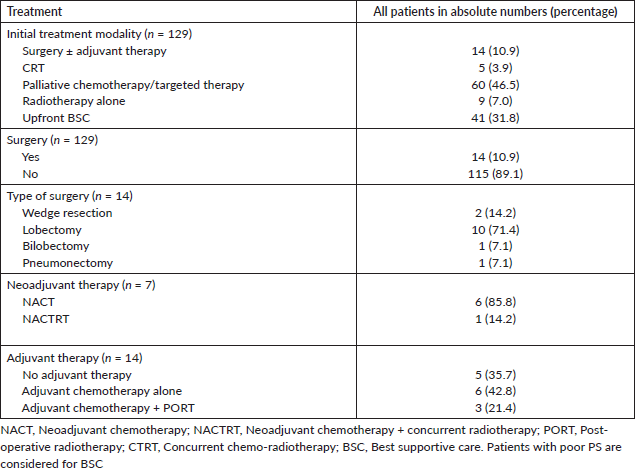
Table 3. Neoadjuvant chemotherapy regimens and the response to chemotherapy.
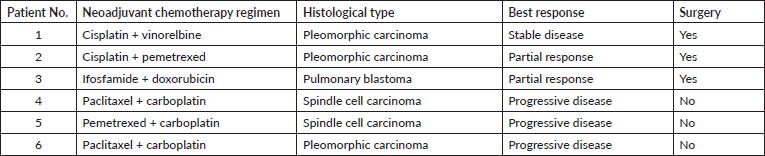
Table 4. Response rate to subsequent lines of chemotherapy.
We found that the median SUV max of the tumour on the baseline PET-CT was 20.2, which is similar to that reported in other studies which reported that PSC has intense SUV uptake values [28, 29]. This suggests that PSCs are F-fluorodeoxyglucose (FDG)-avid tumours and that performing a PET-CT scan would be an appropriate staging test, especially in patients who are being planned for radical therapy. The median tumour size was 7.5 cm and the majority presented with advanced T stages (about 91.4 % were of T3 and T4). In a similar study of 51 cases of PSC, the median T size was 6 cm and the incidence of T3/T4 disease was 60.7%. However, this frequency was relatively higher in our study when compared with case series from other countries. Interestingly, only three patients had T1 disease in our study. Similarly, the percentage of stage III and IV disease was 95.3%, which was markedly higher in contrast with other case series [10, 30].
Most studies on PSC have reported that PSC carries a poor prognosis with the caveat that these are small case series or single-institution studies. In one study from the Mayo Clinic, a matched comparison between PSC and typical NSCLC demonstrated that PSC histology was associated with significantly poorer survival than other NSCLC histologies. In this study, the median OS was 9.9, 25.2 and 16.8 months for PSC, adenocarcinoma and squamous cell carcinoma, respectively [9]. Similarly, a study from the Surveillance, Epidemiology and End Results database on PSC also revealed a significantly worse OS for patients with PSC compared with matched NSCLC controls [31]. The survival in our study was markedly lower when compared to other published case series which had reported a median survival range of 10–19 months. The poor survival in our study, when compared to the other case series, may be attributed to a multitude of causes. A high percentage of patients presented with a very advanced stage, with early-stage disease (stage I and II) accounting for only 4.7%. The percentage of patients who could receive curative-intent therapy was also markedly lower in comparison with other reported case series: surgery in 9.3% and CTRT in 3.3% of patients [9, 31]. Other reasons include a higher percentage of patients with poor PS, high nodal stage, higher incidence of brain metastasis, higher rates of upfront BSC, lower incidence of targetable driver mutation and the lower use of ICIs.
There are conflicting reports on the prevalence of EGFR mutation in sarcomatoid carcinoma of the lung. In a study by Jiang et al [32], the EGFR mutation was seen in 9 out of 32 patients (28.1%). In another study of 22 patients by Italiano et al [33], none had EGFR mutations. Likewise, the efficacy of EGFR TKIs in PSC is unknown. Few reports have suggested that PSC with EGFR mutations respond poorly to TKIs [32, 34]. In our study, the frequency of EGFR mutation was 7% (4/57 patients) and two patients had a reasonable response to EGFR TKI. The prevalence of EGFR mutation in lung sarcomatoid carcinoma is lower than that reported in our patients with adenocarcinoma but similar to that reported in our patients with squamous cell lung cancer [35]. Similarly, the prevalence of ALK rearrangement in PSC is not clear [36]. Chen et al [37], in a study of 82 patients with PSC, found that ALK rearrangement was seen in three patients (3.6%). Gelibter et al [38] reported a case of PSC with ALK rearrangement who had a PFS of 15 months with alectinib. Two patients in our study had ALK rearrangement by IHC and the only patient who received crizotinib had a poor response with a PFS of 3 months.
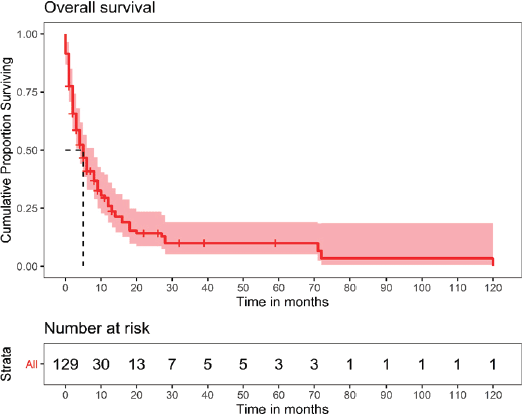
Figure 2. OS of the entire patient cohort.
Table 5. Outcomes for the various treatment modalities in patients with PSC.
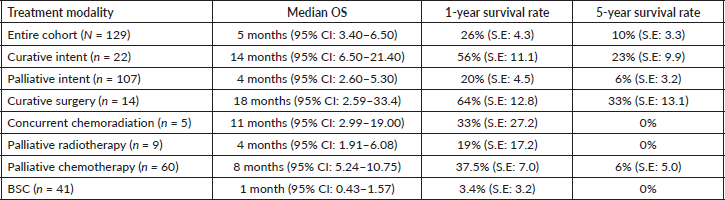
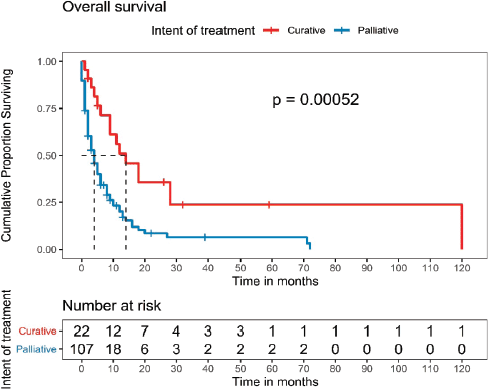
Figure 3. OS of patients according to the intent of treatment.
PSC was historically considered to be resistant to chemotherapy [39–42]. Our study also revealed a poor response of PSC to chemotherapy. The response rates to first-line chemotherapy in our study were 15.2%, 13.3% for the second line, and 0% for the third line. This is similar to a study by Vieira et al [15], in which platinum-based chemotherapy was given to 73% of patients (n = 97), the response rate to chemotherapy was just 16.5% and about 69% of the patients had progressive disease at the time of first evaluation after the initiation of chemotherapy. These rates are much higher when compared with conventional NSCLC. For instance, in a combined analysis of three randomised controlled trials from the Southwest Oncology Group, the response rate to first-line platinum-based chemotherapy was 27% and the progression rate was 38% for NSCLC [43]. Molecular analysis of PSC has revealed a high frequency of mutation in certain genes like TP53 in 60%–80% [20, 44] and KRAS in 30%–35% of the cases [44, 45] and this may be the reason for resistance to chemotherapy [46]. The role of antiangiogenic therapy has been explored in the treatment of PSC. Apatinib, an antiangiogenic TKI which targets the vascular endothelial growth factor receptor 2 (VEGFR2) has been reported to cause a sustained response of 14 months in a case report by LI et al [47]. TP53 mutation has been postulated to be a predictive biomarker of response to antiangiogenic agents [48, 49]. Since these tumours have a high prevalence of TP53 mutations, further studies are required to evaluate the role of VEGF targeting agents.
Very little progress has been made in the treatment of PSC to date. Given the high incidence of KRAS and MET mutation in PSC and with the arrival of novel drugs to target these mutations [50–52], the survival is expected to improve in the future [53, 54]. Bringing down the cost of ICIs would go a long way to improve the outcomes in lower-middle-income countries like India [55]. Ongoing studies in recurrent or metastatic PSC are evaluating the role of dual ICIs like nivolumab + ipilimumab (NCT02834013), durvalumab + tremelimumab (NCT03022500), chemotherapy combined with ICIs (ifosfamide + doxorubicin + durvalumab, NCT04224337), the combination of chemotherapy, ICIs and anti-VEGF agents (toripalimab + bevacizumab + nab-paclitaxel and carboplatin, NCT04725448) and savolitinib in MET exon 14 mutated PSC (NCT02897479).
The limitations of our study include the retrospective nature subject to an inherent bias, a lack of central pathological review and the heterogeneous nature of the chemotherapies used. We also were limited in our ability to perform extensive molecular testing in all patients; hence, we do not have information on the molecular profiles of all the tumours.
Conclusion
PSC presents in advanced stages, has a poor response to the current chemotherapeutic drugs, and is associated with poor survival outcomes. Further studies using molecular characterisation, ICIs and targeted therapy will be indispensable to improve the survival of these patients.
Acknowledgments and funding
No funding or writing assistance was required.
Conflicts of interest
There are no conflicts of interest.
References
1. Baldovini C, Rossi G, and Ciarrocchi A (2019) Approaches to tumor classification in pulmonary sarcomatoid carcinoma Lung Cancer Targets Ther 10 131–149 https://doi.org/10.2147/LCTT.S186779
2. Pelosi G, Sonzogni A, and De Pas T, et al (2010) Review article: pulmonary sarcomatoid carcinomas: a practical overview Int J Surg Pathol 18(2) 103–120 https://doi.org/10.1177/1066896908330049
3. Beasley MB, Brambilla E, and Travis WD (2005) The 2004 World Health Organization classification of lung tumors Semin Roentgenol 40(2) 90–97 https://doi.org/10.1053/j.ro.2005.01.001 PMID: 15898407
4. Rossi G, Cavazza A, and Sturm N, et al (2003) Pulmonary carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements: a clinicopathologic and immunohistochemical study of 75 cases Am J Surg Pathol 27(3) 311–324 https://doi.org/10.1097/00000478-200303000-00004 PMID: 12604887
5. Nemeh F, Kuo AH, and Ross J, et al (2017) The radiologic and pathologic diagnosis of biphasic pulmonary blastoma J Radiol Case Rep 11(9) 10–21
6. Chang YL, Lee YC, and Shih JY, et al (2001) Pulmonary pleomorphic (spindle) cell carcinoma: peculiar clinicopathologic manifestations different from ordinary non-small cell carcinoma Lung Cancer 34(1) 91–97 https://doi.org/10.1016/S0169-5002(01)00224-0 PMID: 11557118
7. Hou J, Xing L, and Yuan Y (2018) A clinical analysis of 114 cases of sarcomatoid carcinoma of the lung Clin Exp Med 18(4) 555–562 https://doi.org/10.1007/s10238-018-0517-2 PMID: 29987681
8. Chaft JE, Sima CS, and Ginsberg MS, et al (2012) Clinical outcomes with perioperative chemotherapy in sarcomatoid carcinomas of the lung J Thorac Oncol 7(9) 1400–1405 https://doi.org/10.1097/JTO.0b013e3182614856 PMID: 22895138 PMCID: 3632635
9. Maneenil K, Xue Z, and Liu M, et al (2018) Sarcomatoid carcinoma of the lung: the mayo clinic experience in 127 patients Clin Lung Cancer 19(3) e323–e333 https://doi.org/10.1016/j.cllc.2017.12.008 PMID: 29454534
10. Venissac N, Pop D, and Lassalle S, et al (2007) Sarcomatoid lung cancer (spindle/giant cells): an aggressive disease? J Thorac Cardiovasc Surg 134(3) 619–623 https://doi.org/10.1016/j.jtcvs.2007.05.031 PMID: 17723808
11. Martin LW, Correa AM, and Ordonez NG, et al (2007) Sarcomatoid carcinoma of the lung: a predictor of poor prognosis Ann Thorac Surg 84(3) 973–980 https://doi.org/10.1016/j.athoracsur.2007.03.099 PMID: 17720411
12. Park JS, Lee Y, and Han J, et al (2011) Clinicopathologic outcomes of curative resection for sarcomatoid carcinoma of the lung Oncology 81(3–4) 206–213 https://doi.org/10.1159/000333095 PMID: 22076573
13. Velcheti V, Rimm DL, and Schalper KA (2013) Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1) J Thorac Oncol 8(6) 803–805 https://doi.org/10.1097/JTO.0b013e318292be18 PMID: 23676558 PMCID: 3703468
14. Kim S, Kim MY, and Koh J, et al (2015) Programmed death-1 ligand 1 and 2 are highly expressed in pleomorphic carcinomas of the lung: comparison of sarcomatous and carcinomatous areas Eur J Cancer 51(17) 2698–2707 https://doi.org/10.1016/j.ejca.2015.08.013 PMID: 26329973
15. Vieira T, Antoine M, and Hamard C, et al (2016) Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1) and strong immune-cell infiltration by TCD3 cells and macrophages Lung Cancer 98 51–58 https://doi.org/10.1016/j.lungcan.2016.05.013 PMID: 27393506
16. Chen P, Yu M, and Zhang JL, et al (2020) Significant benefits of pembrolizumab in treating refractory advanced pulmonary sarcomatoid carcinoma: a case report World J Clin Cases 8(13) 2876–2884 https://doi.org/10.12998/wjcc.v8.i13.2876 PMID: 32742998 PMCID: 7360715
17. Kotlowska MP, Rueda AG, and Olmedo ME, et al (2019) Efficacy of immunotherapy in sarcomatoid lung cancer, a case report and literature review Respir Med Case Rep 26 310–314 PMID: 30931249 PMCID: 6409391
18. Naito M, Tamiya A, and Takeda M, et al (2019) A high PD-L1 expression in pulmonary pleomorphic carcinoma correlates with parietal-pleural invasion and might predict a poor prognosis Intern Med 58(7) 921–927 https://doi.org/10.2169/internalmedicine.1462-18 PMCID: 6478980
19. Sukrithan V, Sandler J, and Gucalp R, et al (2019) Immune checkpoint blockade is associated with durable responses in pulmonary sarcomatoid carcinoma Clin Lung Cancer 20(3) e242–e246 https://doi.org/10.1016/j.cllc.2018.12.013 PMID: 30665873
20. Liang X, Li Q, and Xu B, et al (2019) Mutation landscape and tumor mutation burden analysis of Chinese patients with pulmonary sarcomatoid carcinomas Int J Clin Oncol 24(9) 1061–1068 https://doi.org/10.1007/s10147-019-01454-6 PMID: 31065835
21. Eisenhauer EA, Therasse P, and Bogaerts J, et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer 45(2) 228–247 https://doi.org/10.1016/j.ejca.2008.10.026
22. Chakraborty S (2018) A step-wise guide to performing survival analysis Cancer Res Stat Treat 1(1) 41
23. Dessai S and Patil V (2019) Testing and interpreting assumptions of COX regression analysis Cancer Res Stat Treat 2(1) 108 https://doi.org/10.4103/CRST.CRST_40_19
24. Dessai S, Simha V, and Patil V (2018) Stepwise cox regression analysis in SPSS Cancer Res Stat Treat 1(2) 167
25. Weissferdt A, Kalhor N, and Correa AM, et al (2017) “Sarcomatoid” carcinomas of the lung: a clinicopathological study of 86 cases with a new perspective on tumor classification Hum Pathol 63 14–26 https://doi.org/10.1016/j.humpath.2016.12.010
26. Noronha V, Dikshit R, and Raut N, et al (2012) Epidemiology of lung cancer in India: focus on the differences between non-smokers and smokers: a single-centre experience Indian J Cancer 49(1) 74–81 https://doi.org/10.4103/0019-509X.98925 PMID: 22842172
27. Nishida K, Kobayashi Y, and Ishikawa Y, et al (2002) Sarcomatoid adenocarcinoma of the lung: clinicopathological, immunohistochemical and molecular analyses Anticancer Res 22(6B) 3477–3483
28. Rapicetta C, Lococo F, and Stefani A, et al (2016) Primary sarcomatoid carcinoma of the lung: radiometabolic ((18)F-FDG PET/CT) findings and correlation with clinico-pathological and survival results Lung 194(4) 653–657 https://doi.org/10.1007/s00408-016-9904-1 PMID: 27300448
29. Wu X, Huang Y, and Li Y, et al (2019) 18F-FDG PET/CT imaging in pulmonary sarcomatoid carcinoma and correlation with clinical and genetic findings Ann Nucl Med 33(9) 647–656 https://doi.org/10.1007/s12149-019-01374-5 PMID: 31165974
30. Huang SY, Shen SJ, Li XY (2013) Pulmonary sarcomatoid carcinoma: a clinicopathologic study and prognostic analysis of 51 cases World J Surg Oncol 11 252 https://doi.org/10.1186/1477-7819-11-252 PMID: 24088577 PMCID: 3850921
31. Yendamuri S, Caty L, and Pine M, et al (2012) Outcomes of sarcomatoid carcinoma of the lung: a surveillance, epidemiology, and end results database analysis Surgery 152(3) 397–402 https://doi.org/10.1016/j.surg.2012.05.007 PMID: 22739072
32. Jiang X, Liu Y, and Chen C, et al (2012) The value of biomarkers in patients with sarcomatoid carcinoma of the lung: molecular analysis of 33 cases Clin Lung Cancer 13(4) 288–296. https://doi.org/10.1016/j.cllc.2011.11.004
33. Italiano A, Cortot AB, and Ilie M, et al (2009) EGFR and KRAS status of primary sarcomatoid carcinomas of the lung: implications for anti-EGFR treatment of a rare lung malignancy Int J Cancer 125(10) 2479–2482 https://doi.org/10.1002/ijc.24610 PMID: 19681124
34. Ushiki A, Koizumi T, and Kobayashi N, et al (2009) Genetic heterogeneity of EGFR mutation in pleomorphic carcinoma of the lung: response to gefitinib and clinical outcome Jpn J Clin Oncol [Internet] 39(4) Date accessed: 8/05/21 https://doi.org/10.1093/jjco/hyn155 PMID: 19155283
35. Noronha V, Prabhash K, and Thavamani A, et al (2013) EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy PLoS One 8(4) e61561 https://doi.org/10.1371/journal.pone.0061561 PMID: 23620765 PMCID: 3631198
36. Karim NA, Schuster J, and Eldessouki I, et al (2017) Pulmonary sarcomatoid carcinoma: University of Cincinnati experience Oncotarget 9(3) 4102–4108 https://doi.org/10.18632/oncotarget.23468
37. Chen X, Liang J, and Lu J, et al (2016) Pulmonary sarcomatoid carcinoma with ALK rearrangement: frequency, clinical-pathological characteristics, and response to ALK inhibitor J Clin Oncol [Internet] [https://ascopubs.org/doi/abs/10.1200/JCO.2016.34.15_suppl.e20055] Date accessed: 12/12/20 https://doi.org/10.1200/JCO.2016.34.15_suppl.e20055
38. Gelibter AJ, Siringo M, and Napoli VM, et al J Case Rep Cancer 1 5
39. Hong JY, Choi MK, and Uhm JE, et al (2009) The role of palliative chemotherapy for advanced pulmonary pleomorphic carcinoma Med Oncol 26(3) 287–291 https://doi.org/10.1007/s12032-008-9117-4
40. Ouziane I, Boutayeb S, and Mrabti H, et al (2014) Sarcomatoid carcinoma of the lung: a model of resistance of chemotherapy North Am J Med Sci 6(7) 342–345 https://doi.org/10.4103/1947-2714.136920
41. Ishida T, Tateishi M, and Kaneko S, et al (1990) Carcinosarcoma and spindle cell carcinoma of the lung. Clinicopathologic and immunohistochemical studies J Thorac Cardiovasc Surg 100(6) 844–852. https://doi.org/10.1016/S0022-5223(19)36826-6 PMID: 1701011
42. Yang Z, Xu J, and Li R, et al (2019) PD-L1 and CD47 co-expression in pulmonary sarcomatoid carcinoma: a predictor of poor prognosis and potential targets of future combined immunotherapy J Cancer Res Clin Oncol 145(12) 3055–3065 https://doi.org/10.1007/s00432-019-03023-w PMID: 31522278
43. Lara PN, Redman MW, and Kelly K, et al (2008) Disease control rate at 8 weeks predicts clinical benefit in advanced non–small-cell lung cancer: results from southwest oncology group randomized trials J Clin Oncol 26(3) 463–467 https://doi.org/10.1200/JCO.2007.13.0344 PMID: 18202421
44. Manzotti G, Torricelli F, and Benedetta D, et al (2019) An epithelial-to-mesenchymal transcriptional switch triggers evolution of pulmonary sarcomatoid carcinoma (PSC) and identifies dasatinib as new therapeutic option Clin Cancer Res 25(7) 2348–2360 https://doi.org/10.1158/1078-0432.CCR-18-2364
45. Lococo F, Gandolfi G, and Rossi G, et al (2016) Deep Sequencing analysis reveals that KRAS mutation is a marker of poor prognosis in patients with pulmonary sarcomatoid carcinoma J Thorac Oncol 11(8) 1282–1292 https://doi.org/10.1016/j.jtho.2016.04.020 PMID: 27156442
46. Tao S, Wang S, and Moghaddam SJ, et al (2014) Oncogenic KRAS confers chemoresistance by upregulating NRF2 Cancer Res 74(24) 7430–7441 https://doi.org/10.1158/0008-5472.CAN-14-1439 PMID: 25339352 PMCID: 4268230
47. Li X, He Y, and Zhu J, et al (2018) Apatinib-based targeted therapy against pulmonary sarcomatoid carcinoma: a case report and literature review Oncotarget 9(72) 33734–33738 https://doi.org/10.18632/oncotarget.25989 PMID: 30263099 PMCID: 6154744
48. Koehler K, Liebner D, and Chen JL (2016) TP53 mutational status is predictive of pazopanib response in advanced sarcomas Ann Oncol 27(3) 539–543 https://doi.org/10.1093/annonc/mdv598 PMCID: 5006122
49. Wheler JJ, Janku F, and Naing A, et al (2016) TP53 alterations correlate with response to VEGF/VEGFR inhibitors: implications for targeted therapeutics Mol Cancer Ther 15(10) 2475–2485 https://doi.org/10.1158/1535-7163.MCT-16-0196 PMID: 27466356
50. Hong DS, Fakih MG, and Strickler JH, et al (2020) KRASG12C inhibition with sotorasib in advanced solid tumors N Engl J Med 383(13) 1207–1217 https://doi.org/10.1056/NEJMoa1917239 PMID: 32955176 PMCID: 7571518
51. Wolf J, Seto T, and Han JY, et al (2020) Capmatinib in MET exon 14–mutated or MET-amplified non–small-cell lung cancer N Engl J Med 383(10) 944–957 https://doi.org/10.1056/NEJMoa2002787 PMID: 32877583
52. Paik PK, Felip E, and Veillon R, et al (2020) Tepotinib in non–small-cell lung cancer with MET exon 14 skipping mutations N Engl J Med 383(10) 931–943 https://doi.org/10.1056/NEJMoa2004407 PMID: 32469185 PMCID: 8422679
53. Pelosi G, Scarpa A, and Manzotti M, et al (2004) K-ras gene mutational analysis supports a monoclonal origin of biphasic pleomorphic carcinoma of the lung Mod Pathol 17(5) 538–546 https://doi.org/10.1038/modpathol.3800058 PMID: 14990969
54. Pécuchet N, Vieira T, and Rabbe N, et al (2017) Molecular classification of pulmonary sarcomatoid carcinomas suggests new therapeutic opportunities Ann Oncol Off J Eur Soc Med Oncol 28(7) 1597–1604 https://doi.org/10.1093/annonc/mdx162
55. Mu CY, Huang JA, and Chen Y, et al (2011) High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation Med Oncol 28(3) 682–688 https://doi.org/10.1007/s12032-010-9515-2
Supplementary material
Supplementary Table 1. Univariate and multivariate analyses of factors associated with OS.